Abstract
Maximizing the internal carbon sources in raw wastewater was found to be an alternative option to alleviate the financial burden in external carbon sources (ECS) addition to the biological nutrient removal (BNR) process. Based on previous studies on particulate recovery via fine-sieving technologies, alkali pretreatment was used to improve the short-chain fatty acid (SCFA) production from the fine-sieving fractions (FSF). Hydrolysis performance and methane production were monitored to evaluate the reasons for the SCFA boost. Besides, the microbial community structure was evaluated by high-throughput sequencing. Furthermore, mass balance and financial benefits were preliminarily estimated. The results showed that alkali pretreatment effectively promoted the generation of SCFAs with 234 mg/g volatile suspended solids (VSS), almost double that of the control test. This was partially attributed to the efficient hydrolysis, with soluble polysaccharides and protein increased by 2.1 and 1.2 times compared to that of the control, respectively. Inhibition of methanogens was also devoted to the accumulation of SCFAs, with no methane production until 150 h at high pH value. Finally, a preliminary evaluation revealed that 44.51 kg/d SCFAs could be supplied as the electron donor for denitrification, significantly reducing the cost in ECS addition for most wastewater treatment plants (WWTPs) with carbon insufficiency.
1. Introduction
Recently, with the more stringent effluent standard of nitrogen and phosphorus to alleviate the water eutrophication, the biological nutrient removal (BNR) process has been widely applied in wastewater treatment plants (WWTPs) [1]. However, the inadequate available organic matters has been frequently reported to limit the upgrading of nutrient removal, especially for the de-nitrification process [2]. As a result, a large amount of external carbon sources (ECS) have been dozed to provide enough electron donors for efficient removal of nitrogen, which results in an economic burden for the purchase and transformation of the chemical agents [3]. Consequently, the exploration of alternative carbon sources with low cost and environmental impact has been widely investigated, the use of short-chain fatty acids (SCFAs), for instance [4,5]. What is notable is that the sewage treatment industry has been undergoing a paradigm shift from “waste treatment” to “energy and resources recovery” [6,7]. In addition, increasing attention has been paid to the organic matter contained in sewage and sludge due to its abundant chemical energy (17.7–28.7 kJ per gram of chemical oxygen demand (COD), five times of which is consumed in sewage treatment) [8]. Although waste activated sludge (WAS) has been a common feedstock for SCFA extraction, the cost of pretreatment has been often significant to broaden the limited extraction efficiency (with 30–50% reduction in particulate organics with 20–30 days, even less within 10 days for fermentation) [9], as well as the evitable carbon footprint [10].
In this sense, the maximum recovery of organics in raw sewage has become a research hotspot [11,12,13], with specific attention on fine-sieving utilization, for instance. Ruiken recovered the particulates via a fine sieve <350 μm with an average removal efficiency of 35% of the total COD [12]. Similarly, Remy maximized the extraction of organics via a fine-sieve (100 μm mesh size) assisted by 15–20 mg/L Al addition, with a high yield of 70–80% of the total COD and 70–90% of the total phosphorus [14]. The primary clarifier has been generally removed in most Chinese WWTPs in order to direct more particulate organics into the nutrient removal process. However, the actual utilization of the carbon sources has recently been questioned due to its limited hydrolysis rate [15,16] and a series of problems, including equipment wear, reduced working volume, and lower sludge activity [17]. It is in this light remarkable to recover the particulate organics at the up-stage of the biological process. As revealed, the re-direction of carbon sources suggested lower aeration demand, higher caloric value for electricity production in incineration, resulting at least in 40% reduction of the net energy need compared with those WWTPs without fine-sieving [18]. The benefits could be extended further by more energy extraction combined with anaerobic digestion (AD). As evaluated by Remy, increased extraction of COD with higher biogas yield (600 NL/L) and considerable reduction in energy consumption (54% of the reference) led to a net electricity balance of 0.47 kW/m3 raw water, almost tripling its own requirement [12]. The selectivity of fine-sieving technology (or micro-sieving) with more flexibility by pore size control has also been proved. Besides, it was easy to operate and mainten, with half the total cost of capital and operation cost of the primary clarifier.
Based on the above discussion and the current state of lack of documentation on SCFA recovery from fine-sieving fractions (FSF), our preliminary experiments were undertaken, but with low efficiency to maximize the contained organic resources due to limited solids hydrolysis. The alkaline condition was efficient in sewage sludge decomposition and microbial community monitoring. Under alkaline conditions (usually with a pH value <10), more extracellular and intracellular materials can be released to boost the SCFA production [19,20]. Besides, methanogenesis could be severely restrained by the alkaline conditions, due to its inhibitory effect on methane-producing bacteria [21]. In this sense, an initial alkaline microhabitat was preferable to promote the hydrolysis process of the recovered organics, FSF, as termed by Ruiken [12]. Attributable to the abundant organics and acclimated microbes—in the sewage pipe and controlled by the alkali ecology—the hydrolysis rate was expected to rise significantly with less energy input compared with WAS under the same conditions.
This study addressed the effect of initial alkalic ecology on the SCFA bio-extraction from FSF, compared with the performance in raw FSF. Changes in SCFA concentration and composition were monitored. Also, hydrolysis characteristics were recorded to evaluate the decomposition effect of the initial alkaline environment, including soluble hydrocarbons and soluble proteins. Besides, changes in methane production and pH values were monitored to reveal the contribution of methanogenesis inhibition to the SCFA accumulation. High-throughput sequencing was applied to evaluate the dominant microbes at different levels to acknowledge the change in the microbial community. Finally, the cost reduction in ECS addition was preliminarily evaluated based on the SCFA yield of the FSF in the case of WWTPs.
