Ammonia Borane: An Extensively Studied, Though Not Yet Implemented, Hydrogen Carrier
Abstract
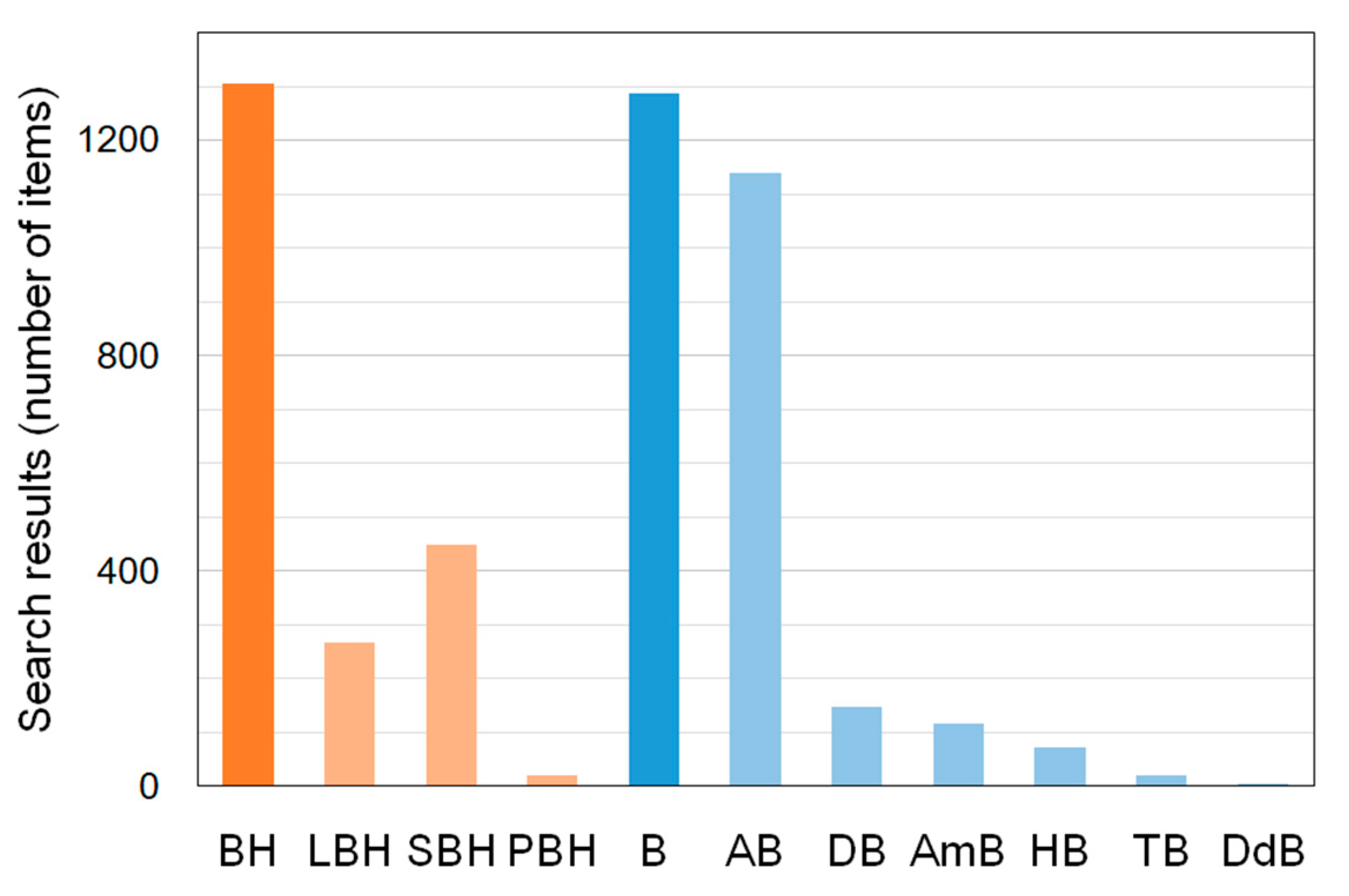
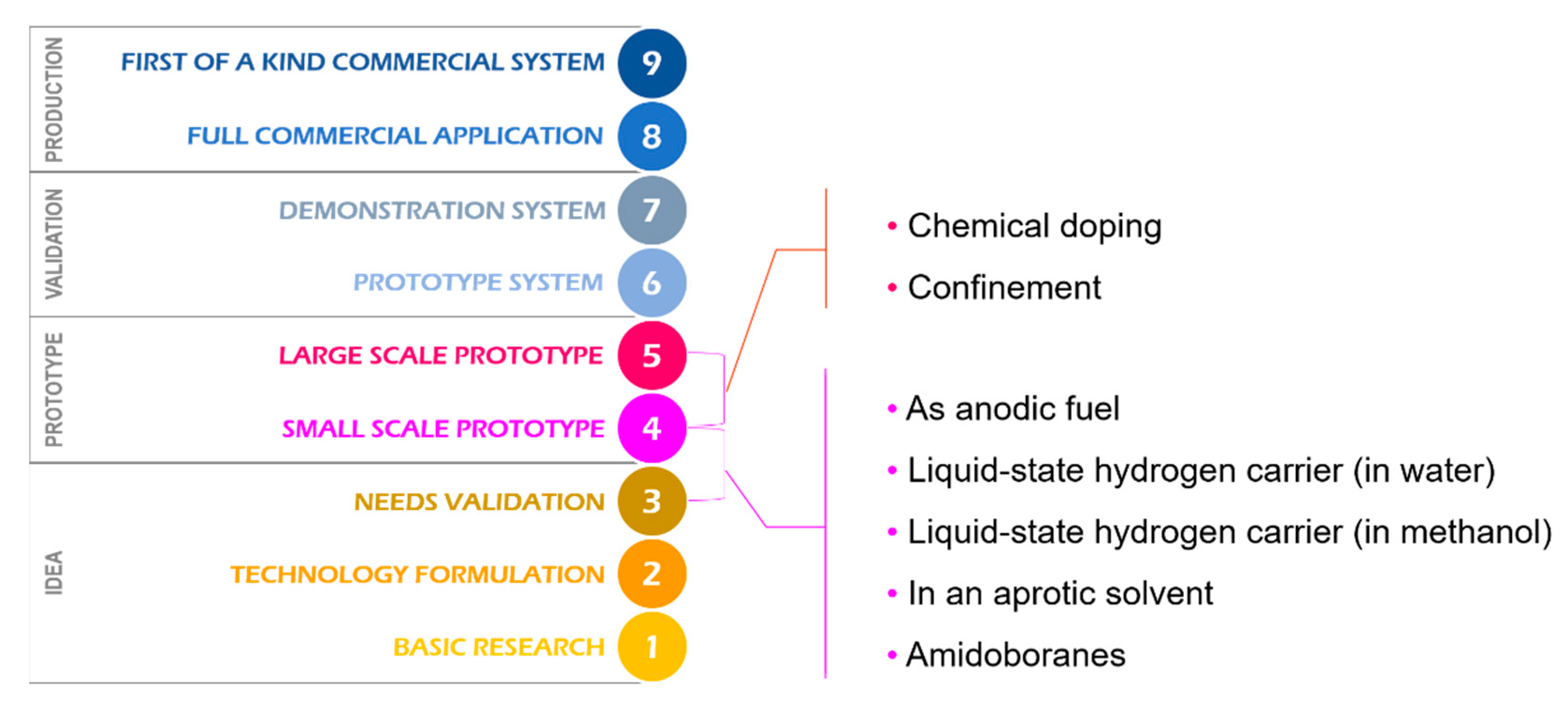
1. Introduction
2. Ammonia Borane, an Old Compound
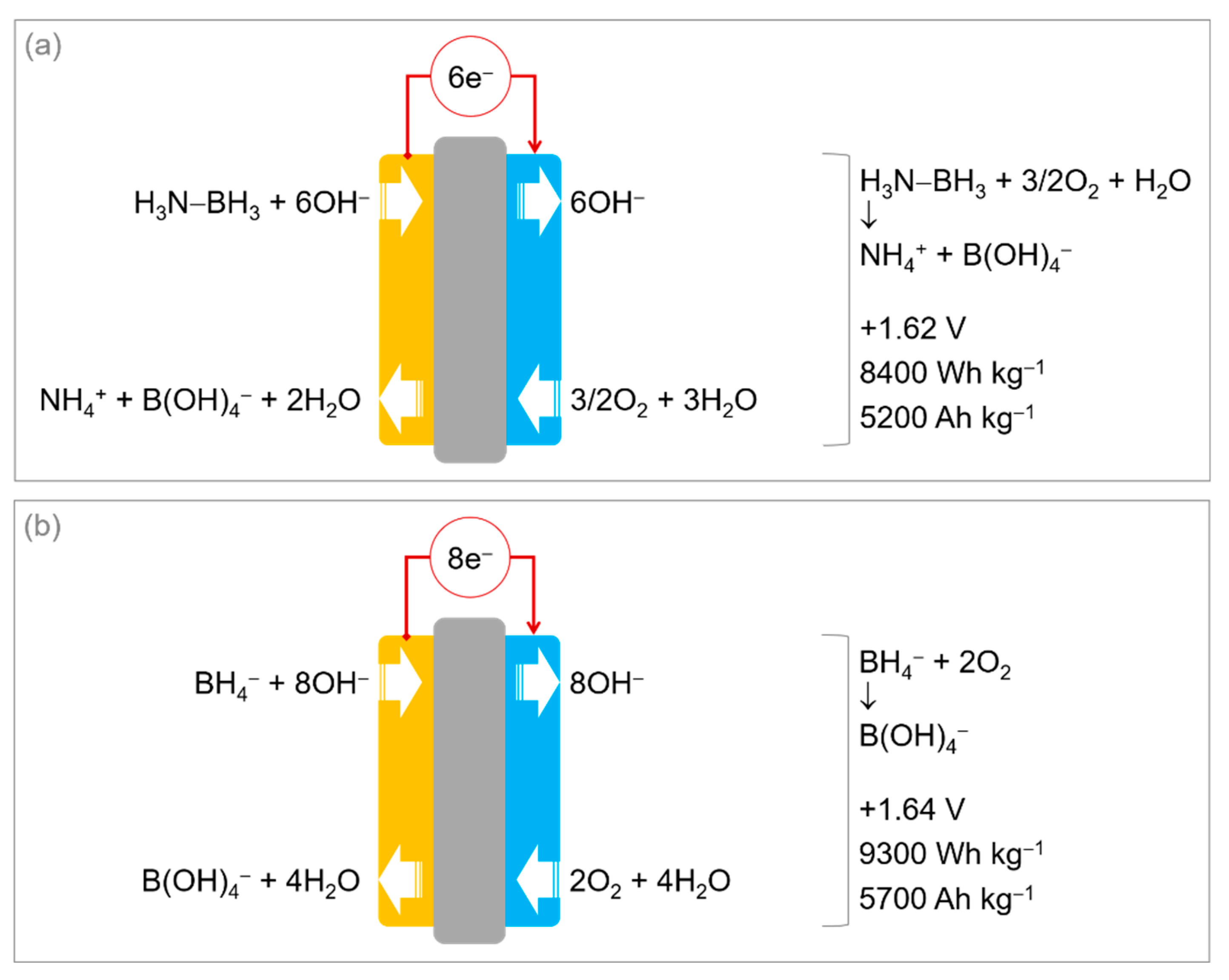
3. Aqueous Ammonia Borane as Anodic Fuel
4. Ammonia Borane in Solution as a Liquid-State Hydrogen Carrier
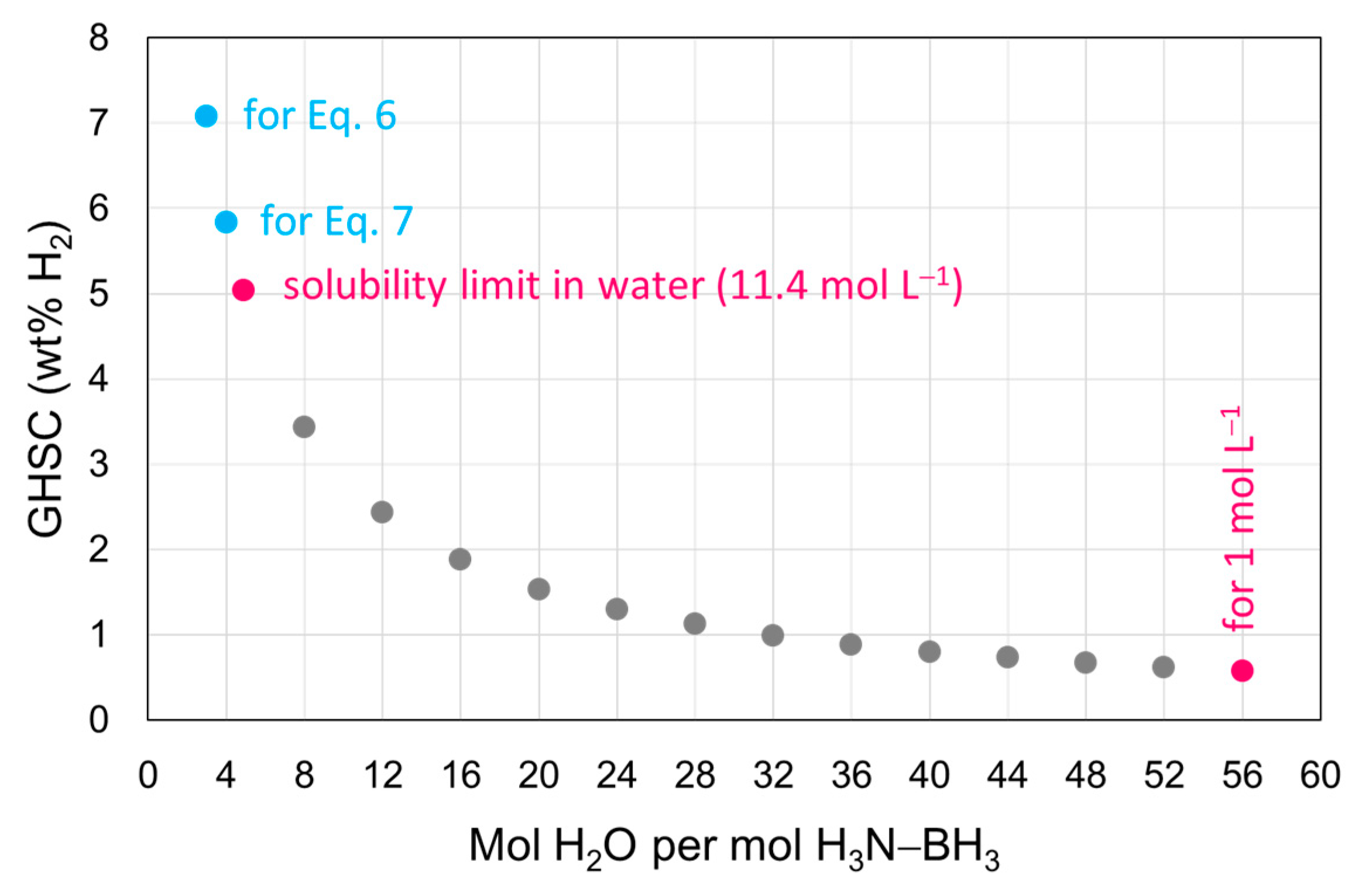
4.1. In Water
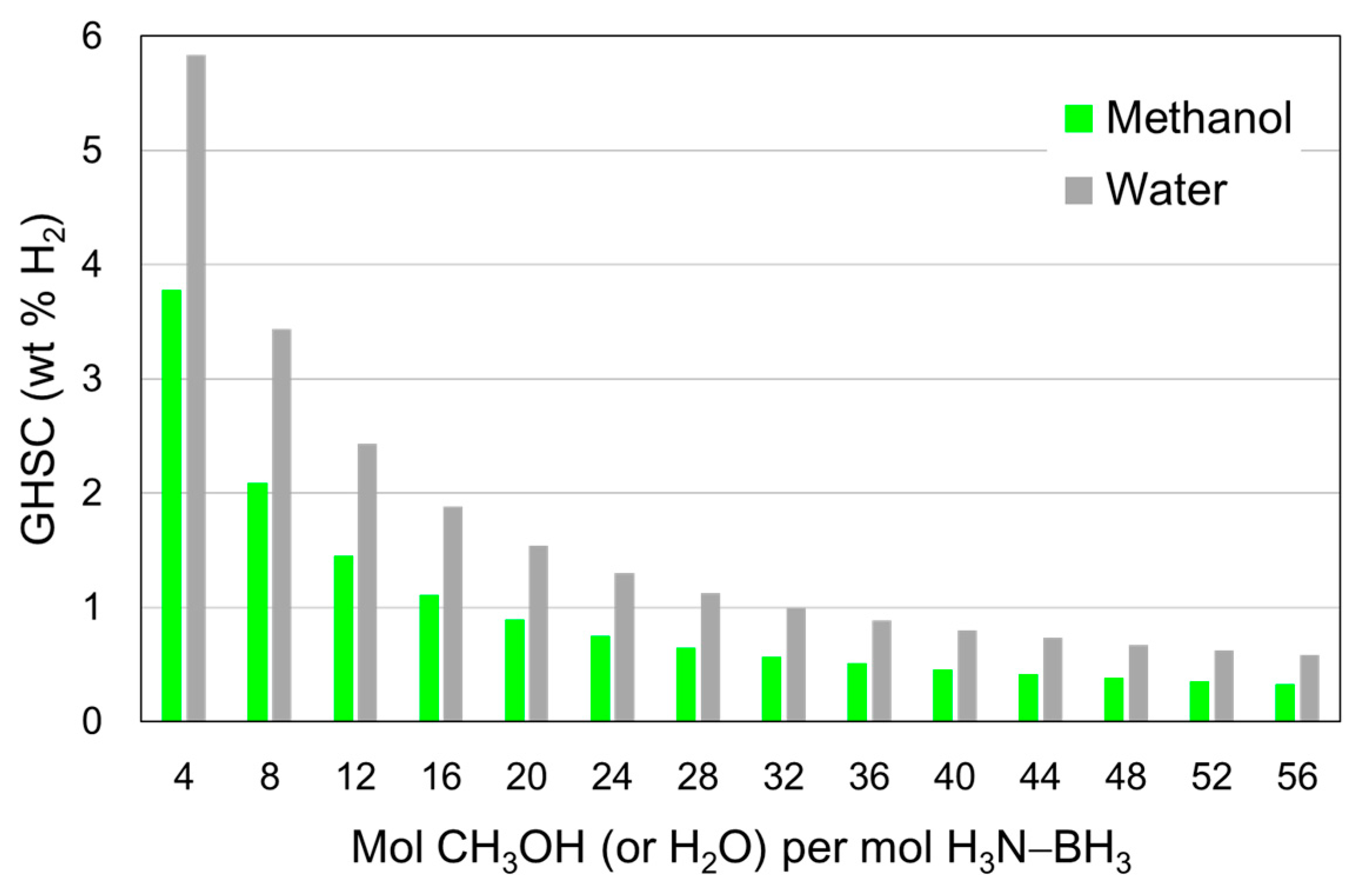
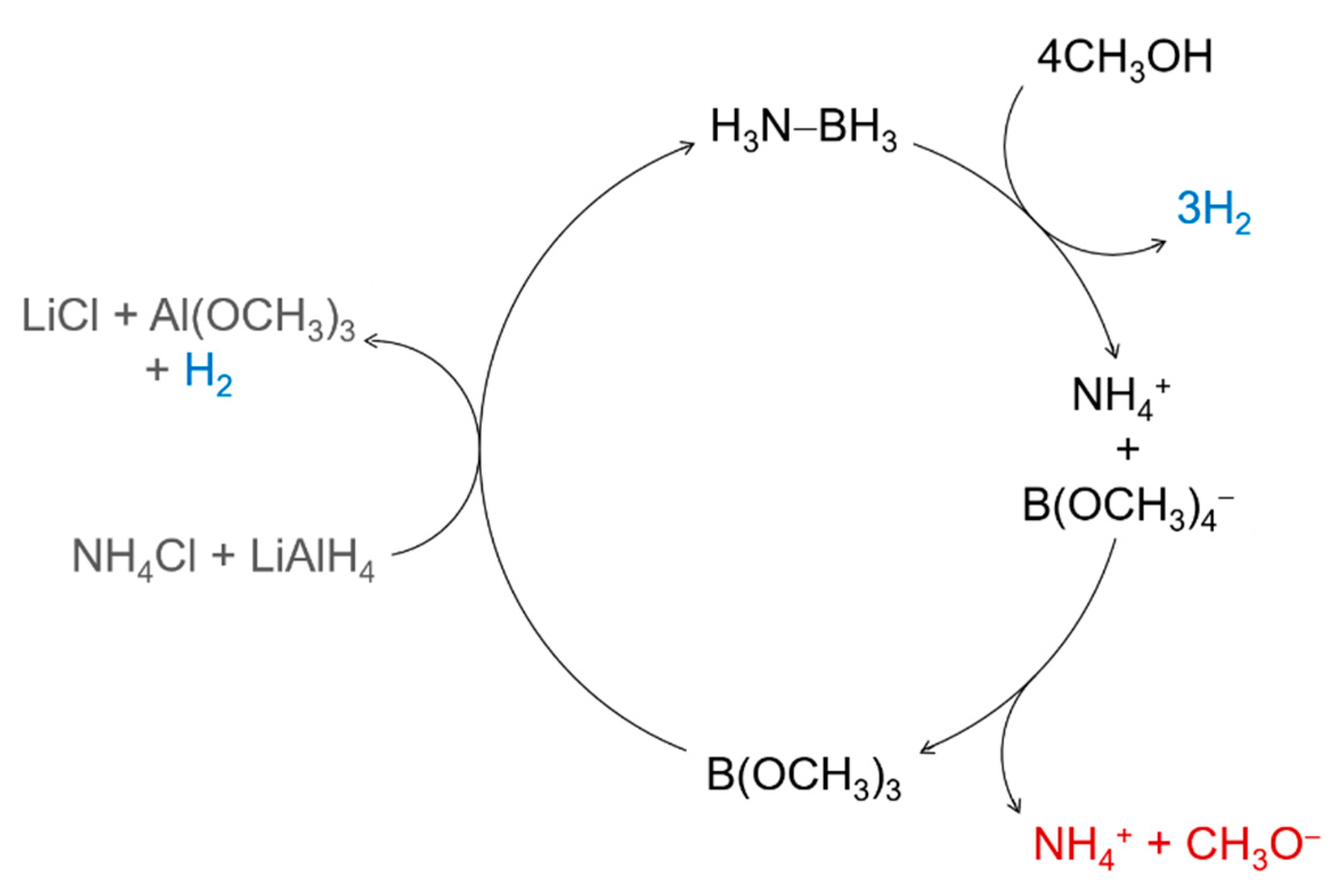
4.2. In Methanol
→ 5H3N−BH3 + 5Al(OCH3)3 + 7CH3OH + H2 + 5LiCl + 5NH3
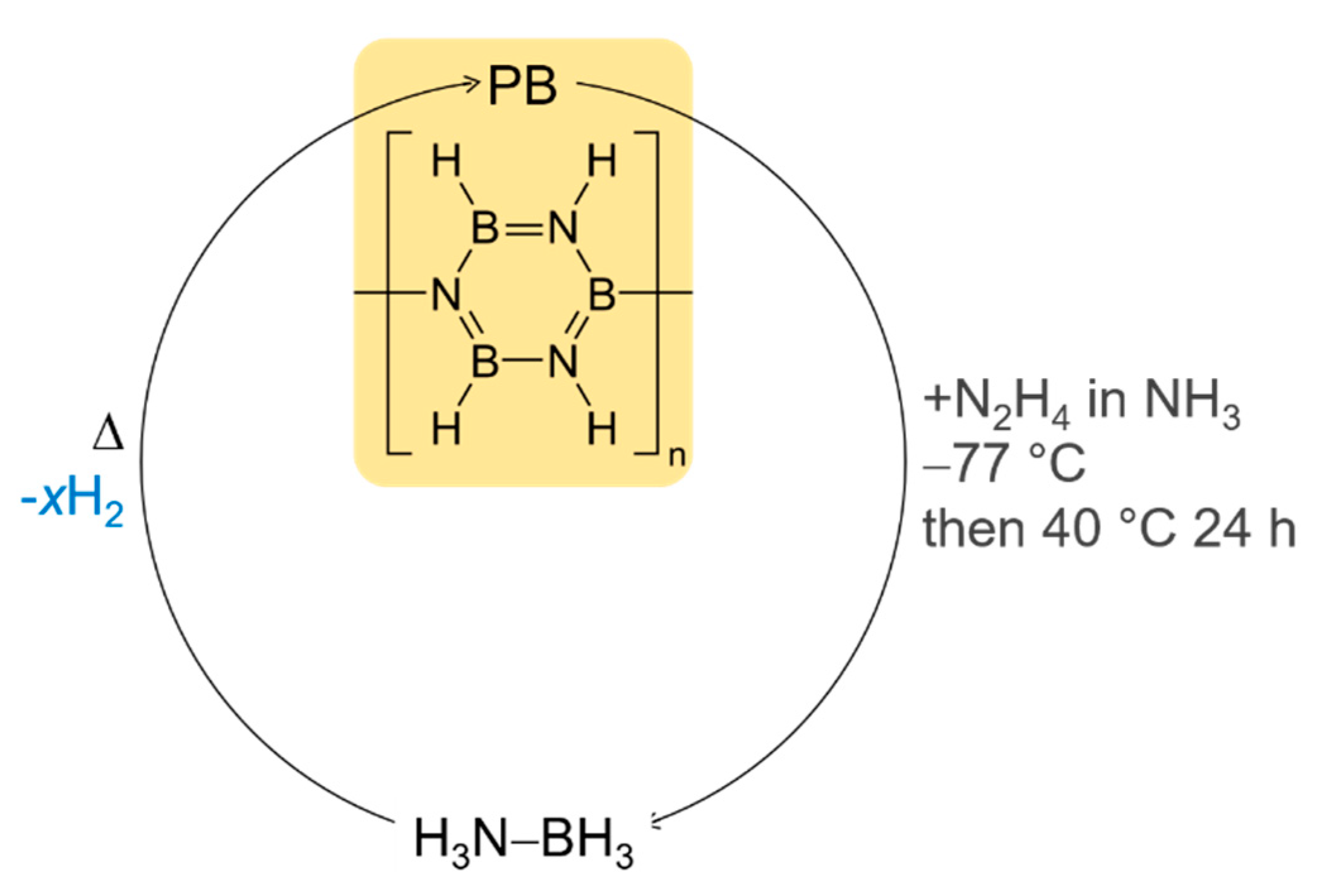
5. Ammonia Borane as a Solid-State Hydrogen Storage Material
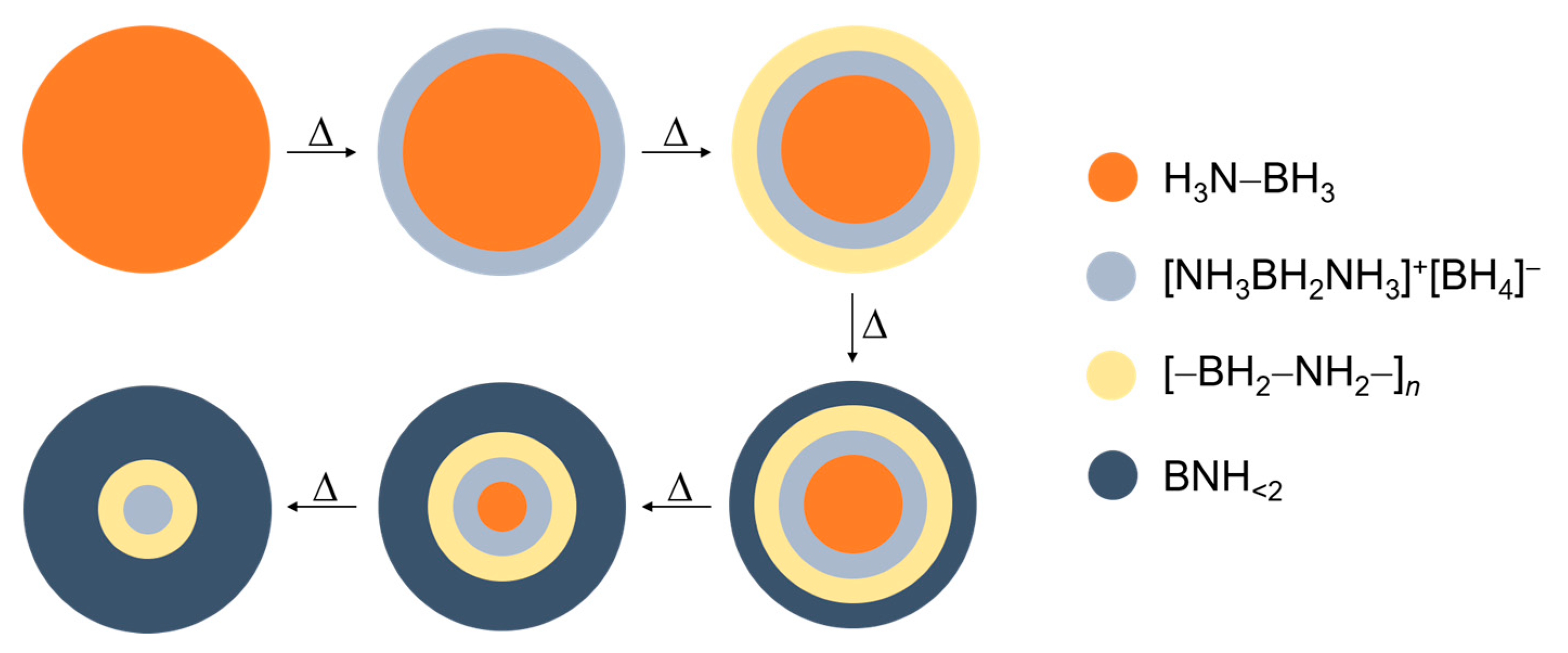
5.1. Pristine Ammonia Borane
5.2. Ammonia Borane in an Aprotic Solvent
5.3. Chemical Doping of Solid Ammonia Borane
5.4. Nanoconfinement of Ammonia Borane
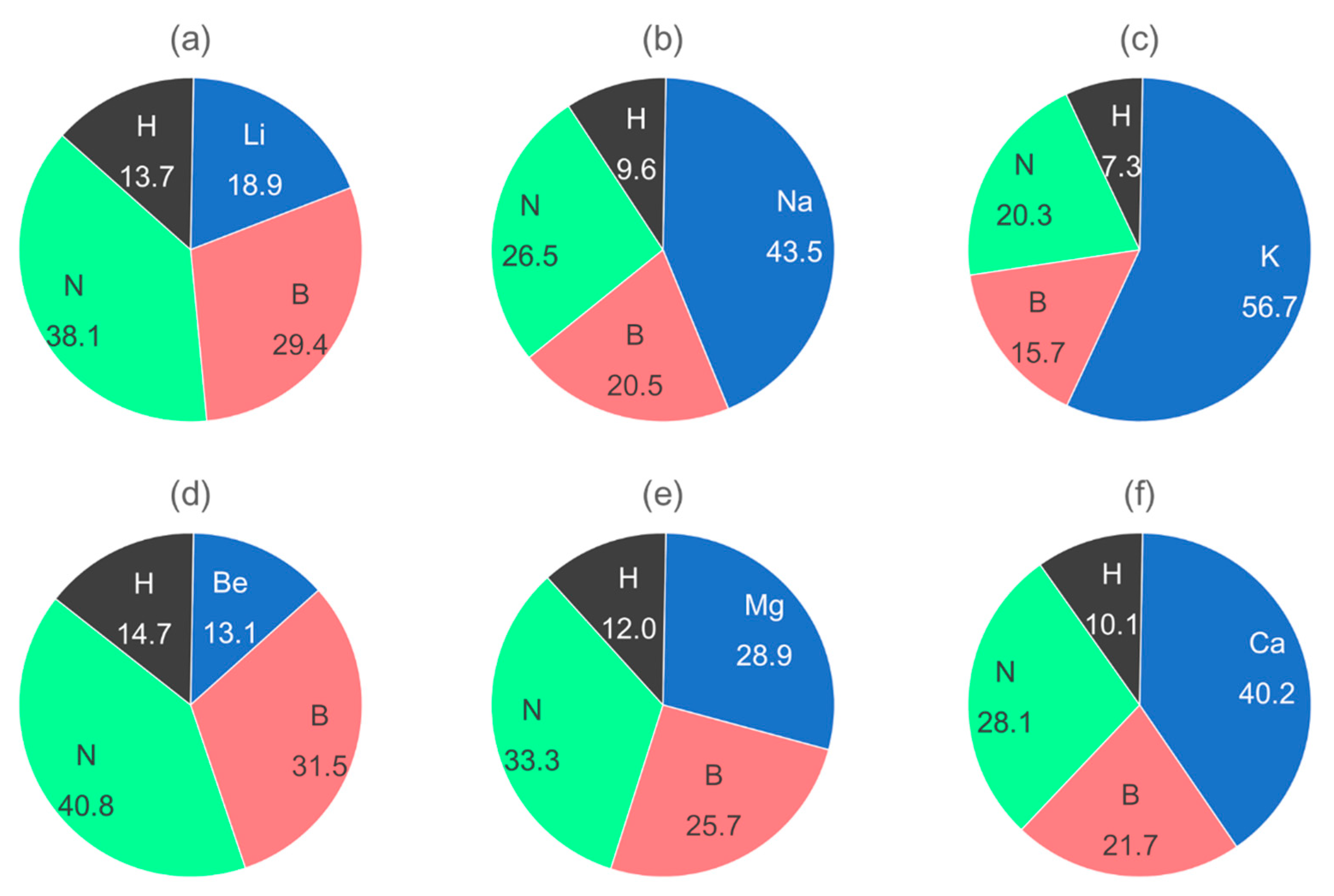
5.5. Alkali and Alkaline-Earth Derivatives of Ammonia Borane
5.6. The Critical Issue of Regeneration
6. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Chapman, A.; Itaoka, A.; Farabi-Asl, H.; Fujii, Y.; Nakahara, M. Societal penetration of hydrogen into the future energy system: Impacts of policy; technology and carbon targets. Int. J. Hydrog. Energy 2020, 45, 3883–3898. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrog. Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Kurtz, J.; Sprik, S.; Bradley, T.H. Review of transportation hydrogen infrastructure performance and reliability. Int. J. Hydrog. Energy 2019, 44, 12010–12023. [Google Scholar] [CrossRef]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrog. Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Current research trends and perspectives on materials-based hydrogen storage solutions: A critical review. Int. J. Hydrog. Energy 2017, 42, 289–311. [Google Scholar] [CrossRef]
- Dimitriou, P.; Tsujimura, T. A review of hydrogen as a compression ignition engine fuel. Int. J. Hydrog. Energy 2017, 42, 24470–24486. [Google Scholar] [CrossRef]
- Alaswad, A.; Baroutaji, A.; Achour, H.; Carton, J.; Al Makky, A.; Olabi, A.G. Developments in fuel cell technologies in the transport sector. Int. J. Hydrog. Energy 2016, 41, 16499–16508. [Google Scholar] [CrossRef]
- Hirscher, M. Hydrogen storage by cryoadsorption in ultrahigh-porosity metal–organic frameworks. Angew. Chem. Int. Ed. 2011, 50, 581–582. [Google Scholar] [CrossRef]
- Lai, Q.; Sun, Y.; Wang, T.; Modi, P.; Cazorla, C.; Demirci, U.B.; Ares Fernandez, J.R.; Leardini, F.; Aguey-Zinsou, K.F. How to design hydrogen storage materials? Fundamentals, synthesis, and storage tanks. Adv. Sust. Syst. 2019, 3, 1900043. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy—Review and discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Milanese, C.; Garroni, S.; Gennari, F.; Marini, A.; Klassen, T.; Dornheim, M.; Pistidda, C. Solid state hydrogen storage in alanates and alanate-based compounds: A review. Metals 2018, 8, 567. [Google Scholar] [CrossRef]
- Kojima, Y. Hydrogen storage materials for hydrogen and energy carriers. Int. J. Hydrog. Energy 2019, 44, 18179–18192. [Google Scholar] [CrossRef]
- Lyu, J.; Lider, A.; Kudiiarov, V. Using ball milling for modification of the hydrogenation/dehydrogenation process in magnesium-based hydrogen storage materials: An overview. Metals 2019, 9, 768. [Google Scholar] [CrossRef]
- Koohi-Fayegh, S.; Rosen, M.A. A review of energy storage types, applications and recent developments. J. Energy Storage 2020, 27, 101047. [Google Scholar] [CrossRef]
- Milanese, C.; Jensen, T.R.; Hauback, B.C.; Pistidda, C.; Dornheim, M.; Yang, Y.; Lombardo, L.; Zuettel, A.; Filinchuk, Y.; Ngene, P.; et al. Complex hydrides for energy storage. Int. J. Hydrog. Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef]
- Kumar, R.; Karkamkar, A.; Bowden, M.; Autrey, M. Solid-state hydrogen rich boron–nitrogen compounds for energy storage. Chem. Soc. Rev. 2019, 48, 5350–5380. [Google Scholar] [CrossRef] [PubMed]
- Demirci, U.B. Sodium borohydride for the near-future energy: A “rough diamond” for Turkey. Turk. J. Chem. 2018, 42, 193–220. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia borane, a material with exceptional properties for chemical hydrogen storage. Int. J. Hydrog. Energy 2017, 42, 9978–10013. [Google Scholar] [CrossRef]
- Moury, R.; Demirci, U.B. Hydrazine borane and hydrazinidoboranes as chemical hydrogen storage materials. Energies 2015, 8, 3118–3141. [Google Scholar] [CrossRef]
- Demirci, U.B. The hydrogen cycle with the hydrolysis of sodium borohydride: A statistical approach for highlighting the scientific/technical issues to prioritize in the field. Int. J. Hydrog. Energy 2015, 40, 2673–2691. [Google Scholar] [CrossRef]
- Demirci, U.B. About the technological readiness of the H2 generation by hydrolysis of B(-N)-H compounds. Energy Technol. 2018, 6, 470–486. [Google Scholar] [CrossRef]
- Chandra, M.; Xu, Q. A high-performance hydrogen generation system: Transition metal-catalyzed dissociation and hydrolysis of ammonia–borane. J. Power Sources 2006, 156, 190–194. [Google Scholar] [CrossRef]
- Gutowska, A.; Liyu, L.; Shin, Y.; Wang, C.M.; Li, X.S.; Linehan, J.C.; Smith, R.S.; Kay, B.D.; Schmid, B.; Shaw, W.; et al. Nanoscaffold mediates hydrogen release and the reactivity of ammonia borane. Angew. Chem. Int. Ed. 2005, 44, 3578–3582. [Google Scholar] [CrossRef] [PubMed]
- Hügle, T.; Kühnel, M.F.; Lentz, D. Hydrazine borane: A promising hydrogen storage material. J. Am. Chem. Soc. 2009, 131, 7444–7446. [Google Scholar] [CrossRef] [PubMed]
- Karahan, S.; Zahmakıran, M.; Özkar, S. Catalytic hydrolysis of hydrazine borane for chemical hydrogen storage: Highly efficient and fast hydrogen generation system at room temperature. Int. J. Hydrog. Energy 2011, 36, 4958–4966. [Google Scholar] [CrossRef]
- Moury, R.; Moussa, G.M.; Demirci, U.B.; Hannauer, J.; Bernard, S.; Petit, E.; van der Lee, A.; Miele, P. Hydrazine borane: Synthesis, characterization, and application prospects in chemical hydrogen storage. Phys. Chem. Chem. Phys. 2012, 14, 1768–1777. [Google Scholar] [CrossRef]
- Demirci, U.B. Impact of H.I. Schlesinger’s discoveries upon the course of modern chemistry on B-(N-)H hydrogen carriers. Int. J. Hydrog. Energy 2017, 42, 21048–21062. [Google Scholar] [CrossRef]
- Burg, A.B.; Schlesinger, H.I. Hydrides of boron. VII. Evidence of the transitory existence of borine (BH3); borine carbonyl and borine trimethylamine. J. Am. Chem. Soc. 1937, 59, 780–787. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Burg, A.B. Hydrides of boron. VIII. The structure of diammoniate of diborane and its relation to the structure of diborane. J. Am. Chem. Soc. 1938, 60, 290–299. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Ritter, D.M.; Burg, A.B. Hydrides of boron. X. The preparation and preliminary study of the new compound B2H7N. J. Am. Chem. Soc. 1938, 60, 2297–2300. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Burg, A.B. Recent developments in the chemistry of the boron hydrides. Chem. Rev. 1942, 31, 1–41. [Google Scholar] [CrossRef]
- Schaeffer, G.W.; Schaeffer, R.; Schlesinger, H.I. The preparation of borazole and its reaction with boron halides. J. Am. Chem. Soc. 1951, 73, 1612–1614. [Google Scholar] [CrossRef]
- Shore, S.G.; Parry, P.W. The crystalline compound ammonia-borane, H3NBH3. J. Am. Chem. Soc. 1955, 77, 6084–6085. [Google Scholar] [CrossRef]
- Shore, S.G.; Parry, P.W. Chemical evidence of the structure of “diammoniate of diborane.” II. The preparation of ammonia-borane. J. Am. Chem. Soc. 1958, 80, 8–12. [Google Scholar] [CrossRef]
- Shore, S.G.; Bödekker, K.W. Large scale synthesis of H2B(NH3)2+BH4- and H3NBH3. Inorg. Chem. 1964, 3, 914–915. [Google Scholar] [CrossRef]
- Hu, M.G.; Van Paasschen, J.M.; Geanangel, R.A. New synthetic approaches to ammonia-borane and its deuterated derivatives. J. Inorg. Nucl. Chem. 1977, 39, 2147–2150. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Gagare, P.D. Preparation of ammonia borane in high yield and purity, methanolysis and regeneration. Inorg. Chem. 2007, 46, 7810–7817. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Karkamkar, A.; Linehan, J.C.; Autrey, T. Synthesis of ammonia borane for hydrogen storage applications. Energy Environ. Sci. 2008, 1, 156–160. [Google Scholar] [CrossRef]
- Figen, A.A.; Pişkin, M.B.; Coşkuner, B.; Imamoğlu, V. Synthesis, structural characterization, and hydrolysis of ammonia borane (NH3BH3) as a hydrogen carrier. Int. J. Hydrog. Energy 2013, 38, 16215–16228. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Kulkarni, A.S. The role of ammonia in promoting ammonia borane synthesis. Dalton Trans. 2016, 45, 16433–16440. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Kulkarni, A.S. Water-promoted, safe and scalable preparation of ammonia borane. Int. J. Hydrog. Energy 2016, 42, 1451–1455. [Google Scholar] [CrossRef]
- Petit, J.F.; Miele, P.; Demirci, U.B. Ammonia borane H3N-BH3 for solid-state chemical hydrogen storage: Different samples with different thermal behaviors. Int. J. Hydrog. Energy 2016, 41, 15462–15470. [Google Scholar] [CrossRef]
- Peyerimhoff, S.D. Further study of umbrella vs bridged geometries, SCF-MO and CI calculations for C2H6++ and ammonia borane. J. Chem. Phys. 1968, 49, 312–325. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Melius, C.F. Thermochemistry of molecules in the B-N-Cl-H system: Ab initio predictions using the BAC-MP4 method. J. Phys. Chem. A 1997, 101, 2670–2680. [Google Scholar] [CrossRef]
