Parametric Study of Various Thermodynamic Cycles for the Use of Unconventional Blends
Abstract
1. Introduction
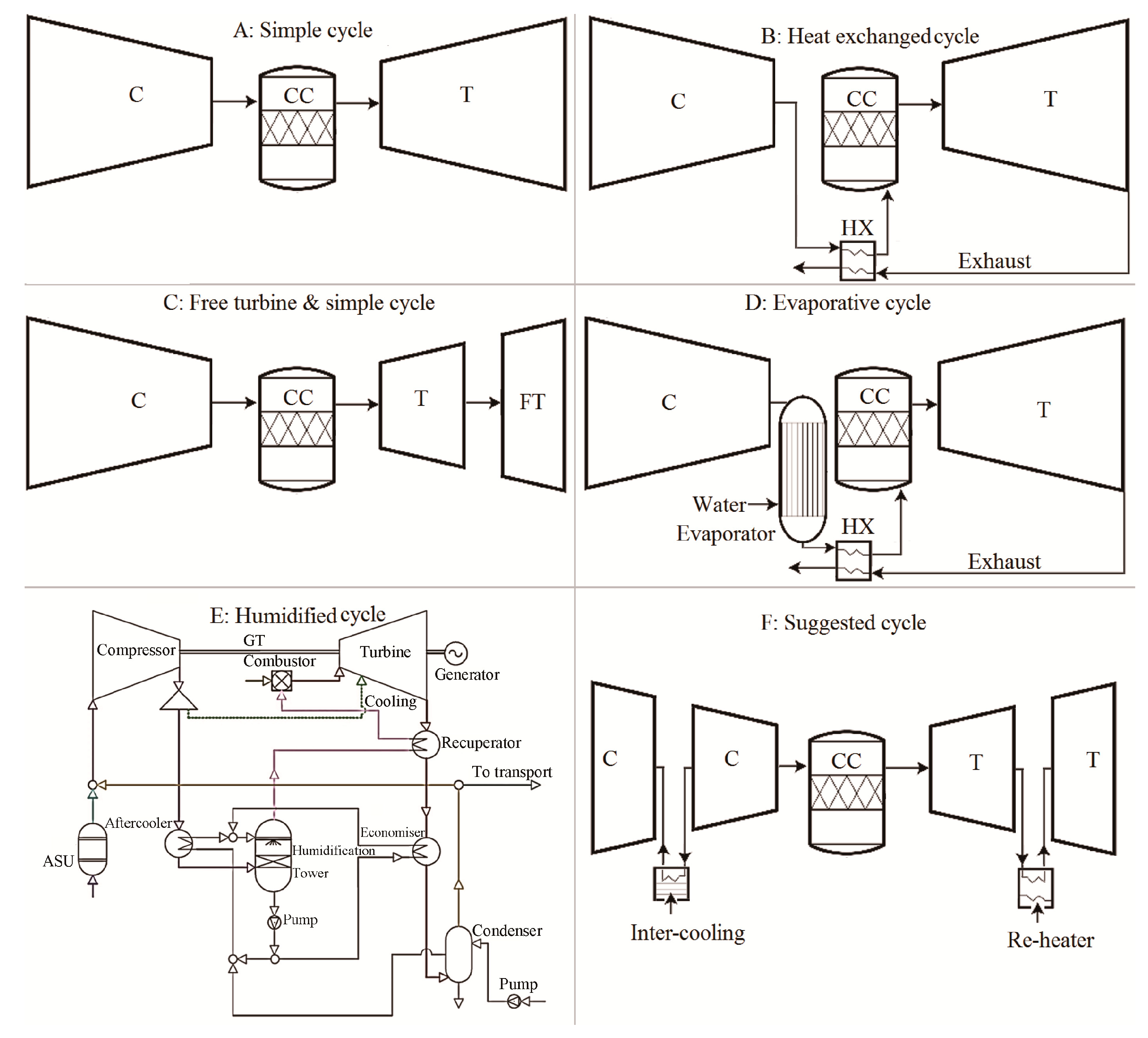
2. Setup
3. Results
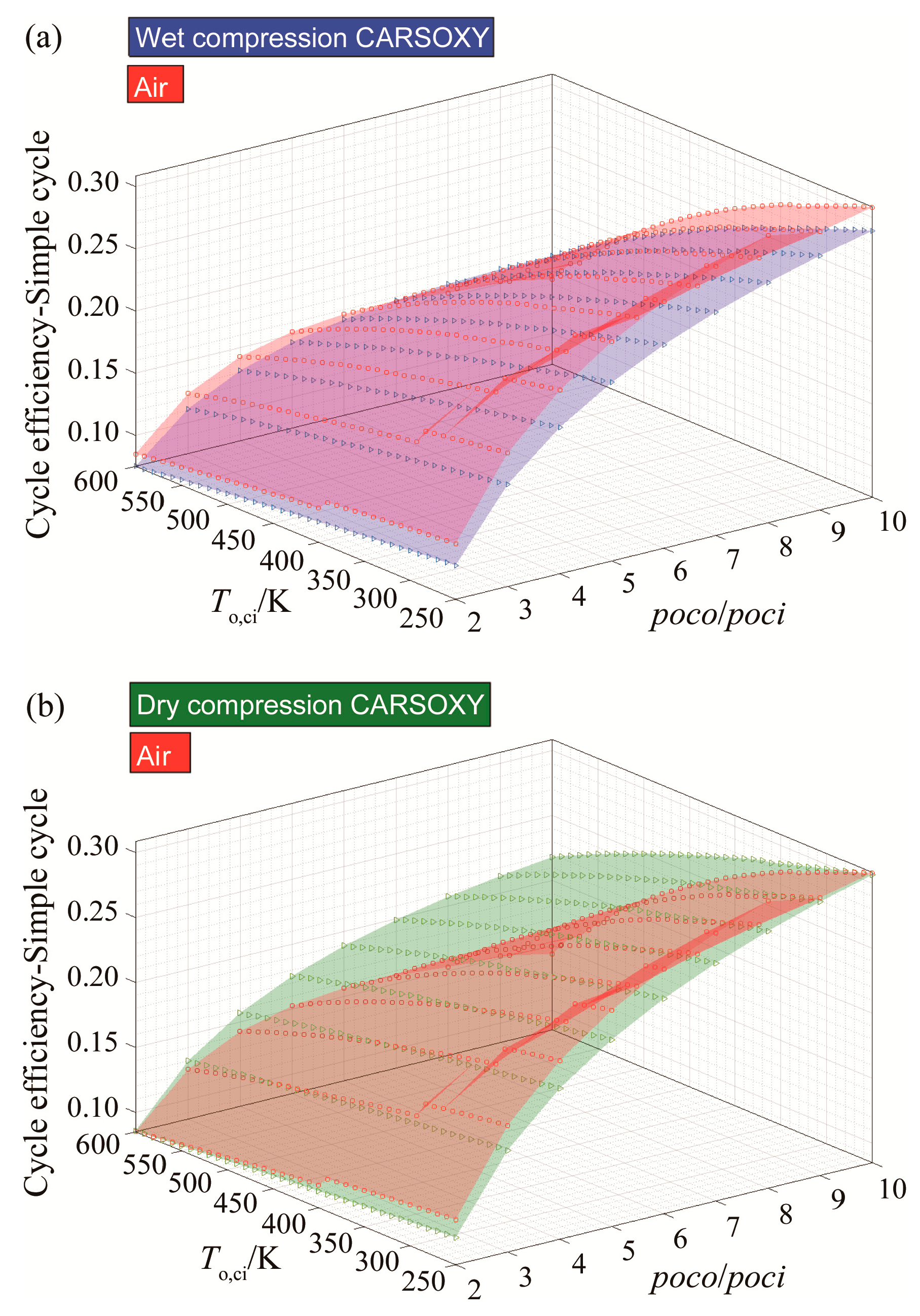
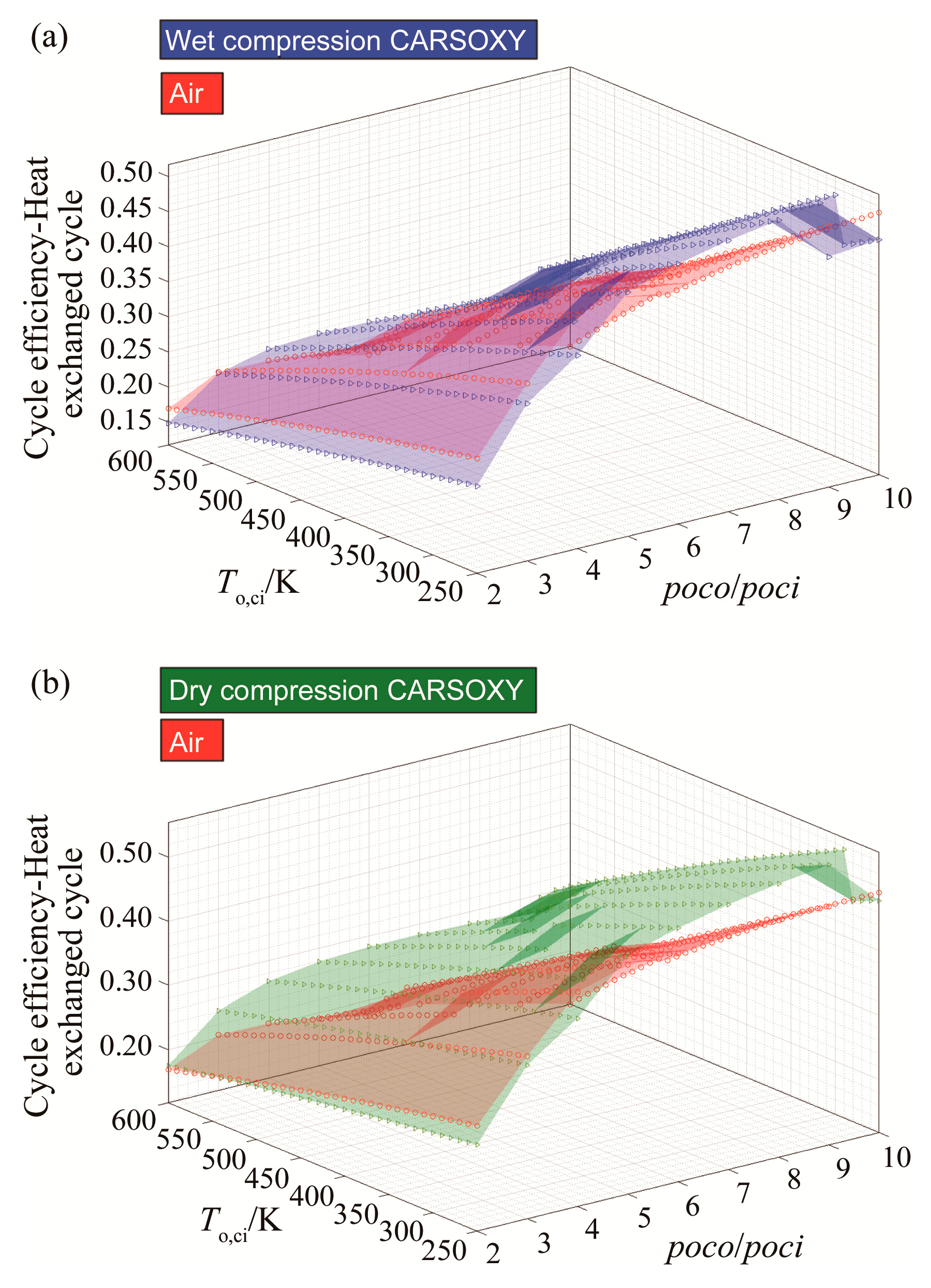
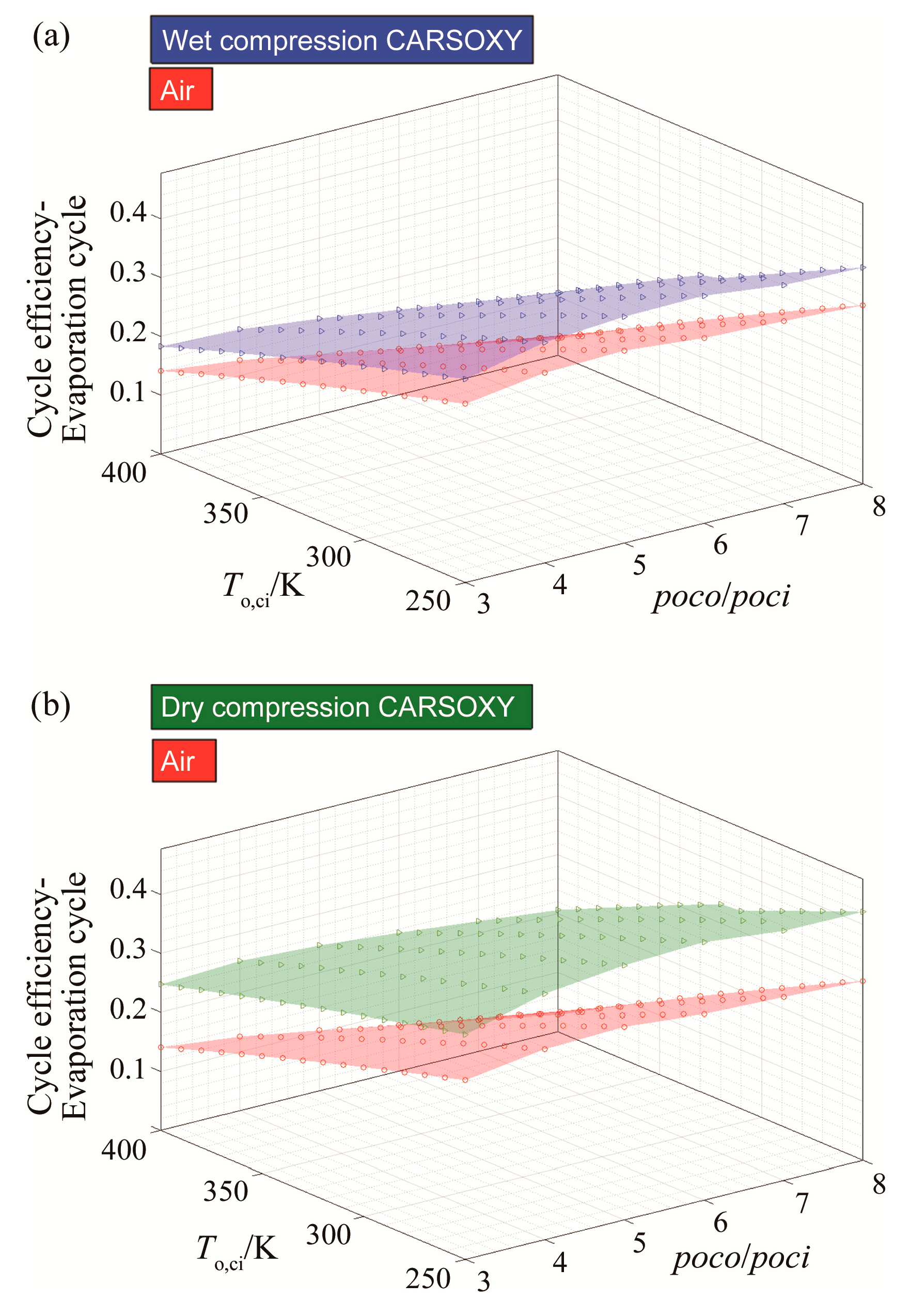
3.1. Efficiency Results with Respect to Variable Compressor Inlet Temperatures and Compressor Pressure Ratio
3.2. Efficiency Results with Respect to Variable Molar Fractions of Ar, CO2, and H2O
3.3. Efficiency Results with Respect to Variable Turbine Inlet Temperatures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| A | Molar fraction of fuel in CARSOXY mixture |
| B | Molar fraction of the oxygen in CARSOXY mixture |
| CxHy | Hydrocarbon fuel, methane (x: 1 and y: 4) |
