Abstract
The changes introduced by both ball milling and the addition of small amounts of TiF in the kinetics of the hydrogen desorption of three different Ca(BH) polymorphs (, and ) have been systematically investigated. The samples with different polymorphic contents, before and after the addition of TiF, were characterized by powder X-ray diffraction and vibrational spectroscopy. The hydrogen desorption reaction pathways were monitored by differential scanning calorimetry. The hydrogen desorption of Ca(BH) depends strongly on the amount of coexistent , and polymorphs as well as additional ball milling and added TiF to the sample. The addition of TiF increased the hydrogen desorption rate without significant dissociation of the fluoride. The combination of an -Ca(BH) rich sample with 10 mol% of TiF and 8 h of milling led to up to 27 decrease of the hydrogen desorption peak temperature.
1. Introduction
The alkali earth metal calcium borohydride, Ca(BH), possesses 11.6 wt.% gravimetric and 130 kgm volumetric hydrogen densities, respectively, and therefore it is often considered an attractive hydrogen storage system either alone or in composites [1,2,3,4,5,6,7]. Moreover, its calculated low reaction enthalpy, 32 kJmolH for equation
that leads to a 9.6 wt.% H yield [8,9,10,11,12] and its possible reversibility at moderate conditions [2,13,14,15,16], make Ca(BH) a particularly attractive hydrogen storage candidate.
However, this is a complicated borohydride to control. There are four known polymorphs of Ca(BH). Three of them are referred to as low-temperature phases, -, -, and -Ca(BH), while only one high-temperature modification, -Ca(BH), has been identified [1,10,17].
Their crystal structures have been assigned to orthorhombic space groups in the cases of - (Fddd [8,18] or F2dd [17]) and -Ca(BH) (Pbca [18]), and to tetragonal space groups for - (I-42d [17]) and -Ca(BH) (P4 [19] or P-4 [17]). Most transitions between the polymorphs are of first-order [20], with the - transition being of second order [17]. Because of the small enthalpy differences between the various polymorphs (H(-) = 13.03 kJmolH, H(-) = 6.08 kJmolH [19]; or H(-) = 8.59 kJmolH, H(-) = 3.38 kJmolH [20]), it is common that they appear mixed in different ratios in batches produced by the same synthesis method. This fact complicates the characterization of the thermodynamics and kinetics of Ca(BH) and the future reproducibility for practical applications [1].
The complete dehydrogenation of Ca(BH) takes place between room temperature (RT) and 600 C [1,10,21]. The process leads to three main endothermic events that can be assigned to the reversible to , low-temperature-to-high-temperature polymorphic transformation (between 160 and 250 ), the decomposition of the high- temperature polymorph (above 350 ), and the decomposition of intermediates formed from metastable phases (CaBH has been proposed [22]). The temperatures at which these events occur depend strongly on experimental conditions such as type of gas flow, heating rate, et cetera, but also on the partial content of the different Ca(BH) polymorphs in the sample [1]. In particular, our previous detailed study of the role of Ca(BH) polymorphs showed increases of the decomposition temperature of up to 15 C in samples containing -Ca(BH) compared to a purer -Ca(BH), and of about 6 C lower decomposition temperatures for a pure - Ca(BH) sample synthesized by a solvent free method compared to the high-temperature modification obtained via the polymorphic transformation of -Ca(BH). The polymorphic content was also found to affect the amount of hydrogen released in each of the decomposition steps, with an increase of the mass loss for samples containing more of the -Ca(BH) polymorph [1]. These results confirm that the pure Ca(BH) polymorphs have different kinetic barriers and that the polymorphic content of a sample determines the decomposition kinetics [1].
On the other hand, it is known that the decomposition temperature of Ca(BH) is decreased both by ball milling and by the addition of additives in small amounts [2,15,21]. Recently, similar effects have been observed in other light metal borohydrides such as NaBH [23,24,25,26] and Mg(BH) [27,28,29], and on reactive composites like the Ca(BH)-MgH system [6]. Moreover, fluoride additives such as TiF and NbF not only have shown that the reversibility of Ca(BH) is possible under moderate conditions (140 bar and near RT), but also that they significantly decrease the decomposition temperature of the borohydride when compared to chlorides [14,15,27,30]. However, the mechanisms by which the improvement happens are not yet known.
Based on these previous works, we present here a study on the effects on the dehydrogenation temperature of the three most common polymorphs of Ca(BH), , and , by both ball milling and the addition of small amounts of TiF.
2. Results and Discussion
2.1. Milling Effects on As-Received Ca(BH)
Before analyzing the influence of TiF on the decomposition properties of Ca(BH) polymorphs, the effects of ball milling on the as-received - and -Ca(BH) were studied (Table 1 and Figure 1). Due to the lack of enough original material these milling experiments were not performed on pure -Ca(BH).
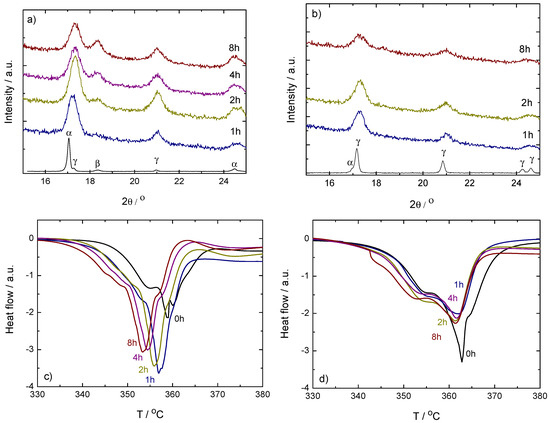
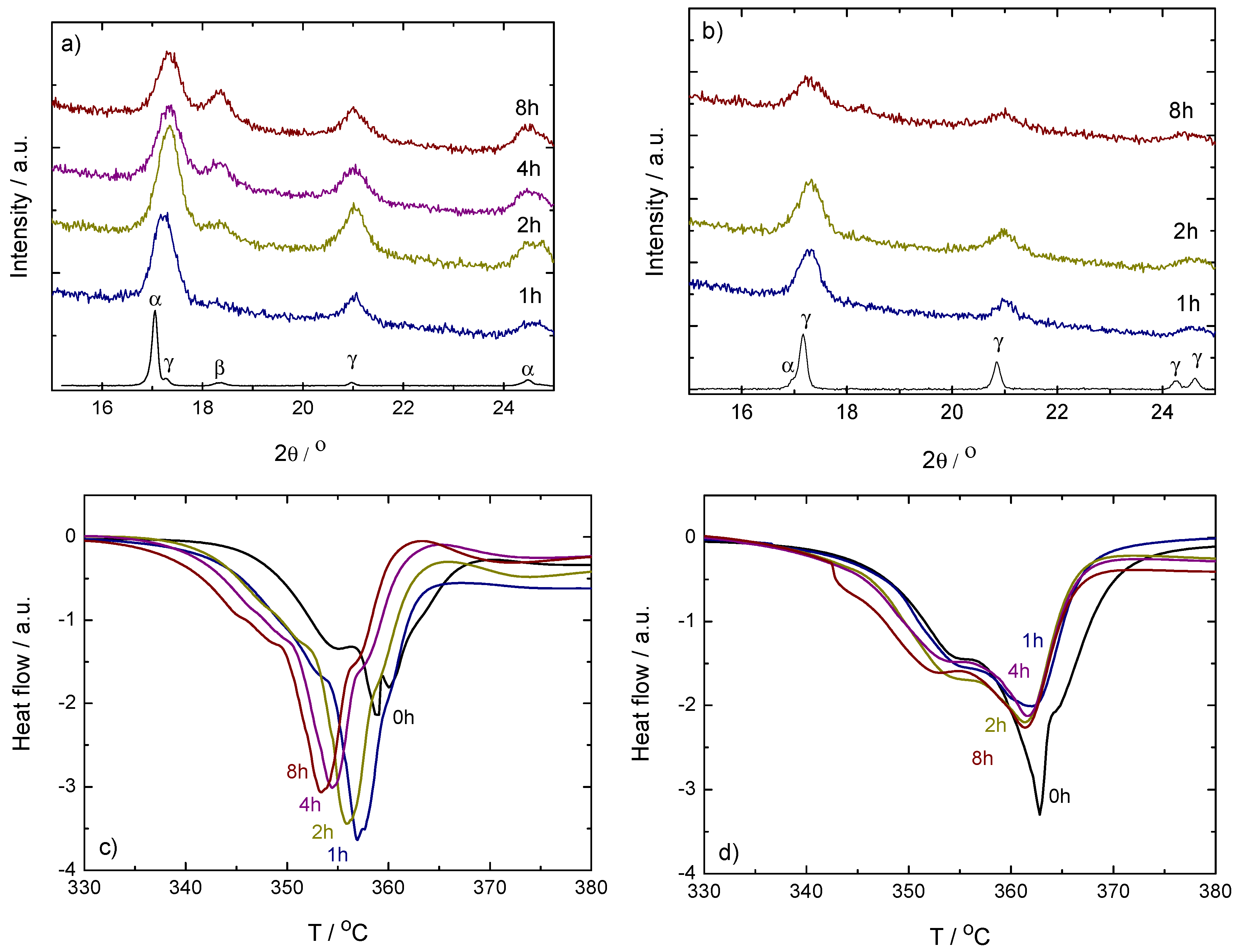
Figure 1.