- Palke, W.E. Calculation of the internal rotation barrier and its derivative in BH3NH3. J. Chem. Phys. 1972, 56, 5308–5312. [Google Scholar] [CrossRef]
- Hoffmann, R. Extended Hückel theory. III. Compounds of boron and nitrogen. J. Chem. Phys. 1964, 40, 2474–2480. [Google Scholar] [CrossRef]
- Dill, J.D.; Schleyer, P.R.; Pople, J.A. Molecular orbital theory of the electronic structure of organic compounds. XXIV. Geometries and energies of small boron compounds. Comparisons with carbocations. J. Am. Chem. Soc. 1975, 97, 3402–3409. [Google Scholar] [CrossRef]
- Staubitz, A.; Robertson, A.P.M.; Manners, I. Ammonia-borane and related compounds as dihydrogen sources. Chem. Rev. 2010, 110, 4079–4124. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Chen, X.; Shore, S.G. Ammonia-borane, past as prolog. J. Organomet. Chem. 2014, 751, 60–66. [Google Scholar] [CrossRef]
- Hugues, E.W. The crystal structure of ammonia-borane, H3NBH3. J. Am. Chem. Soc. 1956, 78, 502–503. [Google Scholar] [CrossRef]
- Lippert, E.L.; Lipscomb, W.N. The structure of H3NBH3. J. Am. Chem. Soc. 1956, 78, 503–504. [Google Scholar] [CrossRef]
- Hoon, C.F.; Reynhardt, E.C. Molecular dynamics and structures of amine boranes of the type R3N-BH3: I. X-ray investigation of H3N-BH3 at 295 K and 110 K. J. Phys. C Solid State Phys. 1983, 16, 6129–6136. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, X.; Zhao, Z.; Li, W.; Jiang, S.; Duan, D.; Bao, K.; Zhou, Q.; Liu, B.; Cui, T. Experimental verification of the high pressure crystal structures in NH3BH3. J. Chem. Phys. 2014, 140, 244507. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Ke, X.; Zhang, J.; Lin, Z.; Vogel, S.C.; Hartl, M.; Sinogeikin, S.; Daemen, L.; Cornelius, A.L.; Chen, C.; et al. Pressure induced structural changes in the potential hydrogen storage compound ammonia borane: A combined X-ray, neutron and theoretical investigation. Chem. Phys. Lett. 2010, 495, 203–207. [Google Scholar] [CrossRef]
- Hamilton, C.W.; Baker, R.T.; Staubitz, A.; Manners, I. B-N compounds for chemical hydrogen storage. Chem. Soc. Rev. 2009, 38, 279–293. [Google Scholar] [CrossRef]
- Olah, G.A.; Kuhn, S.J. Propellant Compositions. U.S. Patent 3,103,782, 17 September 1963. [Google Scholar]
- Chew, W.M.; Murfree, J.A.; Martigoni, P.; Nappier, H.A.; Ayers, O.E. Amine-Boranes as Hydrogen Generating Propellants. U.S. Patent 4,157,927, 12 June 1979. [Google Scholar]
- English, W.D.; Chew, W.M. Solid Propellant Hydrogen Generator. U.S. Patent 4,315,786, 16 February 1982. [Google Scholar]
- Artz, G.D.; Grant, L.R. Solid Propellant Hydrogen Generator. U.S. Patent 4,468,263, 28 August 1984. [Google Scholar]
- Lee, J.G.; Weismiller, M.; Connell, T.L., Jr.; Risha, G.A.; Yetter, R.A.; Peter, G.; Son, S. Ammonia borane-based propellants. In Proceedings of the 44th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Hartford, CT, USA, 21–23 July 2008. [Google Scholar] [CrossRef]
- Pfeil, M.A.; Son, S.F.; Anderson, W.E. Influence of ammonia borane on the stability of a liquid rocket combustor. J. Propuls. Power 2014, 30, 290–298. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zhao, F.Q.; Yi, J.H.; Luo, Y. Applications of hydrogen-storage materials in high-energy solid rocket propellants. Chin. J. Explos. Propellants 2015, 38, 8–14. [Google Scholar]
- Srinivas, D.; Mitchell, L.A.; Parrish, D.A.; Shreeve, J.M. From FOX-7 to H-FOX to insensitive energetic materials with hypergolic properties. Chem. Commun. 2016, 52, 7668–7671. [Google Scholar] [CrossRef]
- Bi, X.; Liu, J. Detonation properties of high explosives containing ammonia borane. Z. Anorg. Allg. Chem. 2016, 642, 773–777. [Google Scholar] [CrossRef]
- Bhosale, V.A.; Jeong, J.; Kwon, S. Ignition of boron-based green hypergolic fuels with hydrogen peroxide. Fuel 2019, 255, 115729. [Google Scholar] [CrossRef]
- Nagle, L.C.; Rohan, J.F. Ammonia borane oxidation at gold microelectrodes in alkaline solutions. J. Electrochem. Soc. 2006, 153, C773–C776. [Google Scholar] [CrossRef]
- Andrieux, J.; Laversenne, L.; Krol, O.; Chiriac, R.; Bouajila, Z.; Tenu, R.; Counioux, J.J.; Goutaudier, C. Revision of the NaBO2-H2O phase diagram for optimized yield in the H2 generation through NaBH4 hydrolysis. Int. J. Hydrog. Energy 2012, 37, 5798–5810. [Google Scholar] [CrossRef]
- Zhang, X.B.; Yan, J.M.; Han, S.; Chandra, M.; Shioyama, H.; Yasuda, K.; Kuriyama, N.; Kobayashi, T.; Xu, Q. A new fuel cell using aqueous ammonia-borane as the fuel. J. Power Sources 2008, 168, 167–171. [Google Scholar] [CrossRef]
- Zhang, X.B.; Yan, J.M.; Han, S.; Shioyama, H.; Yasuda, K.; Kuriyama, N.; Xu, Q. A high performance anion exchange membrane-type ammonia borane fuel cell. J. Power Sources 2008, 182, 515–519. [Google Scholar] [CrossRef]
- Demirci, U.B. Direct liquid-feed fuel cells: Thermodynamic and environmental concerns. J. Power Sources 2007, 169, 239–246. [Google Scholar] [CrossRef]
- Yao, C.; Yang, H.; Zhuang, L.; Ai, X.; Cao, Y.; Lu, J. A preliminary study of direct borazane fuel cell. J. Power Sources 2007, 165, 125–127. [Google Scholar] [CrossRef]
- Zhang, X.B.; Han, S.; Yan, J.M.; Shioyama, H.; Kuriyama, N.; Kobayashi, T.; Xu, Q. Electrochemical oxidation of ammonia borane on gold electrode. Int. J. Hydrog. Energy 2009, 34, 174–179. [Google Scholar] [CrossRef]
- Molina Concha, M.B.; Chatenet, M.; Lima, F.H.B.; Ticianelli, E.A. In situ Fourier transform infrared spectroscopy and on-line differential electrochemical mass spectrometry study of the NH3BH3 oxidation reaction on gold electrodes. Electrochim. Acta 2013, 89, 607–615. [Google Scholar] [CrossRef]
- Nagle, L.C.; Rohan, J.F. Direct oxidation of ammonia borane as an alternative fuel at nanoporous Au. ECS Trans. 2010, 25, 13–35. [Google Scholar]
- Nagle, L.C.; Rohan, J.F. Nanoporous gold catalyst for direct ammonia borane fuel cells. J. Electrochem. Soc. 2011, 158, B772–B778. [Google Scholar] [CrossRef]
- Barsuk, D.; Zadick, A.; Chatenet, M.; Georgarakis, A.; Panagiotopoulos, N.T.; Champion, Y.; Jorge, A.M., Jr. Nanoporous silver for electrocatalysis application in alkaline fuel cells. Mater. Des. 2016, 111, 528–536. [Google Scholar] [CrossRef]
- Karabiberoğlu, Ş.U.; Koçak, Ç.C.; Koçak, S.; Dursun, Z. Polymer film supported bimetallic Au-Ag catalysts for electrocatalytic oxidation of ammonia borane in alkaline media. Nano-Micro Lett. 2016, 8, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.B.; Yan, J.M.; Han, S.; Shioyama, H.; Xu, Q. Magnetically recyclable Fe@Pt core-shell nanoparticles and their use as electrocatalysts for ammonia borane oxidation: The role of crystallinity of the core. J. Am. Chem. Soc. 2009, 131, 2778–2779. [Google Scholar] [CrossRef]
- Kiran, V.; Kalidindi, S.B.; Jagirdar, B.R.; Sampath, S. Electrochemical oxidation of boron containing compounds on titanium carbide and its implications of direct fuel cells. Electrochim. Acta 2011, 56, 10493–10499. [Google Scholar] [CrossRef]
- Olu, P.Y.; Deschamps, F.; Caldarella, G.; Chatenet, M.; Job, N. Investigation of platinum and palladium as potential anodic catalysts for direct borohydride and ammonia borane fuel cells. J. Power Sources 2015, 297, 492–503. [Google Scholar] [CrossRef]
- Zadick, A.; Dubau, L.; Artyushkova, A.; Serov, A.; Atanassov, P.; Chatenet, M. Nickel-based electrocatalysts for ammonia borane oxidation: Enabling materials for carbon-free-fuel direct liquid alkaline fuel cell technology. Nano Energy 2017, 37, 248–259. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Chen, T.; Tan, Y. Ni1-xMxSe2 (M = Fe; Co; Cu) nanowires as anodes for ammonia-borane electrooxidation and the derived Ni1-xMxSe2-y-OOH ultrathin nanosheets as efficient electrocatalysts for oxygen evolution. J. Mater. Chem. A 2019, 7, 16372–16386. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, J.; Wang, H.; Wang, G.; Chen, T.; Tan, Y. Porous Ni1-xCuxO nanowire arrays as noble-metal-free high-performance catalysts for ammonia-borane electrooxidation. ACS Catal. 2020, 10, 721–735. [Google Scholar] [CrossRef]
- Braesch, G.; Bonnefont, A.; Martin, V.; Savinova, E.R.; Chatenet, M. Borohydride oxidation reaction mechanisms and poisoning effects on Au, Pt and Pd bulk electrodes: From model (low) to direct borohydride fuel cell operating (high) concentrations. Electrochim. Acta 2018, 273, 483–494. [Google Scholar] [CrossRef]
- Jiang, H.L.; Singh, S.A.; Yan, J.M.; Zhang, X.B.; Xu, Q. Liquid-phase chemical hydrogen storage: Catalytic hydrogen generation under ambient conditions. ChemSusChem 2010, 3, 541–549. [Google Scholar] [CrossRef]
- Jiang, H.L.; Xu, Q. Catalytic hydrolysis of ammonia borane for chemical hydrogen storage. Catal. Today 2011, 170, 56–63. [Google Scholar] [CrossRef]
- Lu, Z.H.; Xu, Q. Recent progress in boron- and nitrogen-based chemical hydrogen storage. Funct. Mater. Lett. 2012, 5, 1230001. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Zhan, W.W.; Zhu, Q.L.; Xu, Q. Dehydrogenation of ammonia borane by metal nanoparticle catalysts. ACS Catal. 2016, 6, 6892–6905. [Google Scholar] [CrossRef]
- Özkar, S. Enhancement of catalytic activity by increasing surface area in heterogeneous catalysis. Appl. Surf. Sci. 2009, 256, 1272–1277. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özkar, S. Ammonia borane as hydrogen storage materials. Int. J. Hydrog. Energy 2018, 43, 18592–18606. [Google Scholar] [CrossRef]
