Abstract
Over the past several decades, the number of electric vehicles (EVs) has continued to increase. Projections estimate that worldwide, more than 125 million EVs will be on the road by 2030. At the heart of these advanced vehicles is the lithium-ion (Li-ion) battery which provides the required energy storage. This paper presents and compares key components of Li-ion batteries and describes associated battery management systems, as well as approaches to improve the overall battery efficiency, capacity, and lifespan. Material and thermal characteristics are identified as critical to battery performance. The positive and negative electrode materials, electrolytes and the physical implementation of Li-ion batteries are discussed. In addition, current research on novel high energy density batteries is presented, as well as opportunities to repurpose and recycle the batteries.
1. Introduction
Electric vehicles (EVs) were first demonstrated in 1828 [1,2] with the first production electric car introduced in 1884 [3]. These EVs had clear advantages over the competing steam- and gasoline-powered vehicles, such as absence of the loud noise from an un-muffled internal combustion engine, and the difficult starting procedures that in early vehicles required the involvement of specialized staff that would initially heat those engines to operating temperature (the so-called “chauffeur”—i.e., “warmer”) [4].
The inventions of the internal combustion engine muffler is 1897 [5], the electric engine starter in 1911 [6], and the desire for larger vehicle autonomy and faster vehicle re-charging procedures all contributed to internal combustion engine powered vehicles eclipsing the use of EVs except in specialized uses.
Lately, the desire to decrease the negative ramifications associated with the use of internal combustion engine powered transportation [7,8,9], and in particular the drive to decrease carbon emissions [10,11], has led to a significant resurgence in interest in electrified transportation. Of particular importance to enable electrified transportation is the availability of economically and technologically robust batteries.
The Progression of Battery Technologies Used for EV Applications
Many different kinds of batteries exist, and as new systems are developed to commercial maturity, they have been applied to the problem of electrified transportation. A Ragone plot of some of the more common battery technologies is shown in Figure 1 [12,13,14].
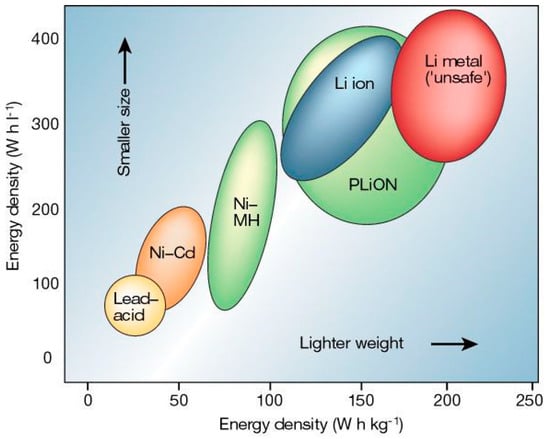
Figure 1.
Ragone plot of several of the battery technologies used in EVs [12].
Early EV applications used the rechargeable Lead-Acid battery developed in 1859 by Gaston Planté [1]. In 1899, Waldemar Jungner introduced the nickel-cadmium battery that made significant improvements in storage capacity but had some drawbacks including a voltage suppression issue that occurs as the battery aged, known as a memory effect [15]. Research continued through the beginning and latter half of the 20th century but it was not until 1985 that the first lithium-ion (Li-ion) batteries were created. It took a further 6 years of research before they were commercialized [15,16]. In the meantime, EVs using ZEBRA batteries and Nickel-Metal Hydride batteries were developed [17]. The current predominant battery energy storage technology for EVs is the Li-ion battery.
Batteries are fundamentally a storage medium made up of two electrodes in an electrolyte. This electrolyte provides a medium for the exchange of ions which produces the electricity [14]. Each of the batteries shown in Figure 1 has their own unique advantages and disadvantages, though recent innovations in Li-ion batteries have propelled them to become the market leader for use in most handheld and portable electronics as well as EVs. This is primarily due to their specific energy (Wh/kg), cycle life and high efficiency [14,15,16,17,18]. They do have downsides which include their high cost and the need for complex safety and monitoring systems [14].
This paper will present an overview of several different types of Li-ion batteries, their advantages, disadvantages, and opportunities with Li-ion energy storage as it relates to EVs. It will conclude with a brief overview of ways to recycle or reuse batteries that have reached their end of life in EVs as well as discuss some additional research opportunities.
2. The Li-Ion Batteries
In general, Li-ion batteries can be characterized as energy storage systems that rely on insertion reactions from both electrodes where lithium ions act as the charge carrier [18]. Given this broad definition, there are several different cell chemistries that make up the Li-ion battery family. Most Li-ion batteries use a negative electrode [19] principally made from carbon (e.g., graphite) or lithium titanate (Li4Ti5O12), with some novel materials under development, namely, Li metal and Li(Si) alloys. The electrolyte used varies based on the choice of electrode materials, but is typically composed of a mixture of lithium salts (e.g., LiPF6) and an organic solvent (e.g., diethyl carbonate) to allow for ion transfer—these components will be discussed in more detail below. A separating membrane is used to allow lithium ions to pass between the electrodes while preventing an internal short circuit [20]. This arrangement is shown conceptually in Figure 2, with the transport aspects of the battery when operating as an energy source (i.e., a galvanic device) illustrated—the electrons travel from the negative electrode to the positive electrode while simultaneously the Li+ ions travel from the negative electrode through the electrolyte to the positive electrode to maintain electroneutrality. When the system is operated in charge mode (i.e., as an electrolytic device) the electron current and Li+ ion flow is reversed.
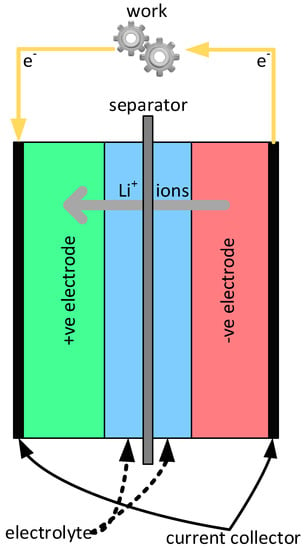
Figure 2.
The schematic construction of a Li-ion battery showing the positive and negative electrodes, the electrode connecting electrolyte that includes a porous separator membrane intended to prevent direct contact between the two electrodes. When the battery acts as a galvanic device, the electrons travel from the negative electrode to a current collector to the load, and then via a second current collector to the positive electrode. Simultaneously, the Li+ ions travel from the negative electrode through the electrolyte to the positive electrode to maintain electroneutrality.
There are therefore many choices of materials for the positive and negative electrode materials, the electrolyte, and the separator. The technological limitations of the various materials are driven by their function, as detailed below.
The electrolyte must offer the highest possible lithium ion transport under use conditions. The batteries must operate in the general environment, likely to extend from, e.g., −30 °C for a vehicle that has been parked for a period of time in extreme cold, to +60 °C for a battery that has heated as a consequence of the combination of environmental conditions and heat generated by charging [21].
The separator must likewise offer the highest possible lithium ion conduction under the same operational conditions and must offer the ability for a rapid thermal shutdown if significant overheating occurs to prevent a thermal runaway process [22,23,24].
A suitable combination of negative and positive electrode materials must exist that leads to a cost-effective high capacity battery. A summary of battery electrode materials and their electrochemical half cell potentials vs. a Li/Li+ reference can be found in Figure 3. The eventual cell voltage is the difference between the chosen pair of electrode materials, and further modified by cell losses, such as necessary overpotentials to achieve current flows, or IR losses due to poor Li+ ion transport through the electrolyte. For example, if LiFePO4 was chosen as the positive half-cell, and Li4Ti5O12 as the negative half-cell, the nominal open circuit voltage would be VOC = V+ − V− = 1.95 V, where V+ represents the half-cell potential of the positive electrode and V− that of the negative electrode.
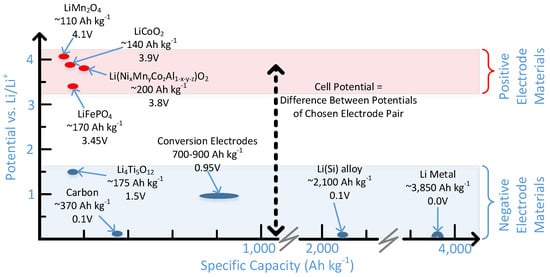
Figure 3.