Top: Powder X-ray diffraction (PXD) results on ball milled (left-hand side, (a)) and (right-hand side, (b)). Samples were taken from the vials after 1, 2, 4 and 8 h milling. The pattern of the as-received sample is included for comparison. Missing 4 h curve in the case due to low signal-to-noise-ratio. Below: Corresponding differential scanning calorimetry (DSC) curves of ball milled (left-hand side, (c)) and (right-hand side, (d)). Only the main decomposition region between 330 and 380 C is shown. Measurements were done under dynamic conditions in protective Ar flow, with 2 C/min heating rates. The curves have been shifted in the vertical axis to show the peak trend.
In addition to the broadening of the diffraction peaks seen for all the milled samples when compared to the as-received material, the PXD analysis (top of Figure 1) also shows an increase of the -Ca(BH) content with increased milling time in the case of the sample. This indicates that the temperatures achieved in the vials during the milling process are enough to induce a phase transformation from the low temperature - to the high temperature -Ca(BH). The transition temperature is about 167 C under a helium gas flow and with a heating rate of 5 C/min [1].
This behavior is in agreement with the fact that -Ca(BH) becomes more stable compared to -Ca(BH) with increasing temperature [19]. The Differential scanning calorimetry (DSC) curves (bottom of Figure 1) show a small decrease of the decomposition temperature of the samples with milling time, from approximately the 357 C of the 1 h milled sample to the 353 C of the 8 h sample, and thus indicates that the -Ca(BH) formed during milling is less stable that the same phase formed during heating. This could be related to smaller crystallites in the milling case. No big changes in the polymorphic ratio with milling time are observed in the PXD patterns of the sample (Figure 1b), leading to no shift of the decomposition temperatures in the DSC curves (Figure 1d). The lack of change in this case agrees with the gradual