- Kalidindi, S.B.; Jagirdar, B. Hydrogen generation from ammonia borane using nanocatalysts. J. Indian Inst. Sci. 2010, 90, 181–187. [Google Scholar]
- Lu, Z.H.; Yao, Q.; Zhang, Z.; Yang, Y.; Chen, X. Nanocatalysts for hydrogen generation from ammonia borane and hydrazine borane. J. Nanomater. 2014, 2014, 729029. [Google Scholar] [CrossRef]
- Umegaki, T.; Xu, Q.; Kojima, Y. Porous materials for hydrolytic dehydrogenation of ammonia borane. Materials 2015, 8, 4512–4534. [Google Scholar] [CrossRef]
- Luconi, L.; Tuci, G.; Giambastiani, G.; Rossin, A.; Peruzzini, M. H2 production from lightweight inorganic hydrides catalyzed by 3d transition metals. Int. J. Hydrog. Energy 2019, 44, 25746–25776. [Google Scholar] [CrossRef]
- Alpaydın, C.Y.; Gülbay, S.A.; Colpan, C.O. A review on the catalysts used for hydrogen production from ammonia borane. Int. J. Hydrog. Energy 2020, 45, 3414–3434. [Google Scholar] [CrossRef]
- Fu, F.; Wang, C.; Wang, Q.; Martinez-Villacorta, A.M.; Escobar, A.; Ching, H.; Wang, X.; Moya, S.; Salmon, L.; Fouquet, E.; et al. Highly selective and sharp volcano-type synergistic Ni2Pt@ZIF-8-catalyzed hydrogen evolution from ammonia borane hydrolysis. J. Am. Chem. Soc. 2018, 140, 10034–10042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, M.; Wen, J.; Li, Y.; Li, A.; Zhang, L.; Ali, A.M.; Li, Y. Sub-3 nm Rh nanoclusters confined within a metal-organic framework for enhanced hydrogen generation. Chem. Commun. 2019, 55, 4699–4702. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Du, L.; Yao, F.; Xu, S.; Deng, X.; He, S.; Zhanng, H.; Zhou, X. Shape-controlled dodecaborate supramolecular organic-framework-supported ultrafine trimetallic PtCoNi for catalytic hydrolysis of ammonia borane. ACS Appl. Mater. Interf. 2019, 11, 23445–23453. [Google Scholar] [CrossRef]
- Sarıca, E.; Akbayrak, S.; Özkar, S. Ruthenium(0) nanoparticles supported on silica coated Fe3O4 as magnetically separable catalysts for hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrog. Energy 2018, 43, 15124–15134. [Google Scholar]
- Umegaki, T.; Yabuuchi, A.; Yoshida, N.; Xu, Q.; Kojima, Y. In situ synthesized hollow spheres of a silica-ruthenium-nickel composite catalyst for the hydrolytic dehydrogenation of ammonia borane. New J. Chem. 2019, 44, 450–455. [Google Scholar] [CrossRef]
- Chen, M.; Xiong, R.; Cui, X.; Wang, Q.; Liu, X. SiO2-encompassed Co@N-doped porous carbon assemblies as recyclable catalysts for efficient hydrolysis of ammonia borane. Langmuir 2019, 35, 671–677. [Google Scholar] [CrossRef]
- Toyama, N.; Nikura, N.; Ito, I.; Umegaki, T.; Kojima, Y. Synthesis of mesoporous silica-zirconia composite hollow spheres with enhanced activity toward hydrolysis of ammonia borane. Microp. Mesop. Mater. 2020, 294, 109839. [Google Scholar] [CrossRef]
- Du, X.; Tai, Y.; Liu, H.; Zhang, J. One-step synthesis of reduced graphene oxide supported CoW nanoparticles as efficient catalysts for hydrogen generation from NH3BH3. React. Kinet. Mech. Catal. 2018, 125, 171–181. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Zhang, Z.; Wang, S.; Williams, N.; Cheng, Y.; Liu, S.; Gu, J. rGO supported PfNi-CeO2 nanocomposite as an efficient catalyst for hydrogen evolution from the hydrolysis of NH3BH3. Int. J. Hydrog. Energy 2018, 43, 18745–18753. [Google Scholar] [CrossRef]
- Xu, M.; Huai, X.; Zhang, H. Highly dispersed CuCo nanoparticles supported on reduced graphene oxide as high-activity catalysts for hydrogen evolution from ammonia borane hydrolysis. J. Nanopart. Res. 2018, 20, 329. [Google Scholar] [CrossRef]
- Uzundurukan, A.; Devrim, Y. Carbon nanotube-graphene hybrid supported platinum as an effective catalyst for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2019, 44, 26773–26782. [Google Scholar] [CrossRef]
- Shang, Y.; Feng, A.; Wang, Y.; Sun, C.; Zhong, J. Carbon nitride supported Ni0.5C0.5O nanoparticles with strong interfacial interaction to enhance the hydrolysis of ammonia borane. RSC Adv. 2019, 9, 11552–11557. [Google Scholar] [CrossRef]
- Zhou, X.; Meng, X.F.; Wang, J.M.; Shang, N.Z.; Feng, T.; Gao, Z.Y.; Zhang, H.X.; Ding, X.L.; Gao, S.T.; Feng, C.; et al. Boron nitride supported NiCoP nanoparticles as noble metal-free catalyst for highly efficient hydrogen generation from ammonia borane. Int. J. Hydrog. Energy 2019, 44, 4764–4770. [Google Scholar] [CrossRef]
- Gao, M.; Yu, Y.; Yang, W.; Li, J.; Xu, S.; Feng, M.; Li, H. Ni nanoparticles supported on graphitic carbon nitride as visible light catalysts for hydrolytic dehydrogenation of ammonia borane. Nanoscale 2019, 11, 3506–3513. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Wang, Y.; Li, G.; Hu, G.; Shiwei, W.; Cao, Z.; Zhang, K. Enhanced catalytic activity of the nanostructured Co-W-B film catalysts for hydrogen evolution from the hydrolysis of sodium borohydride. J. Colloid Interf. Sci. 2018, 524, 25–32. [Google Scholar] [CrossRef]
- Rakap, M.; Abay, B.; Tunç, N. Hydrolysis of ammonia borane and hydrazine borane by poly(N-vinyl-2-pyrrolidone)-stabilized CoPd nanoparticles for chemical hydrogen storage. Turk. J. Chem. 2017, 41, 221–232. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.; Ge, H.; Chen, C.; Yan, W.; Gao, Z.; Gan, J.; Zhang, B.; Duan, X.; Qin, Y. Synergistic effects in atomic-layer-deposited PtCox/CNTs catalysts enhancing hydrolytic dehydrogenation of ammonia borane. Appl. Catal. B Env. 2018, 235, 256–263. [Google Scholar] [CrossRef]
- Coşkuner Filiz, B.; Kantürk Figen, A.; Pişkin, S. The remarkable role of metal promoters on the catalytic activity of Co-Cu based nanoparticles for boosting hydrogen evolution: Ammonia borane hydrolysis. Appl. Catal. B Env. 2018, 238, 365–380. [Google Scholar] [CrossRef]
- Coşkuner Filiz, B.; Kantürk Figen, A. Polymeric and metal oxide structured nanofibrous composites fabricated by electrospinning as highly efficient hydrogen evolution catalyst. J. Colloid Interf. Sci. 2019, 533, 82–94. [Google Scholar]
- Feng, X.; Zhao, Y.; Liu, D.; Mo, Y.; Liu, Y.; Chen, X.; Yan, W.; Jin, X.; Chen, B.; Duan, X.; et al. Towards high activity of hydrogen production from ammonia borane over efficient non-noble Ni5P4 catalyst. Int. J. Hydrog. Energy 2018, 43, 17112–17120. [Google Scholar] [CrossRef]
- Qu, X.; Jiang, R.; Li, Q.; Zeng, F.; Zheng, X.; Xu, Z.; Chen, C.; Peng, J. The hydrolysis of ammonia borane catalyzed by NiCoP/OPC-300 nanocatalysts: High selectivity and efficiency, and mechanism. Green Chem. 2019, 21, 850–860. [Google Scholar] [CrossRef]
- Yang, C.; Men, Y.; Xu, Y.; Liang, L.; Cai, P.; Luo, W. In situ synthesis of NiCoP nanoparticles supported on reduced graphene oxide for the catalytic hydrolysis of ammonia borane. ChemPlusChem 2019, 84, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Xu, C.; Wang, Q.; Wang, Y.; Zhang, Y.; Gao, D.; Bi, J.; Fan, G. Ruthenium nanoclusters distributed on phosphorus-doped carbon derived from hypercrosslinked polymer networks for highly efficient hydrolysis of ammonia-borane. Int. J. Hydrog. Energy 2018, 43, 18253–18260. [Google Scholar] [CrossRef]
- Zhong, F.; Wang, Q.; Xu, C.; Yang, Y.; Wang, Y.; Zhang, Y.; Gao, D.; Bi, J.; Fan, G. Ultrafine and highly dispersed Ru nanoparticles supported on nitrogen-doped carbon nanosheets: Efficient catalysts for ammonia borane hydrolysis. Appl. Surf. Sci. 2018, 455, 326–332. [Google Scholar] [CrossRef]
- Cui, Z.; Guo, Y.; Feng, Z.; Xu, D.; Ma, J. Ruthenium nanoparticles supported on nitrogen-doped carbon as a highly efficient catalyst for hydrogen evolution from ammonia borane. New J. Chem. 2019, 43, 4377–4384. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özçifçi, Z.; Tabak, A. Noble metal nanoparticles supported on activated carbon: Highly recyclable catalysts in hydrogen generation from the hydrolysis of ammonia borane. J. Colloid Interf. Sci. 2019, 546, 324–332. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, S.; Zhang, Z.; Williams, N.; Cheng, Y.; Gu, J. Hollow nickel-cobalt layered double hydroxide supported palladium catalysts with superior hydrogen evolution activity for hydrolysis of ammonia borane. ChemCatChem 2018, 10, 3206–3213. [Google Scholar] [CrossRef]
- Gil-San-Millan, R.; Grau-Atienza, A.; Johnson, D.T.; Rico-Francés, S.; Serrano, E.; Garcia-Martinez, J. Improving hydrogen production from the hydrolysis of ammonia borane by using multifunctional catalysts. Int. J. Hydrog. Energy 2018, 43, 17100–17111. [Google Scholar] [CrossRef]
- Jia, H.; Chen, X.; Song, X.; Zheng, X.; Guan, X.; Liu, P. Graphitic carbon nitride-chitosan composites-anchored palladium nanoparticles as high-performance catalyst for ammonia borane hydrolysis. Int. J. Energy Res. 2019, 43, 535–543. [Google Scholar] [CrossRef]
- Çelik Kazici, H.; Yıldız, F.; İzgi, M.S.; Ulas, B.; Kıvrak, H. Novel activated carbon supported trimetallic PdCoAg nanoparticles as efficient catalysts for the hydrolytic dehydrogenation of ammonia borane. Int. J. Hydrog. Energy 2019, 44, 10561–10572. [Google Scholar] [CrossRef]
- Akbayrak, S.; Tonbul, Y.; Özkar, S. Magnetically separable Rh0/Co3O4 nanocatalyst provides over a million turnovers in hydrogen release from ammonia borane. ACS Sust. Chem. Eng. 2020, 8, 4216–4224. [Google Scholar] [CrossRef]
- Brockman, A.; Zheng, Y.; Gore, J. A study of catalytic hydrolysis of concentrated ammonia borane solutions. Int. J. Hydrog. Energy 2010, 35, 7350–7356. [Google Scholar] [CrossRef]