| Cpmix | Specific heat at constant pressure of a mixture/J∙mol−1∙K−1 |
| Cp(i) | Specific heat at constant pressure of ith component in a mixture/J∙mol−1∙K−1 |
| Cvmix | Specific heat of a mixture at constant volume/J∙mol−1∙K−1 |
| Change in heat capacity at constant pressure/J∙mol−1∙K−1 | |
| f | Fuel to air ratio |
| HHVT0.1′ | Higher heating value at the combustion inlet temperature/kJ∙kg−1 |
| HHVT0.2′ | Higher heating value at the combustion outlet temperature/kJ∙kg−1 |
| Hwf,T0.2′ | Enthalpy of the working fluid at the combustion outlet temperature/kJ∙mol−1 |
| Hwf,T0.1′ | Enthalpy of the working fluid at the combustion inlet temperature/kJ∙mol−1 |
| ∆HReaction, 25 °C | Standard enthalpy change of the combustion reaction |
| ∆Hproducts | Enthalpy of products/kJ∙mol−1 |
| ∆Hreactant | Enthalpy of reactants/kJ∙mol−1 |
| ∆HReaction,T0.1′ | Enthalpy of the combustion reaction at the combustion inlet temperature /kJ∙mol−1 |
| Enthalpy of H2O at the combustion inlet temperature/kJ∙kg−1 | |
| LHVT0.1′ | Lower heating value at the combustion inlet temperature/kJ∙kg−1 |
| Molecular weight of methane (x: 1 and y: 4)/g∙mol−1 | |
| Molecular weight of H2O/g∙mol−1 | |
| Number of moles of H2O produced due to combustion | |
| Number of moles of hydrocarbon fuel in CARSOXY mixture | |
| poco/poci | Compressor pressure ratio |
| Rmix | Gas constant of a gaseous mixture /J∙mol−1∙K−1 |
| ri | Volume fraction of ith |
| SFC | specific fuel consumption |
| To,ci | Compressor inlet temperature/K |
| To,ti | Turbine inlet temperature/K |
| Wt | Turbine specific work/kJ∙kg−1 |
| WC | Specific work required from the turbine to run the compressor/kJ∙kg−1 |
| X | Molar fraction of the Argon in CARSOXY mixture |
| xi | Molar fraction of ith component in a mixture |
| Y | Molar fraction of H2O in CARSOXY mixture |
| γmix | Heat capacity ratio of a mixture |
| η | Cycle efficiency |
| μmix | Average molecular weight of a mixture/g∙mol−1 |
| μi | Molecular weight of ith component in a mixture/g∙mol−1 |
| σ | Molar fraction in products |
References
- Committee on Climate Change. UK regulations: The Climate Change Act—Committee on Climate Change. 2019. Available online: https://www.theccc.org.uk/tackling-climate-change/the-legal-landscape/the-climate-change-act/ (accessed on 5 June 2019).