and broad temperature range in which the to transformation occurs [31] and the fact that -Ca(BH) is metastable in the whole temperature range below the decomposition temperature of the high temperature polymorph [19]. Thus, it seems that under the ball milling conditions used in this work, the to transformation is less probable than the to transformation. The behavior is reproducible and indicates that not only the as-received Ca(BH) polymorphs have different decomposition temperatures [1], but that ball milling effects also depend on the polymorphic composition of the samples.
2.2. Milling Effects on Ca(BH) with TiF
PXD patterns of the samples containing 1.6 and 10 mol% of TiF show that no new species are formed during the milling with Ca(BH) (Figure 2). In particular, no cation or anion substitutions, such as those found for NaBH [24], are observed. In all the cases, Ca(BH) and TiF in the crystalline form are still present in the milled material. The broadening of the Bragg peaks and the loss of signal to noise ratio of the measurements are an indication of the finer crystallite size introduced by ball milling.
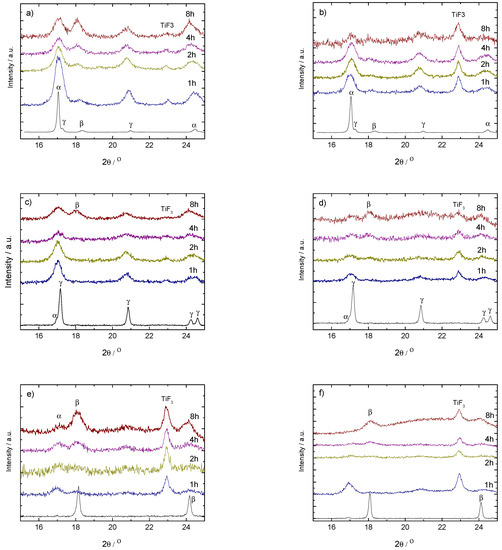
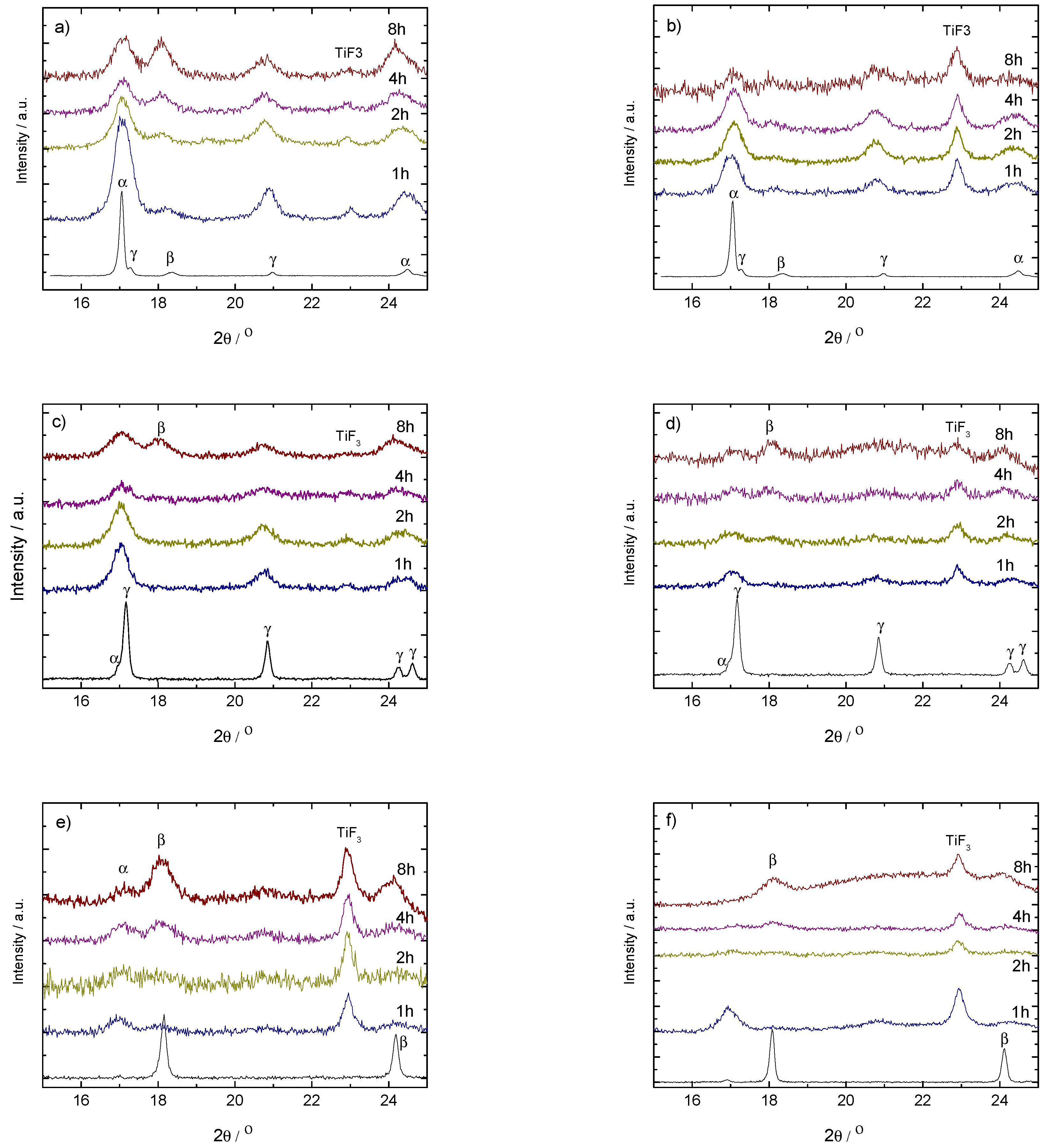
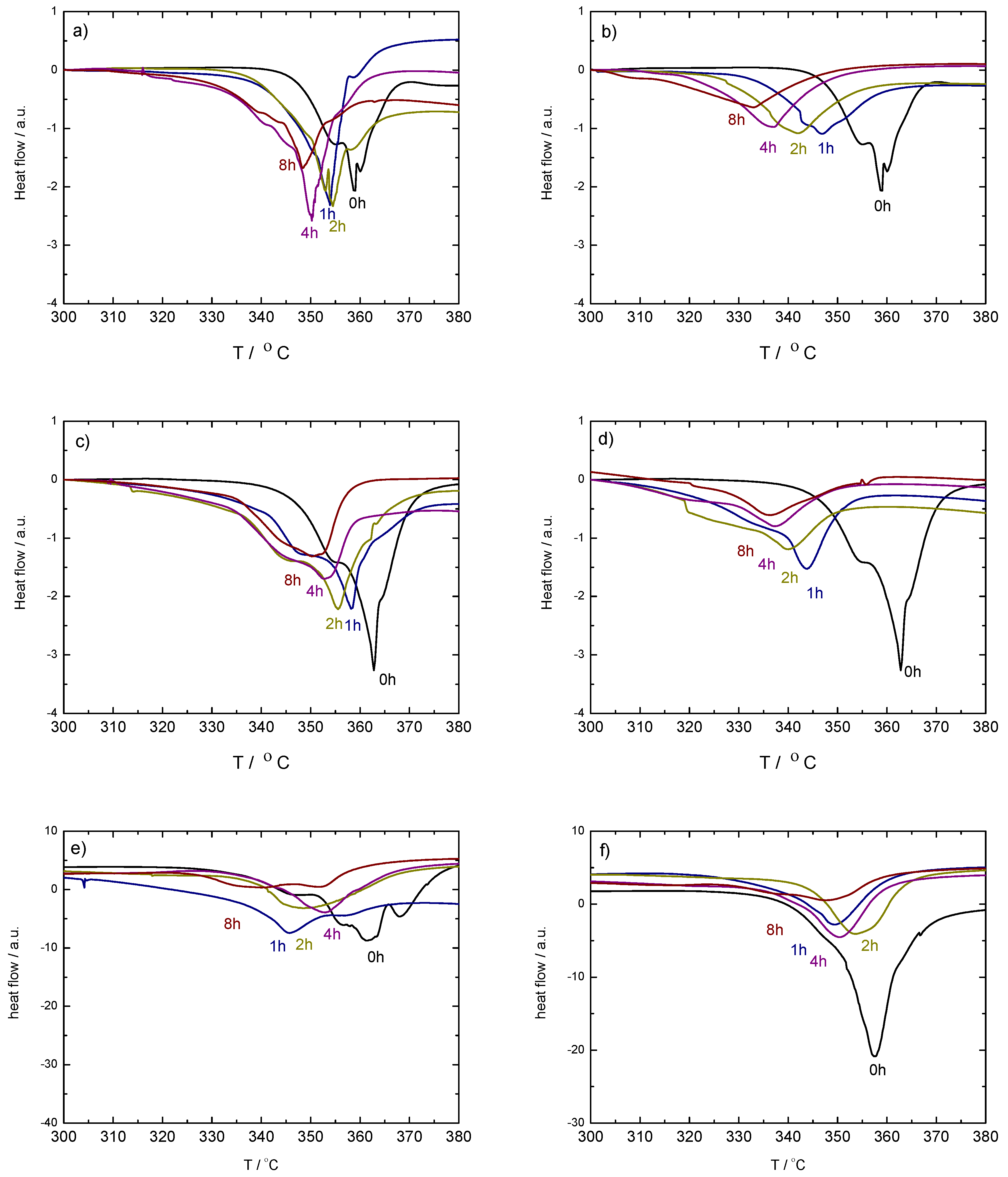
Figure 2.
PXD results on ball milled (up, (a,b)) and (middle, (c,d)) with 1.6 mol% (left-hand side, (a,c)) and 10 mol% (right-hand side, (b,d)) of TiF. Bottom: PXD results on ball milled (left-hand side, (e)) and (right-hand side, (f)) with 10 mol% of TiF. Samples were taken from the vials after 1, 2, 4 and 8 h milling. The pattern of the as-received sample is included for comparison.
The PXD results are confirmed by IR spectroscopy (Figure 3 and Figure 4), where the only differences between the as-received and the samples with TiF are observed in the 400–1000 cm range. In particular, the samples with additive show a prominent feature at approximately 554 cm, which increases with additive amount.
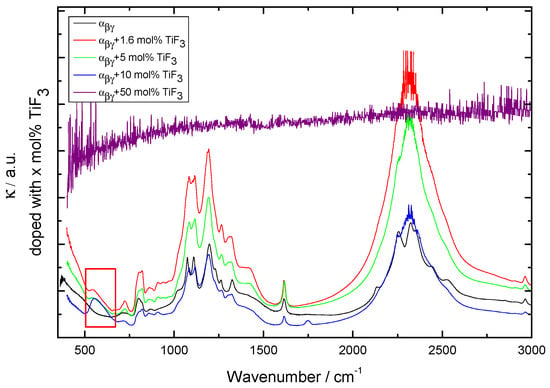
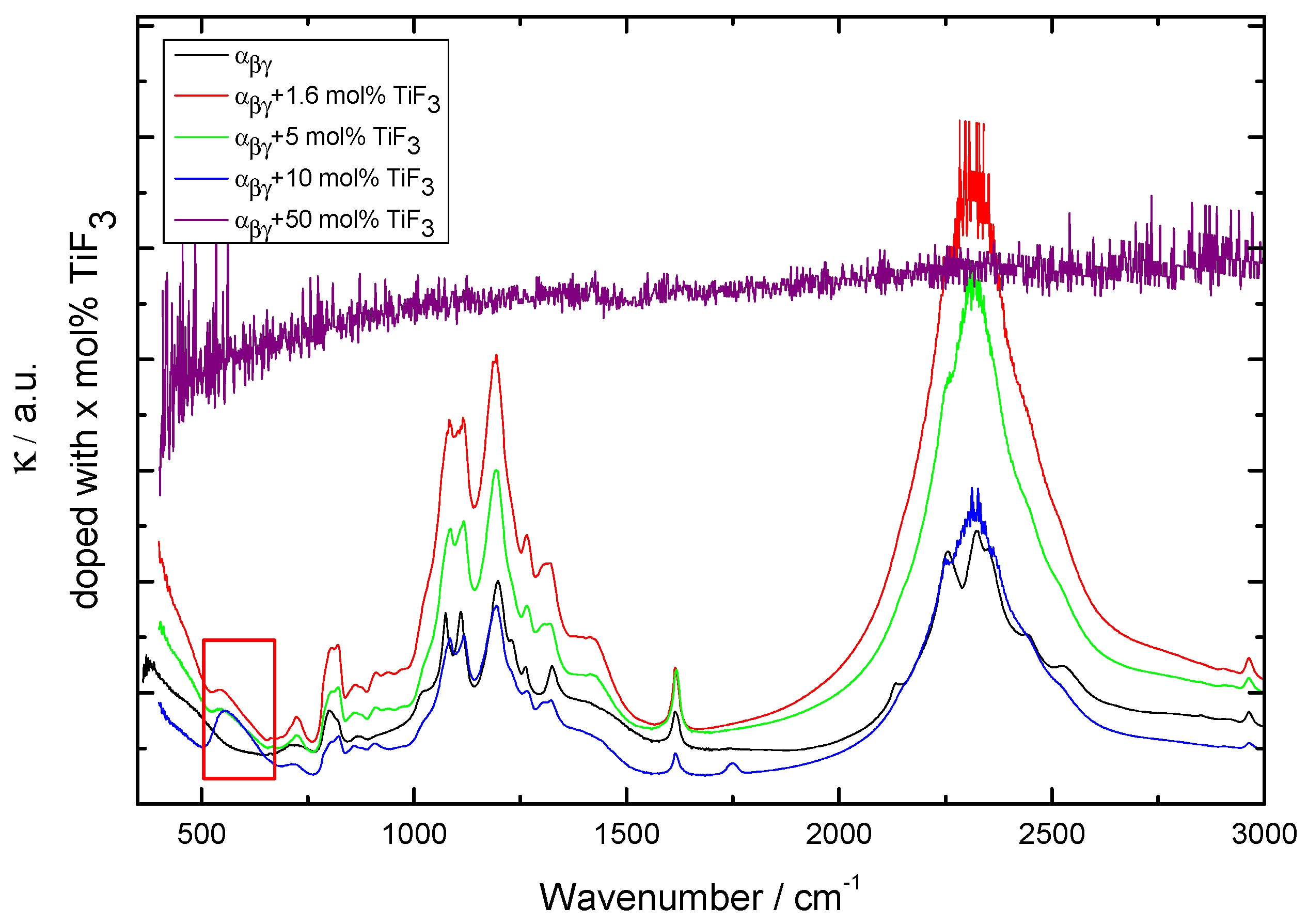
Figure 3.