A summary of some present and future electrode chemistry options for Li-ion batteries. The proposed capacity of the Li(Si) is 50% of the theoretical capacity of the material, similar to the case found for some of the positive electrode materials.
Likewise, the cell capacity is dictated by the specific capacity of each of the electrode materials present in the cell, and the fact that equal capacities are present for the positive and negative electrodes. For example, using the capacities presented in Figure 3 to construct conceptual pairs, and choosing the same LiFePO4 (170 Ah kg−1) paired with Li4Ti5O12 (175 Ah kg−1), assuming an idealized ratio of positive and negative electrode materials in a manner proportionate to their conceptual capacity, the idealized ratio of LiFePO4:Li4Ti5O12 = 170 Ah kg−1:175 Ah kg−1 = 0.97, meaning that an idealized battery with 170 Ah of capacity would contain 1 kg LiFePO4 and 0.97 kg Li4Ti5O12. Therefore, because of the mass of both electrodes combined, and using an approx. 15% weight allowance for the balance of plant shown in Figure 2 (i.e., electrolyte, separator, current collectors) and a battery enclosure, a battery with a specific capacity of ca. ((170 Ah)/(1 kg+0.97 kg))/1.15 = 75 Ah kg−1 would result. Using the same positive electrode paired with a carbon negative electrode (370 Ah kg−1, resulting in an electrode mass ratio of 170 Ah kg−1:370 Ah kg−1 = 0.46), and the same 15% allowance for balance of plant would result in a battery that is about 101 Ah kg−1. The specific energy of the battery would then be obtained by multiplying this resulting capacity by the expected cell voltage for this combination of electrode materials.
Lithium ion batteries have one of the highest coulombic efficiency (CE) ratings in rechargeable batteries (more than 99%). CE describes the charge efficiency by which electrons are transferred in batteries and is the ratio of the total charge extracted from the battery to the total charge put into the battery over a full cycle. The coulombic efficiency of Li-ion batteries improves with cycling. To prove this, Panasonic, E-one Moli, Sony, LG and Samsung Li-ion batteries in 18,650 cell format where cycled. Some cells began with a coulombic efficiency of 99.1% and improved to 99.5% with 15 cycles. Some started at 99.5% and reached 99.9% with 30 cycles. The consistency on repeat tests was high, reflecting in Li-ion being a very stable battery system [25].
2.1. Positive Electrode
Positive electrodes generally are intercalation compounds from which Li+ ions can diffuse out or back in. Well known examples of materials used, as detailed in Figure 3, include LiCoO2 (~140 Ah kg−1, ~3.9 V vs. Li/Li+), LiFePO4 (~170 Ah kg−1, ~3.45 V vs. Li/Li+), Li(Ni1−x−yMnxCoy)O2 (e.g., NMC811 where 1−x−y = 0.8, x = 0.1 and y = 0.1, Li(Ni1−x−yCoxAly)O2 (NCA, ~200 Ah kg−1, ~3.8 V vs. Li/Li+), and LiMn2O4 (~110 Ah kg−1, ~4.1 V vs. Li/Li+) [16]. Individual materials will be discussed briefly below. Generally, the choice of materials is predicated on the desire for battery performance (principally energy/power vs. cycle lifetime and/or safety), and cost.
2.1.1. Lithium Cobalt Oxide (LiCoO2)
First developed by Sony in 1991, the lithium cobalt oxide battery has been the battery of choice for most personal electronics (laptops, cameras, tablets, etc.) due to their high energy density, long life cycle and ease of manufacturing [20]. Lithium cobalt batteries are very reactive and therefore suffer from poor thermal stability and must be monitored during operation to ensure safe use. The limited availability of cobalt also makes it more expensive and difficult to be a viable option for use in EVs. Nevertheless, this high energy dense battery powers the Tesla Roadster and Smart Fortwo electric drive (ED) [26].
2.1.2. Lithium Nickel Oxide (LiNiO2)
LiNiO2 was recognized as a promising material for high voltage batteries (i.e., an approx. 4 V vs. Li/Li+ electrode potential), because it is a lower cost material (it is Co free) and has a high theoretical capacity of 250 Ah kg−1 [27,28,29]. However, difficulties with its use, especially the formation of a self-passivation layer at the surfaces caused difficulties with its use [30]. Because stoichiometric LiNiO2 requires great care in manufacture, and is a somewhat less practical electrode material, solid solutions of this material with Co [31], Fe [32], Mn [33], Al [34], Ti [35], and Mg were developed [36], from which the current “NMC 811” (i.e., “Nickel 0.8 Manganese 0.1 Cobalt 0.1”) and related materials were developed, and discussed below.
2.1.3. Lithium Manganese Oxide (LiMn2O4)
Lithium manganese oxide batteries (LMO) were first introduced in the early 1980’s [37], though they took nearly 15 years to commercialize [16]. The architecture forms a three-dimensional spinel structure that improves ion flow on the electrode, which results in lower internal resistance and improved current handling. Low internal cell resistance enables fast charging and high-current discharging. Li-manganese can be discharged at currents of 20–30 A with moderate heat buildup in an 18,650 package. This chemistry provides better thermal stability than the lithium cobalt oxide battery but results in approximately 33% lower capacity and a lower life span [38].
Most Li-manganese batteries blend with lithium manganese cobalt oxide (NMC) to improve the specific energy and prolong the life span. The LMO-NMC has been used by multiple EV manufacturers in the past including Nissan Leaf, Chevy Volt and BMW i3 [20].
One of the main research efforts in the field of lithium-manganese oxide electrodes for Li-ion batteries involves developing composite electrodes using structurally integrated layered Li2MnO3 and spinel LiMn2O4, with a chemical formula of xLi2MnO3 (1−x)Li1+yMn2−yO4. The combination of both structures provides increased structural stability during electrochemical cycling while achieving higher capacity and rate-capability. A rechargeable capacity in excess of 250 Ah kg−1 was reported in 2005 using this material, which has nearly twice the capacity of current commercialized rechargeable batteries of the same dimensions [39].
2.1.4. Lithium Iron Phosphate (LiFePO4)
In 1996, researchers at the University of Texas in Austin found that phosphate materials could be used in Li-ion battery positive electrodes [40,41]. LiFePO4 (LFP) offers good electrochemical performance with low resistance, besides high current rating and long cycle life [40]. The phosphate helps to stabilize the electrode against overcharging and provides a higher tolerance to heat which limits the breakdown of the material [20]. These batteries have a wide temperature range and can operate between +60 °C to −30 °C and are much less likely to suffer from a thermal runaway [20]. LiFePO4 has a higher self-discharge than other Li-ion batteries, which can cause balancing issues with aging. This can be mitigated through the use of sophisticated control electronics, at increased battery pack cost. Further, moisture seems to significantly limit the lifetime of the battery [40].
With the efficient power-to-weight ratios, high safety features and the chemistry’s resistance to thermal runaway, LiFePO4 batteries are achieving popularity for use in motorhomes. A consortium of German companies (ElektroFahrzeuge Stuttgart and WOF, located in Baden-Wurttemberg, Germany) has teamed up to bring to market the first all-electric motorhome: the Iridium E Mobil, and this electric powertrain includes a lithium iron phosphate battery [42].
2.1.5. Lithium Nickel Manganese Cobalt Oxide (Li(NixMnyCo1−x−y)O2)
Lithium Nickel Manganese Cobalt Oxide (NMC) electrodes can be designed for high specific energy or power with high density. The secret of NMC lies in combining nickel and manganese: nickel is known for its high specific energy but poor stability; manganese has the benefit of forming a spinel structure to achieve low internal resistance but offers a low specific energy [37]. The mix of the various metals (nickel and manganese) varies by manufacturer and is a very closely guarded formula. Researchers are using nickel-rich electrodes to increase energy density, while reduction in cobalt is also helpful since it lowers costs. Companies have switched from NMC111 (discharge capacity: 154 Ah kg−1 at 0.1 C) to NMC442 to NMC622, and now NMC811 (discharge capacity: >185 Ah kg−1 at 0.1 C) is slated for introduction. NMC111 means equal parts nickel, manganese and cobalt [43].
