The Influence of Fe on the Structure and Hydrogen Sorption Properties of Ti-V-Based Metal Hydrides
Abstract
1. Introduction
2. Results and Discussion
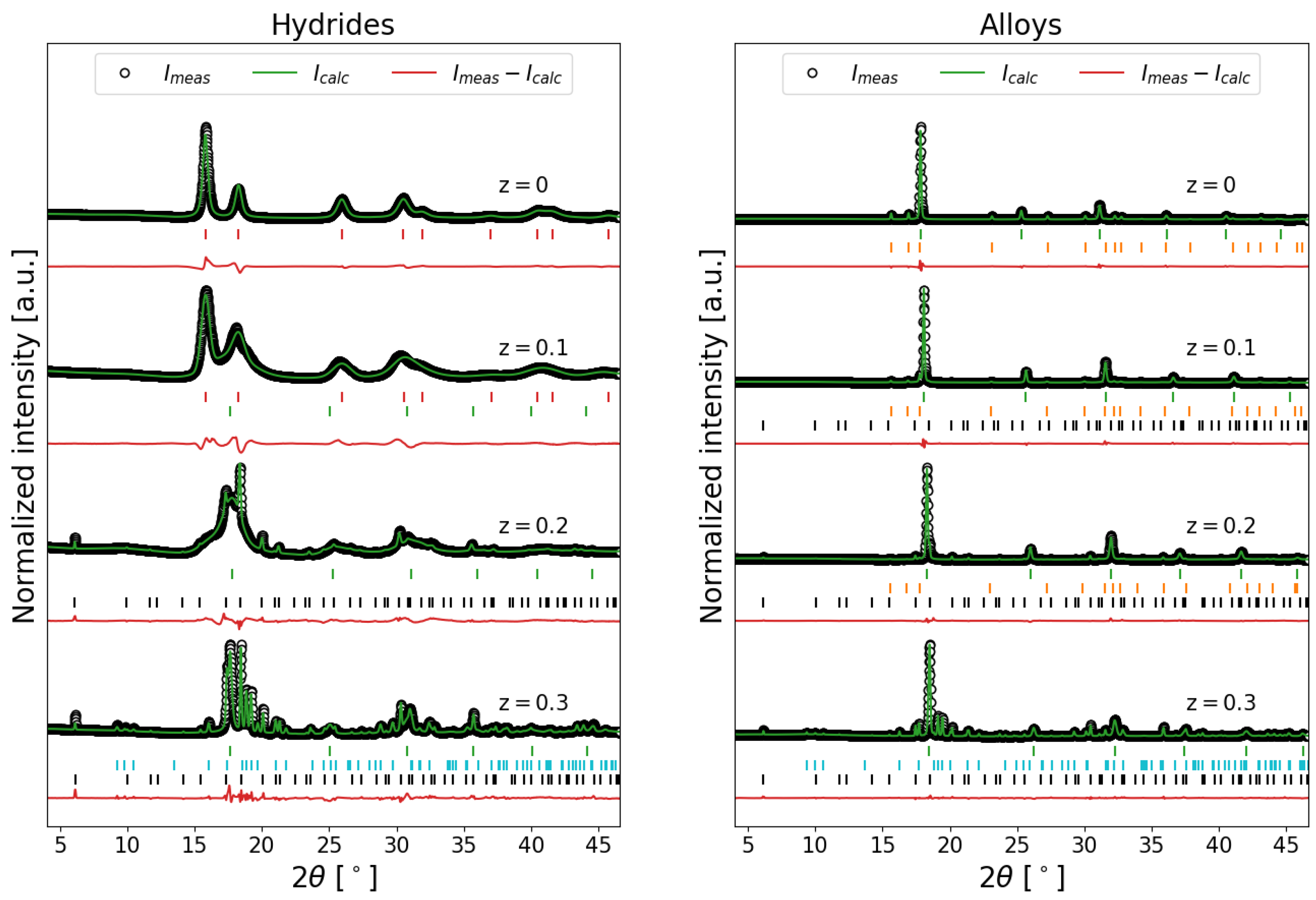
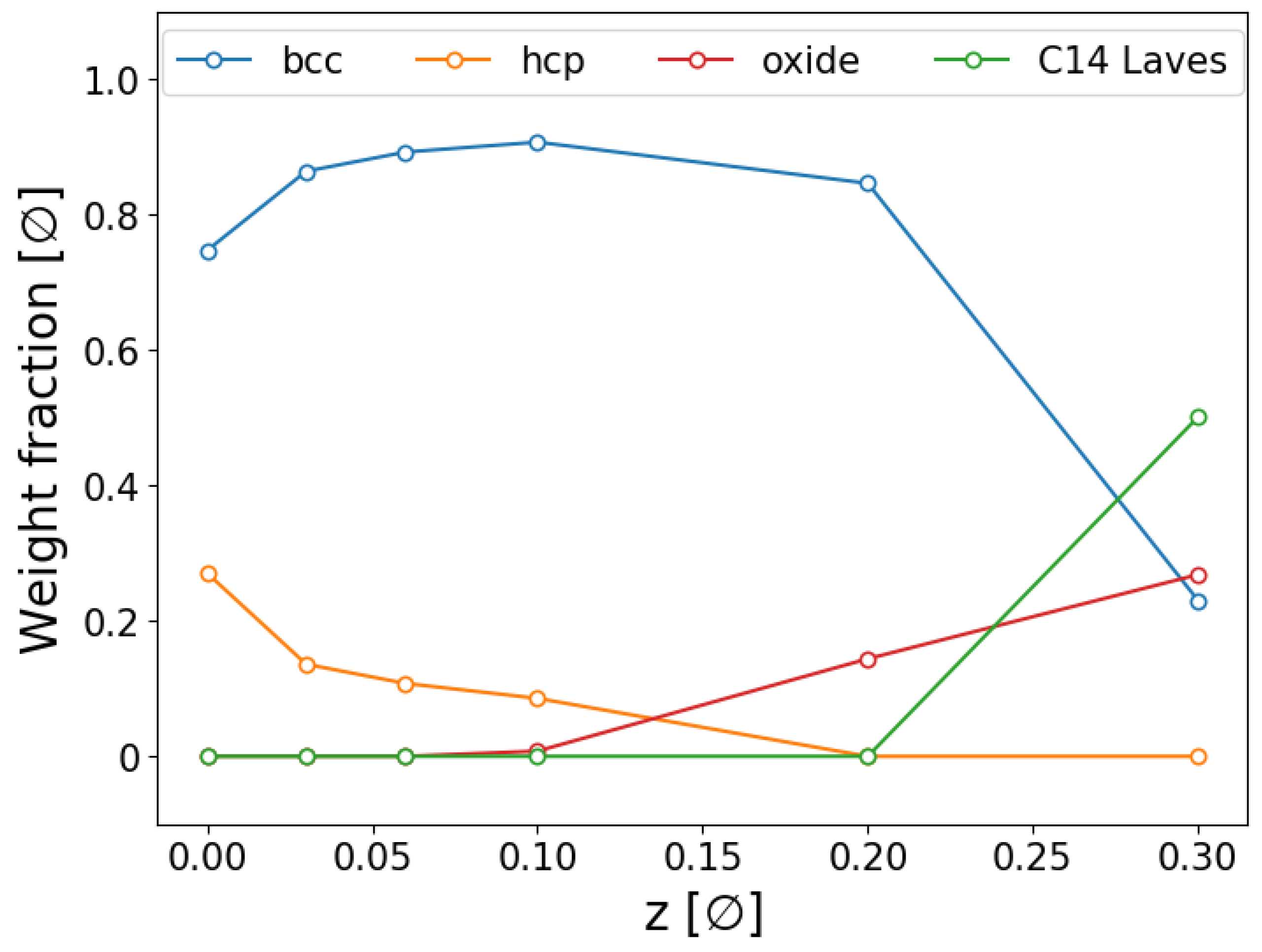
2.1. Ex Situ SR-PXD Structural Analysis
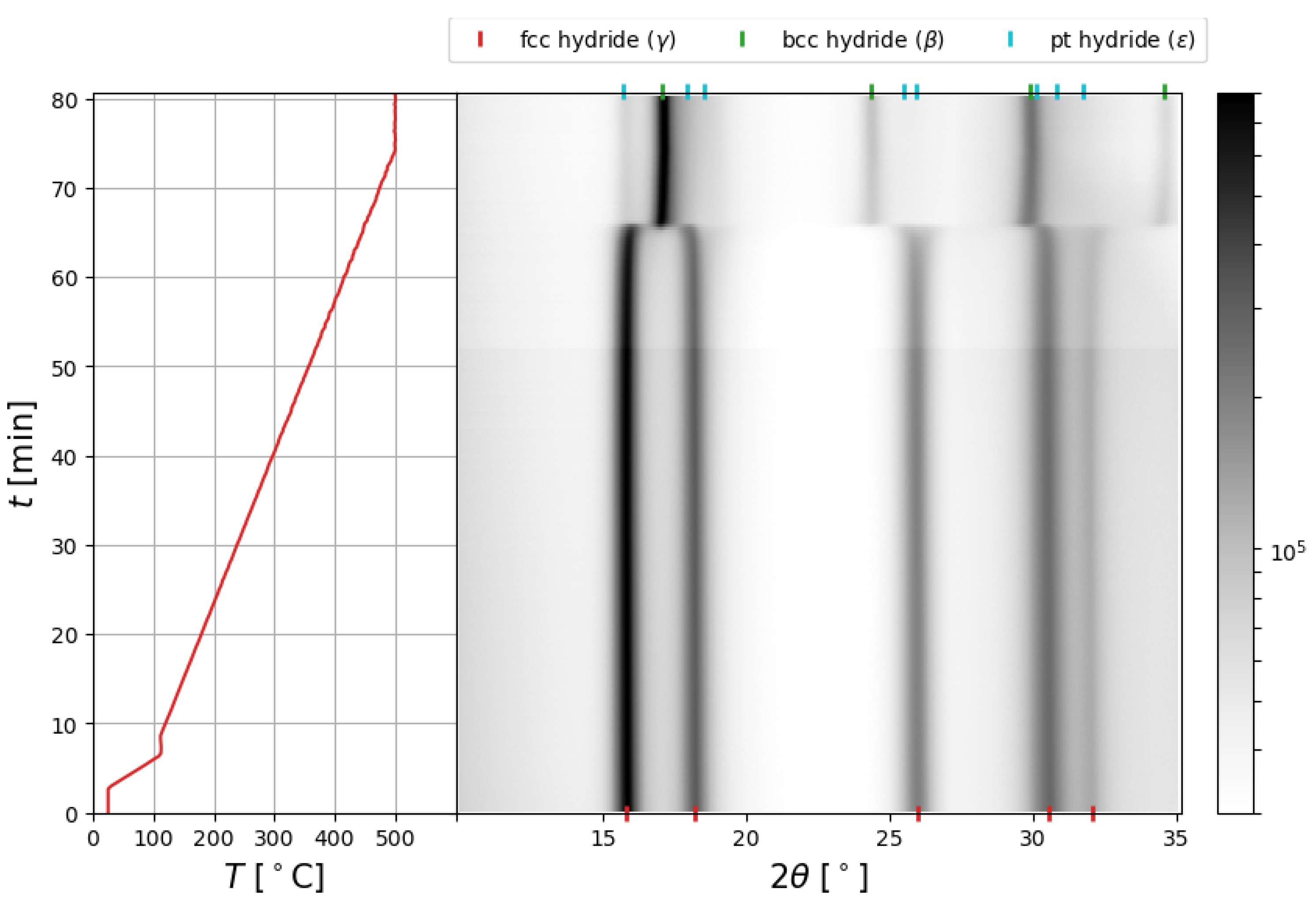
2.2. In Situ SR-PXD Analysis
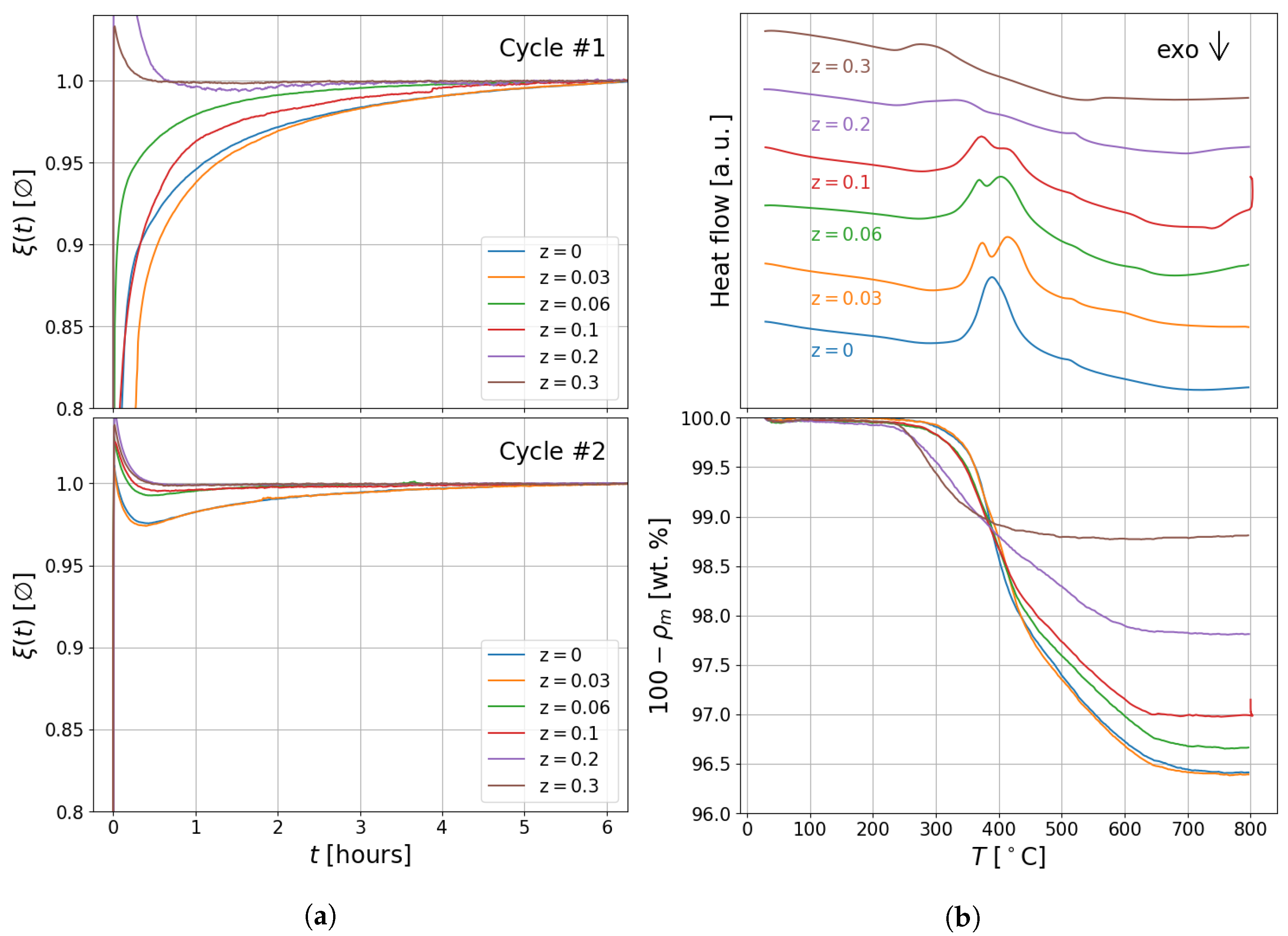
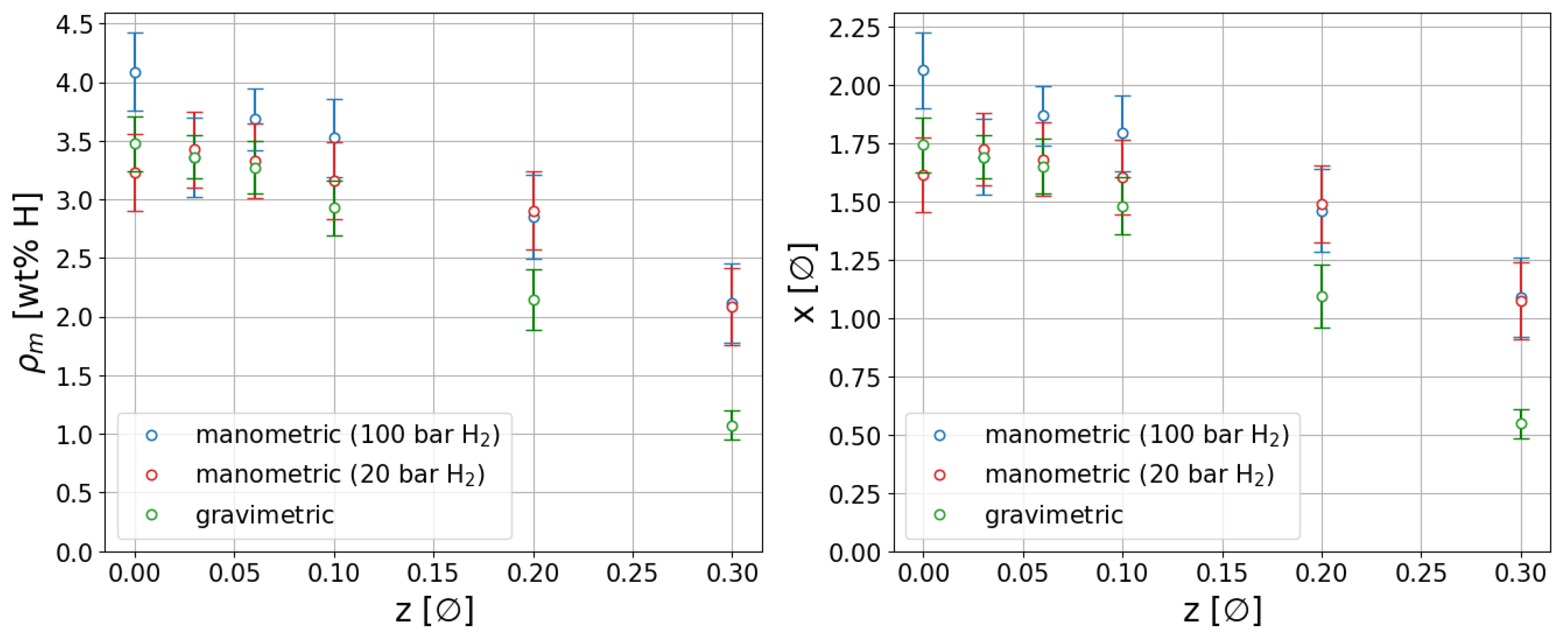
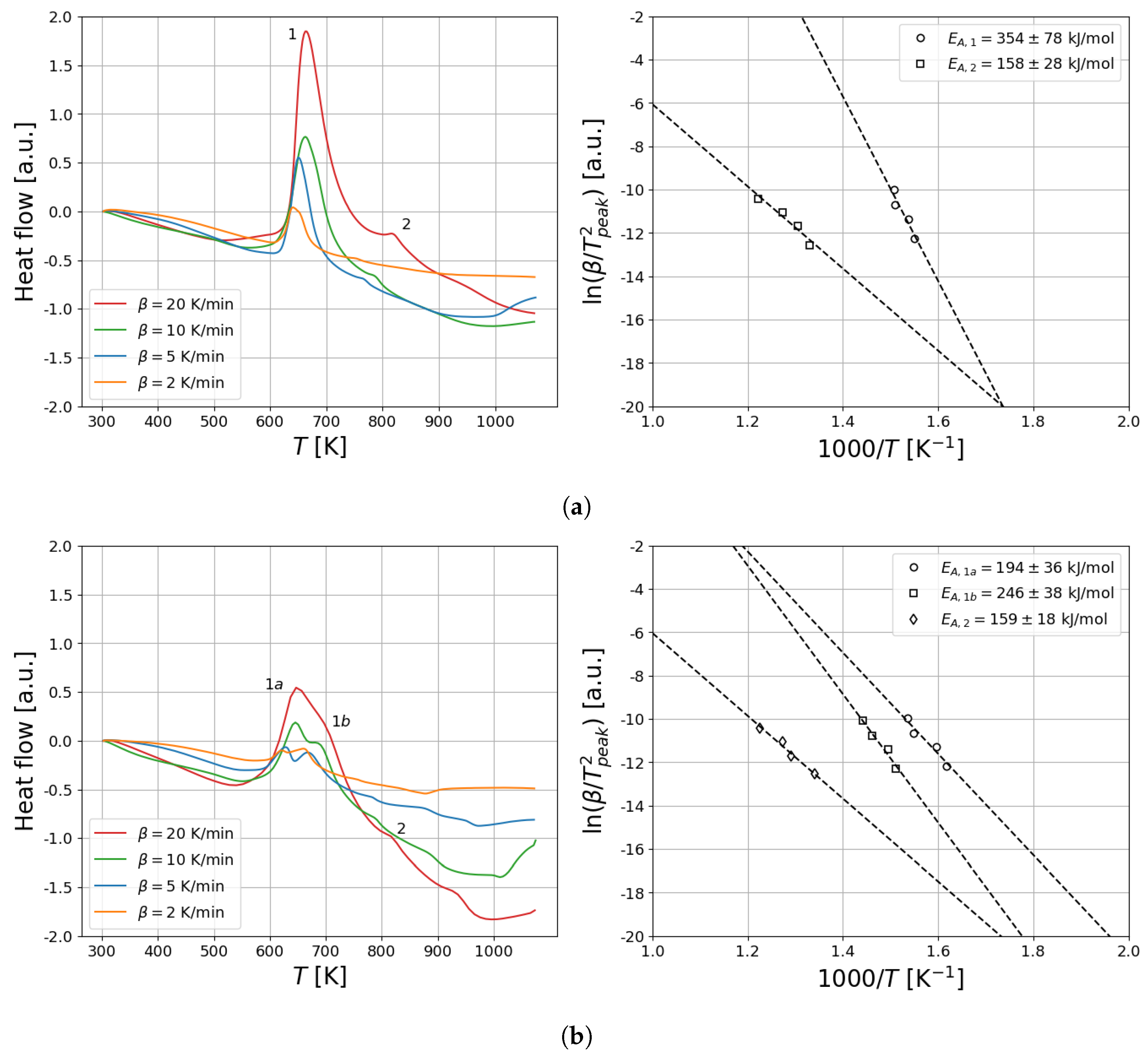
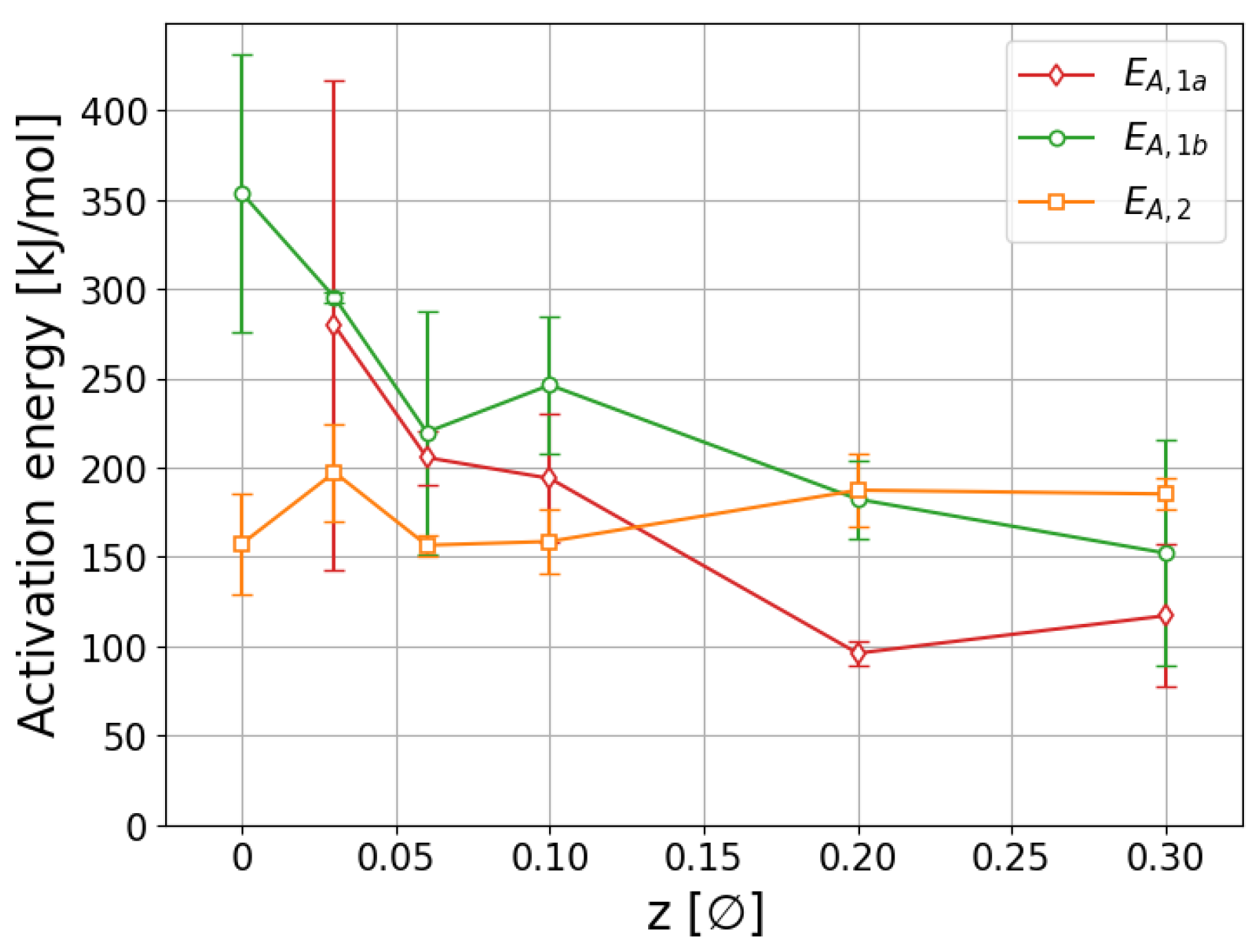
2.3. Thermal Analysis and Sieverts Measurements
3. Materials and Methods
3.1. Synthesis
3.2. SR-PXD Experiments
3.3. Thermal Analysis
3.4. Sieverts Measurements
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohtadi, R.; Orimo, S.-I. The renaissance of hydrides as energy materials. Nat. Rev. Mater 2016, 2, 1–15. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Bellosta von Colbe, J.M.