Infrared (IR) spectra for -Ca(BH) samples with additive. External modes corresponding to lattice vibrations in TiF are marked with a red box. The spectrum of a sample prepared by milling + 50 mol% TiF is also included.
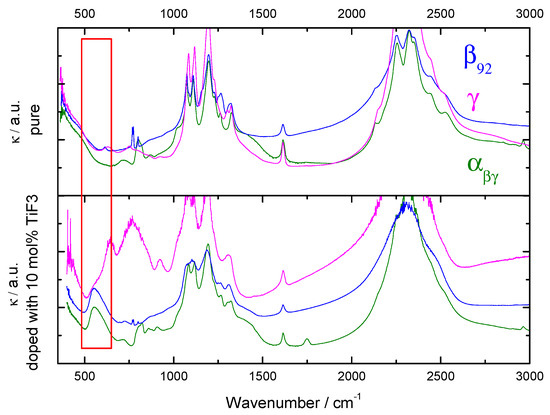
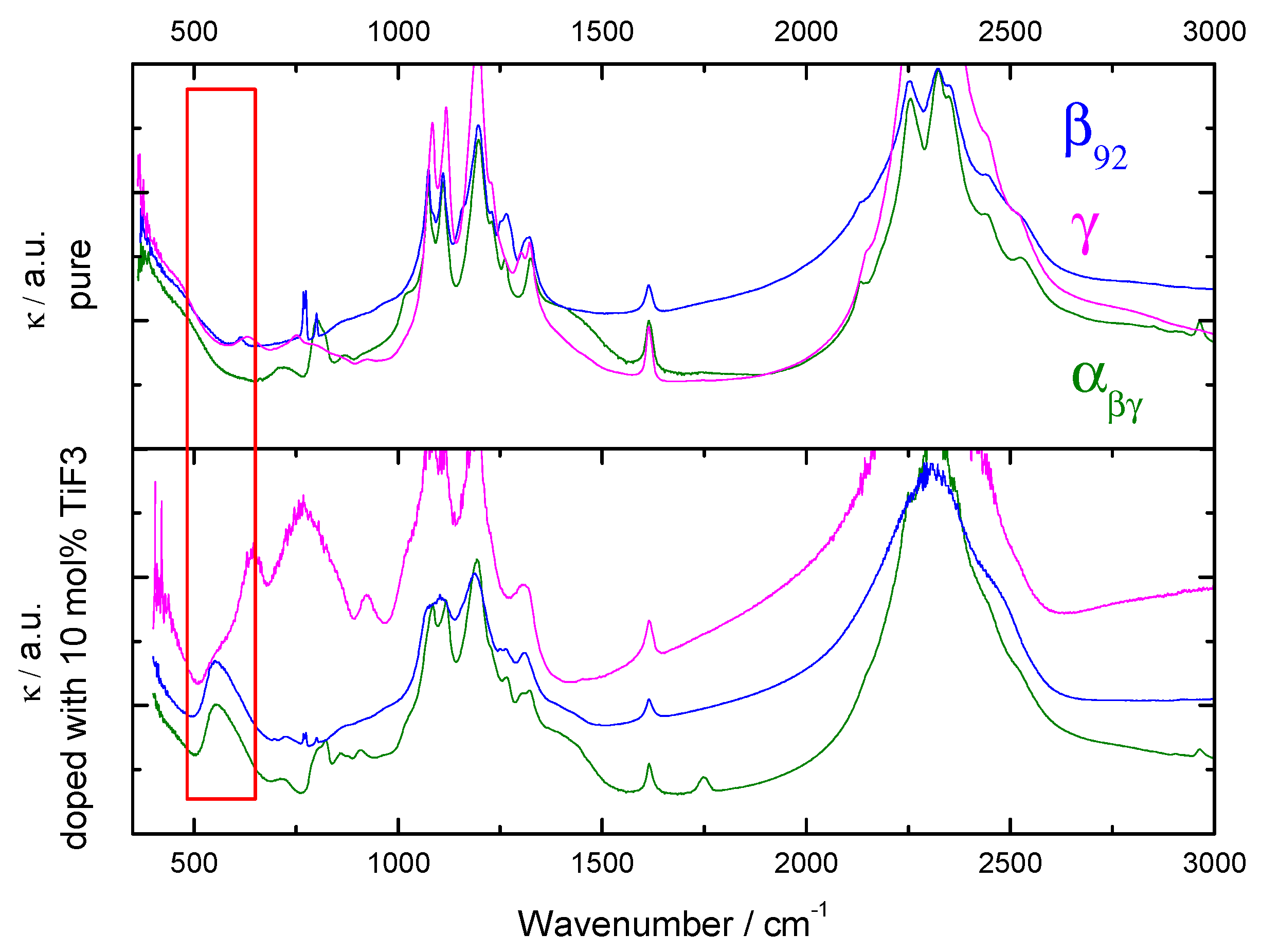
Figure 4.
IR spectra for as-received (upper panel) and -, and with 10 mol% of TiF. External modes corresponding to lattice vibrations in TiF are marked with a red box.
According to symmetry selection rules, the two IR active vibrational modes in borohydrides are the BH stretching and HBH bending modes at 2200–2300 cm and 1100 cm, respectively. Other bands corresponding to overtones and combinations are also seen experimentally [1]. Below 1000 cm, external and librational modes between BH and Ca can be distinguished in the IR spectra [1].
The addition of TiF changes the decomposition behavior of the samples after milling compared to the as-received materials. Thus, while -Ca(BH) + 1.6 mol% TiF (Figure 2a) transforms into -Ca(BH) with increasing milling time, the addition of 10 mol% of TiF (Figure 2b) seems to have no effect. This indicates that, for small amounts of TiF, the -Ca(BH) samples are mostly affected by the polymorph nature and the milling process and not by the additive. Increasing the additive content makes the milling conditions harsher for the high temperature polymorph as well as for the original borohydride, as indicated by the loss of crystallinity of the polymorph in Figure 2a.
On the other hand, for -Ca(BH) (Figure 2c,d), the addition of TiF in both 1.6 and 10 mol% leads to an increase of the content of the high temperature polymorph -Ca(BH) with milling time. This shows that in the case, TiF has a more prominent role in improving the kinetic decomposition of the borohydride than for the polymorph. For -Ca(BH) the addition of 10 mol% of TiF has different effects depending on the polymorphic purity of the sample. In the case of (Figure 2e), an increase of the -Ca(BH) content is induced by the additive, while for (Figure 2d), the crystalline borohydride is affected strongly by the milling time, with the disappearance and formation of - and -Ca(BH) at different stages, respectively.