- Gibbins, J.; Chalmers, H. Carbon capture and storage. Energy Policy 2008, 36, 4317–4322. [Google Scholar] [CrossRef]
- Pioro, I.; Duffey, R.B.; Kirillov, P.L.; Pioro, R.; Zvorykin, A.; Machrafi, R. Current Status and Future Developments in Nuclear-Power Industry of the World. J. Nucl. Eng. Radiat. Sci. 2019, 5, 024001. [Google Scholar] [CrossRef]
- Gad-Briggs, A.; Pericles, P.; Nikolaidis, T. Analyses of the Costs Associated with Very High Turbine Entry Temperatures in Helium Recuperated Gas Turbine Cycles for Generation IV Nuclear Power Plants. J. Nucl. Eng. Radiat. Sci. 2019, 5, 011019. [Google Scholar] [CrossRef]
- Ubald, B.N.; Tucker, P.G.; Cui, J.; Watson, R.; Shahpar, S. Numerical Analysis of an Instrumented Turbine Blade Cascade. J. Turbomach. 2019, 141, 051013. [Google Scholar] [CrossRef]
- Yilmaz, I.; Yilmaz, H.; Cam, O. An experimental study on premixed CNG/H2/CO2 mixture flames. Open Eng. 2018, 8, 32–40. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon capture and utilization update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Kosowska-Golachowska, M. Thermal analysis and kinetics of coal during oxy-fuel combustion. J. Therm. Sci. 2017, 26, 355–361. [Google Scholar] [CrossRef]
- Lele, F.; Wu, Y.; Xu, K.; Zhang, H.; Zhang, Y.; Zhang, M. Coal-Derived Soot Behaviors in O2/N2 and O2/CO2 Atmospheres, Studied through a 1-D Transient Coal Combustion Model. Energy Fuels 2019, 33, 3620–3629. [Google Scholar]
- Saeed, M.; Goodarzi, M.; Safaei, M.R. Comparative study of the performance of air and geothermal sources of heat pumps cycle operating with various refrigerants and vapor injection. Alexandria Eng. J. 2020. [Google Scholar] [CrossRef]