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage—Past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Milanese, C.; Jensen, T.R.; Hauback, B.C.; Pistidda, C.; Dornheim, M.; Yang, H.; Lombardo, L.; Züttel, A.; Filinchuk, Y.; Ngene, P.; et al. Complex hydrides for energy storage. Int. J. Hydrog. Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef]
- Yartys, V.A.; Lototskyy, M.V.; Akiba, E.; Albert, R.; Antonov, V.E.; Ares, J.R.; Baricco, M.; Bourgeois, N.; Buckley, C.E.; Bellosta von Colbe, J.M.; et al. Magnesium based materials for hydrogen based energy storage: Past, present and future. Int. J. Hydrog. Energy 2019, 44, 7809–7859. [Google Scholar] [CrossRef]
- Maeland, A.J.; Gibb, T.R.P.; Schumacher, D.P. A novel hydride of vanadium. J. Am. Chem. Soc. 1961, 83, 3728–3729. [Google Scholar] [CrossRef]
- Maeland, A.J. Investigation of the vanadium-hydrogen system by X-ray diffraction techniques. J. Am. Chem. Soc. 1961, 68, 2197–2200. [Google Scholar]
- Nagel, H.; Perkins, R.S. Crystallographic investigation of ternary titanium vanadium hydrides. Z. Für Met. 1961, 66, 362–366. [Google Scholar]
- Hagi, T.; Sato, Y.; Yasuda, M.; Tanaka, K. Structure and phase diagram of Ti−V−H system at room temperature. Trans. Jpn. Inst. Met. 1987, 28, 198–204. [Google Scholar] [CrossRef]
- Kagawa, A. Absorption of hydrogen by vanadium-titanium alloys. Rep. Fac. Eng. Nagasaki Univ. 1995, 25, 233–239. [Google Scholar]
- Kumar, S.; Jain, A.; Ichikawa, T.; Kojima, Y.; Dey, G.K. Development of vanadium based hydrogen storage material: A review. Renew. Sustain. Energy Rev. 2017, 72, 791–800. [Google Scholar] [CrossRef]
- Maeland, A.J.; Libowitz, G.G.; Lynch, J.F.; Rak, G. Hydride formation rates of bcc group V metals. J. Less Common Met. 1984, 104, 133–139. [Google Scholar] [CrossRef]
- Maeland, A.J.; Libowitz, G.G.; Lynch, J.P. Hydride formation rates of titanium-based bcc solid solution alloys. J. Less Common Met. 1984, 104, 361–364. [Google Scholar] [CrossRef]