2. Materials and Methods
2.1. Sludge Collection
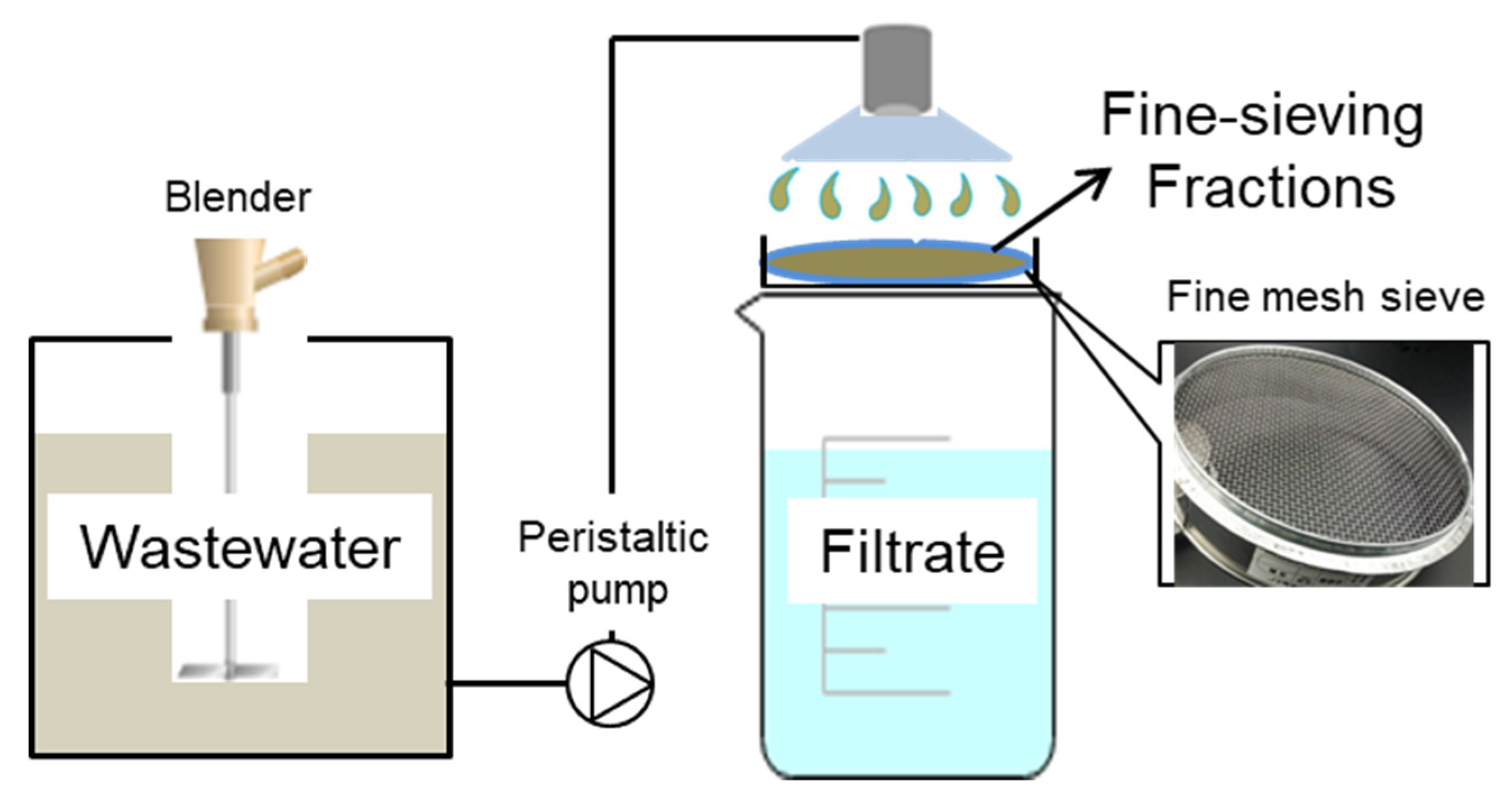
The FSF was collected on-site at the end of the sand sedimentation tank in Zhengyang municipal WWTP in Shanxi Province, China. The WWTP was operated with a daily capacity of 20,000 m3 and a sequencing batch reactor (SBR) was used for biological nutrient removal. A fine-sieve with a pore size of 131 microns was used to recover the organics from the sewage. As demonstrated in Figure 1, the sewage was pumped up and split into different branch pipes before flowing through sieves with different pore sizes. A constant low flow rate of 1.25 m3/h was maintained until a thick filter mat had been developed with a sharp decrease of flux (Figure S1). The sieving fractions were scraped and large impurities removed via a sieve with 380 microns before storing them in the refrigerator at 4 °C. The characteristic of the FSF fed into the fermenters was as follows: TCOD 20,472 ± 432 mg/L, soluble COD (SCOD) 2531 ± 432 mg/L, TSS 22,733 ± 127 mg/L, volatile suspended solids (VSS) 11,279 ± 106 mg/L, pH value 6.9 ± 0.2. Seed sludge was collected from 5-days fermented waste activated sludge (WAS) from the same WWTPs. The basic characteristics were listed as follows: pH value 6.95–7.02, TSS 18147 ± 216 mg/L, VSS 12837 ± 165 mg/L, TCOD 18,018 ± 257 mg/L, SCOD 447 ± 43 mg/L, SCFAs 164.8 ± 16 mg/L, total polysaccharides 4293 ± 41 mg/L, total protein 9072 ± 73 mg/L, soluble carbohydrates 46.9 ± 3.7 mg/L, soluble protein 132.1 ± 11 mg/L.
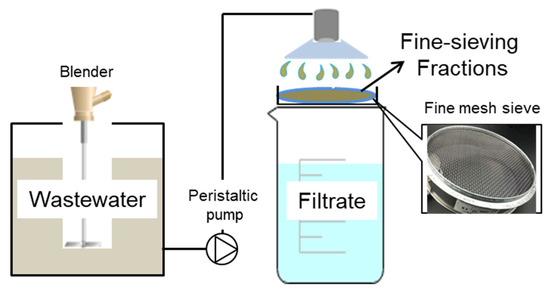
Figure 1.
Schematic for fine-sieving fractions (FSF) recovery by a fine mesh sieve with pore size of 131 microns.
2.2. Batch Fermentation of Alkaline Pretreated FSF
Eight bottles with sealed caps were used as anaerobic fermenters to perform the batch fermentations (Figure S2). Each fermenter was fed with 300 mL raw sludge and 30 mL seed sludge. The pH value was adjusted to a constant pH 10 via dosing 0.4 M/L sodium hydroxide into the first four bottles to maintain an initial alkaline environment. For the description, it was grouped as F-10.0 test. The other four tests were grouped as a control test fed with raw FSF with an initial pH value of 7.0 (F-7.0). Oxygen was stripped by nitrogen to maintain a strict anaerobic environment before the experiments. All fermenters were fermented for 10 days in an air bath oscillator with 20 °C at 120 rpm/min. One bottle in each group was chosen to regularly measure the change in VSS. The others (with three replications) were sampled every day to determine the pH value, gas production and components, volatile acid production and components, as well as soluble polysaccharides and protein content.
2.3. Analysis of Microbial Community Structure
To figure out the shaping effect of alkalic ecology on the structure of the microbial community, high-throughput sequencing was used to detect the prominent population in the fermented sludge samples. This was operated by a third-party (Biozeron, Shanghai, China). Polymerase chain reaction (PCR) reactions were completed using the primers 16S rRNA V3-V4: 341F–806R [22] soon after the DNA was extracted using a Soil DNA Isolation kit (Thermo Scientific, Shanghai, China) [23]. Then, the PCR products were mixed and purified for Illumina PE250 sequencing, where sequencing libraries were generated. The raw sequences data were submitted to the NCBI Short Read Archive database with an accession number of SRR 11474140 and SRR 11474143. Sequences were trimmed shorter than 350 bp to eliminate the effects of random sequencing errors. Finally, the taxonomic analysis was carried out at different taxonomy levels (phylum, class, and genus).