- Lai, Q.; Aguey-Zinsou, F.K.; Demirci, U.B. Nanosizing ammonia borane with nickel – An all-solid and all-in-one approach for H2 generation by hydrolysis. Int. J. Hydrog. Energy 2018, 43, 14498–14506. [Google Scholar] [CrossRef]
- Keskin, E.; Coşkuner Filiz, B.; Kılıç Depren, S.; Kantürk Figen, A. Recommendations for ammonia borane composite pellets as a hydrogen storage medium. Int. J. Hydrog. Energy 2018, 43, 20354–20371. [Google Scholar] [CrossRef]
- Chandra, M.; Xu, Q. Room temperature hydrogen generation from aqueous ammonia-borane using noble metal nano-clusters as highly active catalysts. J. Power Sources 2007, 168, 135–142. [Google Scholar] [CrossRef]
- Chrysostomou, D.; Flowers, J.; Zaera, F. The thermal chemistry of ammonia on Ni(110). Surf. Sci. 1999, 439, 34–48. [Google Scholar] [CrossRef]
- Liu, C.H.; Wu, Y.C.; Chou, C.C.; Chen, B.H.; Hsueh, C.L.; Ku, J.R.; Tsau, F. Hydrogen generated from hydrolysis of ammonia borane using cobalt and ruthenium based catalysts. Int. J. Hydrog. Energy 2012, 37, 2950–2959. [Google Scholar] [CrossRef]
- Lan, R.; Tao, S. Ammonia as a suitable fuel for fuel cells. Front. Energy Res. 2014, 2, 35. [Google Scholar] [CrossRef]
- Petit, E.; Miele, P.; Demirci, U.B. By-product carrying humidified hydrogen: An underestimated issue in the field of hydrolysis of sodium borohydride NaBH4. ChemSusChem 2016, 9, 1777–1780. [Google Scholar] [CrossRef]
- Lapena-Rey, N.; Blanco, J.A.; Ferreyra, E.; Lemus, J.L.; Pereira, S.; Serrot, E. A fuel cell powered unmanned aerial vehicle for low altitude surveillance missions. Int. J. Hydrog. Energy 2017, 42, 6926–6940. [Google Scholar] [CrossRef]
- Kildahl, N.K. Bond energy data summarized. J. Chem. Educ. 1995, 72, 423–424. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrog. Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Mohajeri, N.; T-Raissi, A.; Adebiyi, O. Hydrolytic cleavage of ammonia-borane complex for hydrogen production. J. Power Sources 2007, 167, 482–485. [Google Scholar] [CrossRef]
- Spessard, J.E. Investigations of borane equilibria in neutral salt solutions. J. Inorg. Nucl. Chem. 1970, 32, 2607–2613. [Google Scholar] [CrossRef]
- Mesmer, R.E.; Baes, C.F.; Sweeton, F.H. Acidity measurements at elevated temperatures. VI. Boric acid equilibria. Inorg. Chem. 1972, 11, 537–543. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Fang, C.H.; Fang, Y.; Zhu, F.Y.; Cao, L.D. Polyborates in aqueous borate solution at 298.15 K. Asian J. Chem. 2012, 24, 29–32. [Google Scholar]
- Rachiero, G.P.; Demirci, U.B.; Miele, P. Bimetallic RuCo and RuCu catalysts supported on γ-Al2O3. A comparative study of their activity in hydrolysis of ammonia-borane. Int. J. Hydrog. Energy 2011, 36, 7051–7065. [Google Scholar] [CrossRef]
- Valero-Pedraza, M.J.; Alligier, D.; Petit, E.; Cot, D.; Granier, D.; Adil, K.; Yot, P.G.; Demirci, U.B. Diammonium tetraborate dihydrate as hydrolytic by-product of ammonia borane in aqueous alkaline conditions. Int. J. Hydrog. Energy 2020, 45, 9926–9935. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Liu, C.H.; Chen, B.H. The concept about the regeneration of spent borohydrides and used catalysts from green electricity. Materials 2015, 8, 3456–3466. [Google Scholar] [CrossRef]
- Monteverde, M.; Magistri, L. Hydrogen from sodium borohydride and fossil source: An energetic and economical comparison. Int. J. Hydrog. Energy 2012, 37, 5452–5460. [Google Scholar] [CrossRef]
- Özkar, S. Transition metal nanoparticle catalysts in releasing hydrogen from the methanolysis of ammonia borane. Int. J. Hydrog. Energy 2020, 45, 7881–7891. [Google Scholar] [CrossRef]
- Kalidindi, S.B.; Sanyal, U.; Jagirdar, B.R. Nanostructured Cu and Cu@Cu2O core shell catalysts for hydrogen generation from ammonia-borane. Phys. Chem. Chem. Phys. 2008, 10, 5870–5874. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Huang, M.; Lu, Z.H.; Yang, Y.; Zhang, Y.; Chen, X.; Yang, Z. Methanolysis of ammonia borane by shape-controlled mesoporous copper nanostructures for hydrogen generation. Dalton Trans. 2014, 44, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Yurderi, M.; Bulut, A.; Ertaş, İ.E.; Zahmakıran, M.; Kaya, M. Supported copper–copper oxide nanoparticles as active; stable and low-cost catalyst in the methanolysis of ammonia-borane for chemical hydrogen storage. Appl. Catal. B Env. 2015, 165, 169–175. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Li, Q.; Hou, C.C.; Wang, C.; Peng, C.Y.; Lopez, N.; Chen, Y. Enhancing electrostatic interactions to activate polar molecules: Ammonia borane methanolysis on Cu/Co(OH)2 nanohybrid. Catal. Sci. Technol. 2019, 9, 2828–2835. [Google Scholar] [CrossRef]
- Kalidindi, S.B.; Vernekar, A.A.; Jagirdar, B.R. Co-Co2B, Ni-Ni3B and Co-Ni-B nanocomposites catalyzed ammonia-borane methanolysis for hydrogen generation. Phys. Chem. Chem. Phys. 2009, 11, 770–775. [Google Scholar] [CrossRef]
- Sun, D.; Mazumder, V.; Metin, Ö.; Sun, S. Methanolysis of ammonia borane by CoPd nanoparticles. ACS Catal. 2012, 2, 1290–1295. [Google Scholar] [CrossRef]
- Murathan, H.B.; Özkan, G.; Akkuş, M.S.; Özgür, D.Ö.; Özkan, G. Hydrogen production from the methanolysis of ammonia borane by Pd-Co/Al2O3 coated monolithic catalyst. Int. J. Hydrog. Energy 2018, 43, 10728–10733. [Google Scholar] [CrossRef]
- Özhava, D.; Kiliçaslan, N.Z.; Özkar, S. PVP-stabilized nickel(0) nanoparticles as catalyst in hydrogen generation from the methanolysis of hydrazine borane or ammonia borane. Appl. Catal. B Env. 2015, 162, 573–582. [Google Scholar] [CrossRef]
- Kantürk Figen, A. Improved catalytic performance of metal oxide catalysts fabricated with electrospinning in ammonia borane methanolysis for hydrogen production. Int. J. Hydrog. Energy 2019, 44, 28451–28462. [Google Scholar] [CrossRef]
- Erdoğan, H.; Metin, Ö.; Özkar, S. In situ-generated PVP-stabilized palladium(0) nanocluster catalyst in hydrogen generation from the methanolysis of ammonia-borane. Phys. Chem. Chem. Phys. 2009, 11, 10519–10525. [Google Scholar] [CrossRef] [PubMed]
- Karataş, Y.; Gülcan, M.; Çelebi, M.; Zahmakıran, M. Pd(0) nanoparticles decorated on graphene nanosheets (GNS): SynthesiS, definition and testing of the catalytic performance in the methanolysis of ammonia borane at room conditions. ChemistrySelect 2017, 2, 9628–9635. [Google Scholar] [CrossRef]
- Eghbali, P.; Gürbüz, M.U.; Ertürk, A.S.; Metin, Ö. In situ synthesis of dendrimer-encapsulated palladium(0) nanoparticles as catalysts for hydrogen production from the methanolysis of ammonia borane. Int. J. Hydrog. Energy 2020, 45. in press. [Google Scholar] [CrossRef]
- Çalışkan, S.; Zahmakıran, M.; Özkar, S. Zeolite confined rhodium(0) nanoclusters as highly active; reusable; and long-lived catalyst in the methanolysis of ammonia-borane. Appl. Catal. B Env. 2010, 93, 387–394. [Google Scholar] [CrossRef]
- Özhava, D.; Özkar, S. Rhodium(0) nanoparticles supported on hydroxyapatite nanospheres and further stabilized by dihydrogen phosphate ion: A highly active catalyst in hydrogen generation from the methanolysis of ammonia borane. Int. J. Hydrog. Energy 2015, 40, 10491–10501. [Google Scholar] [CrossRef]
- Özhava, D.; Özkar, S. Nano-alumina-supported rhodium(0) nanoparticles as catalyst in hydrogen generation from the methanolysis of ammonia borane. Mol. Catal. 2017, 439, 50–59. [Google Scholar] [CrossRef]
- Luo, W.; Cheng, W.; Hu, M.; Wang, Q.; Cheng, X.; Zhang, Y.; Wang, Y.; Gao, D.; Bi, J.; Fan, G. Ultrahigh catalytic activity of L-proline-functionalized Rh nanoparticles for methanolysis of ammonia borane. ChemSusChem 2019, 12, 535–541. [Google Scholar] [CrossRef]
- Dai, H.B.; Kang, X.D.; Wang, P. Ruthenium nanoparticles immobilized in montmorillonite used as catalyst for methanolysis of ammonia borane. Int. J. Hydrog. Energy 2010, 35, 10317–10323. [Google Scholar] [CrossRef]
- Erdoğan, H.; Metin, Ö.; Özkar, S. Hydrogen generation from the methanolysis of ammonia borane catalyzed by in situ generated polymer stabilized ruthenium(0) nanoclusters. Catal. Today 2011, 170, 93–98. [Google Scholar] [CrossRef]
- Peng, S.; Liu, J.; Zhang, J.; Wang, F. An improved preparation of graphene supported ultrafine ruthenium(0) NPs: Very active and durable catalysts for H2 generation from methanolysis of ammonia borane. Int. J. Hydrog. Energy 2015, 40, 10856–10866. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Raju, B.C.; Gagare, P.D. One-pot synthesis of ammonia-borane and trialkylamine-boranes from trimethyl borate. Org. Lett. 2012, 14, 6119–6121. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E. Conversion of dihydridodiammineboron(III) borohydride to ammonia-borane without hydrogen evolution. Inorg. Chem. 1973, 12, 1954–1955. [Google Scholar] [CrossRef]
- Rassat, S.D.; Aardahl, C.L.; Autrey, T.; Smith, R.S. Thermal stability of ammonia borane: A case study for exothermic hydrogen storage materials. Energy Fuels 2010, 24, 2596–2606. [Google Scholar] [CrossRef]
- Bluhm, M.E.; Bradley, M.G.; Butterick, R., III; Kusari, U.; Sneddon, L.G. Amineborane-based chemical hydrogen storage: Enhanced ammonia borane dehydrogenation in ionic liquids. J. Am. Chem. Soc. 2006, 128, 7748–7749. [Google Scholar] [CrossRef] [PubMed]
- Baitalow, F.; Baumann, J.; Wolf, G.; Jaenicke-Rössler, A.; Leitner, G. Thermal decomposition of B-N-H compounds investigated by using combined thermoanalytical methods. Thermochim. Acta 2002, 391, 159–168. [Google Scholar] [CrossRef]