- Lynch, J.F.; Maeland, A.J.; Libowitz, G.G. Lattice parameter variation and thermodynamics of dihydride formation in the vanadium-rich V−Ti−Fe/H2 system. Z. Für Phys. Chem. 1985, 145, 51–59. [Google Scholar] [CrossRef]
- Kagawa, A.; Ono, E.; Kusakabe, T.; Sakamoto, Y. Absorption of hydrogen by vanadium-rich V−Ti-based alloys. J. Less Common Met. 1991, 172, 64–70. [Google Scholar] [CrossRef]
- Mauroy, H.; Klyukib, K.; Shelyapina, M.G.; Thøgersen, A.; Keen, D.; Hauback, B.C.; Sørby, M.H. Short-range structure of Ti0.63V0.27Fe0.10D1.73 from neutron total scattering and Reverse Monte Carlo modelling. Energies 2020, 13, 1947. [Google Scholar] [CrossRef]
- Wood, R.M. The lattice constants of high purity alpha titanium. Proc. Phys. Soc. 1962, 80, 783. [Google Scholar] [CrossRef]
- Makinson, J.D.; Lee, J.S.; Magner, S.H.; De Angelis, R.J.; Weins, W.N.; Hieronymus, A.S. X-ray diffraction signatures of defects in nanocrystalline materials. Adv. X-ray Anal. 2000, 42, 407–411. [Google Scholar]
- Balogh, L.; Ribárik, G.; Ungár, T. Stacking faults and twin boundaries in fcc crystals determined by X-ray diffraction profile analysis. J. Appl. Phys. 2006, 100, 023512. [Google Scholar] [CrossRef]
- Matsuda, J.; Akiba, E. Lattice defects in V−Ti bcc alloys before and after hydrogenation. J. Alloys Compd. 2013, 581, 369–372. [Google Scholar] [CrossRef]
- Numakura, H.; Koiwa, M. Hydride precipitation in titanium. Acta Metall. 1984, 32, 1799–1807. [Google Scholar] [CrossRef]
- Irving, P.E.; Beevers, C.A. Some metallographic and lattice parameter observations on titanium hydride. Metall. Trans. 1971, 2, 613–615. [Google Scholar] [CrossRef]
- Ma, M.; Liang, L.; Wang, L.; Wang, Y.; Cheng, Y.; Tang, B.; Xiang, W.; Tan, X. Phase transformations of titanium hydride in thermal desorption process with different heating rates. Int. J. Hydrog. Energy 2015, 40, 8926–8934. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, L.; Liu, S. Effect of surface morphology on initial hydrogen diffusion in vanadium alloys. Mater. Lett. 2019, 241, 100–103. [Google Scholar] [CrossRef]
- Suwarno, S.; Solberg, J.K.; Maehlen, J.P.; Krogh, B.; Yartys, V.A. Influence of Cr on the hydrogen storage properties of Ti-rich Ti−V−Cr alloys. Int. J. Hydrog. Energy 2012, 37, 7624–7628. [Google Scholar] [CrossRef]
- Brinks, H.W.; Fossdal, A.; Bowman, R.C.; Hauback, B.C. Pressure-composition isotherms of TbNiAlHx. J. Alloys Compd. 2006, 417, 92–95. [Google Scholar] [CrossRef]
- Dyadkin, V.; Pattison, P.; Dmitriev, V.; Chernyshov, D. A new multipurpose diffractometer PILATUS @ SNBL. J. Synchrotron Radiat. 2016, 23, 825–829. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. General Structure Analysis System (GSAS); Los Alamos National Laboratory: Santa Fe, NM, USA, 1994; pp. 86–748.
- Toby, B.H. EXPGUI, a graphical user interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general—Purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
| z | VEC | [Å] | [Å] | [Å] | [Å] | [Å] |
|---|---|---|---|---|---|---|
| 0 | 3.18291(11) | 2.95913(12) | 4.7365(2) | - | - | |
| 0.03 | 3.17578(11) | 2.95945(15) | 4.7385(3) | - | - | |
| 0.06 | 3.16162(8) | 2.96280(14) | 4.7469(3) | - | - | |
| 0.1 | 3.14036(10) | 2.96392(19) | 4.7487(5) | - | - | |
| 0.2 | 3.10136(10) | - | - | - | - | |
| 0.3 | 3.07307(8) | - | - | 4.93242(12) | 8.0163(3) |
| z | VEC | [Å] | [Å] | [Å] | [Å] |
|---|---|---|---|---|---|
| 0 | 4.39387(18) | - | - | - | |
| 0.03 | 4.3903(2) | - | - | - | |
| 0.06 | 4.3904(4) | - | - | - | |
| 0.1 | 4.3918(4) | 3.220(3) | - | - | |
| 0.2 | - | 3.1889(6) | - | - | |