- Nima, B.; KazımSömek, S.; Özalevli, C.C.; Baker, D.; Tarı, İ. Numerical analysis of phase change material characteristics used in a thermal energy storage device. Heat Transf. Eng. 2018, 39, 268–276. [Google Scholar]
- JiHo, A.; Jeong, J.H.; Kim, T.S. Performance Enhancement of a Molten Carbonate Fuel Cell/Micro Gas Turbine Hybrid System with Carbon Capture by Off-Gas Recirculation. J. Eng. Gas Turbines Power 2019, 141, 041036. [Google Scholar]
- Georgescu-Roegen, N. Energy analysis and economic valuation. In Green Accounting; Routledge: Morgantown, WV, USA, 2018; pp. 75–110. [Google Scholar]
- Ibrahim Thamir, K.; Mohammed, M.K.; Awad, O.I.; Abdalla, A.N.; Basrawi, F.; Mohammed, M.N.; Najafi, G.; Mamat, R. A comprehensive review on the exergy analysis of combined cycle power plants. Renew. Sustain. Energy Rev. 2018, 90, 835–850. [Google Scholar] [CrossRef]
- Tailu, L.; Liu, J.; Wang, J.; Meng, N.; Zhu, J. Combination of two-stage series evaporation with non-isothermal phase change of organic Rankine cycle to enhance flue gas heat recovery from gas turbine. Energy Convers. Manag. 2019, 185, 330–338. [Google Scholar]
- Roberto, C.; Giordano, L. Upgrading existing gas-steam combined cycle power plants through steam injection and methane steam reforming. Energy 2019, 173, 229–243. [Google Scholar]
- Mahdiyeh, K.; Mohamed, A.A.; Brandauer, W. Recuperation of regenerative braking energy in electric rail transit systems. IEEE Trans. Intell. Transp. Syst. 2019. [Google Scholar] [CrossRef]
- Ali, A.; Gutesa, M.; Valera-Medina, A.; Syred, N.; Ng, J.; Chong, C.T. CO2-Argon-Steam oxy-fuel (CARSOXY) combustion for CCS inert gas atmospheres in gas turbines. Appl. Therm. Eng. 2017, 122, 350–358. [Google Scholar]