- Wolf, G.; Baumann, J.; Baitalow, F.; Hoffmann, F.P. Calorimetric process monitoring of thermal decomposition of B-N-H compounds. Thermochim. Acta 2000, 343, 19–25. [Google Scholar] [CrossRef]
- Stowe, A.C.; Shaw, W.J.; Linehan, J.C.; Schmid, B.; Autrey, T. In situ solid state 11B MAS-NMR studies of the thermal decomposition of ammonia borane: Mechanistic studies of the hydrogen release pathways from a solid state hydrogen storage material. Phys. Chem. Chem. Phys. 2007, 9, 1831–1836. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Karkamkar, A.; Hess, N.J.; Bowden, M.; Rassat, S.; Zheng, F.; Rappe, K.; Autrey, T. The effects of chemical additives on the induction phase in solid-state thermal decomposition of ammonia borane. Chem. Mater. 2008, 20, 5332–5336. [Google Scholar] [CrossRef]
- Hu, M.G.; Geanangel, R.A.; Wendlandt, W.W. The thermal decomposition of ammonia borane. Thermochim. Acta 1978, 23, 249–255. [Google Scholar] [CrossRef]
- Al-Kukhun, A.; Hwang, H.T.; Varma, A. Mechanistic studies of ammonia borane dehydrogenation. Int. J. Hydrog. Energy 2013, 38, 169–179. [Google Scholar] [CrossRef]
- Kang, X.; Fang, Z.; Kong, L.; Cheng, H.; Yao, X.; Lu, G.; Wang, P. Ammonia borane destabilized by lithium hydride: An advanced on-board hydrogen storage material. Adv. Mater. 2008, 20, 2756–2759. [Google Scholar] [CrossRef] [PubMed]
- Sit, V.; Geanangel, R.A.; Wendlandt, W.W. The thermal dissociation of NH3BH3. Thermochim. Acta 1987, 113, 379–382. [Google Scholar] [CrossRef]
- Frueh, S.; Kellett, R.; Mallery, C.; Moller, T.; Willis, W.S.; King’ondu, C.; Suib, S.L. Pyrolytic decomposition of ammonia borane to boron nitride. Inorg. Chem. 2011, 50, 783–792. [Google Scholar] [CrossRef]
- Baumann, J.; Baitalow, F.; Wolf, G. Thermal decomposition of polymeric aminoborane (H2BNH2)x under hydrogen release. Thermochim. Acta 2005, 430, 9–14. [Google Scholar] [CrossRef]
- Wolstenholme, D.J.; Traboulsee, K.T.; Hua, Y.; Calhoun, L.A.; McGrady, G.S. Thermal desorption of hydrogen from ammonia borane: Unexpected role of homopolar B-H···H-B interactions. Chem. Commun. 2012, 48, 2597–2599. [Google Scholar] [CrossRef]
- Roy, B.; Hajari, A.; Manna, J.; Sharma, P. Supported ammonia borane decomposition through enhanced homopolar B-B coupling. Dalton Trans. 2018, 47, 6570–6579. [Google Scholar] [CrossRef]
- Petit, J.F.; Demirci, U.B. Mechanistic insights into dehydrogenation of partially deuterated ammonia borane NH3BD3 being heating to 200 °C. Inorg. Chem. 2019, 58, 489–494. [Google Scholar] [CrossRef]
- Petit, J.F.; Dib, E.; Gaveau, P.; Miele, P.; Alonso, B.; Demirci, U.B. 11B MAS NMR study of the thermolytic dehydrocoupling of two ammonia boranes upon the release of one equivalent of H2 at isothermal conditions. ChemistrySelect 2017, 2, 9396–9401. [Google Scholar] [CrossRef]
- Summerscales, O.T.; Gordon, J.C. Regeneration of ammonia borane from spent fuel materials. Dalton Trans. 2013, 42, 10075–10084. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Gupta, S.; Caporini, M.A.; Pecharsky, V.A.; Pruski, M. Mechanism of solid-state thermolysis of ammonia borane: A 15N NMR study using fast magic-ange spinning and dynamic nuclear polarization. J. Phys. Chem. C 2014, 118, 19548–19555. [Google Scholar] [CrossRef]
- Nakagawa, T.; Burrell, A.A.; Del Sesto, R.E.; Janicke, M.T.; Nekimken, A.L.; Purdy, G.M.; Paik, B.; Zhong, R.Q.; Semelsberger, T.A.; Dais, B.L. Physical, structural, and dehydrogenation properties of ammonia borane in ionic liquids. RSC Adv. 2014, 4, 21681–21687. [Google Scholar] [CrossRef]
- Himmelberger, D.W.; Alden, L.R.; Bluhm, M.E.; Sneddon, L.G. Ammonia borane hydrogen release in ionic liquids. Inorg. Chem. 2009, 48, 9883–9889. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Zheng, Y.; Gore, J.P. An experimental study of neat and ionic liquid-aided ammonia borane thermolysis. J. Power Sources 2011, 196, 734–740. [Google Scholar] [CrossRef]
- Ahluwalia, R.A.; Peng, J.A.; Hua, T.Q. Hydrogen release from ammonia borane dissolved in an ionic liquid. Int. J. Hydrog. Energy 2011, 36, 15689–15697. [Google Scholar] [CrossRef]
- Sahler, S.; Strum, S.; Kessler, M.T.; Prechtl, M.H.G. The role of ionic liquids in hydrogen storage. Chem. Eur. J. 2014, 20, 8934–8941. [Google Scholar] [CrossRef]
- Valero-Pedraza, M.J.; Martin-Cortes, A.; Navarrete, A.; Bermejo, M.D.; Martín, A. Kinetics of hydrogen release from dissolutions of ammonia borane in different ionic liquids. Energy 2015, 91, 742–750. [Google Scholar] [CrossRef]
- Koska, J.F.; Schellenberg, R.; Baitalow, F.; Smolinka, T.; Mertens, F. Concentration-dependent dehydrogenation of ammonia-borane/triglyme mixtures. Eur. J. Inorg. Chem. 2012, 2012, 49–54. [Google Scholar] [CrossRef]
- Shaw, W.J.; Linehan, J.C.; Szymczak, N.A.; Heldebrant, D.J.; Yonker, C.; Camaioni, D.M.; Baker, R.T.; Autrey, T. In situ multinuclear NMR spectroscopic studies of the thermal decomposition of ammonia borane in solution. Angew. Chem. Int. Ed. 2008, 47, 7493–7496. [Google Scholar] [CrossRef]
- Denney, M.C.; Pons, V.; Hebden, T.J.; Heinekey, D.M.; Goldberg, K.I. Efficient catalysis of ammonia borane dehydrogenation. J. Am. Chem. Soc. 2006, 128, 12048–12049. [Google Scholar] [CrossRef] [PubMed]
- Rossin, A.; Caporali, M.; Gonsalvi, L.; Guerri, A.; Lledós, A.; Peruzzini, M.; Zanobini, F. Selective B–H versus N–H bond activation in ammonia borane by [Ir(dppm)2]OTf. Eur. J. Inorg. Chem. 2009, 2009, 3055–3059. [Google Scholar] [CrossRef]
- Keaton, R.J.; Blacquiere, J.M.; Baker, R.T. Base metal catalyzed dehydrogenation of ammonia-borane for chemical hydrogen storage. J. Am. Chem. Soc. 2007, 129, 1844–1845. [Google Scholar] [CrossRef] [PubMed]
- Conley, B.L.; Williams, T.J. Dehydrogenation of ammonia-borane by Shvo’s catalyst. Chem. Commun. 2010, 46, 4815–4817. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.T.; Gordon, J.C.; Hamilton, C.W.; Henson, N.J.; Lin, P.H.; Maguire, S.; Murugesu, M.; Scott, B.L.; Smythe, N.C. Iron complex-catalyzed ammonia-borane dehydrogenation. A potential route toward B-N-containing polymer motifs using earth-abundant metal catalysts. J. Am. Chem. Soc. 2012, 134, 5598–5609. [Google Scholar] [CrossRef]
- Glüer, A.; Förster, M.; Celinski, V.R.; Schmedt Auf Der Günne, J.; Holthausen, M.C.; Schneider, S. Highly active iron catalyst for ammonia borane dehydrocoupling at room temperature. ACS Catal. 2015, 5, 7214–7217. [Google Scholar] [CrossRef]
- Marziale, A.N.; Friedrich, A.; Klopsch, I.; Drees, M.; Celinski, V.R.; Schmedt Auf Der Günne, J.; Schneider, S. The mechanism of borane-amine dehydrocoupling with bifunctional ruthenium catalysts. J. Am. Chem. Soc. 2013, 135, 13342–13355. [Google Scholar] [CrossRef]
- Rossin, A.; Rossi, A.; Peruzzini, M.; Zanobini, F. Chemical hydrogen storage: Ammonia borane dehydrogenation catalyzed by NP3 ruthenium hydrides (NP3 = N(CH2CH2PPh2)3). ChemPlusChem 2014, 79, 1316–1325. [Google Scholar] [CrossRef]
- Zhang, X.; Kam, L.; Williams, T.J. Dehydrogenation of ammonia borane through the third equivalent of hydrogen. Dalton Trans. 2016, 45, 7672–7677. [Google Scholar] [CrossRef]
- Kim, S.A.; Han, W.S.; Kim, T.J.; Kim, T.Y.; Nam, S.W.; Mitoraj, M.; Piekos, L.; Michalak, A.; Hwang, S.J.; Kang, S.O. Palladium catalysts for dehydrogenation of ammonia borane with preferential B-H activation. J. Am. Chem. Soc. 2010, 132, 9954–9955. [Google Scholar] [CrossRef]
- Rossin, A.; Bottari, G.; Lozana-Vila, A.M.; Paneque, M.; Peruzzini, M.; Rossi, A.; Zanobini, F. Catalytic amine-borane dehydrogenation by a PCP-pincer palladium complex: A combined experimental and DFT analysis of the reaction mechanism. Dalton Trans. 2013, 42, 3533–3541. [Google Scholar] [CrossRef] [PubMed]
- Rossin, A.; Peruzzini, M. Ammonia-borane and amine-borane dehydrogenation mediated by complex metal composites. Chem. Rev. 2016, 116, 8848–8872. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kam, L.; Trerise, R.; Williams, T.J. Ruthenium-catalyzed ammonia borane dehydrogenation: Mechanism and utility. Acc. Chem. Res. 2017, 50, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Shimbayashi, T.; Fujita, K.I. Metal-catalyzed hydrogenation and dehydrogenation reactions for efficient hydrogen storage. Tetrahedron 2020, 2020, 130946. [Google Scholar] [CrossRef]
- Hasenbeck, M.; Becker, J.; Gellrich, U. Efficient organocatalytic dehydrogenation of ammonia borane. Angew. Chem. Int. Ed. 2020, 59, 1590–1594. [Google Scholar] [CrossRef]
- Benedetto, S.D.; Carewska, M.; Cento, C.; Gislon, P.; Pasquali, M.; Scaccia, S.; Prosini, P.P. Effect of milling and doping on decomposition of NH3BH3 complex. Thermochim. Acta 2006, 441, 184–190. [Google Scholar] [CrossRef]
- He, T.; Xiong, Z.; Wu, G.; Chu, H.; Wu, C.; Zhang, T.; Chen, P. Nanosized Co- and Ni-catalyzed ammonia borane for hydrogen storage. Chem. Mater. 2009, 21, 2315–2318. [Google Scholar] [CrossRef]
- Benzouaa, R.; Demirci, U.B.; Chiriac, R.; Toche, F.; Miele, P. Metal chloride-doped ammonia borane thermolysis: Positive effect on induction period as well as hydrogen and borazine release. Thermochim. Acta 2010, 509, 81–86. [Google Scholar] [CrossRef]
- Toche, F.; Chiriac, R.; Demirci, U.B.; Miele, P. Ammonia borane thermolytic decomposition in the presence of metal (II) chlorides. Int. J. Hydrog. Energy 2012, 37, 6749–6755. [Google Scholar] [CrossRef]
- Chiriac, R.; Toche, F.; Demirci, U.B.; Miele, P. Instability of the CuCl2-NH3BH3 mixture followed by TGA and DSC. Thermochim. Acta 2013, 567, 100–106. [Google Scholar] [CrossRef]