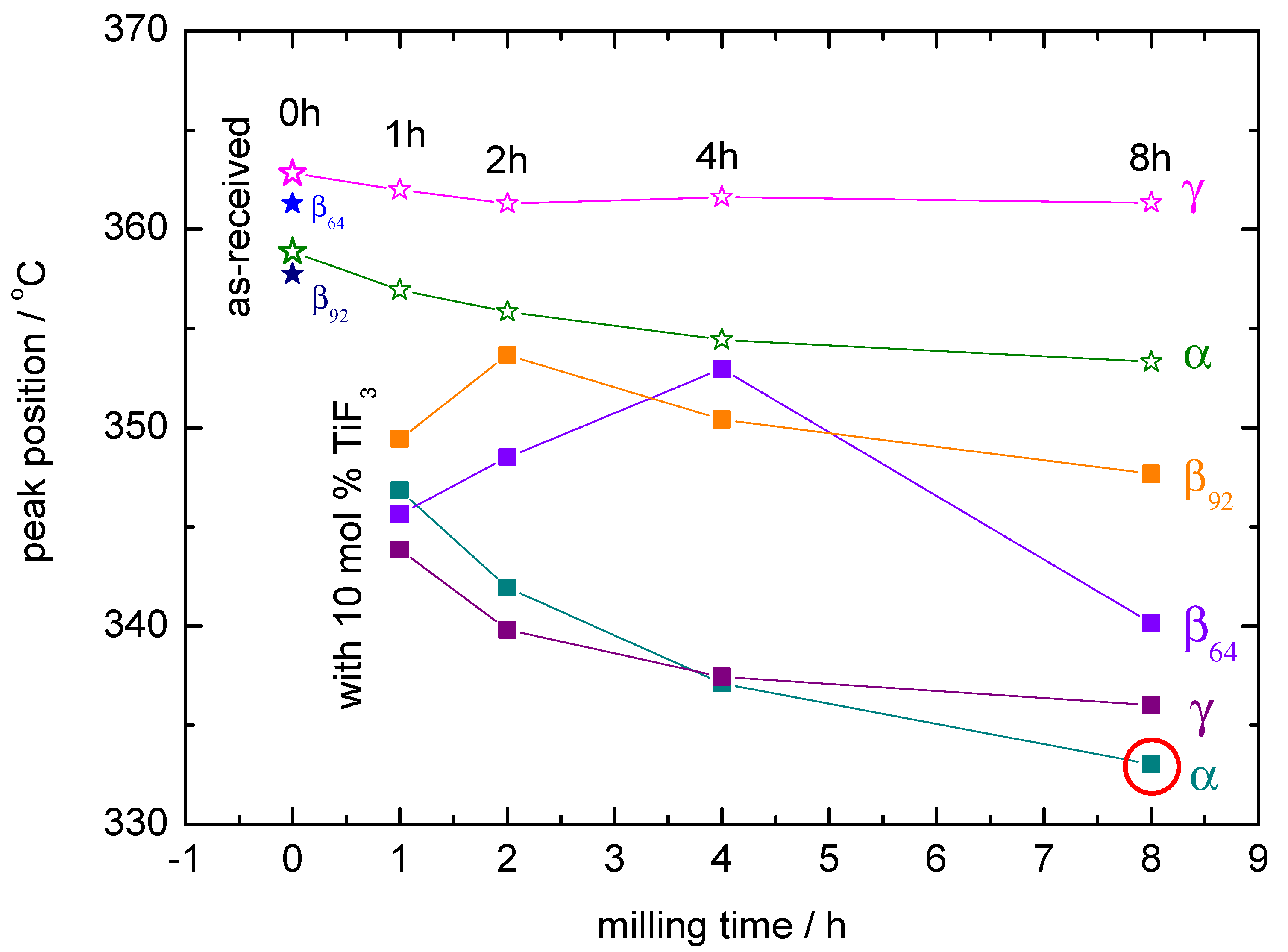
The PXD results strongly correlate with the DSC curves in the decomposition region of the high temperature polymorph (Figure 5). In some cases, these decomposition curves show more steps than the expected main two peaks corresponding to the formation of an intermediate compound and the subsequent decomposition of the high temperature polymorph [32]. This is due to the presence of mixed-polymorphs in the samples formed during milling and their different transformation and decomposition temperatures [1]. The DSC curves also show that a decrease of the decomposition temperature with increasing milling time takes place in all the cases containing TiF. Exceptions are explained by the presence or absence of -Ca(BH) in the samples and by the ratio between - and -Ca(BH). In most cases, larger / ratios lead to lower decomposition temperatures. This agrees with the results by Llamas-Jansa et al. [1] on pure Ca(BH) polymorphs and shows the impact of a milling process. From the present results, the sample with the lowest desorption temperature is -Ca(BH) + 10 mol% TiF, with a temperature shift of approx. 27 C compared to the as-received material (Figure 6).
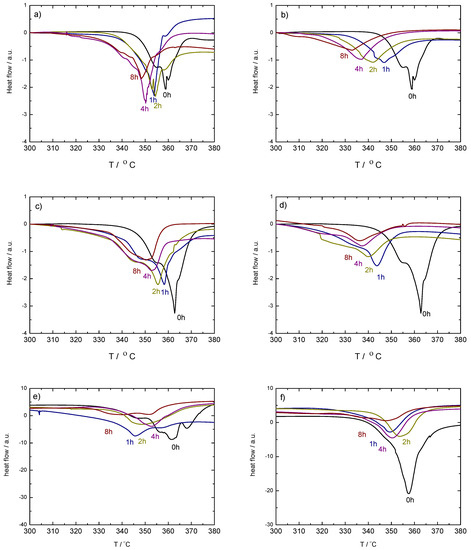
Figure 5.
Corresponding DSC curves of ball milled (top, (a,b)) and (middle, (c,d)), with 1.6 mol% (left-hand side, (a,c)) and 10 mol% (rigth-hand side, (b,d)) of TiF, respectively. Bottom: Corresponding DSC curves of the ball milled (left-hand side, (e)) and (right-hand side, (f)) with 10 mol% of TiF. Only the main decomposition region between 330 and 380 C is shown. Measurements were done under dynamic conditions in protective Ar flow, with 2 C/min heating rates. The curves have been shifted to show the peak trend.
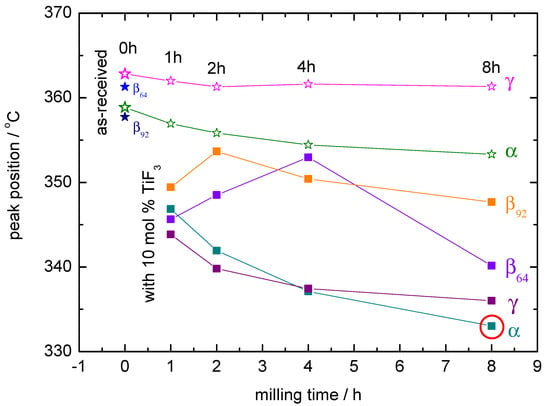
Figure 6.
Position of the decomposition peaks as observed by DSC. The lowest decomposition temperature corresponds to + 10 mol% TiF milled for 8 h and is marked by a red circle. 0 h means the sample was measured as-received.
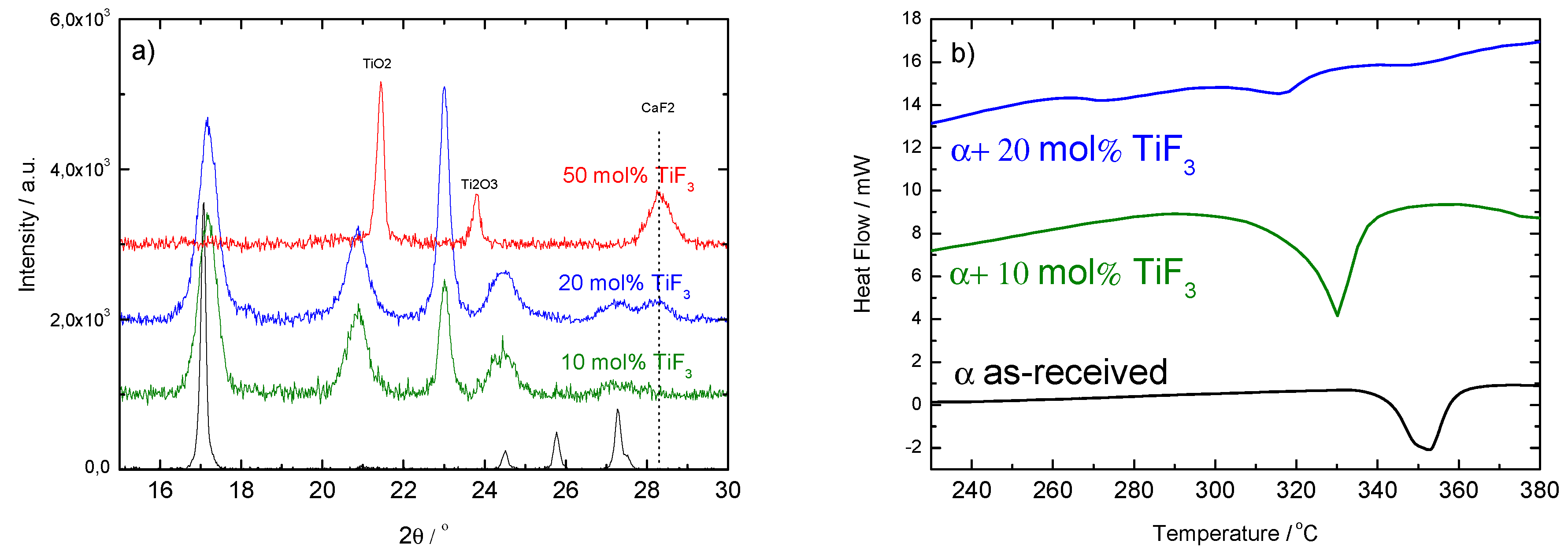
Finally, the additive amount was increased to 20 and 50 mol% of TiF (Figure 7). Increasing the amount of TiF led to the formation of CaF and TiO and, therefore, other effects than merely kinetics are introduced in the behavior of the Ca(BH) samples. The presence of oxygen in Ca(BH) is expected and is related to contamination during the handling of the materials or as part of the commercial products. The fact that the sample containing 50 mol% of TiF led to CaF and TiO also indicates that the milling conditions were harsh enough to decompose the original Ca(BH) and TiF. This was confirmed by the corresponding DSC curves, that showed a decrease of the decomposition peak for 10 and 20 mol%, but no decomposition peaks in the 50 mol% case (not plotted). The lack of borohydride in this sample was confirmed by IR spectroscopy (as shown in Figure 3).