2.4. Mass Balance and Potential Benefits Evaluation
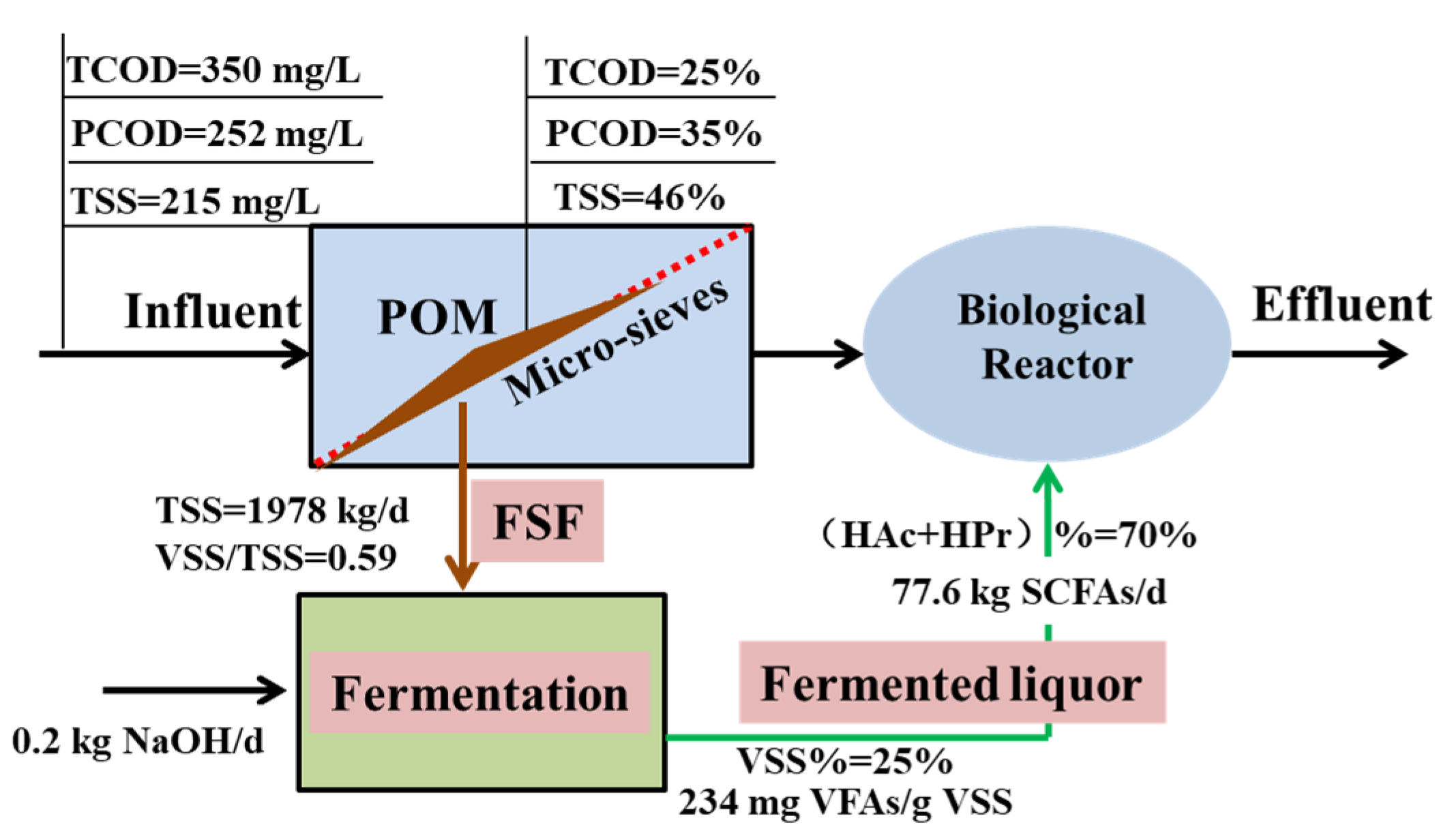
Taking the studied WWTP as an example (with raw sewage characteristics of TCOD 350 mg/L, PCOD 252 mg/L, TSS 215 mg/L), a preliminary evaluation for the mass balance of carbon conversion was made, based on the previous results of FSF recovery (the average removal efficiency for a micro- sieve with 131 microns, TCOD% = 25%, PCOD% = 35%, TSS% = 46%) and the fermentation performance was obtained in this experiment. The produced SCFAs were calculated by the reduced VSS (VS%) and SCFA yield in each group. Economic and environmental profits were evaluated under the assumption that the equivalent COD of methanol addition could be reduced by supplementation of the produced VFAs as carbon sources. Methanol was defined as the typical carbon source with a market price of 1425 RMB/ton (https://www.sci99.com/targetprice/products/methanol/). The cost of caustic soda addition was also covered, with a market price of 3300 RMB/ton with purity of a 51% (http://jiage.molbase.cn/hangqing/4540).
The potential environmental benefits of FSF fermentation motivated by initial alkaline conditions were evaluated from the perspective of life cycle assessment. The functional unit was set as the covered FSF from the case WWTP with 1978 kg/d (35.4 m3). Under the assumption that the total energy demand in sludge heating and agitation was the same for the two fermentation systems, only the potential profits for reduced ECS addition were evaluated. The background data of environmental impact involved in chemical agents were obtained from the local database of e-balance developed by the company of Integrated Knowledge for Our Environment (IKE) (http://www.ike-global.com/) [24].
2.5. Analytical Methods and Statistical Analysis
The sampled sludge was centrifuged at 10,000 rpm (9392× g) and then filtered via a cellulose membrane filter of 0.45 μm and stored at 4 °C before analysis. The filtrate was used for the determination of pH value, SCOD, soluble polysaccharides, soluble proteins, SCFAs, and biogas, as performed in our previous study [25]. A pH meter was used to detect the change in pH value. The potassium dichromate oxidation method was used to determine the TCOD and SCOD. The PCOD was calculated by the difference between the TCOD and SCOD. Soluble polysaccharides and soluble proteins were determined by the phenol sulfate method and a special protein kit (Sangon, Shanghai, China), respectively. The gravimetric method was used to determine the concentration of TSS, VSS, and MLVSS [26]. A gas chromatography (7890, Agilent) was used to determine individual SCFA concentration (equipped with a flame ionization detector, FID). A chromatographic column with type KB-FFAP was used. Nitrogen (N2) was used as carrier gas with a flow rate of 25 mL/min. The detector temperature was set as 240 °C for 5 min. The oven temperature was programmed from 50 °C to 140 °C and then to 240 °C with a rate of 25 °C/min. The total GC run time was about 18 min. After being centrifuged at 8000× g and filtering through a 0.45 μm membrane, 1 mL filtrate from the sludge sample was collected and acidified by mixing with 10 mL of formic acid. Then 1 mL of the mixture liquid was injected into the detector for testing. Gas composition was also detected by gas chromatography. The specific chromatographic conditions were as follows: 2 m packed column (TDX-01) with column temperature of 100 °C; the injector and detector temperature were also set at 100 °C; the bridge current was 100 mA. Then, 200 μL gas was injected into the detector with a peak order of hydrogen, methane, and carbon dioxide. Data were analyzed using the nonlinear regression model of SigmaPlot (SigmaPlot version 12.0, from Systat Software, Inc., San Jose, CA, USA). A three-parameter sigmoid function was used for curve-fitting to the SCFAs vs. time profiles. The formulation was as follows:
where CSCFAs is the SCFA concentration (mg COD/L) at time t (h); CSCFA∞ is the maximum SCFA concentration (mg COD/L); T50 is the time (h) required for 50% of maximum SCFA concentration; Crate indicates the difference in time between which 75% and 25% of the maximum SCFA concentration occurs; and t is the fermentation time.