- Chiriac, R.; Toche, F.; Demirci, U.B.; Krol, O.; Miele, P. Ammonia borane decomposition in the presence of cobalt halides. Int. J. Hydrog. Energy 2011, 36, 12955–12964. [Google Scholar] [CrossRef]
- Li, Y.; Fang, F.; Song, Y.; Li, Y.; Zhang, Q.; Ouyang, L.; Zhu, M.; Sun, D. Enhanced dehydrogenation of ammonia borane by reaction with alkaline earth metal chlorides. Int. J. Hydrog. Energy 2012, 37, 4274–4279. [Google Scholar] [CrossRef]
- Biliskov, N.; Vojta, D.; Kótai, L.; Szilágyi, I.M.; Hunyadi, D.; Pasinszki, T.; Grgac, S.F.; Borgschulte, A.; Züttel, A. High influence of potassium bromide on thermal decomposition of ammonia borane. J. Phys. Chem. C 2016, 120, 25276–25288. [Google Scholar] [CrossRef]
- Kang, X.; Ma, L.; Fang, Z.; Gao, L.; Luo, J.; Wang, S.; Wang, P. Promoted hydrogen release from ammonia borane by mechanically milling with magnesium hydride: A new destabilizing approach. Phys. Chem. Chem. Phys. 2009, 11, 2507–2513. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Kang, X.; Wang, P. Renewed insight into the promoting mechanism of magnesium hydride on ammonia borane. ChemPhysChem 2010, 11, 2152–2157. [Google Scholar] [CrossRef]
- Weng, B.; Wu, Z.; Li, Z.; Leng, H. Dehydrogenation performance of NH3BH3 with Mg2NiH4 addition. Thermochim. Acta 2011, 524, 23–28. [Google Scholar] [CrossRef]
- Kang, X.D.; Luo, J.H.; Wang, P. Efficient and highly rapid hydrogen release from ball-milled 3NH3BH3/MMgH3 (M = Na, K, Rb) mixtures at low temperatures. Int. J. Hydrog. Energy 2012, 37, 4259–4266. [Google Scholar] [CrossRef]
- Choi, Y.J.; Xu, Y.; Shaw, W.J.; Rönnebro, E.C.E. Hydrogen storage properties of new hydrogen-rich BH3NH3-metal hydride (TiH2; ZrH2; MgH2; and/or CaH2) composite systems. J. Phys. Chem. C 2012, 116, 8349–8358. [Google Scholar] [CrossRef]
- Kobayashi, T.; Hlova, I.Z.; Singh, N.A.; Pecharsky, V.A.; Pruski, M. Solid-state NMR study of Li-assisted dehydrogenation of ammonia borane. Inorg. Chem. 2012, 51, 4108–4115. [Google Scholar] [CrossRef]
- Wan, L.; Chen, J.; Tan, Y.; Gu, Q.; Yu, X. Ammonia borane destabilized by aluminium hydride: A mutual enhancement for hydrogen release. Int. J. Hydrog. Energy 2015, 40, 1047–1053. [Google Scholar] [CrossRef]
- Neiner, D.; Karkamkar, A.; Linehan, J.C.; Arey, B.; Autrey, T.; Kauzlarich, S.M. Promotion of hydrogen release from ammonia borane with mechanically activated hexagonal boron nitride. J. Phys. Chem. C 2009, 113, 1098–1103. [Google Scholar] [CrossRef]
- Neiner, D.; Luedtke, A.; Karkamkar, A.; Shaw, W.; Wang, J.; Browning, N.D.; Autrey, T.; Kauzlarich, S.M. Decomposition pathway of ammonia borane on the surface of nano-BN. J. Phys. Chem. C 2010, 114, 13935–13941. [Google Scholar] [CrossRef]
- Gangal, A.C.; Kale, P.; Edla, R.; Manna, J.; Sharma, P. Study of kinetics and thermal decomposition of ammonia borane in presence of silicon nanoparticles. Int. J. Hydrog. Energy 2012, 37, 6741–6748. [Google Scholar] [CrossRef]
- Jin, J.H.; Shin, S.; Jung, J.; Attia, N.F. Solid-phase hydrogen storage based on NH3BH3-SiO2 nanocomposite for thermolysis. J. Nanomater. 2019, 2019, 6126031. [Google Scholar] [CrossRef]
- Kumar, D.; Mangalvedekar, H.A.; Mahajan, S.K. Nano-nickel catalytic dehydrogenation of ammonia borane. Mater. Renew. Sust. Energy 2014, 3, 23. [Google Scholar] [CrossRef][Green Version]
- Hwang, H.T.; Varma, A. Effect of boric acid on thermal dehydrogenation of ammonia borane: Mechanistic studies. Int. J. Hydrog. Energy 2013, 38, 1925–1931. [Google Scholar] [CrossRef]
- Ergüven, H.; Kantürk Figen, A.; Pişkin, S. Ammonia borane-boron composites for hydrogen release: Thermolysis kinetics. Energy Sources A 2017, 39, 613–617. [Google Scholar] [CrossRef]
- Shin, S.; Jin, J.H.; Jung, J. Sugar acid-assisted thermolysis of all-solid-state ammonia borane hydrogen fuel. Energy Technol. 2020, 8, 1901195. [Google Scholar] [CrossRef]
- Kim, G.J.; Boone, A.M.; Chesnut, M.; Shin, J.H.; Jung, J.; Hwang, H.T. Enhanced thermal dehydrogenation of ammonia borane by D-mannitol. Ind. Eng. Chem. Res. 2020, 59, 620–626. [Google Scholar] [CrossRef]
- Kim, Y.; Baek, H.; Lee, J.H.; Yeo, S.; Kim, K.; Hwang, S.J.; Eun, B.; Nam, S.W.; Lim, T.H.; Yoon, C.W. Metal-free; polyether-mediated H2-release from ammonia borane: Roles of hydrogen bonding interactions in promoting dehydrogenation. Phys. Chem. Chem. Phys. 2013, 15, 19584–19594. [Google Scholar] [CrossRef]
- Yeo, S.; Kim, Y.; Lee, J.H.; Kim, A.; Jang, J.H.; Hong, S.A.; Nam, S.W.; Yoon, C.W. Promotional effects of oxygen-containing additives on ammonia borane dehydrogenation for polymer electrolyte membrane fuel cell applications. Int. J. Hydrog. Energy 2014, 39, 21786–21795. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.; Yeo, S.; Kim, A.; Koh, J.E.; Seo, J.E.; Shin, S.J.; Choi, D.K.; Yoon, C.W.; Nam, S.W. Development of a continuous hydrogen generator fueled by ammonia borane for portable fuel cell applications. J. Power Sources 2013, 229, 170–178. [Google Scholar] [CrossRef]
- de Jongh, P.E.; Adelhelm, P. Nanosizing and nanoconfinement: New strategies towards meeting hydrogen storage goals. ChemSusChem 2010, 3, 1332–1348. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.A.; Zhao, H.; Yao, X.D. Nano-confined ammonia borane for chemical hydrogen storage. Front. Chem. Sci. Eng. 2012, 6, 27–33. [Google Scholar] [CrossRef]
- Rossin, A.; Tuci, G.; Luconi, L.; Giambastiani, G. Metal-organic frameworks as heterogenous catalysts in hydrogen production from lightweight inorganic hydrides. ACS Catal. 2017, 7, 5035–5045. [Google Scholar] [CrossRef]
- Rueda, M.; Sanz-Moral, L.M.; Martin, A. Innovative methods to enhance the properties of solid hydrogen storage materials based on hydrides through nanoconfinement: A review. J. Supercrit. Fluids 2018, 141, 198–217. [Google Scholar] [CrossRef]
- Lai, S.W.; Lin, H.L.; Yu, T.L.; Lee, L.P.; Weng, B.J. Hydrogen release from ammonia borane embedded in mesoporous silica scaffolds: SBA-15 and MCM-41. Int. J. Hydrog. Energy 2012, 37, 14393–14404. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Akins, D.L.; Lee, J.W. Effect of composition on dehydrogenation of mesoporous silica/ammonia borane nanocomposites. Ind. Eng. Chem. Res. 2011, 50, 10024–10028. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Herron, R.; Phillips, A.D. Towards an understanding of the beneficial effect of mesoporous materials on the dehydrogenation characteristics of NH3BH3. Appl. Catal. B Env. 2017, 201, 182–188. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, X.; Yang, S.; Li, D.; Cheng, F.; Tao, Z.; Chen, J. Silica hollow nanospheres as new nanoscaffold materials to enhance hydrogen releasing from ammonia borane. Phys. Chem. Chem. Phys. 2011, 13, 18592–18599. [Google Scholar] [CrossRef]
- Rueda, M.; Sanz-Moral, L.M.; Segovia, J.J.; Martín, A. Improvement of the kinetics of hydrogen release from ammonia borane confined in silica aerogel. Microp. Mesop. Mater. 2017, 237, 189–200. [Google Scholar] [CrossRef]
- Colognesi, D.; Ulivi, L.; Zoppi, M.; Ramirez-Cuesta, A.J.; Orecchini, A.; Karkamkar, A.J.; Fichtner, M.; Gil Bardaji, E.; Zhao-Karger, Z. Hydrogen-storage materials dispersed into nanoporous substrates studied through incoherent inelastic neutron scattering. J. Alloys Compd. 2012, 538, 91–99. [Google Scholar] [CrossRef]
- Kim, H.; Karkamkar, A.; Autrey, T.; Chupas, P.; Proffen, T. Determination of structure and phase transition of light element nanocomposites in mesoporous silica: Case study of NH3BH3 in MCM-41. J. Am. Chem. Soc. 2009, 131, 13749–13755. [Google Scholar] [CrossRef]
- Paolone, A.; Palumbo, O.; Rispoli, P.; Cantelli, R.; Autrey, T.; Karkamkar, A. Absence of the structural phase transition in ammonia borane dispersed in mesoporous silica: Evidence of novel thermodynamic properties. J. Phys. Chem. C 2009, 113, 10319–10321. [Google Scholar] [CrossRef]
- Valero-Pedraza, M.J.; Gascon, V.; Carreon, M.; Leardini, F.; Ares, J.R.; Martin, A.; Sanchez-Sanchez, M.; Banares, M. Operando Raman-mass spectrometry investigation of hydrogen release by thermolysis of ammonia borane confined in mesoporous materials. Microp. Mesop. Mater. 2016, 226, 454–465. [Google Scholar] [CrossRef]
- Rueda, M.; Sanz-Moral, L.M.; Nieto-Márquez, A.; Longone, P.; Mattea, F.; Martin, A. Production of silica aerogel microparticles loaded with ammonia borane by batch and semicontinuous supercritical drying techniques. J. Supercrit. Fluids. 2014, 92, 299–310. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, X.; Yang, J.H.; Gao, X.; Yin, L.; Zhao, Y.; Zhang, B. Encapsulation of ammonia borane in Pd/halloysite nanotubes for efficient thermal dehydrogenation. ACS Sust. Chem. Eng. 2010, 8, 2122–2129. [Google Scholar] [CrossRef]
- Richard, J.; Cid, S.L.; Rouquette, J.; van de Lee, A.; Bernard, S.; Haines, J. Pressure-induced insertion of ammonia borane in the siliceous zeolite; silicalite-1F. J. Phys. Chem. C 2016, 120, 9334–9340. [Google Scholar] [CrossRef]
- Feaver, A.; Sepehri, S.; Shamberger, P.; Stowe, A.; Autrey, T.; Cao, G. Coherent carbon cryogel-ammonia borane nanocomposites for H2 storage. J. Phys. Chem. B 2007, 111, 7469–7472. [Google Scholar] [CrossRef]
- Sepehri, S.; García, B.B.; Cao, G. Influence of surface chemistry on dehydrogenation in carbon cryogel ammonia borane nanocomposites. Eur. J. Inorg. Chem. 2009, 2009, 599–603. [Google Scholar] [CrossRef]