- Alrebei, O.; Valera-Madeina, A. Techno-Economics of CO2-Argon-Steam Oxy-Fuel (CARSOXY) Gas Turbines, 3rd ed.; ICCE: Stavanger, Norway, 2019. [Google Scholar]
- Luo, X.; Wang, M. Optimal operation of MEA-based post-combustion carbon capture for natural gas combined cycle power plants under different market conditions. Int. J. Green. Gas Control 2016, 48, 312–320. [Google Scholar] [CrossRef]
- Mohamed, K.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Jean-Marc, A.; Bouallou, C. Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar]
- Saravanamuttoo, H.I.H.; Frederick, G.; Rogers, C.; Cohen, H. Gas Turbine Theory; Pearson Education: London, UK, 2001. [Google Scholar]
- Biskamp, D. Magnetohydrodynamic Turbulence; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Davidson, P.A. An Introduction to Magnetohydrodynamics; Cambridge University Press: Cambridge, UK, 2002; p. 781. [Google Scholar]
- Freidberg, J.P. Plasma Physics and Fusion Energy; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Sarafraz, M.M.; Safaei, M.R.; Jafarian, M.; Goodarzi, M.; Arjomandi, M. High Quality Syngas Production with Supercritical Biomass Gasification Integrated with a Water–Gas Shift Reactor. Energies 2019, 12, 2591. [Google Scholar] [CrossRef]
- Reza, S.M.; Leon, A.S.; Khaled, U.; Goodarzi, M.; Meer, R. Energetic Analysis of Different Configurations of Power Plants Connected to Liquid Chemical Looping Gasification. Processes 2019, 7, 763. [Google Scholar]
- Sarafraz, M.M.; Jafarian, M.; Arjomandi, M.; Nathan, G.J. Potential use of liquid metal oxides for chemical looping gasification: A thermodynamic assessment. Appl. Energy 2017, 195, 702–712. [Google Scholar] [CrossRef]