Combining nickel and manganese enhances each other’s strengths, making NMC the most successful Li-ion system and suitable for EV powertrains. These batteries are currently in high demand given the high specific energy and excellent thermal characteristics. As mentioned above, NMC has been used by many EV manufacturers, including Nissan Leaf, Chevy Volt and BMW i3 [20].
2.1.6. Lithium Nickel Cobalt Aluminum Oxide (Li(NixCoyAl1−x−y)O2)
Lithium Nickel Cobalt Aluminum Oxide (NCA) has been around since 1999 for special applications. It shares similarities with NMC by offering high specific energy and specific power (the rate at which the battery can deliver energy), and a long life span [20]. NCA is not as safe as the others listed above and as such, require special safety monitoring measures to be employed for use in EVs. They are also more costly to manufacture, limiting their viability for use in other applications [44].
So far, Tesla is known as the only EV manufacturer who uses NCA chemistry, and claims their NCA battery in production has even less Cobalt than NMC811. The NCA batteries used in the Tesla Model 3 and the first Model S in 2012 had only 15% Cobalt content [45,46].
2.2. Negative Electrode
Two main types of negative electrodes being used include lithium titanate and carbon-based electrodes, and new types of electrodes under development include lithium metal and lithium-metal alloys with a special focus on lithium-silicon alloys, and conversion electrodes [47].
2.2.1. Carbon Based Electrodes
Carbon, and usually synthetic graphite, still remains the active material of choice for the negative electrode, due to its relatively high specific capacity of ~370 Ah kg−1, low average voltage (150 mV vs. Li/Li+) and a relatively flat voltage rendering a high overall cell voltage and high roundtrip energy efficiency [48]. Further, because it is a very abundant, low cost and non-toxic material, it is a particularly good choice of electrode and therefore widely used. Regrettably, under some specific conditions, carbon reacts with atmospheric oxygen, and in the case of a thermal runaway event, the electrode can catch fire.
2.2.2. Lithium Titanate (Li4Ti5O12)
Batteries with lithium titanate negative electrodes have been known since the 1980s. Li-titanate (LTO) replaces the graphite in the negative electrode of a typical Li-ion battery and the material forms into a spinel structure. The counter-electrode can be lithium manganese oxide or NMC [37]. Spinel lithium titanate has been regarded as a highly useful electrode material because of the zero volume change during lithiation, leading to an extremely long operational lifetime for the electrode, coupled with the improved safety owing to an extremely flat discharge and charge plateau at about 1.55 V vs. Li/Li+. This material has low electronic conductivity and the Li+ diffusion coefficient of this material can result in poor performance at high power levels, though this can be improved through the reduction of the lithium ion transport path lengths through proper nanostructuring, and the improvement of the electronic conductivity through doping, surface coating, and forming composites with better electronic conductors such as carbon materials [49].
Titanate batteries are used in certain Japanese-only versions of Mitsubishi’s i-MiEV electric vehicle [50], and Honda uses them in its Fit EV [51]. LTO are also used in the Tosa concept electric bus [52]. Due to their high level of safety, lithium titanate batteries are used in mobile medical devices [53].
2.2.3. Lithium Metal
With a very large capacity (3860 Ah kg−1) and the lowest negative electrochemical potential, it is natural to consider Li metal electrodes for the negative electrode in a Li-ion battery since the significant electrode capacity may decrease the mass of the negative electrode by an order of magnitude, and possibly decrease the mass of the overall battery by about a third. Unfortunately, Li metal electrodes in secondary batteries have proved challenging due to the growth of metallic dendrites during Li plating/stripping, with short circuit caused by the dendrites leading to thermal runaway and a risk of fire/explosion [54]. Nonetheless, research in this electrode option continues [55], and attempts to create safe lithium metal electrodes may eventually prove practical, greatly enhancing the performance of EVs [56,57].
2.2.4. Alloy Based Electrodes
Constructing the negative electrode from metals that electrochemically alloy with lithium at close to room temperature presents opportunities for the creation of Li-ion batteries with higher specific capacity than that offered by conventional graphite electrodes. For this reason, a number of metals and metalloids, like aluminum, tin, and silicon which react with lithium to form alloys by electrochemical processes that are partially reversible, are under study [58]. Unfortunately, the accommodation of so much lithium is accompanied by enormous volume changes in the host metal plus phase transitions. The mechanical strain generated during the alloying/de-alloying processes leads to cracking and crumbling of the metal electrode and a marked loss of capacity to store charge, in the course of a few cycles [59].
2.2.5. Silicon Based Electrodes
The lithium–silicon alloy has, in its fully lithiated composition, Li15Si4, a theoretical specific capacity of 4200 Ah kg−1 which is even higher than the 3860 Ah kg−1 for metallic lithium [60]. The major issue with this electrode chemistry is the significant volumetric change of the electrode material, where the transition between Si and Li15Si4 causes a 280% volumetric change, generating high internal strain in the active materials [61]. The resulting strain leads to cracking and eventual disintegration of the Si material leading to significant reversible capacity fade [62].
Additional drawbacks of Si are a low Li+ diffusion coefficient and high electrical resistivity [63]. Manufacture of composite electrodes composed of nanostructured Si, better able to accommodate volume expansion [64], and using heavily doped Si embedded in conductive matrices appears to lead to significantly improved mechanical and electrical properties [65,66]. The combination of these issues has prevented the practical use of Si-based electrodes in EVs to date, though Si(Li) based battery technology seems to be approaching commercialization [67].
2.2.6. Conversion Electrodes
A different type of electrode material than the lithium intercalation metal oxide based electrodes is the conversion electrode [68,69,70]. In a conversion electrode, an actual chemical reaction takes place, as opposed to the mere intercalation of the Li+ ions into the lattice of a host material. The reaction is generally of the type
where M denotes a transition metal and X an anionic species. Anions such as oxides and sulfides have shown promise as high-theoretical-capacity (generally from 500 to 1500 Ah kg−1) materials [71]. Because of their stability, these electrodes would again add to the safety of the battery system, decreasing the risks associated with thermal runaway, and also decrease the overall mass of the battery.
MaXb + (b × c)Li+ + (b × c)e− ⇄ aM + bLicX
2.3. Electrolytes
As mentioned, the electrolyte is an essential part of the battery, providing ionic conductivity enabling Li+ ions to shuttle between the two electrodes, while not being electronically conductive. Two major classes of electrolytes exist: liquid (aqueous and organic) and solid (polymer and ceramic) [72].
2.3.1. Aqueous Electrolytes
As in all electrochemical systems, the cell potential is limited by the electrochemical window of the electrolyte. Although aqueous electrolytes may conceptually be safer and have lower potential environmental impacts, the restricted electrochemical voltage window (1.23 V) precludes its use in Li-ion batteries—indeed, all negative electrode materials spontaneously react with water to produce free hydrogen. While it has been demonstrated that by creating “water in salt” electrolytes (aqueous mixtures of salts and water at high salt concentrations of the salt, and in which the number of salt particles exceeds the number of water molecules) can increase this electrochemical window and enable demonstration Li-ion batteries, this approach is not close to commercialization [73].
2.3.2. Organic Liquid Electrolytes
In order to achieve a wider electrochemical window, Li-ion battery electrolytes are usually based on an organic solvent loaded with a lithium salt. A common system is the use of an organic carbonate, such as Ethyl carbonate, propylene carbonate, and dimethyl carbonate, with dissolved LiPF6, LiBF4 or LiClO4 [74]. Using appropriate blends of these solvents and salts, electrolytes with good electrochemical stability and with suitably high Li+ ion conductivities of approx. 10 mS/cm are needed for a high-performance battery. A new development in the field is the use of Room Temperature Ionic Liquids (RTILs) which mitigates the flammability and volatility of organic electrolytes issue [75].
2.3.3. Polymer Electrolytes
Because the use of an organic liquid electrolyte poses an environmental contamination risk due to leakage, coupled with a flammability issue [76], the development of solid polymer electrolytes (SPEs) are promising alternatives to enhance the safety performance of batteries [77]. Composite electrolytes based on POE (poly(oxyethylene)) and similar matrices have been developed that can have gel-like (low molecular weight) or solid (high polymer molecular weight) properties for Li-ion cell applications.
2.3.4. Ceramic Electrolytes
Recent advances in battery technology involve using ceramics as the electrolytes, in particular the use of LiSICONs (Lithium Super Ion Conductors) and including glassy materials with similar compositions [78], result in higher conductivities overall due to higher conductivity at grain boundaries. Work to improve the conductivity of ceramic electrolytes to yield materials with performance similar to that of liquid electrolytes continues, with some promising results [79].