3. Results and Discussion
3.1. Enhanced SCFAs Production and Composition under Alkaline Condition
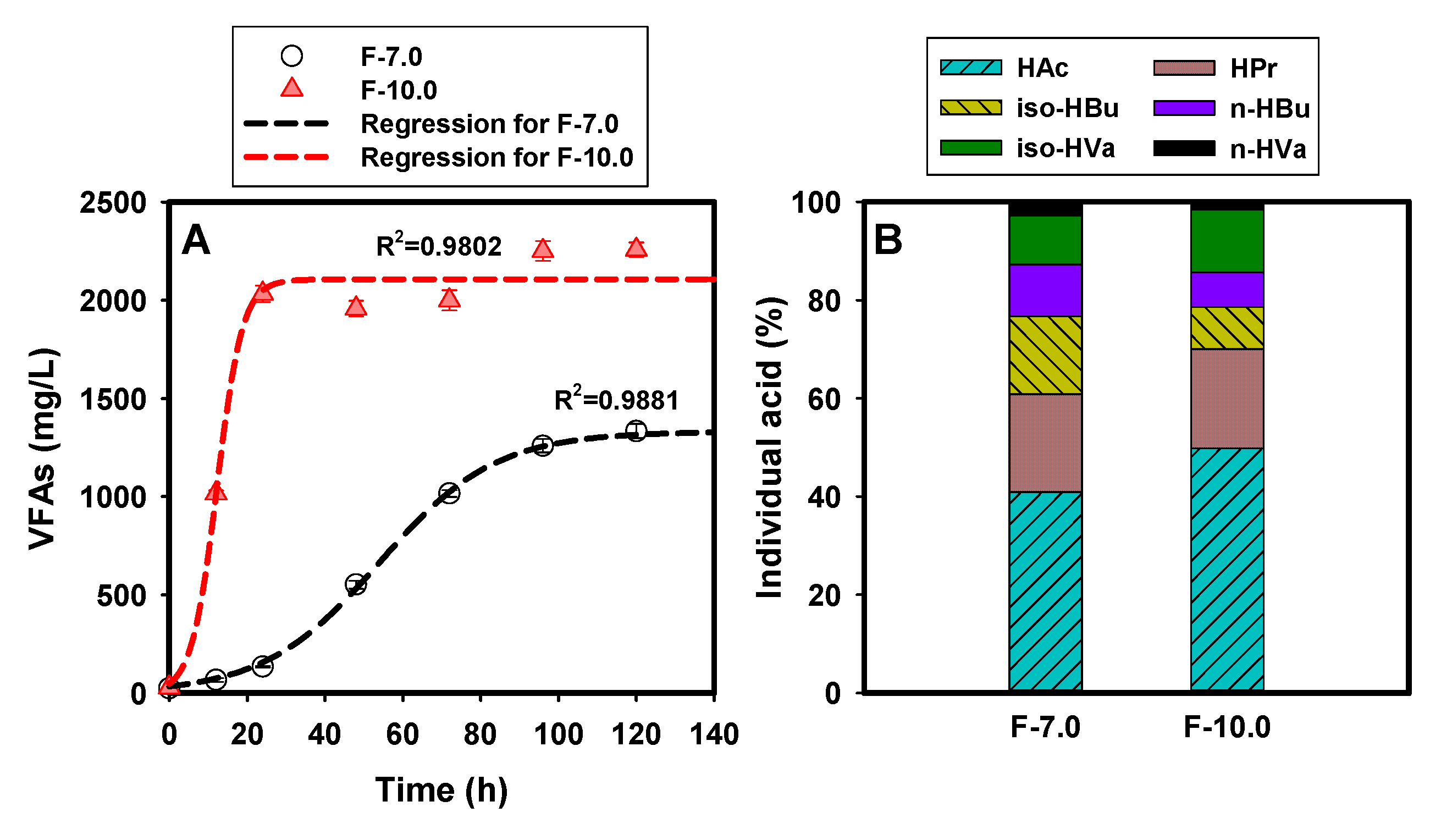
Figure 2 demonstrates the effect of initial alkaline condition on the performance of SCFA production from FSF. As revealed in Figure 2A, higher initial pH significantly promoted the SCFA production with a sharp increase within 24 h compared with the slow increase trend in F-7.0. The peak concentrations of SCFAs were obtained at 120 h in both fermentation groups, with 2258 ± 41 mg/L and 1334 ± 36 mg/L in the F-10.0 and F-7.0 test, respectively. Accordingly, the SCFA yield was 234 mg VFA-COD/g VSS and 118 mg VFA-COD/g VSS. A curve fitting for the time profiles of SCFA production (Figure S3) revealed that the acid-producing rate increased by 75% under alkaline condition, with 14.87 ± 0.78 and 3.22 ± 0.65 for the fitted data of Crate in F-7.0 and F-10.0, respectively (Table 1). The directional accumulation effect of alkaline condition on short-chain acid production (acetate, for instance) was also confirmed by distribution of the individual acids. As depicted in Figure 2B, acetate (HAc) accounted for the highest proportion in the total SCFAs, with 49.8% in F- 10.0, 22% higher than that in the F-7.0 test. A slight difference in the propionic acid (HPr) proportion was observed, with an average of 20% in both groups. It was reported that alkalic ecology was beneficial for the transformation of butyric acid (HBu) into HAc, which could be proved by the lower proportion of 15.6% in the F-10.0 test, 41% lower than that in the F-7.0 test. This observation was consistent with the results obtained by Liu [27].
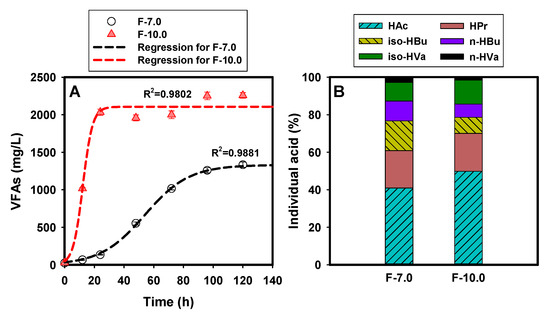
Figure 2.
SCFA production from FSF fermentation: (A) for curve fitting of SCFA generation; (B) for proportion of individual acids in the total peak SCFA yields at 120 h.

Table 1.
Modeling statistics of sigmoid 3 parameter equations for the fitting SCFA-time profiles for the alkali-pretreated FSF.
The FSF disintegration indicated by the changes in VSS concentration is recorded in Table 2. Within 24 h of the alkali addition, a sharp decrease in VSS concentration was observed of 15.6%, from 11.28 ± 1.2 g/L to 9.52 ± 0.4 g/L. In contrast, a mild reduction with only 7.1% was recorded in the F-7.0 test. Before the peak value of the SCFAs (within 120 h), the VSS concentration was reduced by 28.4% in the F-10.0 test, double that in the untreated group (13.2%). At the end of the experimental procedure, 45% VSS had disintegrated in the former group, suggesting an efficient transformation of organics by stimulation of the alkaline agents. Due to limited hydrolysis, the F-7.0 group only obtained a 25% reduction in VSS concentration.

Table 2.
Time profiles of VSS reduction during the anaerobic fermentation (g/L).