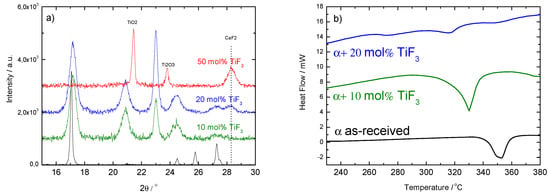
Figure 7.
PXD (left-hand panel, (a)) and DSC (right-hand panel, (b)) results of ball milled with 10, 20 and 50 mol% of TiF after 8 h continuous milling. The results for the as-received material are included for comparison.
2.3. Activation Energies
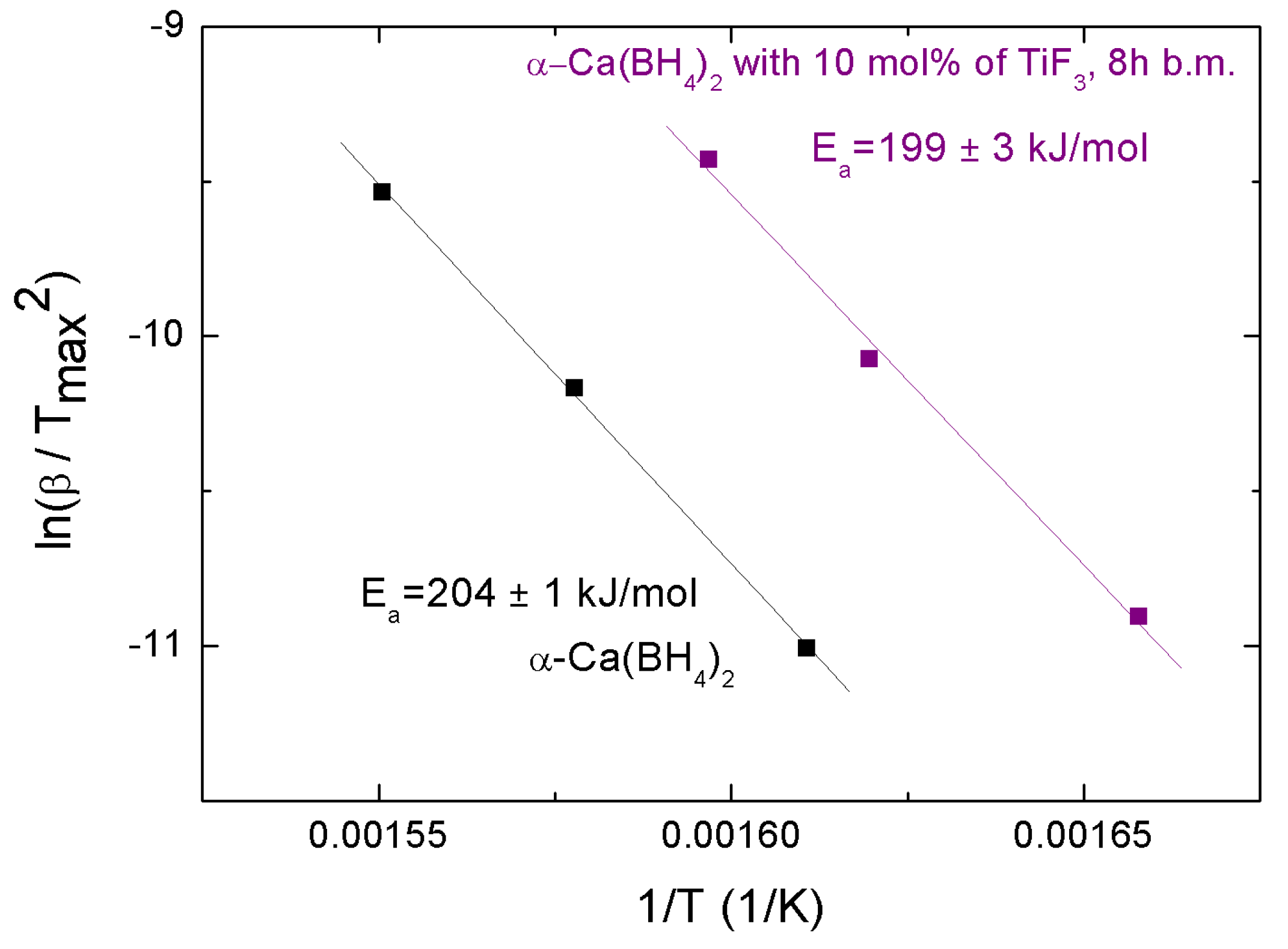
Sample , containing 98% of the -Ca(BH) polymorph (Table 1), was ball milled for 8 h without stop, with and without 10 mol% TiF. The milling products were separated in smaller samples and then heated at different heating rates in order to calculate their activation energies from a generalization of the Kissinger equation [33]:
where is the heating rate and is the temperature of the endothermic peak at its maximum; R is the gas constant and is the activation energy. Activation energies calculated from the graphical representation of this equation lead to values of 199 ± 3 and 204 ± 1 kJmol for the samples with additive and without additive, respectively (Figure 8). The small difference indicates some success by TiF to destabilize Ca(BH), in agreement with the observed decomposition behavior.
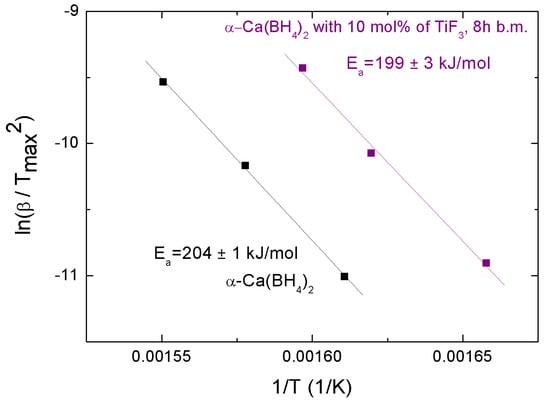
Figure 8.
Kissinger plot corresponding to as-received and + 10 mol% TiF milled for 8 h.
3. Materials and Methods
The present study was performed on Ca(BH) samples containing different amounts of the , , and polymorphs (Table 1). All polymorphs were crystalline and stable at room temperature (RT). Samples and were prepared from commercial Ca(BH).2THF by heating in vacuum up to 160 C for 1 h, while was synthesized by wet chemistry of CaH + 2EtN.BH. The and samples containing 64 and 92 mol% of phase, respectively, were synthesized by a solvent-free method based on the reaction of metal hydrides with diborane and subsequent ball milling. The details of the synthesis methods are described elsewhere [8,34,35,36]. Sample handling was carried out in MBraun Unilab glove boxes filled with purified argon (<1 ppm O, HO) and transportable glove bags filled with Ar.