2.3.5. Solid Electrolyte Interphase
A final resistance to Li+ ion transport in the batteries that must be considered to understand the performance of the Li-ion battery is the presence of the Solid Electrolyte Interphase (SEI) [80,81]. At the electrode surfaces, this passivation layer (SEI) is formed from decomposition products of the electrolytes. The SEI allows Li+ transport while blocking further electrolyte decomposition. While not completely understood, this nanometer scale SEI film is of paramount importance to the performance of the battery [82], but materials modelling is starting to reveal its nature [83].
2.4. Physical Implementation of Li-Ion Batteries
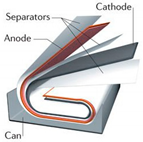
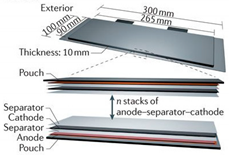
Individual cell design can come in several different forms and shapes that can be seen in Table 1 [18]. This flexibility helps to broaden the uses for Li-ion batteries as they can be designed around multiple different form factors for each specific need [18].

Table 1.
Cell designs and Relative Strengths and Weaknesses. Figures adapted from [84] with permission.
2.5. Comparisons of Different Types of Li-Ion Batteries Used in Electric Vehicles
There are no ideal contenders for the electric powertrain, and Li-ion remains a good choice. Figure 4 graphically compares different types of Li-ion batteries used in EVs considering several characteristics, with the larger colored area being more desirable. The major factors considered are specific energy, specific power, safety, performance, life span, and cost. Specific energy demonstrates how much energy a battery can hold per unit weight, which reflects the driving range. Specific power is the ability to deliver high current on demand and demonstrates potential vehicle acceleration. Safety is naturally one of the most important aspects when choosing a battery for the EV—an incident could significantly affect public opinion. Performance reflects the condition of the battery when driving the EV in extreme temperature conditions. Life Span reflects cycle count and longevity. Cost naturally presents technology feasibility, with necessary ancillary systems for safety, battery management for state of charge status monitoring, climate control for longevity, and the 8–10-year warranty adding to this challenge [26].
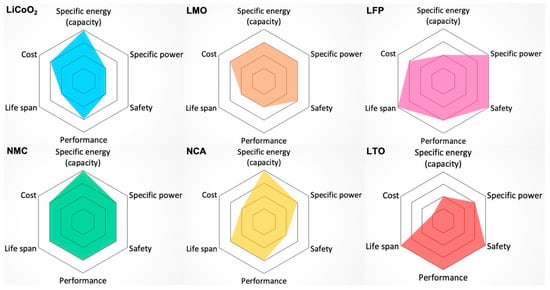
Figure 4.
Comparisons of different types of Li-ion batteries used in EVs from the following perspectives: specific energy (capacity), specific power, safety, performance, life span, and cost (the outer hexagon is most desirable). Lithium Nickel Manganese Cobalt Oxide (NMC), Lithium Iron Phosphate (LiFePO4) and Lithium Manganese Oxide stand out as being superior among these six candidates [26].
3. Li-Ion Battery Lifespan
There are several factors that affect the health and lifespan of Li-ion batteries [85]. The performance degradation of Li-ion batteries can be characterized by the loss of either capacity (i.e., available energy) or power (i.e., reaction rate). Capacity is lost when the active material has been transformed into inactive phases as a result of parasitic chemical reactions [86], though the issue is complicated and not straightforward to model from fundamental approaches [87]. Power is likewise reduced when parasitic reactions occur that convert battery materials to other compounds that act as transport barriers, increasing the cell’s internal impedance, and which in turn reduces the operating voltage at each discharge rate [88,89]. A recent report highlights the high correlation between capacity fade and energy efficiency of the cell (i.e., low hysteresis), presumably due to the low impedance of the solid electrolyte interphase of the new cell, and the rate at which this interphase therefore changes [90,91]. This section reviews some of the key issues that can affect the overall State of Health (SOH) of those batteries, e.g., the ability to deliver power compared to a new pack, and provides a review of several current research areas that help address the issues. Note, SOH is an important indicator of battery functionality that predicts the number of times the battery can be charged and discharged before its life is terminated [92,93].
3.1. Temperature
The temperature of the batteries, especially during charging and discharging is one of the key factors that affect the performance and lifespan of a Li-ion battery [94]. Overheating of the batteries can lead to a thermal runaway where temperatures can reach as high as 500 °C [24]. The thermal runaway of even a single cell can lead to a chain reaction with other cells potentially causing fire and loss of life or property. There have been several high-profile cases of battery fires that have cost companies millions of dollars to rectify [95,96,97].
To improve the lifespan of the battery and solve the greater safety issue, all Li-ion batteries have a battery management system (BMS) which regulates and controls all aspects of the batteries including charging, discharging, and cell equalization and monitoring as well as controlling the overall temperature of the system [20]. In EVs, a BMS can perform data logging, report to a Supervisory Control Module (SCM) and improve battery performance and optimize vehicle operation including the following perspectives: (1) protecting from safety hazards such as fire and shock [98]; (2) maintaining an optimal operating environment (30–40 °C), state of charge (SOC), depth of discharge (DOD), SOH, charge/discharge power and battery cell balancing for the enhancement of the battery life and efficiency [99]; (3) and accurately predicting the remaining driving distance that the battery can support [100]. The components of BMS and their roles are listed below in Table 2.

Table 2.
Components of BMS and their roles [101].
The temperature range for each battery varies based on the chemistry used for that specific battery. Operating the batteries outside of their ideal temperature range can adversely affect the maximum charge capacity and reduce the total number of cycles a battery can provide [102].
To help reduce the drastic change in temperatures experienced during charging and discharging, most EVs employ one or more thermal management systems. These systems can include both passive and active cooling systems and may also include one or more heating sources to warm the batteries in colder climates [20]. These systems are designed to maintain the temperatures of the entire battery system, but can extend to monitoring the temperature of groups of cells or even individual cells to prevent performance degradation, given that failure of a single cell can significantly impair the performance of the entire battery. The BMS adds additional complexity and power demands from the batteries they are maintaining but the benefit far outweighs the additional weight and power costs.
The Open Circuit Voltage (OCV) curve represents the battery’s electrochemical processes and thermodynamics at various SOCs. It is generally a nonlinear monotonic function relating SOC and OCV Li-ion and is hence widely used in battery management systems (BMS) for correcting SOC calculations [103]. The accuracy of the OCV curve has a great influence on the estimations of SOC value and battery capacity [104]. The OCV curve is naturally heavily dependent on the different battery chemistries of various Li-ion batteries, as shown below in Figure 5 [103].
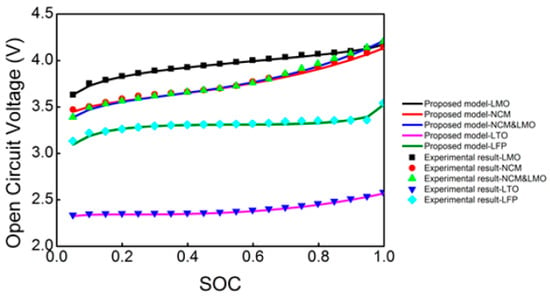
Figure 5.
The estimated and experimental OCV curves for different Li-ion batteries: LiMn2O4 (LMO), Li(NixMnyCo1−x−y)O2 (NCM or LNMCO), NCM&LMO, LiTi5O12 (LTO), LiFePO4 (LFP) [103].
3.2. Charge/Discharge Rate
Most manufacturers recommend a standard charging convention known as constant current, constant voltage (CC-CV). The typical CC-CV charge cycle can be seen in Figure 6 [105]. When charging using this profile, the system provides a constant current until the battery reaches the maximum charging voltage then drops the current to maintain this charging voltage to prevent overcharging of the cells [106].
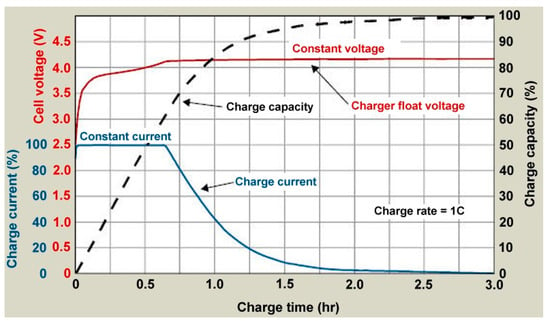
Figure 6.