3.2. Acceleration of FSF Hydrolysis and Inhibition of Methane Generation
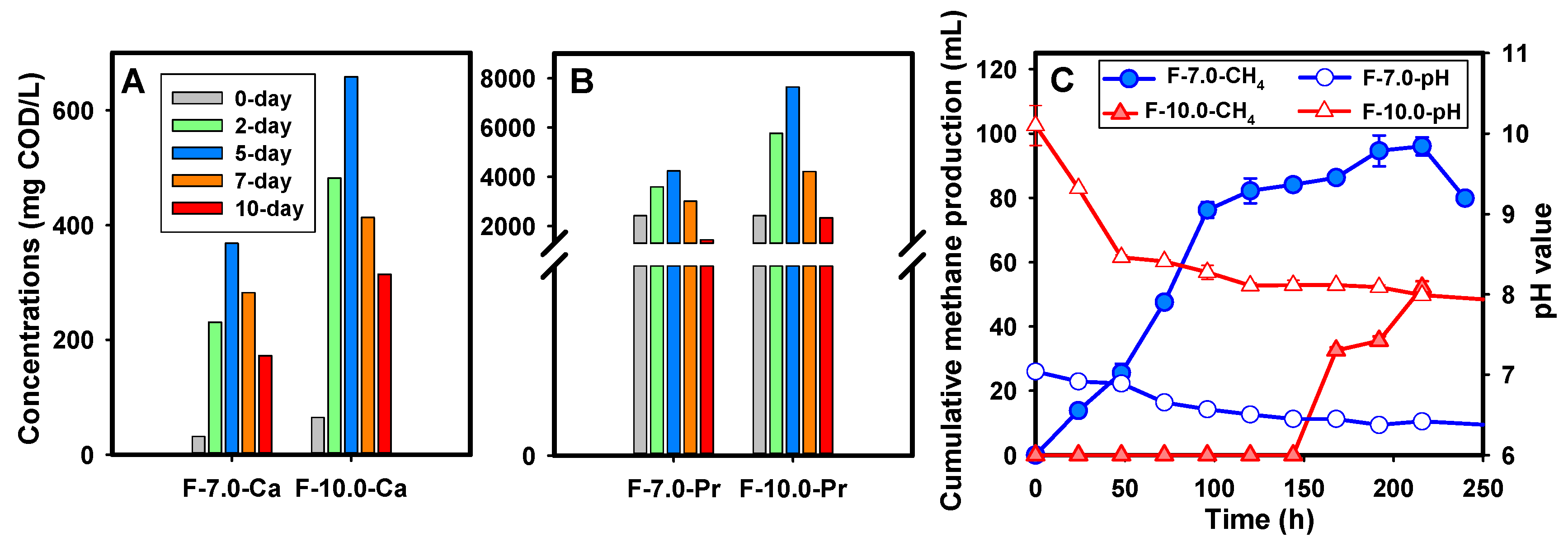
The effect of alkaline conditions on the SCFAs production was significantly dependent on the strong decomposition of the particulates in FSF, leading to the acceleration of the hydrolysis procedure. Figure 3A shows the hydrolysis performance of the F-10.0 test, indicated by time-course profiles of soluble polysaccharides and protein concentrations during the fermentation. As depicted, a sustained promotion effect via alkaline environment on the release of soluble polysaccharides and proteins was observed. Attributed to the strong destruction, a boost in both soluble polysaccharides and proteins was observed within 24 h in the F-10.0 test [20,28]. As revealed by the calculated dissolution rate, the polysaccharide dissolution rate in the Al-FSF test was as high as 17.4 mg/L∙h, 2.1 times higher than that obtained in the control test (8.3 mg/L∙h). Similarly, the protein dissolution rate was 139 mg/L∙h and 49 mg/L∙h in the F-10.0 and F-7.0 test, respectively. Consequently, the abundant soluble organics boost the SCFA production benefiting from sufficient substrates, which could be proved by the rapid consumption of soluble polysaccharides and proteins after the peak value.
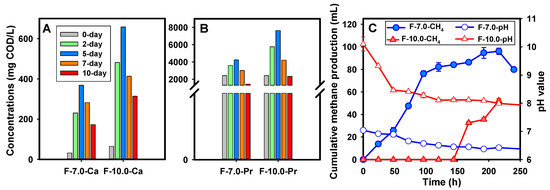
Figure 3.
Performance of FSF hydrolysis and methane inhibition, (A) for soluble polysaccharides release; (B) for soluble protein release under alkaline condition; (C) for changes in cumulative methane production and pH value (Note: Error bars represent standard deviation).
The SCFAs accumulation in the F-10.0 test was also attributed to the inhibition of methane production, which was the main contribution of SCFA consumption. Figure 3B demonstrates the time-course profile of methane production during the alkaline fermentation. As revealed, methane production was detected immediately on the first day in the F-7.0 group, and it continued to increase with the fermentation procedure. Methane production in the F-10.0 test was inhibited with an inhibition ratio of 60–100% all through the fermentation procedure. In detail, low methane content was detected before the 8th day. Yuan investigated the effect of different acid–base conditions on the acid production from WAS and believed that alkaline conditions severely restrained the methanogenesis. This inhibition was most likely attributed to the alkalic ecology as confirmed by changes in pH value. As acknowledged, the optimal pH value for methanogens was in the range of 6.6–7.8. Higher or lower than this threshold, methane production could be inhibited. As depicted in Figure 3B, the pH value in the F-10.0 test was much higher, far beyond the optimal range, which meant severely constrained microbial activity under the alkalic ecology.
3.3. Microbial Community Structure
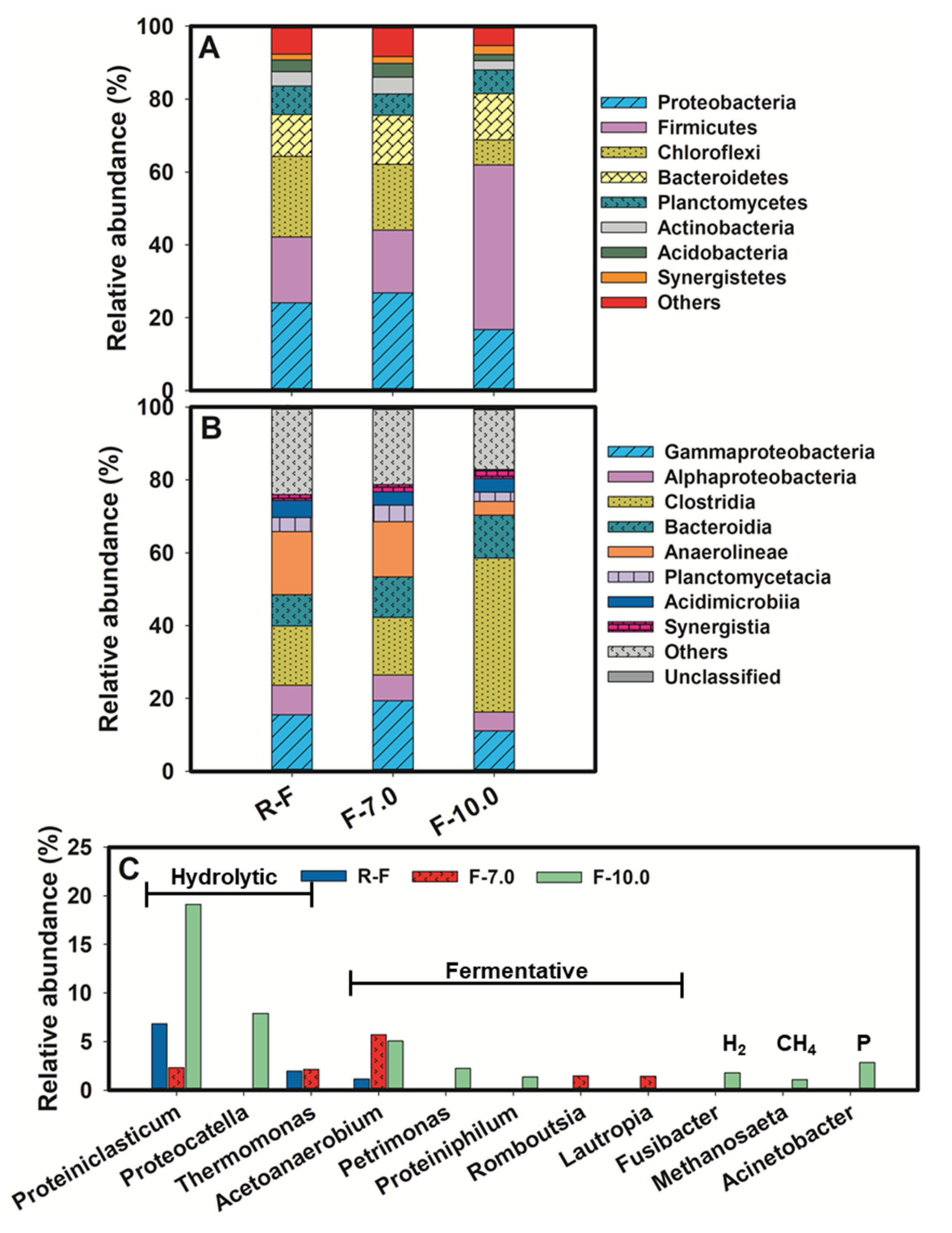
Changes in microbial community structure were highly responsive to the performance in hydrolysis and acidification under alkaline conditions, as depicted in Figure 4. Firmicutes was a typical phylum with capacity for hydrolysis and fermentation [29]. As revealed, the abundance of Firmicutes increased sharply to 45.20% after alkaline fermentation with an initial pH value of 10.5, 2.5–2.6 times higher than that in the raw FSF (RF) and neutral fermented group, which might be the underlying reason for its remarkable performance in SCFA production. A sharp decrease in abundance of Chloroflexi was also observed, with 6.81% in F-10.0, 69% lower than that in RF. Proteobacteria, as a common fermentative phylum, accounted for the largest proportion in both RF and F-7.0. However, it decreased by 31% in alkalic ecology. Further analysis at class level (Figure 4B) demonstrated that Clostridia made a great contribution to the climbing abundance of Firmicutes, with 42.35% in F-10.0, 61.7% higher than that in RF. While, Anaerolineae, being subjected to Chloroflexi, took up only 3.73% in the alkaline group, 75% lower than that in the F-7.0.
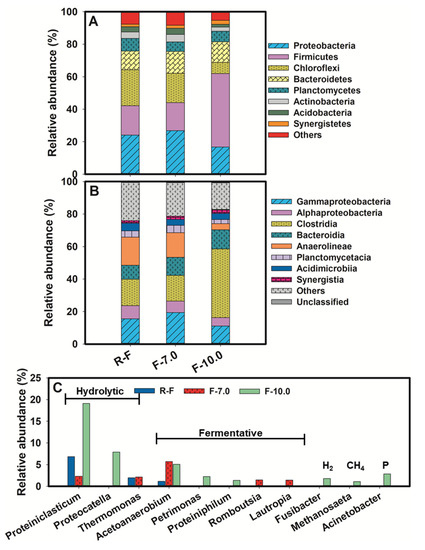
Figure 4.