- Moussa, G.; Bernard, S.; Demirci, U.B.; Chiriac, R.; Miele, P. Room-temperature hydrogen release from activated carbon-confined ammonia borane. Int. J. Hydrog. Energy 2012, 37, 13437–13445. [Google Scholar] [CrossRef]
- Bravo Diaz, L.; Hanlon, J.M.; Bielewski, M.; Milewska, A.; Gregory, D.H. Ammonia borane based nanocomposites as solid-state hydrogen stores for portable power applications. Energy Technol. 2018, 6, 583–594. [Google Scholar] [CrossRef]
- Li, L.; Yao, X.; Sun, C.; Du, A.; Cheng, L.; Zhu, Z.; Yu, C.; Zou, J.; Simth, S.C.; Wang, P.; et al. Lithium-catalyzed dehydrogenation of ammonia borane within mesoporous carbon framework for chemical hydrogen storage. Adv. Funct. Mater. 2009, 19, 265–271. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, D.; Chen, B.; Liu, Z.; Xia, Q.; Zhu, Y.; Xia, Y. Improved hydrogen release from ammonia borane confined in microporous carbon with narrow pore size distribution. J. Mater. Chem. A 2017, 5, 15395–15400. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, G.; Ge, Y.; Wang, C.; Guo, Z.; Li, X.; Yu, X. Ammonia borane confined by nitrogen-containing carbon nanotubes: Enhanced dehydrogenation properties originating from synergetic catalysis and nanoconfinement. J. Mater. Chem. A 2015, 3, 20494–20499. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, H.; Chen, X.; Wu, L.; Yu, X. Graphene oxide based recyclable dehydrogenation of ammonia borane within a hybrid nanostructure. J. Am. Chem. Soc. 2012, 134, 5464–5467. [Google Scholar] [CrossRef]
- So, S.H.; Jang, J.H.; Sung, S.J.; Yang, S.J.; Nam, K.T.; Park, C.R. Demonstration of the nanosize effect of carbon nanomaterials on the dehydrogenation temperature of ammonia borane. Nanoscale Adv. 2019, 1, 4697–4703. [Google Scholar] [CrossRef]
- Klooster, W.T.; Koetzle, T.F.; Siegbahn, P.E.M.; Richardson, T.B.; Crabtree, R.H. Study of the N-H···H-B dihydrogen bond including the crystal structure of BH3NH3 by neutron diffraction. J. Am. Chem. Soc. 1999, 121, 6337–6343. [Google Scholar] [CrossRef]
- Kuang, A.; Liu, T.; Kuang, M.; Yang, R.; Huang, R.; Wang, G.; Yuan, H.; Chen, H.; Yang, X. Hydrogen bonding-mediated dehydrogenation in the ammonia borane combined graphene oxide systems. Phys. E 2018, 97, 75–81. [Google Scholar] [CrossRef]
- Sepehri, S.; Feaver, A.; Shaw, W.J.; Howard, C.J.; Zhang, Q.; Autrey, T.; Cao, G. Spectroscopic studies of dehydrogenation of ammonia borane in carbon cryogel. J. Phys. Chem. B 2007, 111, 14285–14289. [Google Scholar] [CrossRef]
- Sepehri, S.; Garcia, B.B.; Zhang, Q.; Cao, G. Influences of surface chemistry on dehydrogenation of ammonia borane in porous carbon scaffold. Adv. Mater. Res. 2010, 132, 19–28. [Google Scholar] [CrossRef]
- Champet, S.; van den Berg, J.; Szczesny, R.; Godula-Jopek, A.; Gregory, D.H. Nano-inclusion in one step: Spontaneous ice-templating of porous hierarchical nanocomposites for selective hydrogen release. Sust. Energy Fuels 2019, 3, 396–400. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, G.; Lu, G.; Qiu, S.; Yao, X. Ammonia borane confined by a metal-organic framework for chemical hydrogen storage: Enhancing kinetics and eliminating ammonia. J. Am. Chem. Soc. 2010, 132, 1490–1491. [Google Scholar] [CrossRef] [PubMed]
- Gadipelli, S.; Ford, J.; Zhou, W.; Wu, H.; Udovic, T.J.; Yildirim, T. Nanoconfinement and catalytic dehydrogenation of ammonia borane by magnesium-metal-organic-framework-74. Chem. Eur. J. 2011, 17, 6043–6047. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.; Travis, W.; Ford, J.; Wu, H.; Guo, Z.X.; Yildirim, T. Nanoconfined ammonia borane in a flexible metal-organic framework Fe–MIL-53: Clean hydrogen release with fast kinetics. J. Mater. Chem. A 2013, 1, 4167–4172. [Google Scholar] [CrossRef]
- Si, X.L.; Sun, L.X.; Xu, F.; Jiao, C.L.; Li, F.; Liu, S.S.; Zhang, J.; Song, L.F.; Jiang, C.H.; Wang, S.; et al. Improved hydrogen desorption properties of ammonia borane by Ni-modified metal-organic frameworks. Int. J. Hydrog. Energy 2011, 36, 6698–6704. [Google Scholar] [CrossRef]
- Jeong, H.M.; Shin, W.H.; Park, J.H.; Choi, J.H.; Kang, J.K. A metal–organic framework as a chemical guide to control hydrogen desorption pathways of ammonia borane. Nanoscale 2014, 6, 6526–6530. [Google Scholar] [CrossRef]
- Yang, H.; Li, Z.; Liu, A.; Meng, F.; Niu, C. Clean hydrogen release from ammonia borane in a metal-organic framework with unsaturated coordinated Tm3+. J. Phys. Chem. C 2015, 119, 2260–2265. [Google Scholar] [CrossRef]
- Chung, J.Y.; Liao, C.W.; Chang, Y.W.; Chang, B.A.; Wang, H.; Li, J.; Wang, C.Y. Influence of metal-organic framework porosity on hydrogen generation from nanoconfined ammonia borane. J. Phys. Chem. C 2017, 121, 27369–27378. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Yang, H.; Sun, T.; Liu, K.; Wang, Z.; Niu, C. Improved thermal dehydrogenation of ammonia borane by MOF-5. RSC Adv. 2015, 5, 10746–10750. [Google Scholar] [CrossRef]
- Zhong, R.Q.; Zou, R.Q.; Nakagawa, T.; Janicke, M.; Semelsberger, T.A.; Burrell, A.K.; Del Sesto, R.E. Improved hydrogen release from ammonia-borane with ZIF-8. Inorg. Chem. 2012, 51, 2728–2730. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.; Ford, J.; Zhou, W.; Yildirim, T. Zn-MOF assisted dehydrogenation of ammonia borane: Enhanced kinetics and clean hydrogen generation. Int. J. Hydrog. Energy 2012, 37, 3633–3638. [Google Scholar] [CrossRef]
- Kong, S.; Dai, R.; Li, H.; Sun, W.; Wang, Y. Microwave hydrothermal synthesis of Ni-based metal-organic frameworks and their derived yolk-shell NiO for Li-ion storage and supported ammonia borane for hydrogen desorption. ACS Sust. Chem. Eng. 2015, 3, 1830–1838. [Google Scholar] [CrossRef]
- Wu, Y.J.; Wang, C.Y. Insight into the catalytic effects of open metal sites in metal-organic frameworks on hydride dehydrogenation via nanoconfinement. ACS Sust. Chem. Eng. 2019, 7, 16013–16025. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, J.; Zhang, X.; Cheng, F.; Liang, J.; Tao, Z.; Chen, J. A soft hydrogen storage material: Poly(methyl acrylate)-confined ammonia borane with controllable dehydrogenation. Adv. Mater. 2009, 22, 394–397. [Google Scholar] [CrossRef]
- Li, S.F.; Tang, Z.W.; Tan, Y.B.; Yu, X.B. Polyacrylamide blending with ammonia borane: A polymer supported hydrogen storage composite. J. Phys. Chem. C 2012, 116, 1544–1549. [Google Scholar] [CrossRef]
- Seemaladinne, R.; Pati, S.; Kharel, A.; Bafana, A.; Al-Wahish, A.; Wujcik, E.K.; Günaydin-Sen, Ö. Ammonia borane with polyvinylpyrrolidone as a hydrogen storage material: Comparison of different molecular weights. J. Phys. Chem. Solids 2017, 110, 394–400. [Google Scholar] [CrossRef]
- Tang, Z.; Li, S.; Yang, W.; Yu, X. Hypercrosslinked porous poly(styrene-co-divinylbenzene) resin: A promising nanostructure-incubator for hydrogen storage. J. Mater. Chem. 2012, 22, 12752–12758. [Google Scholar] [CrossRef]
- Kurban, Z.; Lovell, A.; Bennington, S.M.; Jenkins, D.W.A.; Ryan, K.R.; Jones, M.O.; Skipper, N.T.; David, W.I.F. A solution selection model for coaxial electrospinning and its application to nanostructured hydrogen storage materials. J. Phys. Chem. C 2010, 114, 21201–21213. [Google Scholar] [CrossRef]
- Tang, Z.; Li, S.; Yang, Z.; Yu, X. Ammonia borane nanofibers supported by poly(vinyl pyrrolidone) for dehydrogenation. J. Mater. Chem. 2011, 21, 14616–14621. [Google Scholar] [CrossRef]
- Alipour, J.; Shoushtari, A.M.; Kaflou, A. Electrospun PMMA/AB nanofiber composites for hydrogen storage applications. e-Polymers 2014, 14, 305–311. [Google Scholar] [CrossRef]
- Nathanson, A.S.; Ploszajski, A.R.; Billing, M.; Cook, J.P.; Jenkins, D.W.K.; Headen, T.F.; Kurban, Z.; Lovell, A.; Bennington, S.M. Ammonia borane–polyethylene oxide composite materials for solid hydrogen storage. J. Mater. Chem. A 2015, 3, 3683–3691. [Google Scholar] [CrossRef]
- Kharel, A.; Gangineni, R.; Ware, L.; Lu, Y.; Wujcik, E.K.; Wei, S.; Günaydin-Sen, Ö. Dehydrogenation properties of ammonia borane-polyacrylamide nanofiber hydrogen storage composites. J. Mater. Sci. 2017, 52, 4894–4902. [Google Scholar] [CrossRef]
- Ploszajski, A.R.; Billing, M.; Nathanson, A.S.; Vickers, S.; Bennington, S.M. Freeze-dried ammonia borane-polyethylene oxide composites: Phase behaviour and hydrogen release. Int. J. Hydrog. Energy 2018, 43, 5645–5656. [Google Scholar] [CrossRef]
- Ploszajski, A.R.; Billing, M.; Cockcroft, J.A.; Skipper, N.T. Crystalline structure of an ammonia borane-polyethylene oxide cocrystal: A material investigated for its hydrogen storage potential. CrystEngComm 2018, 20, 4436–4440. [Google Scholar] [CrossRef]
- Moussa, G.; Demirci, U.B.; Malo, S.; Bernard, S.; Miele, P. Hollow core@mesoporous shell boron nitride nanopolyhedron-confined ammonia borane: A pure B–N–H composite for chemical hydrogen storage. J. Mater. Chem. A 2014, 2, 7717–7722. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, J.; Cheng, F.; Tao, Z.; Chen, J. Porous MnO2 hollow cubes as new nanoscaffold materials for the dehydrogenation promotion of ammonia-borane (AB). Microp. Mesop. Mater. 2012, 161, 40–47. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, H.; Li, H.; Li, F.; Qijing, H.; Zhang, Y. Enhancing the thermal dehydrogenation properties of ammonia borane (AB) by using monodisperse MnO2 hollow spheres (MHS). J. Alloys Compd. 2019, 781, 111–117. [Google Scholar] [CrossRef]
- Niedenzu, P.M. Studies on Polyboron Hydride Anions and Ammine-Borane. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 1990. [Google Scholar]