Example Constant Current, Constant Voltage (CC-CV) battery charging approaches [106].
The ratio at which a battery can be charged or discharged relative to its maximum capacity is called the Crate:
where Pch is the total charge power and Ecap is the total capacity of the battery. If a battery has a capacity of 75 kWh, then at 1 C it would indicate 75 kW for one hour to fully charge the battery [107]. Rated capacity in ampere-hour (Ah) is typically used to describe a battery’s ability to deliver current. For a fixed time period, the higher the Crate, the lower the rated Ah capacity [108].
While the battery is being charged, the charger analyzes the battery condition and adjusts the charge current as needed. An ultra-fast charger may operate at up to 10 C at the start of the charge process, and gradually reduce this to 1 C or even less as the battery approaches the normal operating voltage. This additional current during the initial charge time increases the stress on the battery which increases the battery temperature. The goal is to maintain a normal working temperature and avoid lithium plating of the electrodes. As such, it is recommended to use ultra-fast chargers only when necessary [109].
Some research has been done by Abdullah, et al. [106] into different charge profiles that change the profile of the CC-CV standard charging profile by adding resting periods to the process. This process does add additional time to the charge cycle but helps to reduce stress on the cells by reducing temperature fluctuations during the charging process [106]. An alternative approach to achieve very fast re-energizing of electric vehicles is through the use of the “battery swap” approach, in which the batteries are physically removed and a freshly recharged pack mounted on the vehicle, which also results in not requiring stressing the batteries by charging at accelerated rates [110].
3.3. Charge/Discharge Depth
From [111], an EV Li-ion battery has reached the end of its useful life when it will no longer charge above 80% of its original capacity [112]. While Li-ion batteries are not as prone to the “memory effect” of older battery types, the depth of discharge (DoD) can affect the total number of charge cycles that a battery can accommodate during its useful life. DOD is an alternate method to indicate a battery’s state of charge (SOC). The DOD is the complement of SOC: as one increases, the other decreases. Just as mechanical wear on gears and pistons will shorten the life of the part, so does the level to which the battery is discharged due to the secondary chemical processes taking place within the battery during the discharge and subsequent charge cycle [112]. Table 3 shows the number of cycles that can be expected from a typical Li-ion battery [113] with 100% DoD indicating a full drain and charge of the battery.

Table 3.
Cycle life 1 as a function of Depth of Discharge [114].
To help maintain the battery life, manufacturers have typically added software controls into their BMS to mitigate this issue by not allowing the battery to be drained or charged beyond specific set points. This allows a bit of an emergency “limp home” power should the driver need it but helps to control and extend the battery life [114].
3.4. Additonal Ways to Extend the Life of Li-Ion Batteries
It is also possible to improve on the design of the electrodes and increase the battery capacity reviewing the design and/or size of the materials used for the construction of the battery.
Within Li-ion batteries, the chemical reactions take place at the electrodes. By reducing the internal resistance of the electrodes, it is possible to reduce the heat generated during use, and therefore increase the cell capacity and lifespan. Zhao, et al. [94] noted in their research that the electrode sizes could be reduced to improve efficiency. They found that there were limits to the reduction, such as if the electrode thickness fell below 130 μm then the battery was no longer able to meet the needed energy specifications. There are additional tradeoffs with the thinner material size including an increased cost of manufacturing. Zhao, et al. recommended that the electrode size can be included as a key design factor in the overall design of the entire system [94].
Another area of improvement can be made in the construction of the electrodes. By modifying the material as they are produced, it is possible to optimize the transport distances, or route the ion travel to improve the overall conductivity and thermal performance [25]. This can be done by coating, doping or adding deposits to the electrodes as they are being produced. In some cases, these changes have had the added benefit of improving the strength of the material as well as improving thermal efficiency or overall capacity. Materials added to the electrodes have included gold, graphene, yttrium, and zinc among others. Many different factors affect this process and further research in the area is ongoing [25].
4. Recycling and Repurposing
With the large increase in battery-powered devices and vehicles over the past 20 years, efforts have been made to determine ways to reduce or reuse the batteries and components.
4.1. Recycling
Older lead-acid battery recycling programs have been in place for many years. The recycling rate for these batteries in the US alone is almost 99%, in part due to existing laws and the existence of the infrastructure to collect the used batteries as part of the sale of replacements [115]. The use of Li-ion batteries is still relativity new and therefore the infrastructure is not yet in place to match the high success rate of lead-acid batteries. It is possible to recycle many of the materials used in the electrode production with some studies showing nearly a 96% recovery rate for the copper used as part of the batteries [116].
A recent study on the economic viability of recycling Li-ion batteries found that while pyrometallurgical and/or hydrometallurgical recycling processes, which reduce cells back to their elemental products, do not show significant carbon emissions advantages beyond the mere economic advantage of the recycling process, direct material recycling, where the positive electrode material is reconditioned for use in new batteries and which requires only a fraction of the energy required by the metallurgical processes, has the potential to also significantly reduce emissions [117].
The European Union has target recycling rates of 65% for lead-acid, 75% for nickel-cadmium and 50% for all other batteries. As of 2013, Unicom, a company based out of Belgium, has been able to achieve effective recycling of 60% for the steel casings and 51% for synthetic cased batteries which is encouraging but additional work and research needs to be done in this area [118].
4.2. Repurposing for Power Grid
Another promising research topic involves reusing the existing batteries from EVs to provide additional stability and redundancy to the existing utility grids. These second-life batteries are taken from the vehicles when they are no longer able to be charged above 70–80% of their rated capacity [108], tested and re-built to provide modules that can be deployed to supplement wind, solar, or other areas of heavy grid usage. These batteries can help augment the generation capability and provide needed load shaping during times of limited production [111]. Second-life batteries will not have the original full capacity and would need to be monitored by BMS systems but are able to provide peak load shifting and stabilization for stationary applications where the higher capacity required for EVs is not needed.
5. Conclusions
This paper has provided an overview of Li-ion batteries as a method for energy storage for EVs. Different materials for positive and negative electrodes, various types of electrolytes and the physical implementation of Li-ion batteries are presented and compared, and components of battery management systems are described. The performance of existing lithium batteries is heavily dependent on material and thermal characteristics. As discussed, most of the heat from the battery is generated at the electrodes and additional research in various cooling methods and electrode design criteria is needed to reduce or compensate for the heat, therefore, improving the battery life and capacity. As EV batteries reach the end of their useful life, research is showing the different approaches to repurpose them as a supplement to the existing power grid or recycle the battery materials when they are no longer viable.
Author Contributions
Conceptualization, P.H., A.v.J. and A.Y.; methodology, P.H., A.v.J. and A.Y.; validation, P.H., A.v.J., Y.M. and A.Y.; data curation, Y.M. and A.Y.; Writing—Original Draft preparation, P.H.; Writing—Review and Editing, Y.M., A.v.J. and A.Y.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References and Note
- Morimoto, M. Which is the first electric vehicle? Electr. Eng. Jpn. 2015, 192, 31–38. [Google Scholar] [CrossRef]
- History of the Electric Vehicle. Available online: https://en.wikipedia.org/wiki/History_of_the_electric_vehicle (accessed on 16 February 2019).
- World’s First Electric Car Built by Victorian Inventor in 1884. Available online: https://www.telegraph.co.uk/news/newstopics/howaboutthat/5212278/Worlds-first-electric-car-built-by-Victorian-inventor-in-1884.html (accessed on 16 February 2019).
- Thomas, C. Objects to “Chauffeur”. The New York Times. Available online: https://timesmachine.nytimes.com/timesmachine/1902/01/22/101930350.pdf (accessed on 11 February 2019).