Taxonomic classification of pyrosequences from bacterial communities of sludge samples, (A) for bacterial community at phylum level, (B) for bacterial community at class level, (C) for bacterial community at genus level.
The changes in the microbial community were further elaborated at the genus level, as shown in Figure 4C. The high abundance of genus correlating with protein hydrolysis was recorded, providing a firm basis for the remarkable protein hydrolysis, as discussed in Section 3.2. Proteiniclasticum, a specific bacterial genus associated with proteolysis [30], climbed to 19.12% in F- 10.0, 2.8 times and 1.2 times higher than that in RF and F-7.0, respectively. The abundance of Proteocatella was also boosted in F-10.0 to 7.9%. It was a genus characterized by proteolytic, chitinolytic, and amylolytic activities [31]. Note that it was not detected in both RF and F-7.0. Thermomonas, which showed up positive in enzyme activities of β-glucosidase and trypsin with mesophilic growth at the temperature range of 28–37 °C [32], seemed more suitable in neutral ecology, with 1.94% and 2.13% in RF and F-7.0, but with no detection in F-10.0 due to efficient hydrolysis to provide sufficient substrate for acidification, fermentative genus boost. Proteiniphilum is a proteophilic fermentative genus, and Petrimonas is capable of degrading polysaccharides and proteins to produce HAc, HPr and low amounts of valeric acid [33]. As revealed, the proportion of Petrimonas and Proteiniphilum slightly increased to 2.22% and 1.34%, respectively, with the pH value rise. Acetoanaerobium, with the capacity of lignin degradation [34], was more abundant in F-7.0 than that in F-10.0, with 5.71% and 5.06%, respectively. Some polysaccharide-fermentative genus were exclusive in F-7.0, including Romboutsia [35] and Lautropia [36], with a relative abundance of 1.47% and 1.42%, respectively. While attributable to enough SCFAs, especially for HAc, hydrogen and methane-producing genus appeared in F-10.0, although with lower abundance, like Fusibacter [37] (1.77%) and Methanosaeta [38] (1.07%). Note that a higher concentration of phosphorus was released accompanied by FSF disintegration might incur growth of phosphorus-accumulating bacteria, Acinetobacter [39], for instance (with 2.82% in F-10.0).
3.4. Significance and Potential Benefits
The scheme integrated FSF recovery up-stage of the biological treatment with improved SCFA production under alkaline conditions was addressed to maximize the carbon sources available to the BNR process, to alleviate the financial burden for ECS purchase. As revealed in Figure 5, 1978 kg FSF could be recovered, with a higher solid content of 5.4%. The calculated volume for the FSF was 35.4 m3 divided by the solid content and density of 1035 kg/m3. According to the fermentation performance of raw FSF with SCFA yield of 118 mg/g VSS and VSS reduction of 25%, the total production of SCFAs was estimated to be 18.2 kg/d. In detail, it meant 7.3 kg/d HAc and 3.6 kg/d HPr, based on the proportion of individual acids with 40% and 20%, respectively. The initial alkaline condition boosted the SCFA production to 77.6 kg/d, with 31.0 kg/d 15.5 kg/d for HAc and HPr, respectively.
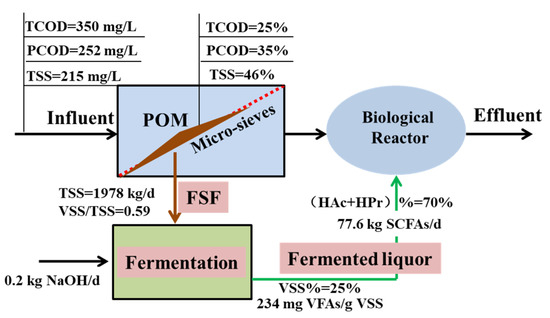
Figure 5.
Preliminary evaluation of carbon conversion by FSF recovery and fermentation under alkaline condition.
The economic and environmental benefits for internal carbon source exploration were further estimated, under the assumption that all the produced SCFAs could be recovered and replace the ECS addition. As listed in Table 3, 18.2 kg SCFAs-COD/d indicated 12.1 kg methanol addition, with a cost reduction of 17.3 RMB/d. Due to more SCFA production, this reduction raised it to 72.3 RMB for the alkaline pretreated FSF fermentation, deducting the cost for alkali agents. Furthermore, the potential environmental benefits were indicated by two environmental impact types with a greater contribution to the life cycle inventory. As revealed, 2580 MJ of primary energy demand (PED) and 54.6 kg CO2 eq of global warming potential (GWP) were reduced in F-10.0, respectively, 3.3-fold and 3.2-fold more than that obtained in F-7.0.