Milling with and without additive was carried out in Ar using a Fritsch Pulverisette 7 (P7) planetary mill at 505 rpm. Steel balls 10 mm in diameter and tempered steel vials were used. Small amounts of milled material were extracted from the vials after 1, 2, 4, and 8 h, always keeping the ball-to-powder ratio constant to 50:1. TiF powder (Aldrich, 99%) was added to the different polymorphs in 1.6, 5, 10, 20 and 50 mol% amounts (Table 2). Tests with 5 mol% of ScH and AlF showed little effect on the decomposition temperature of Ca(BH) and are not discussed here. As shown by Llamas et al. [24], stoichiometric mixtures may lead to new compounds that alter the decomposition path (thermodynamics) of a borohydride. In this study, only kinetic effects were investigated.

Table 1.
Sample list. Phase fractions were obtained from PXD patterns using F2dd [17], Pbca [18] and P-4 [17] for -, - and -Ca(BH), respectively. Other phases, except for CaH and FeNi in , where not taken into account.
Table 1.
Sample list. Phase fractions were obtained from PXD patterns using F2dd [17], Pbca [18] and P-4 [17] for -, - and -Ca(BH), respectively. Other phases, except for CaH and FeNi in , where not taken into account.
| Sample Name | Highest Content Polymorph | Phase Fraction // (mol%) | Synthesis Method |
|---|---|---|---|
| 67/23/10 | from Ca(BH).2THF a | ||
| 98/–/2 | from Ca(BH).2THF a | ||
| 8/–/92 | from CaH + 2EtN.BH b | ||
| –/64/– d | reactive milling in BH c | ||
| –/92/– | reactive milling in BH c |
a Reference [8,34]; b Reference [35]; c Reference [36]; d also containing Ca3(BH4)3(BO3).

Table 2.
Sample preparation conditions.
Table 2.
Sample preparation conditions.
| Sample Name | Highest Content Polymorph | Milling Time/h | TiF Content/mol% |
|---|---|---|---|
| 1, 2, 4, 8 | 0, 1.6, 5, 10 | ||
| 8, 24 | 10, 20, 50 | ||
| 1, 2, 4, 8 | 0, 1.6, 5, 10 | ||
| 1, 2, 4, 8 | 10 | ||
| 1, 2, 4, 8 | 10 |
Powder X-ray diffraction (PXD) patterns were collected in transmission mode using CuK radiation ( = 1.5418 Å) in a Bruker AXS D8 Advance Diffractometer equipped with a Göbbel mirror and a LynxEye™ 1D strip detector. Diffraction patterns were obtained at RT in rotating boron containing glass capillaries (0.8 mm ) filled and sealed under Ar atmosphere. Data acquisition was restricted to 2 = 5–80, with 2 = 0.02 and 2 s/step scanning rates. The phase fraction of the different samples was obtained from the PXD patterns using GSAS [37].
Differential scanning calorimetry (DSC) was carried out between RT and 500 in Al crucibles with pierced lid using a Setaram Sensys DSC. Additional DSC measurements were carried out in a STA449F3 Jupiter from NETZSCH using corundum (AlO) crucibles and pierced lids. In both cases measurements were done under dynamic conditions in protective Ar flow with 2 /min heating rates. Additional heating rates of 5 and 10 /min were used in the STA449F3 Jupiter to determine sample activation energies. Approximately 30 mg of fresh material was used in every case.
Infrared (IR) spectroscopy was carried out in a Bruker IFS 66v spectrometer with a DTGS detector. The samples were embedded in KBr pellets (7 mm ), with a 0.5% mass concentration. The transmission spectra of the KBr pellets were recorded in the 400–8000 cm region, with a 4 cm resolution. Scattering contribution to the spectra due to the pellets was considered to be negligible at these wavenumbers. Mass absorption coefficients () were calculated from the transmission (T) data as:
where A is the area of the pellet in cm, and m is the mass of the absorbing sample in g [38]. The spectroscopic curves were further normalized to the strongest peak in both the bending and the stretching regions to allow a better visualization of the peak shifts introduced by the different samples. Peak analysis was carried out in Origin 7.5 using Gaussian curves.
4. Conclusions
The hydrogen desorption peak temperature of Ca(BH) depends strongly on the composition of the coexistent , and polymorphs, which is determined by the synthesis method. Additional ball milling affects the kinetics of the material and leads to a lowering of the peak temperature, which is more pronounced by increasing the content of the polymorph in the sample. The kinetic effect is further decreased by the addition of TiF to the sample, that is, TiF increases the hydrogen desorption rate. The improved kinetics by the addition of TiF was observed without significant dissociation of TiF and, therefore, must be related to the reduction of all the crystallite sizes (including those of TiF) due to the ball milling process.
Author Contributions
Conceptualization and methodology for this article as well as experimental investigation, validation, formal analysis, data curation, project administration, provision of materials, supervision, visualization and writing of original draft was carried out by I.L.J., O.F., M.F. and E.G.B. provided samples, while A.Z. and B.C.H. reviewed the original manuscript. Overall resources and funding acquisition by B.C.H. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from the Research Council of Norway, the FLYHY project (contract no. 226943) and the ERA-NET project Hy-CO (grant#0327791) under the FP7 Program in the European Commission are also gratefully acknowledged.
Acknowledgments
The authors acknowledge the contributions of Claus Nielsen at the Chemistry Department of the University of Oslo for IR spectroscopy and the project team at the SNBL Beam Line, ESRF, Grenoble.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Llamas-Jansa, I.; Friedrichs, O.; Fichtner, M.; Bardaji, E.G.; Züttel, A.; Hauback, B.C. The Role of Ca(BH4)(2) Polymorphs. J. Phys. Chem. C 2012, 116, 13472–13479. [Google Scholar] [CrossRef]
- Kim, J.H.; Jin, S.A.; Shim, J.H.; Cho, Y.W. Reversible hydrogen storage in calcium borohydride Ca(BH4)(2). Scr. Mater. 2008, 58, 481–483. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, Y.S.; Suh, J.Y.; Shim, J.H.; Cho, Y.W. Metal halide doped metal borohydrides for hydrogen storage: The case of Ca(BH4)(2)-CaX2 (X = F, Cl) mixture. J. Alloys Compd. 2010, 506, 721–727. [Google Scholar] [CrossRef]
- Ibikunle, A.A.; Goudy, A.J. Kinetics and modeling study of a Mg(BH4)2/Ca(BH4)2 destabilized system. Int. J. Hydrog. Energy 2012, 37, 12420–12424. [Google Scholar] [CrossRef]
- Ibikunle, A.A.; Sabitu, S.T.; Goudy, A.J. Kinetics and modeling studies of the CaH2/LiBH4, MgH2/LiBH4, Ca(BH4)2 and Mg(BH4)2 systems. J. Alloys Compd. 2013, 556, 45–50. [Google Scholar] [CrossRef]
- Minella, C.B.; Garroni, S.; Pistidda, C.; Baro, M.D.; Gutfleisch, O.; Klassen, T.; Dornheim, M. Sorption properties and reversibility of Ti(IV) and Nb(V)-fluoride doped-Ca(BH4)2–MgH2 system. J. Alloys Compd. 2015, 622, 989–994. [Google Scholar] [CrossRef][Green Version]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Bellosta Colbe, J.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage—Past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Miwa, K.; Aoki, M.; Noritake, T.; Ohba, N.; Nakamori, Y.; Towata, S.i.; Züttel, A.; Orimo, S.I. Thermodynamical stability of calcium borohydride Ca(BH4)(2). Phys. Rev. B 2006, 74, 155122. [Google Scholar] [CrossRef]