- Reeves, M.O.; Reeves, M.T. Exhaust Muffler for Engines. U.S. Patent 582485A, 5 November 1897. [Google Scholar]
- Kettering, C.F. Engine Starting, Lighting and Ignition System. U.S. Patent 1254811A, 29 January 1918. [Google Scholar]
- Mitropoulos, L.K.; Prevedouros, P.D.; Kopelias, P. Total cost of ownership and externalities of conventional, hybrid and electric vehicle. Transp. Res. Proc. 2017, 24, 267–274. [Google Scholar] [CrossRef]
- Maciel, M.; Rosa, L.; Correa, F.; Maruyama, U. Energy, pollutant emissions and other negative externality savings from curbing individual motorized transportation (IMT): A low cost, low technology scenario analysis in Brazilian urban areas. Energies 2012, 5, 835–861. [Google Scholar] [CrossRef]
- Holzman, D.C. Driving up the cost of clean air. Environ. Health Perspect. 2005, 113, A246–A249. [Google Scholar] [CrossRef]
- Samaras, C.; Meisterling, K. Life cycle assessment of greenhouse gas emissions from plug-in hybrid vehicles: Implications for policy. Environ. Sci. Technol. 2008, 42, 3170–3176. [Google Scholar] [CrossRef] [PubMed]
- Holdway, A.R.; Williams, A.R.; Inderwildi, O.R.; King, D.A. Indirect emissions from electric vehicles: Emissions from electricity generation. Energy Environ. Sci. 2010, 3, 1825–1832. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Ragone, D. Review of battery systems for electrically powered vehicles. SAE Tech. Pap. 1968. [Google Scholar] [CrossRef]
- Keshan, H.; Thornburg, J.; Ustun, T.S. Comparison of lead-acid and lithium ion batteries for stationary storage in off-grid energy systems. In Proceedings of the 4th IET Clean Energy and Technology Conference (CEAT 2016), Kuala Lumpur, Malaysia, 14–15 November 2016; Institution of Engineering and Technology (IET): Kuala Lumpur, Malaysia, 2016; pp. 1–7. [Google Scholar]
- Rechargeable Battery. Available online: https://en.wikipedia.org/wiki/Rechargeable_battery (accessed on 17 February 2019).
- Tarascon, J.M. The Li-Ion Battery: 25 Years of Exciting and Enriching Experiences. Electrochem. Soc. Interface 2016, 25, 79–83. [Google Scholar] [CrossRef]
- Sudworth, J.L. The sodium/nickel chloride (ZEBRA) battery. J. Power Sources 2001, 100, 149–163. [Google Scholar] [CrossRef]
- Horiba, T. Li-ion battery systems. Proc. IEEE 2014, 102, 939–950. [Google Scholar] [CrossRef]
- Because the role of anode and cathode reverses between charging (electrolytic) and discharging (galvanic) modes, we will address the electrodes in the context of this paper as the positive electrode and the negative electrode. In some work the positive electrode is generally named the cathode, and the negative electrode the anode regardless of the battery operating mode.
- Hannan, M.A.; Hoque, M.M.; Hussain, A.; Yusof, Y.; Ker, P.J. State-of-the-art and energy management systems of Li-ion batteries in EV applications: Issues and recommendations. IEEE Access 2018, 6, 19362–19378. [Google Scholar] [CrossRef]
- Tesla. Model S Owner’s Manual. Version 2018.48.12. Available online: https://www.tesla.com/sites/default/files/model_s_owners_manual_north_america_en_us.pdf (accessed on 11 February 2019).
- Arora, P.; Zhang, Z. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, M.F.; Zahn, R.; Wood, V. Characterization and performance evaluation of Li-ion battery separators. Nat. Energy 2019, 4, 16–25. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A critical review of thermal issues in Li-ion batteries. J. Electrochem. Soc. 2011, 158, R1–R25. [Google Scholar] [CrossRef]
- BU-808c: Coulombic and Energy Efficiency with the Battery. Available online: https://batteryuniversity.com/learn/article/bu_808c_coulombic_and_energy_efficiency_with_the_battery (accessed on 26 February 2019).
- Is Li-Ion the Solution for the Electric Vehicle? Available online: https://batteryuniversity.com/learn/archive/is_li_ion_the_solution_for_the_electric_vehicle (accessed on 5 May 2019).
- Yoon, C.S.; Jun, D.-W.; Myung, S.-T.; Sun, Y.-K. Structural Stability of LiNiO2 Cycled above 4.2 V. ACS Energy Lett. 2017, 2, 1150–1155. [Google Scholar] [CrossRef]
- Kalyani, P.; Kalaiselvi, N.; Muniyandi, N. Microwave assisted synthesis of LiNiO2—A preliminary investigation. J. Power Sources 2003, 123, 53–60. [Google Scholar] [CrossRef]
- Dahn, J.R.; von Sacken, U.; Juzkow, M.W.; Al-Janaby, H. Rechargeable LiNiO2/Carbon Cells. J. Electrochem. Soc. 1991, 138, 2207–2211. [Google Scholar] [CrossRef]
- Muto, S.; Sasano, Y.; Tatsumi, K.; Sasaki, T.; Horibuchi, K.; Takeuchi, Y.; Ukyo, Y. Capacity-Fading Mechanisms of LiNiO2-Based Li-ion Batteries II. Diagnostic Analysis by Electron Microscopy and Spectroscopy. J. Electrochem. Soc. 2009, 156, A371–A377. [Google Scholar] [CrossRef]
- Delmas, C.; Saadoune, I.; Rougier, A. The Cycling Properties of the LixNi1–yCoyO2 Electrode. J. Power Sources 1993, 44, 595–602. [Google Scholar] [CrossRef]
- Reimers, J.N.; Rossen, E.; Jones, C.D.; Dahn, J.R. Structure and Electrochemistry of LixFeyNi1-yO2. Solid State Ion. 1993, 61, 335–344. [Google Scholar] [CrossRef]
- Rossen, E.; Jones, C.D.W.; Dahn, J.R. Structure and Electrochemistry of LixMnyNi1−yO2. Solid State Ion. 1992, 57, 311–318. [Google Scholar] [CrossRef]
- Ohzuku, T.; Ueda, A.; Kouguchi, M. Synthesis and Characterization of LiAl1/4Ni3/4O2(R3m) for Li-ion (Shuttlecock) Batteries. J. Electrochem. Soc. 1995, 142, 4033–4039. [Google Scholar] [CrossRef]
- Subramanian, V.; Fey, G.T.-K. Preparation and Characterization of LiNi0.7Co0.2Ti0.05M0.05O2 (M = Mg, Al and Zn) Systems as Cathode Materials for Lithium Batteries. Solid State Ion. 2002, 148, 351–358. [Google Scholar] [CrossRef]
- Pouillerie, C.; Croguennec, L.; Delmas, C. The LixNi1–yMgyO2 (y = 0.05, 0.10) System: Structural Modifications Observed upon Cycling. Solid State Ion. 2000, 132, 15–29. [Google Scholar] [CrossRef]
- Thackeray, M.M.; David, W.I.F.; Bruce, P.G.; Goodenough, J.B. Lithium insertion into manganese spinels. Mater. Res. Bull. 1983, 18, 461–472. [Google Scholar] [CrossRef]
- BU-205: Types of Li-Ion. Available online: https://batteryuniversity.com/learn/article/types_of_lithium_ion (accessed on 5 May 2019).
- Johnson, C.S.; Li, N.; Vaughey, J.T.; Hackney, S.A.; Thackeray, M.M. Lithium-manganese oxide electrodes with layered-spinel composite structures xLi2MnO3∙(1 − x)Li1+yMn2−yO4 (0 < x < 1, 0 ≤ y ≤ 0.33) for lithium batteries. Electrochem. Commun. 2005, 7, 528–536. [Google Scholar] [CrossRef]
- Sun, C.; Rajasekhara, S.; Goodenough, J.B.; Zhou, F. Monodisperse porous LiFePO4 microspheres for a high power Li-ion battery cathode. J. Am. Chem. Soc. 2011, 133, 2132–2135. [Google Scholar] [CrossRef]
- Harrison, K.L.; Bridges, C.A.; Paranthaman, M.P.; Segre, C.U.; Katsoudas, J.; Maroni, V.A.; Idrobo, J.C.; Goodenough, J.B.; Manthiram, A. Temperature dependence of aliovalent-vanadium doping in LiFePO4 cathodes. Chem. Mater. 2013, 25, 768–781. [Google Scholar] [CrossRef]
- All-electric Iridium E Mobil Motorhome Is Coming to Market. Available online: https://electrek.co/2018/12/10/electric-motorhome-iridium-e-mobil/ (accessed on 5 May 2019).