Table 3.
Preliminary evaluation of the environmental benefits of FSF under alkaline condition.
4. Conclusions
An initial alkaline condition can significantly increase SCFA extraction from FSF, with the maximum SCFA yield being 234 mg SCFAs/g VSS. This promotion was mainly due to both the efficient hydrolysis of high molecular organic compounds such as polysaccharides and proteins by strong alkalis, and the strong inhibition of the activity of methanogens. A preliminary evaluation of the mass balance and economic benefit further confirmed the outstanding advantage of alkaline fermentation in the exploration of cheap internal carbon sources. The maximum reduction in ECS was evaluated with 39.6 kg/d methanol for the case of WWTP. Consequently, this strategy by particulate organics re-direction and the enhanced transformation were promising in addressing the carbon insufficiency for the BNR process. The economic and environmental profits were also notable by reduced ECS addition, aeration power, and sludge disposal.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1073/13/18/4690/s1, Figure S1: Time-course profiles of flux in POM recovery by sieves with pore size of 131 μm, Figure S2: Picture of batch fermenter, Figure S3: Time-course profiles of total SCFAs production in F-10.0 and F-7.0 groups, Table S1: Environmental impact of chemical agents manufacture.
Author Contributions
Conceptualization, X.Y.; methodology, Y.D. and Z.Z.; software, Y.G.; validation, Y.L.; formal analysis, Y.L.; investigation, Y.D.; resources, Z.Z.; data curation, Z.Z. and Y.G.; writing—original draft preparation, Y.D. and A.Z.; writing—review and editing, A.Z. and X.Y.; visualization, Y.D.; supervision, X.Y.; project administration, A.Z.; funding acquisition, X.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Ecology and Environment of the People’s Republic of China (Major Science and Technology Program, No. 2019YFC0408601 and No. 2019YFC0408602), by the National Natural Science Foundation of China (NSFC, Nos. 51708386, 21501129 and 21707099), by the China Postdoctoral Science Foundation (No. 2016M591416), by the State Key Laboratory of Pollution Control and Resource Reuse Foundation (No. PCRRF17021), by the Scientific and Technological Project of Shanxi Province (No. 201701D221230) and by the Key Research and Development (R&D) Project of Shanxi Province (No. 201903D321057 and 201903D321055).
Acknowledgments
We are very grateful to Yang and Li in Zhengyang WWTP for sample collection. Also, special thanks are given to Wang for offering help in the data collection. In addition, we appreciate the help of Wang in the laboratory for her patient guidance in the calibration and commissioning of the testing instruments.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| Abbreviation | Name |
| BNR | Biological nutrient removal |
| WWTPs | Wastewater treatment plants |
| ECS | External carbon sources |
| SCFAs | Short chain fatty acids |
| COD | Chemical oxygen demand |
| WAS | Waste activated sludge |
| AD | Anaerobic digestion |
| FSF | Fine-sieving fractions |
| TCOD | Total COD |
| PCOD | Particulate COD |
| TSS | Total suspended solids |
| SBR | Sequencing batch reactor |
| SCOD | Soluble COD |
| VSS | Volatile suspended solids |
| ISS | Inorganic suspended solids |
| PCR | Polymerase chain reaction |
References
- Li, C.; Liu, S.; Ma, T.; Zheng, M.; Ni, J. Simultaneous nitrification, denitrification and phosphorus removal in a sequencing batch reactor (SBR) under low temperature. Chemosphere 2019, 229, 132–141. [Google Scholar] [CrossRef]
- Hu, X.; Wisniewski, K.; Czerwionka, K.; Zhou, Q.; Xie, L.; Makinia, J. Modeling the Effect of External Carbon Source Addition under Different Electron Acceptor Conditions in Biological Nutrient Removal Activated Sludge Systems. Environ. Sci. Technol. 2016, 50, 1887–1896. [Google Scholar] [CrossRef]
- Flores-Alsina, X.; Corominas, L.; Snip, L.; Vanrolleghem, P.A. Including greenhouse gas emissions during benchmarking of wastewater treatment plant control strategies. Water Res. 2011, 45, 4700–4710. [Google Scholar] [CrossRef]
- Guo, L.; Guo, Y.; Sun, M.; Gao, M.; Zhao, Y.; She, Z. Enhancing denitrification with waste sludge carbon source: The substrate metabolism process and mechanisms. Environ. Sci. Pollut. Res. 2018, 25, 13079–13092. [Google Scholar] [CrossRef] [PubMed]
- Zubrowska-Sudol, M.; Walczak, J. Enhancing combined biological nitrogen and phosphorus removal from wastewater by applying mechanically disintegrated excess sludge. Water Res. 2015, 76, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Gu, J.; Zhao, Q.; Liu, Y. COD capture: A feasible option towards energy self-sufficient domestic wastewater treatment. Sci. Rep. 2016, 6, 25054. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, Y.; Li, X.; Luo, P.; Wang, H.; Robinson, Z.P.; Wang, X.; Wu, J.; Li, F. The feasibility and challenges of energy self-sufficient wastewater treatment plants. Appl. Energy 2017, 204, 1463–1475. [Google Scholar] [CrossRef]
- Heidrich, E.S.; Curtis, T.P.; Dolfing, J. Determination of the internal chemical energy of wastewater. Environ. Sci. Technol. 2011, 45, 827–832. [Google Scholar] [CrossRef]
- Ruffino, B.; Campo, G.; Genon, G.; Lorenzi, E.; Novarino, D.; Scibilia, G.; Zanetti, M. Improvement of anaerobic digestion of sewage sludge in a wastewater treatment plant by means of mechanical and thermal pre-treatments: Performance, energy and economical assessment. Bioresour. Technol. 2015, 175, 298–308. [Google Scholar] [CrossRef]
- Yi, L.; Luo, X.; Huang, X.; Wang, D.; Zhang, W. Life Cycle Assessment of a municipal wastewater treatment plant: A case study in Suzhou, China. J. Clean. Prod. 2013, 57, 221–227. [Google Scholar]
- Lin, L.; Li, R.H.; Li, X.Y. Recovery of organic resources from sewage sludge of Al-enhanced primary sedimentation by alkali pretreatment and acidogenic fermentation. J. Clean. Prod. 2018, 172, 3334–3341. [Google Scholar] [CrossRef]