- Nakamori, Y.; Miwa, K.; Ninomiya, A.; Li, H.W.; Ohba, N.; Towata, S.I.; Züttel, A.; Orimo, S.I. Correlation between thermodynamical stabilities of metal borohydrides and cation electronegativites: First-principles calculations and experiments. Phys. Rev. B 2006, 74, 045126. [Google Scholar] [CrossRef]
- Aoki, M.; Miwa, K.; Noritake, T.; Ohba, N.; Matsumoto, M.; Li, H.W.; Nakamori, Y.; Towata, S.; Orimo, S. Structural and dehydriding properties of Ca(BH4)(2). Appl. Phys. Mater. Sci. Process. 2008, 92, 601–605. [Google Scholar] [CrossRef]
- Noritake, T.; Aoki, M.; Matsumoto, M.; Miwa, K.; Towata, S.; Li, H.-W.; Orimo, S. Crystal structure and charge density analysis of Ca(BH4)2. J. Alloys Compd. 2010, 491, 57–62. [Google Scholar] [CrossRef]
- Mao, J.; Guo, Z.; Poh, C.K.; Ranjbar, A.; Guo, Y.; Yu, X.; Liu, H. Study on the dehydrogenation kinetics and thermodynamics of Ca(BH4)2. J. Alloys Compd. 2010, 500, 200–205. [Google Scholar] [CrossRef]
- Rönnebro, E.; Majzoub, E.H. Calcium borohydride for hydrogen storage: Catalysis and reversibility. J. Phys. Chem. B 2007, 111, 12045–12047. [Google Scholar] [CrossRef] [PubMed]
- Rongeat, C.; D’Anna, V.; Hagemann, H.; Borgschulte, A.; Züttel, A.; Schultz, L.; Gutfleisch, O. Effect of additives on the synthesis and reversibility of Ca(BH4)(2). J. Alloys Compd. 2010, 493, 281–287. [Google Scholar] [CrossRef]
- Minella, C.B.; Garroni, S.; Pistidda, C.; Gosalawit-Utke, R.; Barkhordarian, G.; Rongeat, C.; Lindemann, I.; Gutfleisch, O.; Jensen, T.R.; Cerenius, Y.; et al. Effect of Transition Metal Fluorides on the Sorption Properties and Reversible Formation of Ca(BH4)(2). J. Phys. Chem. C 2011, 115, 2497–2504. [Google Scholar] [CrossRef]
- Riktor, M.D.; Sørby, M.H.; Muller, J.; Bardaji, E.G.; Fichtner, M.; Hauback, B.C. On the rehydrogenation of decomposed Ca(BH4)2. J. Alloys Compd. 2015, 632, 800–804. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Rönnebro, E.; Chandra, D. Crystal structures and phase transformations in Ca(BH4)(2). Acta Mater. 2009, 57, 732–738. [Google Scholar] [CrossRef]
- Buchter, F.; Lodziana, Z.; Remhof, A.; Friedrichs, O.; Borgschulte, A.; Mauron, P.; Züttel, A.; Sheptyakov, D.; Barkhordarian, G.; Bormann, R.; et al. Structure of Ca(BD4)(2) beta-phase from combined neutron and synchrotron X-ray powder diffraction data and density functional calculations. J. Phys. Chem. B 2008, 112, 8042–8048. [Google Scholar] [CrossRef]
- Buchter, F.; Lodziana, Z.; Remhof, A.; Friedrichs, O.; Borgschulte, A.; Mauron, P.; Züttel, A.; Sheptyakov, D.; Palatinus, L.; Chlopek, K.; et al. Structure of the orthorhombic gamma-phase and phase transitions of Ca(BD4)(2). J. Phys. Chem. C 2009, 113, 17223–17230. [Google Scholar] [CrossRef]
- Borgschulte, A.; Gremaud, R.; Züttel, A.; Martelli, P.; Remhof, A.; Ramirez-Cuesta, A.J.; Refson, K.; Bardaji, E.G.; Lohstroh, W.; Fichtner, M.; et al. Experimental evidence of librational vibrations determining the stability of calcium borohydride. Phys. Rev. B 2011, 83, 024102. [Google Scholar] [CrossRef]
- Rongeat, C.; Lindemann, I.; Borgschulte, A.; Schultz, L.; Gutfleisch, O. Effect of the presence of chlorides on the synthesis and decomposition of Ca(BH4)(2). Int. J. Hydrog. Energy 2011, 36, 247–253. [Google Scholar] [CrossRef]
- Riktor, M.D.; Filinchuk, Y.; Vajeeston, P.; Bardaji, E.G.; Fichtner, M.; Fjellvåg, H.; Sørby, M.H.; Hauback, B.C. The crystal structure of the first borohydride borate, Ca3(BD4)3(BO3). J. Mater. Chem. 2011, 21, 7188–7193. [Google Scholar] [CrossRef]
- Llamas Jansa, I.; Kalantzopoulos, G.N.; Nordholm, K.; Hauback, B.C. Destabilization of NaBH4 by Transition Metal Fluorides. Molecules 2020, 25, 780. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Jansa, I.; Aliouane, N.; Deledda, S.; Fonneløp, J.E.; Frommen, C.; Humphries, T.; Lieutenant, K.; Sartori, S.; Sørby, M.H.; Hauback, B.C. Chloride substitution induced by mechano-chemical reactions between NaBH4 and transition metal chlorides. J. Alloys Compd. 2012, 530, 186–192. [Google Scholar] [CrossRef]
- Humphries, T.D.; Kalantzopoulos, G.N.; Llamas-Jansa, I.; Olsen, J.E.; Hauback, B.C. Reversible Hydrogenation Studies of NaBH4 Milled with Ni-Containing Additives. J. Phys. Chem. C 2013, 117, 6060–6065. [Google Scholar] [CrossRef]
- Kalantzopoulos, G.N.; Guzik, M.N.; Deledda, S.; Heyn, R.H.; Muller, J.; Hauback, B.C. Destabilization effect of transition metal fluorides on sodium borohydride. Phys. Chem. Chem. Phys. 2014, 16, 20483–20491. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Wang, H.; Liu, J.W.; Zhu, M. Thermal decomposition behaviors of magnesium borohydride doped with metal fluoride additives. Thermochim. Acta 2013, 560, 82–88. [Google Scholar] [CrossRef]
- Saldan, I.; Frommen, C.; Llamas-Jansa, I.; Kalantzopoulos, G.N.; Hino, S.; Arstad, B.; Heyn, R.H.; Zavorotynska, O.; Deledda, S.; Sørby, M.H.; et al. Hydrogen storage properties of γ–Mg(BH4)2 modified by MoO3 and TiO2. Int. J. Hydrog. Energy 2015, 40, 12286–12293. [Google Scholar] [CrossRef]
- Zavorotynska, O.; El-Kharbachi, A.; Deledda, S.; Hauback, B.C. Recent progress in magnesium borohydride Mg(BH4)2: Fundamentals and applications for energy storage. Int. J. Hydrog. Energy 2016, 41, 14387–14403. [Google Scholar] [CrossRef]
- Shi, B.; Song, Y. Influence of transition metals Fe, Ni, and Nb on dehydrogenation characteristics of Mg(BH4)2: Electronic structure mechanisms. Int. J. Hydrog. Energy 2013, 38, 6417–6424. [Google Scholar] [CrossRef]
- Nickels, E.A. Structural and Thermogravimetric Studies of Group I and II Borohydrides. Ph.D. Thesis, Sommerville College, Oxford, UK, 2010. [Google Scholar]
- Kim, J.H.; Jin, S.A.; Shim, J.H.; Cho, Y.W. Thermal decomposition behavior of calcium borohydride Ca(BH4)2. J. Alloys Compd. 2008, 461, L20–L22. [Google Scholar] [CrossRef]
- Llopiz, J.; Romero, M.M.; Jerez, A.; Laureiro, Y. Generalization of the Kissinger equation for several kinetic models. Thermochim. Acta 1995, 256, 205–211. [Google Scholar] [CrossRef]
- Riktor, M.D.; Sørby, M.H.; Chlopek, K.; Fichtner, M.; Buchter, F.; Züttel, A.; Hauback, B.C. In situ synchrotron diffraction studies of phase transitions and thermal decomposition of Mg(BH4)(2) and Ca(BH4)(2). J. Mater. Chem. 2007, 17, 4939–4942. [Google Scholar] [CrossRef]
- Fichtner, M.; Chlopek, K.; Longhini, M.; Hagemann, H. Vibrational spectra of Ca(BH4)(2). J. Phys. Chem. C 2008, 112, 11575–11579. [Google Scholar] [CrossRef]
- Friedrichs, O.; Remhof, A.; Borgschulte, A.; Buchter, F.; Orimo, S.I.; Züttel, A. Breaking the passivation-the road to a solvent free borohydride synthesis. Phys. Chem. Chem. Phys. 2010, 12, 10919–10922. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.; Dreele, R. General Structure Analysis System (GSAS); Report Laur 86-748 ed.; Los Alamos National Laboratory: Los Alamos, NM, USA, 2000.
- Llamas-Jansa, I. Experimental Study of the Optical and Structural Properties of Carbon Nanoparticles. Ph.D. Thesis, Friedrich-Schiller-Universität Jena, Jena, Germany, 2006. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).