- Irvin, G.; Yetter, R.A.; Glumac, N.G. Combustion; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Kalyan, A.; Puri, I.K.; Jog, M.A. Advanced Thermodynamics Engineering; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- McDonald Colin, F.; Wilson, D.G. The utilization of recuperated and regenerated engine cycles for high-efficiency gas turbines in the 21st century. Appl. Therm. Eng. 1996, 16, 635–653. [Google Scholar] [CrossRef]


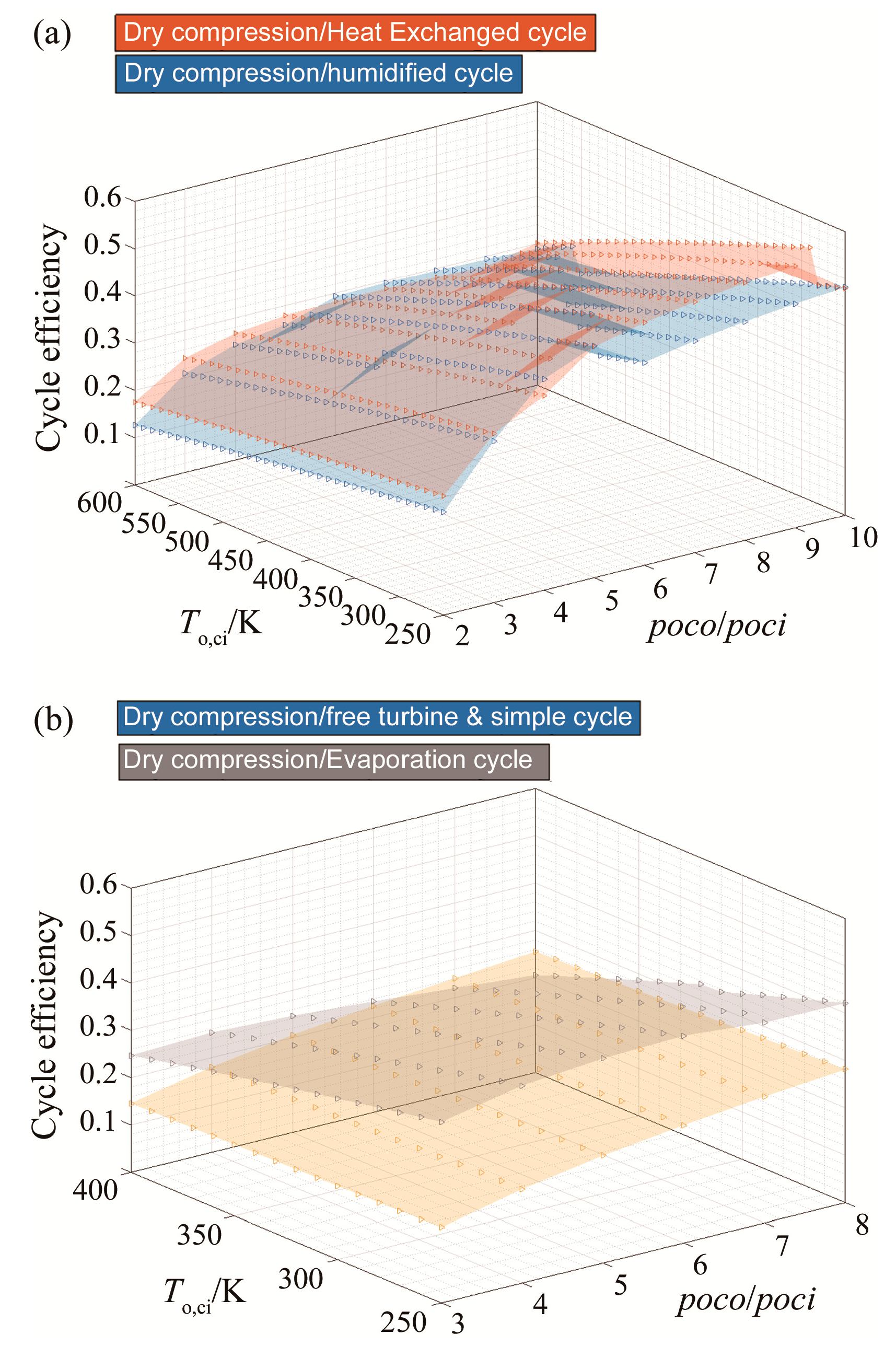

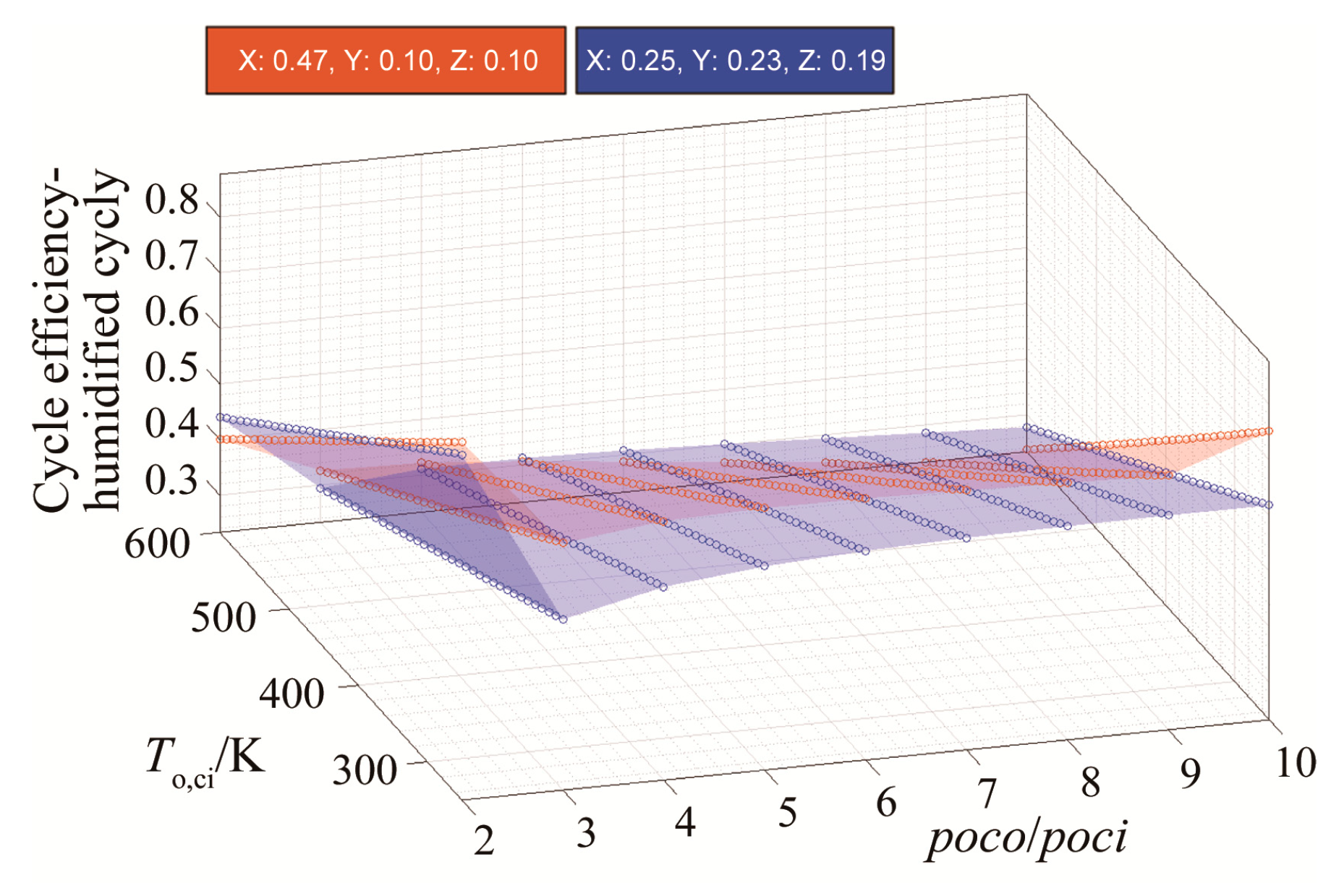
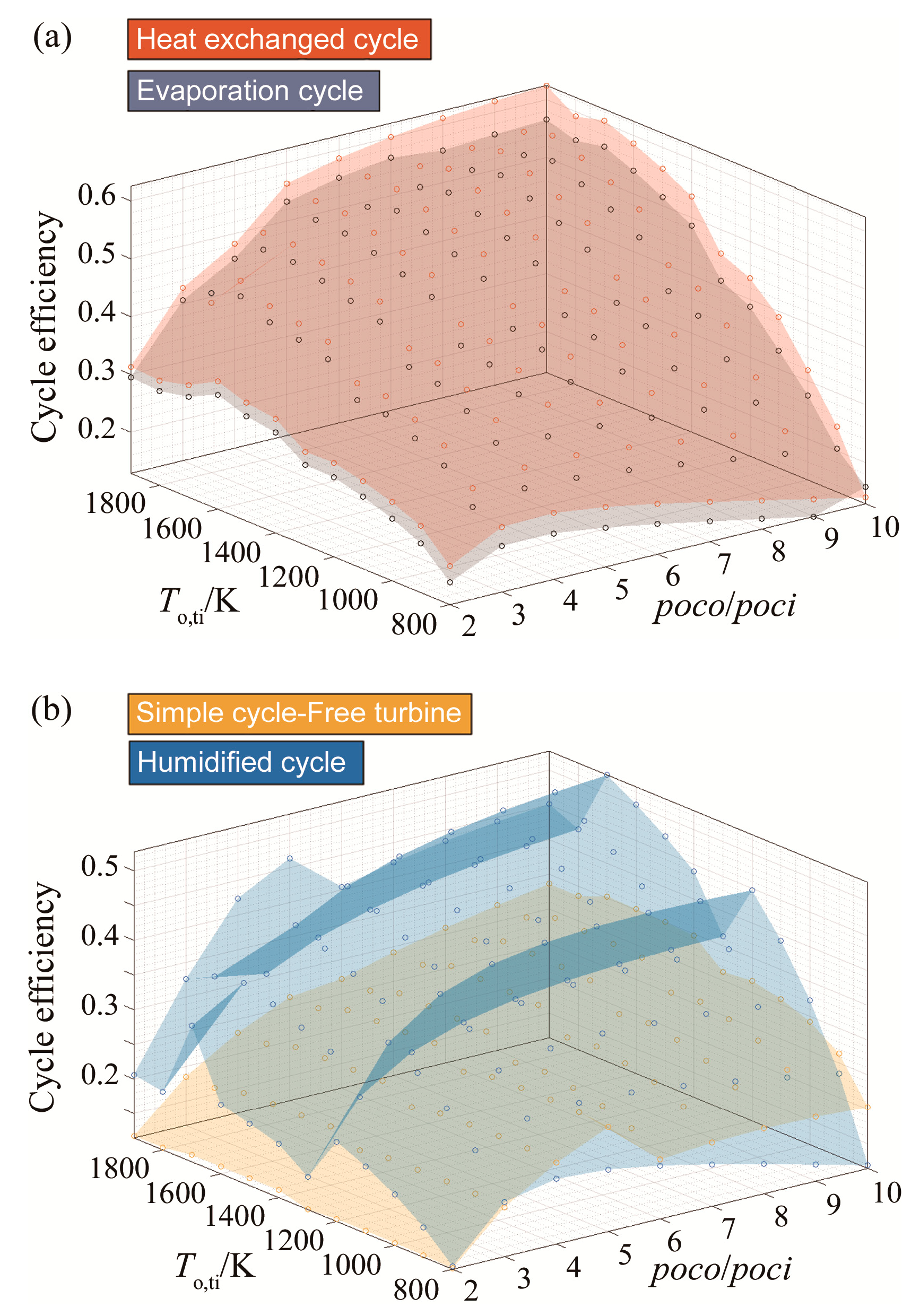
| Blend | Molar Fractions of (Argon, CO2, H2O, fuel, O2)/% |
|---|---|
| 58 | (25, 23, 19, 11, 22) |
| 79 | (24, 19, 12.67, 25, 33) |
| 27 | (30, 24, 16, 10, 20) |
| Cycle | Approximate Efficiency Increase |
|---|---|
| simple cycle | Negligible |
| heat exchanged cycle | 14% |
| free turbine and simple cycle | Negligible |
| evaporative cycle | 15% |
| humidified gas turbine cycle | 14% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrebei, O.F.; Bowen, P.; Valera Medina, A. Parametric Study of Various Thermodynamic Cycles for the Use of Unconventional Blends. Energies 2020, 13, 4656. https://doi.org/10.3390/en13184656
Alrebei OF, Bowen P, Valera Medina A. Parametric Study of Various Thermodynamic Cycles for the Use of Unconventional Blends. Energies. 2020; 13(18):4656. https://doi.org/10.3390/en13184656
Chicago/Turabian StyleAlrebei, Odi Fawwaz, Philip Bowen, and Agustin Valera Medina. 2020. "Parametric Study of Various Thermodynamic Cycles for the Use of Unconventional Blends" Energies 13, no. 18: 4656. https://doi.org/10.3390/en13184656
APA StyleAlrebei, O. F., Bowen, P., & Valera Medina, A. (2020). Parametric Study of Various Thermodynamic Cycles for the Use of Unconventional Blends. Energies, 13(18), 4656. https://doi.org/10.3390/en13184656