- Ruiken, C.J.; Breuer, G.; Klaversma, E.; Santiago, T.; Loosdrecht, M.C.M.V. Sieving wastewater—Cellulose recovery, economic and energy evaluation. Water Res. 2013, 47, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Crutchik, D.; Frison, N.; Eusebi, A.L.; Fatone, F. Biorefinery of cellulosic primary sludge towards targeted Short Chain Fatty Acids, phosphorus and methane recovery. Water Res. 2018, 136, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Remy, C.; Boulestreau, M.; Lesjean, B. Proof of concept for a new energy-positive wastewater treatment scheme. Water Sci. Technol. 2014, 70, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Wang, B.; Jiao, D.; Sun, G.; Wang, B.; Wang, X.C. Characterization of microflora and transformation of organic matters in urban sewer system. Water Res. 2015, 84, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.H.; Jin, P.K.; Ngo, H.H.; Shi, X.; Guo, W.S.; Yang, S.J.; Wang, X.C.; Wang, X.; Dzakpasu, M.; Yang, W.N.; et al. Transformation and utilization of slowly biodegradable organic matters in biological sewage treatment of anaerobic anoxic oxic systems. Bioresour. Technol. 2016, 218, 53–61. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ji, F.-y.; He, X.-l.; Zhou, W.-W.; Xu, X.; Lai, M.-S. Validation of accumulation models for inorganic suspended solids of different particle size in an activated sludge system. Bioresour. Technol. 2013, 149, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Rusten, B.; Razafimanantsoa, V.A.; Andriamiarinjaka, M.A.; Otis, C.L.; Sahu, A.K.; Bilstad, T. Impact of fine mesh sieve primary treatment on nitrogen removal in moving bed biofilm reactors. Water Sci. Technol. 2016, 73, 337–344. [Google Scholar] [CrossRef]
- Jie, W.; Peng, Y.; Ren, N.; Li, B. Volatile fatty acids (VFAs) accumulation and microbial community structure of excess sludge (ES) at different pHs. Bioresour. Technol. 2014, 152, 124–129. [Google Scholar] [CrossRef]
- Wu, H.; Gao, J.; Yang, D.; Zhou, Q.; Liu, W. Alkaline fermentation of primary sludge for short-chain fatty acids accumulation and mechanism. Chem. Eng. J. 2010, 160, 1–7. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, Y.; Zhang, H.; Jiang, S.; Zhou, Q.; Gu, G. Improved bioproduction of short-chain fatty acids (SCFAs) from excess sludge under alkaline conditions. Environ. Sci. Technol. 2006, 40, 2025–2029. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, A.; Liu, H.; Wang, S.; Liu, W.; Wang, A.; Yue, X. Extracellular polymeric substance decomposition linked to hydrogen recovery from waste activated sludge: Role of peracetic acid and free nitrous acid co-pretreatment in a prefermentation-bioelectrolysis cascading system. Water Res. 2020, 176, 115724. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shang, Y.; Gong, T.; Hu, Z.; Yang, K.; Shao, S. High concentration of Mn2+ has multiple influences on aerobic granular sludge for aniline wastewater treatment. Chemosphere 2020, 240, 124945–124953. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wang, X.; Huppes, G.; Zhao, X.; Ren, N. Using site-specific life cycle assessment methodology to evaluate Chinese wastewater treatment scenarios: A comparative study of site generic and site-specific methods. J. Clean. Prod. 2017, 144, 1–7. [Google Scholar] [CrossRef]
- Duan, Y.; Zhou, A.; Wen, K.; Liu, Z.; Liu, W.; Wang, A.; Yue, X. Upgrading VFAs bioproduction from waste activated sludge via co-fermentation with soy sauce residue. Front. Environ. Sci. Eng. 2019, 13, 3. [Google Scholar] [CrossRef]
- Carranzo, I.V. Standard Methods for examination of water and wastewater. An. De Hidrol. Médica 2012, 5, 185–186. [Google Scholar]
- Liu, H.; Wang, J.; Liu, X.; Fu, B.; Chen, J.; Yu, H.-Q. Acidogenic fermentation of proteinaceous sewage sludge: Effect of pH. Water Res. 2012, 46, 799–807. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, X.; Xiao, K.; Shen, N.; Zeng, R.J.; Zhou, Y. Enhanced volatile fatty acids (VFAs) production in a thermophilic fermenter with stepwise pH increase—Investigation on dissolved organic matter transformation and microbial community shift. Water Res. 2017, 112, 261–268. [Google Scholar] [CrossRef]
- He, J.; Wang, X.; Yin, X.; Li, Q.; Li, X.; Zhang, Y.; Deng, Y. Insights into biomethane production and microbial community succession during semi-continuous anaerobic digestion of waste cooking oil under different organic loading rates. AMB Express 2018, 8, 92. [Google Scholar] [CrossRef]
- Zhang, K.; Song, L.; Dong, X. Proteiniclasticum ruminis gen. nov., sp. nov., a strictly anaerobic proteolytic bacterium isolated from yak rumen. Int. J. Syst. Evol. Microbiol. 2010, 60, 2221–2225. [Google Scholar] [CrossRef]
- Pikuta, E.V.; Hoover, R.B.; Marsic, D.; Whitman, W.B.; Lupa, B.; Tang, J.; Krader, P. Proteocatella sphenisci gen. nov., sp. nov., a psychrotolerant, spore-forming anaerobe isolated from penguin guano. Int. J. Syst. Evol. Microbiol. 2009, 5, 2302–2307. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, S.; Wang, D.; Wang, L.; Wang, G. Thermomonas carbonis sp. nov., isolated from the soil of a coal mine. Int. J. Syst. Evol. Microbiol. 2014, 64, 3631–3635. [Google Scholar] [CrossRef]
- Hahnke, S.; Langer, T.; Koeck, D.E.; Klocke, M. Description of Proteiniphilum saccharofermentans sp. nov., Petrimonas mucosa sp. nov. and Fermentimonas caenicola gen. nov., sp. nov. isolated from mesophilic laboratory-scale biogas reactors and emended description of the genus Proteiniphilum. Int. J. Syst. Evol. Microbiol. 2016, 66, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Huo, X.; Du, W.J.; Liang, J.D.; Wang, D.Q.; Yang, S.C. Biodegradation of kraft lignin by a newly isolated anaerobic bacterial strain, Acetoanaerobium sp. WJDL-Y2. Lett. Appl. Microbiol. 2016, 62, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.; Fuentes, S.; Grievink, W.; Niftrik, L.v.; Tindall, B.J.; Timmerman, H.M.; Rijkers, G.T.; Smidt, H. Characterization of Romboutsia ilealis gen. nov., sp. nov., isolated from the gastro-intestinal tract of a rat, and proposal for the reclassification of five closely related members of the genus Clostridium into the genera Romboutsia gen. nov., Intestinibacter gen. nov., Terrisporobacter gen. nov. and Asaccharospora gen. nov. Int. J. Syst. Evol. Microbiol. 2014, 64, 1600–1616. [Google Scholar]
- Daneshvar, M.I.; Douglas, M.P.; Weyant, R.S. Cellular fatty acid composition of Lautropia mirabilis. J. Clin. Microbiol. 2001, 39, 4160–4162. [Google Scholar] [CrossRef] [PubMed]
- He, Z.W.; Liu, W.Z.; Gao, Q.; Tang, C.C.; Wang, L.; Guo, Z.C.; Zhou, A.J.; Wang, A.J. Potassium ferrate addition as an alternative pre-treatment to enhance short-chain fatty acids production from waste activated sludge. Bioresour. Technol. 2017, 247, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Garrity, G.M.; Labeda, D.P.; Oren, A. Judicial Commission of the International Committee on Systematics of Prokaryotes XIIth International (IUMS) Congress of Bacteriology and Applied Microbiology. Int. J. Syst. Evol. Microbiol. 2011, 61, 2775–2780. [Google Scholar] [CrossRef]
- Zuo, N.; He, J.; Ma, X.; Peng, Y.; Li, X. Phosphorus removal performance and population structure of phosphorus-accumulating organisms in HA-A/A-MCO sludge reduction process. Bioengineered 2016, 7, 327–333. [Google Scholar] [CrossRef][Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).