- Salupo, T. Preparations of Ytterbium and Europium Borides from Yb(II) and Eu(II) Boron Hydride Precursors. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 1993. [Google Scholar]
- DeGraffenreid, A.L. Studies on Boron—Nitrogen and Boron—Gadolinium Compounds. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 1995. [Google Scholar]
- Myers, A.G.; Yang, B.H.; Kopecky, D.J. Lithium amidotrihydroborate; a powerful new reductant. Transformation of tertiary amides to primary alcohols. Tetrahedron Lett. 1996, 37, 3623–3626. [Google Scholar] [CrossRef]
- Diyabalanage, H.V.A.; Shrestha, R.P.; Semelsberger, T.A.; Scott, B.L.; Bowden, M.E.; Davis, B.L.; Burrell, A.K. Calcium amidotrihydroborate: A hydrogen storage material. Angew. Chem. Int. Ed. 2007, 46, 8995–8997. [Google Scholar] [CrossRef]
- Chua, Y.S.; Chen, P.; Wu, G.; Xiong, Z. Development of amidoboranes for hydrogen storage. Chem. Commun. 2011, 47, 5116–5129. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhang, J.G.; Man, T.T.; Wu, M.; Chen, C.C. Recent progress and development of metal amidoborane. Chem. Asian J. 2013, 8, 1076–1089. [Google Scholar] [CrossRef] [PubMed]
- Owarzany, R.; Leszczyński, P.J.; Fijalkowski, K.J.; Grochala, W. Mono- and bimetallic amidoboranes. Crystals 2016, 6, 88. [Google Scholar] [CrossRef]
- Castilla-Martinez, C.A.; Moury, R.; Demirci, U.B. Amidoboranes and hydrazinidoboranes: State of the art, potential for hydrogen storage; and other prospects. Int. J. Hydrog. Energy 2020. submitted. [Google Scholar]
- Liu, X.; Wu, Y.; Wang, S.; Li, Z.; Guo, X.; Ye, J.; Jiang, L. Current progress and research trends on lithium amidoborane for hydrogen storage. J. Mater. Sci. 2020, 55, 2645–2660. [Google Scholar] [CrossRef]
- Xiong, Z.; Yong, C.A.; Wu, G.; Chen, P.; Shaw, W.; Karkamkar, A.; Autrey, T.; Jones, M.O.; Johnson, S.R.; Edwards, P.P.; et al. High-capacity hydrogen storage in lithium and sodium amidoboranes. Nat. Mater. 2008, 7, 138–141. [Google Scholar] [CrossRef]
- Wu, C.; Wu, G.; Xiong, Z.; David, W.I.F.; Ryan, K.R.; Jones, M.O.; Edwards, P.P.; Chu, H.; Chen, P. Stepwise phase transition in the formation of lithium amidoborane. Inorg. Chem. 2010, 49, 4319–4323. [Google Scholar] [CrossRef]
- Liu, L.; Wang, S.; Wu, Y.; Li, Z.; Jiang, L.; Guo, X.; Ye, J. Dehydrogenation of two phases of LiNH2BH3. Int. J. Hydrog. Energy 2020, 45, 2127–2134. [Google Scholar] [CrossRef]
- Luedtke, A.T.; Autrey, T. Hydrogen release studies of alkali amidoboranes. Inorg. Chem. 2010, 49, 3905–3910. [Google Scholar] [CrossRef]
- Shimoda, A.; Doi, A.; Nakagawa, T.; Zhang, Y.; Miyaoka, H.; Ichikawa, T.; Tansho, M.; Shimizu, T.; Burrell, A.K.; Kojima, Y. Comparative study of structural changes in NH3BH3, LiNH2BH3, and KNH2BH3 during dehydrogenation process. J. Phys. Chem. C 2012, 116, 5957–5964. [Google Scholar] [CrossRef]
- Xiong, Z.; Wu, G.; Chua, Y.S.; Hu, J.; He, T.; Xu, W.; Chen, P. Synthesis of sodium amidoborane (NaNH2BH3) for hydrogen production. Energy Environ. Sci. 2008, 1, 360–363. [Google Scholar] [CrossRef]
- Sandra, F.P.R.; Demirci, U.B.; Chiriac, R.; Moury, R.; Miele, P. A simple preparation method of sodium amidoborane; highly efficient derivative of ammonia borane dehydrogenating at low temperature. Int. J. Hydrog. Energy 2011, 36, 7423–7430. [Google Scholar] [CrossRef]
- Harder, S. Molecular early main group metal hydrides: Synthetic challenge, structures and applications. Chem. Commun. 2012, 48, 11165–11177. [Google Scholar] [CrossRef] [PubMed]
- Fijałkowski, K.J.; Grochala, W. Substantial emission of NH3 during thermal decomposition of sodium amidoborane; NaNH2BH3. J. Mater. Chem. 2009, 19, 2043–2050. [Google Scholar] [CrossRef]
- Diyabalanage, H.V.A.; Nakagawa, T.; Shrestha, R.P.; Semelsberger, T.A.; Davis, B.L.; Scott, B.L.; Burell, A.K.; David, W.I.F.; Ryan, K.R.; Jones, M.O.; et al. Potassium(I) amidotrihydroborate: Structure and hydrogen release. J. Am. Chem. Soc. 2010, 132, 11836–11837. [Google Scholar] [CrossRef]
- Owarzany, R.; Jaroń, T.; Leszczyński, P.J.; Fijalkowski, K.J.; Grochala, W. Amidoborane of rubidium and caesium: The last missing members of the alkali metal amidoborane family. Dalton Trans. 2017, 46, 16315–16320. [Google Scholar] [CrossRef]
- Kazakov, I.V.; Butlak, A.V.; Shelyganov, P.A.; Suslonov, V.V.; Timoshkin, A.Y. Reversible structural transformations of rubidium and cesium amidoboranes. Polyhedron 2017, 127, 186–190. [Google Scholar] [CrossRef]
- Ramzan, M.; Silverav, F.; Blomqvist, A.; Scheicher, R.H.; Lebègue, S. Structural and energetic analysis of the hydrogen storage materials LiNH2BH3 and NaNH2BH3 from ab initio calculation. Phys. Rev. B 2009, 79, 132102. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, W.; Yildirim, T. Alkali, and alkaline-earth metal amidoboranes: Structure, crystal chemistry, and hydrogen storage properties. J. Am. Chem. Soc. 2008, 130, 14834–14839. [Google Scholar] [CrossRef]
- Davydova, E.I.; Lisovenko, A.S.; Timoshkin, A.Y. Complex beryllium amidoboranes: Structures, stability, and evaluation of their potential as hydrogen storage materials. J. Comput. Chem. 2017, 38, 401–405. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Isobe, S.; Ikarashi, Y.; Ohnuki, S. AB-MH (ammonia borane-metal hydride) composites: Systematic understanding of dehydrogenation properties. J. Mater. Chem. A 2014, 2, 3926–3931. [Google Scholar] [CrossRef]
- Luo, J.; Kang, X.; Wang, P. Synthesis, formation mechanism, and dehydrogenation properties of the long-sought Mg(NH2BH3)2 compound. Energy Environ. Sci. 2013, 6, 1018–1025. [Google Scholar] [CrossRef]
- Leardini, F.; Ares, J.R.; Bodega, J.; Valero-Pedraza, M.J.; Bañares, M.A.; Fernandez, J.F.; Sanchez, C. Hydrogen desorption behavior of calcium amidoborane obtained by reactive milling of calcium hydride and ammonia borane. J. Phys. Chem. C 2012, 116, 24430–24435. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, C.; Fang, C.; Fang, F.; Sun, D.; Ouyang, L.; Zhu, M. Synthesis, crystal structure; and thermal decomposition of strontium amidoborane. J. Phys. Chem. C 2010, 114, 1709–1714. [Google Scholar] [CrossRef]
- Shcherbina, N.A.; Kazakov, I.V.; Timoshkin, A.Y. Synthesis and characterization of barium amidoborane. Russ. J. Gen. Chem. 2017, 87, 2875–2877. [Google Scholar] [CrossRef]
- Smythe, N.C.; Gordon, J.C. Ammonia borane as a hydrogen carrier: Dehydrogenation and regeneration. Eur. J. Inorg. Chem. 2010, 2010, 509–521. [Google Scholar] [CrossRef]
- Hausdorf, S.; Baitalow, F.; Wolf, G.; Mertens, F.O.R.L. A procedure of the regeneration of ammonia borane from BNH-waste products. Int. J. Hydrog. Energy 2008, 33, 608–614. [Google Scholar] [CrossRef]
- Reller, C.; Mertens, F.O.R.L. A self-containing regeneration scheme for spent ammonia borane on the catalytic hydrodechlorination of BCl3. Angew. Chem. Int. Ed. 2012, 51, 11731–11735. [Google Scholar] [CrossRef]
- Davis, D.L.; Dixon, D.A.; Garner, E.B.; Gordon, J.C.; Matus, M.H.; Scott, B.; Stephens, F.H. Efficient regeneration of partially spent ammonia borane fuel. Angew. Chem. Int. Ed. 2009, 48, 6812–6816. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, L.; Chen, X.; Yu, X. Reductive dechlorination of BCl3 for efficient ammonia borane regeneration. Dalton Trans. 2015, 44, 753–757. [Google Scholar] [CrossRef]
- Sutton, A.D.; Burrell, A.A.; Dixon, D.A.; Garner, E.B., III; Gordon, J.C.; Nakagawa, T.; Ott, K.C.; Robinson, J.P.; Vasiliu, M. Regeneration of ammonia borane spent fuel by direction reaction with hydrazine and liquid ammonia. Science 2011, 331, 1426–1429. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.Q.; Ahluwalia, R.K. Off-board regeneration of ammonia borane for use as a hydrogen carrier for automotive fuel cells. Int. J. Hydrog. Energy 2012, 37, 14382–14392. [Google Scholar] [CrossRef]
- Tang, Z.; Tan, Y.; Chen, X.; Yu, X. Regenerable hydrogen storage in lithium amidoborane. Chem. Commun. 2012, 48, 9296–9298. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhang, L.; Wan, L.; Huang, Z.; Liu, H.; Guo, Z.; Yu, X. Regeneration of alkaline metal amidoboranes with high purity. Int. J. Hydrog. Energy 2016, 41, 407–412. [Google Scholar] [CrossRef]
- Marder, T.B. Will we soon be fueling our automobiles with ammonia borane? Angew. Chem. Int. Ed. 2007, 46, 8116–8118. [Google Scholar] [CrossRef]
- Companies House. Available online: https://beta.companieshouse.gov.uk/company/09019506/insolvency (accessed on 2 May 2020).
- First UAV test flight with Cella solid-state hydrogen storage. Fuel Cells Bull. 2016, 3, 4–5.
- Energy.gov. Available online: https://www.energy.gov/sites/prod/files/2017/05/f34/fcto_targets_onboard_hydro_storage_explanation.pdf (accessed on 2 May 2020).














© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirci, U.B. Ammonia Borane: An Extensively Studied, Though Not Yet Implemented, Hydrogen Carrier. Energies 2020, 13, 3071. https://doi.org/10.3390/en13123071
Demirci UB. Ammonia Borane: An Extensively Studied, Though Not Yet Implemented, Hydrogen Carrier. Energies. 2020; 13(12):3071. https://doi.org/10.3390/en13123071
Chicago/Turabian StyleDemirci, Umit Bilge. 2020. "Ammonia Borane: An Extensively Studied, Though Not Yet Implemented, Hydrogen Carrier" Energies 13, no. 12: 3071. https://doi.org/10.3390/en13123071
APA StyleDemirci, U. B. (2020). Ammonia Borane: An Extensively Studied, Though Not Yet Implemented, Hydrogen Carrier. Energies, 13(12), 3071. https://doi.org/10.3390/en13123071