- Exciting Developments in NMC811 Lithium Battery Technology. Available online: https://cleantechnica.com/2018/03/04/exciting-developments-nmc-811-lithium-battery-technology/ (accessed on 5 May 2019).
- Six Li-ion Battery Chemistries: Not All Batteries are Created Equal. Available online: https://www.powerelectronics.com/alternative-energy/six-Li-ion-battery-chemistries-not-all-batteries-are-created-equal (accessed on 5 May 2019).
- Why Tesla’s Grid Batteries Will Use Two Different Chemistries. Available online: http://fortune.com/2015/05/18/tesla-grid-batteries-chemistry/ (accessed on 11 February 2019).
- Tesla Panasonic Quietly Outmaneuver All Lithium Battery Manufacturers. Available online: https://insideevs.com/tesla-panasonic-quietly-outmaneuvers-all-lithium-battery-manufacturers/ (accessed on 5 May 2019).
- Kinoshita, K.; Zaghib, K. Negative Electrodes for Li-ion Batteries. J. Power Sources 2002, 110, 416–423. [Google Scholar] [CrossRef]
- Mao, C.; Wood, M.; David, L.; An, S.J.; Sheng, Y.; Du, Z.; Meyer, H.M., III; Ruther, R.E.; Wood, D.L., III. Selecting the Best Graphite for Long-Life, High-Energy Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A1837–A1845. [Google Scholar] [CrossRef]
- Shuai, C. Self-supported Li4Ti5O12 nanosheet arrays for lithium ion batteries with excellent rate capability and ultralong cycle life. Energy Environ. Sci. 2014, 7, 1924–1930. [Google Scholar] [CrossRef]
- Mitsubishi Choose Super-Efficient Toshiba SCiB Battery for EVs. Available online: https://integrityexports.com/blog/mitsubishi-chooses-toshiba-scib-battery-for-evs/ (accessed on 5 May 2019).
- Toshiba’s SCiB Battery for the Fit EV. Available online: https://www.greencarcongress.com/2011/11/scib-20111117.html (accessed on 5 May 2019).
- Auge, O. TOSA concept: A full electric large capacity urban bus system. In Proceedings of the 17th European Conference on Power Electronics and Applications (EPE’15 ECCE-Europe), Geneva, Switzerland, 8–10 September 2015. [Google Scholar]
- All About Batteries, Part 12: Lithium Titanate (LTO). Available online: https://www.eetimes.com/author.asp?section_id=36&doc_id=1325358# (accessed on 5 May 2019).
- Zhang, J.; Xu, W.; Henderson, W.A. Lithium Metal Anodes and Rechargeable Lithium Metal Batteries; Springer: Basel, Switzerland, 2016; pp. 5–43. [Google Scholar]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.-G. Lithium Metal Anodes for Rechargeable Batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, Q. Dendrite-Free Lithium Metal Anodes: Stable Solid Electrolyte Interphases for High-Efficiency Batteries. J. Mater. Chem. A 2015, 3, 7207–7209. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, F.; Lee, C.-S.; Tang, Y. Low-Cost Metallic Anode Materials for High Performance Rechargeable Batteries. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Nazar, L.F.; Crosnier, O. Anodes and Composite Anodes: An Overview. In Lithium Batteries Science and Technology; Nazri, G.-A., Pistoia, G., Eds.; Springer: New York, NY, USA, 2004; pp. 112–143. [Google Scholar]
- Lai, S.-C. Solid Lithium-Silicon Electrode. J. Electrochem. Soc. 1976, 123, 1196–1197. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Christensen, L. Structural Changes in Silicon Anodes during Lithium Insertion/Extraction. Electrochem. Solid-State Lett. 2004, 7, A93–A96. [Google Scholar] [CrossRef]
- Gu, M.; He, Y.; Zheng, J.M.; Wang, C.M. Nanoscale silicon as anode for Li-ion batteries: The fundamentals, promises, and challenges. Nano Energy 2015, 17, 366–383. [Google Scholar] [CrossRef]
- Ding, N.; Xu, J.; Yao, Y.X.; Wegner, G.; Fang, X.; Chen, C.H.; Lieberwirth, I. Determination of the Diffusion Coefficient of Lithium Ions in Nano-Si. Solid State Ion. 2009, 180, 222–225. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-Dependent Fracture of Silicon Nanoparticles during Lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Domi, Y.; Usui, H.; Yamaguchi, K.; Yodoya, S.; Sakaguchi, H. Silicon-Based Anodes with Long Cycle Life for Li-ion Batteries Achieved by Significant Suppression of their Volume Expansion in Ionic-Liquid Electrolyte. ACS Appl. Mater. Interfaces 2019, 11, 2950–2960. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Krause, L.J. Reversible Cycling of Crystalline Silicon Powder. J. Electrochem. Soc. 2007, 154, A103–A108. [Google Scholar] [CrossRef]
- Schneider, D. To Boost Li-ion Battery Capacity by up to 70%, Add Silicon. 2019. Available online: https://spectrum.ieee.org/energy/renewables/to-boost-lithiumion-battery-capacity-by-up-to-70-add-silicon (accessed on 12 February 2019).
- Yu, S.-H.; Feng, X.; Zhang, N.; Seok, J.; Abruña, H.D. Understanding Conversion-Type Electrodes for Lithium Rechargeable Batteries. Acc. Chem. Res. 2018, 51, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, Q.; Sun, D.; Li, N.; Ju, H.; Feng, J.; Zhu, J.; Mai, L.; Cairns, E.J.; Guo, J. Conversion reaction of vanadium sulfide electrode in the Li-ion cell: Reversible or not reversible? Nano Energy 2018, 51, 391–399. [Google Scholar] [CrossRef]
- Ren, Q.-Q.; Wang, Z.-B.; Ke, K.; Zhang, S.-W.; Yin, B.-S. NiCO2O4 nanosheets and nanocones as additive-free anodes for high-performance Li-ion batteries. Ceram. Int. 2017, 43, 13710–13716. [Google Scholar] [CrossRef]
- Yu, S.-H.; Lee, S.H.; Lee, D.J.; Sung, Y.-E.; Hyeon, T. Conversion Reaction-Based Oxide Nanomaterials for Lithium Ion Battery Anodes. Small 2016, 12, 2146–2172. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2016, 2. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous Li-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Younesi, R.; Veith, G.M.; Johansson, P.; Edström, K.; Vegge, T. Lithium salts for advanced lithium batteries: Li–metal, Li–O2, and Li–S. Energy Environ. Sci. 2015, 8, 1905–1922. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Howlett, P.C.; Kar, M.; Passerini, S.; Pringle, J.M.; Ohno, H.; Watanabe, M.; Yan, F.; Zheng, W.; et al. Ionic liquids and their solid-state analogues as materials for energy generation and storage. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Grugeon, S.; Laruelle, S.; Boyanov, S.; Lecocq, A.; Bertrand, J.-P.; Marlair, G. In-depth safety-focused analysis of solvents used in electrolytes for large scale lithium ion batteries. Phys. Chem. Chem. Phys. 2013, 15, 9145–9155. [Google Scholar] [CrossRef] [PubMed]
- Que, M.; Tong, Y.; Wei, G.; Yuan, K.; Wei, J.; Jiang, Y.; Zhu, H.; Chen, Y. Safe and flexible ion gel based composite electrolyte for lithium batteries. J. Mater. Chem. A 2016, 4, 14132–14140. [Google Scholar] [CrossRef]
- Zheng, F.; Masashi Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state Li-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Kamaya, N. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Peled, E.; Golodnitsky, D.; Ardel, G. Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes. J. Electrochem. Soc. 1997, 144, L208–L210. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Levi, M.D.; Levi, E.; Schechter, A.; Moshkovich, M.; Cohen, Y. New insights into the interactions between electrode materials and electrolyte solutions for advanced nonaqueous batteries. J. Power Sources 1999, 81, 95–111. [Google Scholar] [CrossRef]
- Winter, M. The Solid Electrolyte Interphase—The Most Important and the Least Understood Solid Electrolyte in Rechargeable Li Batteries. Z. Phys. Chem. 2009, 223, 1395–1406. [Google Scholar] [CrossRef]