| 0.3 | - | 3.2145(2) | 4.9962(2) | 8.1543(7) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nygård, M.M.; Sørby, M.H.; Grimenes, A.A.; Hauback, B.C. The Influence of Fe on the Structure and Hydrogen Sorption Properties of Ti-V-Based Metal Hydrides. Energies 2020, 13, 2874. https://doi.org/10.3390/en13112874
Nygård MM, Sørby MH, Grimenes AA, Hauback BC. The Influence of Fe on the Structure and Hydrogen Sorption Properties of Ti-V-Based Metal Hydrides. Energies. 2020; 13(11):2874. https://doi.org/10.3390/en13112874
Chicago/Turabian StyleNygård, Magnus M., Magnus H. Sørby, Arne A. Grimenes, and Bjørn C. Hauback. 2020. "The Influence of Fe on the Structure and Hydrogen Sorption Properties of Ti-V-Based Metal Hydrides" Energies 13, no. 11: 2874. https://doi.org/10.3390/en13112874
APA StyleNygård, M. M., Sørby, M. H., Grimenes, A. A., & Hauback, B. C. (2020). The Influence of Fe on the Structure and Hydrogen Sorption Properties of Ti-V-Based Metal Hydrides. Energies, 13(11), 2874. https://doi.org/10.3390/en13112874