- Wang, A.; Kadam, S.; Li, H.; Shi, S.; Qi, Y. Review on modeling of the anode solid electrolyte interphase (SEI) for Li-ion batteries. Comput. Mater. 2018, 4, 15. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-Li-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
- Palacín, M.R.; de Guibert, A. Why do batteries fail? Science 2016, 351. [Google Scholar] [CrossRef]
- Hall, J.; Lin, T.; Brown, G.; Biensan, P.; Bonhomme, F. Decay Processes and Life Predictions for Lithium Ion Satellite Cells. In Proceedings of the 4th International Energy Conversion Engineering Conference and Exhibit (IECEC), San Diego, CA, USA, 26–29 January 2006; pp. 2006–4078. [Google Scholar]
- El Mejdoubi, A.; Chaoui, H.; Gualous, H.; Van Den Bossche, P.; Omar, N.; Van Mierlo, J. Li-ion Batteries Health Prognosis Considering Aging Conditions. IEEE Trans. Power Electron. 2019. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C. Hammouche Ageing mechanisms in Li-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Broussely, M.; Biensan, P.; Bonhomme, F.; Blanchard, P.; Herreyre, S.; Nechev, K.; Staniewicz, R.J. Main aging mechanisms in Li ion batteries. J. Power Sources 2005, 146, 90–96. [Google Scholar] [CrossRef]
- Redondo-Iglesias, E.; Venet, P.; Pelissier, S. Efficiency Degradation Model of Li-ion Batteries for Electric Vehicles. IEEE Trans. Ind. Appl. 2019. [Google Scholar] [CrossRef]
- Lin, Y.-X.; Liu, Z.; Leung, K.; Chen, L.-Q.; Lu, P.; Qi, Y. Connecting the irreversible capacity loss in Li-ion batteries with the electronic insulating properties of solid electrolyte interphase (SEI) components. J. Power Sources 2016, 309, 221–230. [Google Scholar] [CrossRef]
- Rahimi-Eichi, H.; Ojha, U.; Baronti, F.; Chow, M.-Y. Battery management system: An overview of its application in the smart grid and electric vehicles. IEEE Ind. Electron. Mag. 2013, 7, 4–16. [Google Scholar] [CrossRef]
- Topan, P.A.; Ramadan, M.N.; Fathoni, G.; Cahyadi, A.I.; Wahyunggoro, O. State of charge (SOC) and state of health (SOH) estimation on lithium polymer battery via Kalman filter. In Proceedings of the 2nd International Conference on Science and Technology-Computer (ICST), Yogyakarta, Indonesia, 27–28 October 2016; pp. 93–96. [Google Scholar]
- Zhao, R.; Zhang, S.; Liu, J.; Gu, J. A review of thermal performance improving methods of lithium ion battery: Electrode modification and thermal management system. J. Power Sources 2015, 299, 557–577. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Boeing 787 Dreamliner Battery Problems. Available online: https://en.wikipedia.org/wiki/Boeing_787_Dreamliner_battery_problems (accessed on 17 February 2019).
- Plug-in Electric Vehicle Fire Incidents. Available online: https://en.wikipedia.org/wiki/Plug-in_electric_vehicle_fire_incidents (accessed on 17 February 2019).
- Burke, A.; Jungers, B.; Yang, C.; Ogden, J. Battery Electric Vehicles: An Assessment of the Technology and Factors Influencing Market Readiness; Advanced Energy Pathway (AEP) Project; Public Interest Energy Research (PIER) Program California Energy Commission: Sacramento, CA, USA, 2007.
- Haiying, W.; Feng, W.; Ying, F.; Ran, L.; Qian, Z. Study on key technologies of lithium battery for electric vehicle. In Proceedings of the 6th International Forum on Strategic Technology (IFOST), Harbin, China, 22–24 August 2011; pp. 291–294. [Google Scholar] [CrossRef]
- Corrigan, D.A.; Masias, A. Batteries for electric and hybrid vehicles. In Linden’s Handbook of Batteries, 4th ed.; Reddy, T.B., Ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Cheng, K.W.E.; Divakar, B.P.; Wu, H.; Ding, K.; Ho, H.F. Battery-management system (BMS) and SOC development for electric vehicles. IEEE Trans. Veh. Technol. 2011, 60, 76–88. [Google Scholar] [CrossRef]
- Leng, F.; Tan, C.M.; Pecht, M. Effect of Temperature on the Aging rate of Li Ion Battery Operating above Room Temperature. Sci. Rep. 2015, 5, 12967. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, J.; Zhang, L.; Liu, S.; Wang, L.; Loh, P.C. A generalized SOC-OCV model for Li-ion batteries and the SOC estimation for LNMCO battery. Energies 2016, 9, 900. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.Y.; Li, X.; Chen, W.; Yin, G.; Jiang, J. Robust and adaptive estimation of state of charge for Li-ion batteries. IEEE Trans. Ind. Electron. 2015, 62, 4948–4957. [Google Scholar] [CrossRef]
- BU-409: Charging Li-Ion. Available online: https://batteryuniversity.com/learn/article/charging_lithium_ion_batteries (accessed on 17 February 2019).
- Al-karakchi, A.A.; Lacey, G.; Putrus, G. A method of electric vehicle charging to improve battery life. In Proceedings of the 50th International Universities Power Engineering Conference (UPEC), Stoke on Trent, UK, 1–4 September 2015; pp. 1–3. [Google Scholar] [CrossRef]
- Battery Lifetime: How Long Can Electric Vehicle Batteries Last? Available online: https://cleantechnica.com/2016/05/31/battery-lifetime-long-can-electric-vehicle-batteries-last/ (accessed on 17 February 2019).
- BU-402: What Is C-Rate? Available online: https://batteryuniversity.com/learn/article/what_is_the_c_rate (accessed on 26 February 2019).
- Collin, R.; von Jouanne, A.; Yokochi, A.; Enjeti, P. Advanced Electric Vehicle Fast-charging Technologies. Energies 2019, in press. [Google Scholar]
- Adegbohun, J.; von Jouanne, A.; Lee, K. Autonomous Battery Swapping System and Methodologies of Electric Vehicles. Energies 2019, 12, 667. [Google Scholar] [CrossRef]
- Abdel-Monem, M.; Hegazy, O.; Omar, N.; Trad, K.; Van den Bossche, P.; Van Mierlo, J. Li-ion batteries: Comprehensive technical analysis of second-life batteries for smart grid applications. In Proceedings of the 19th European Conference on Power Electronics and Applications (EPE’17 ECCE Europe), Warsaw, Poland, 11–14 September 2017; pp. 1–16. [Google Scholar]
- Croy, J.R.; Balasubramanian, M.; Gallagher, K.G.; Burrell, A.K. Review of the U.S. Department of Energy’s “Deep Dive” Effort to Understand Voltage Fade in Li- and Mn-Rich Cathodes. Acc. Chem. Res. 2015, 48, 2813–2821. [Google Scholar] [CrossRef]
- BU-808: How to Prolong Lithium-Based Batteries. Available online: https://batteryuniversity.com/learn/article/how_to_prolong_lithium_based_batteries (accessed on 17 February 2019).
- EV Tech Explained: Why Do EVs Restrict the Amount of Battery Capacity That Can Be Used for Driving? Available online: https://chargedevs.com/newswire/ev-tech-explained-why-do-evs-restrict-the-amount-of-battery-capacity-that-can-be-used-for-driving/ (accessed on 17 February 2019).
- Lead–Acid Battery. Available online: https://en.wikipedia.org/wiki/Lead–acid_battery (accessed on 17 February 2019).
- Zhou, X.; He, W.-Z.; Li, G.-M.; Zhang, X.-J.; Zhu, S.-G.; Huang, J.W.; Zhu, S.-G. Recycling of Electrode Materials from Spent Li-ion Batteries. In Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Ciez, R.E.; Whitacre, J.F. Examining different recycling processes for Li-ion batteries. Nat. Sustain. 2019, 2, 148–156. [Google Scholar] [CrossRef]
- Tytgat, J. The recycling efficiency of Li-ion EV batteries according to the European Commission regulation, and the relation with the end-of-life vehicles directive recycling rate. In Proceedings of the World Electric Vehicle Symposium and Exhibition (EVS27), Barcelona, Spain, 17–20 November 2013; pp. 1–9. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).