Process Integration of Green Hydrogen: Decarbonization of Chemical Industries
Abstract
:1. Introduction
2. Economic Trends
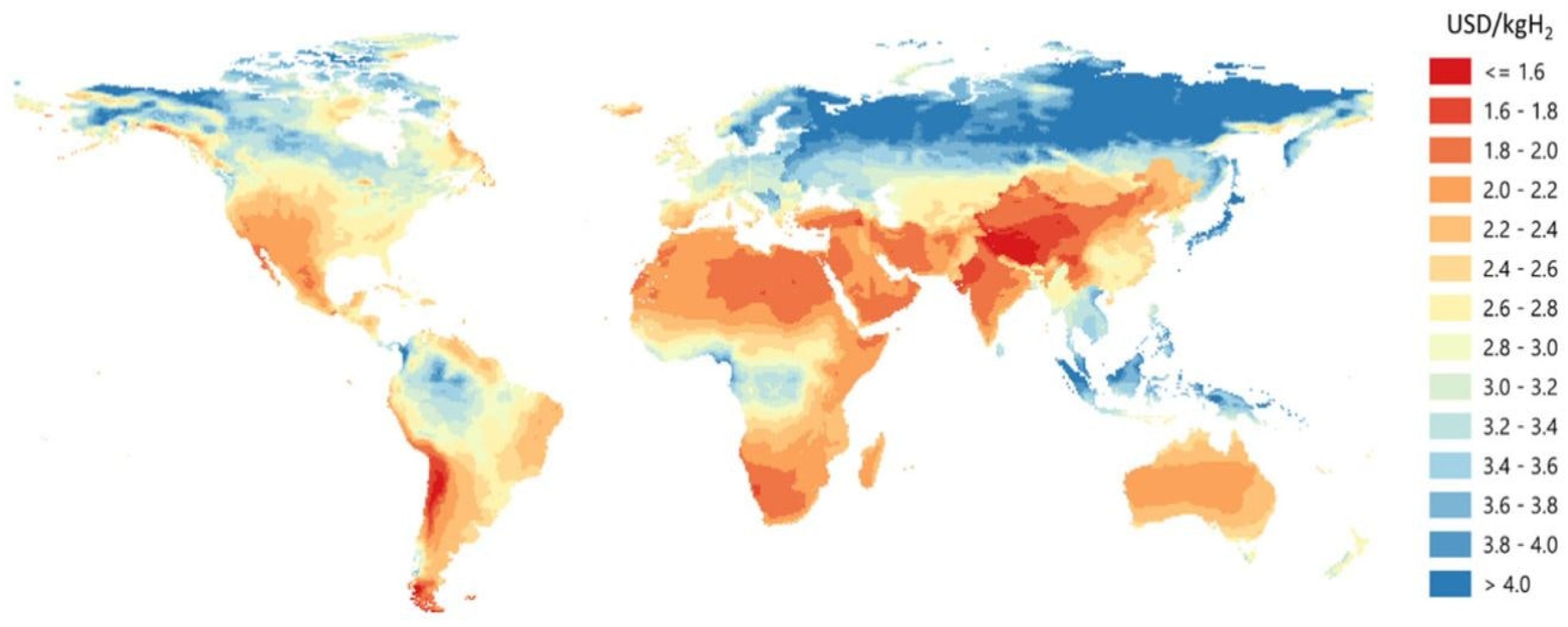
2.1. Renewable Energy
2.2. Electrolysis
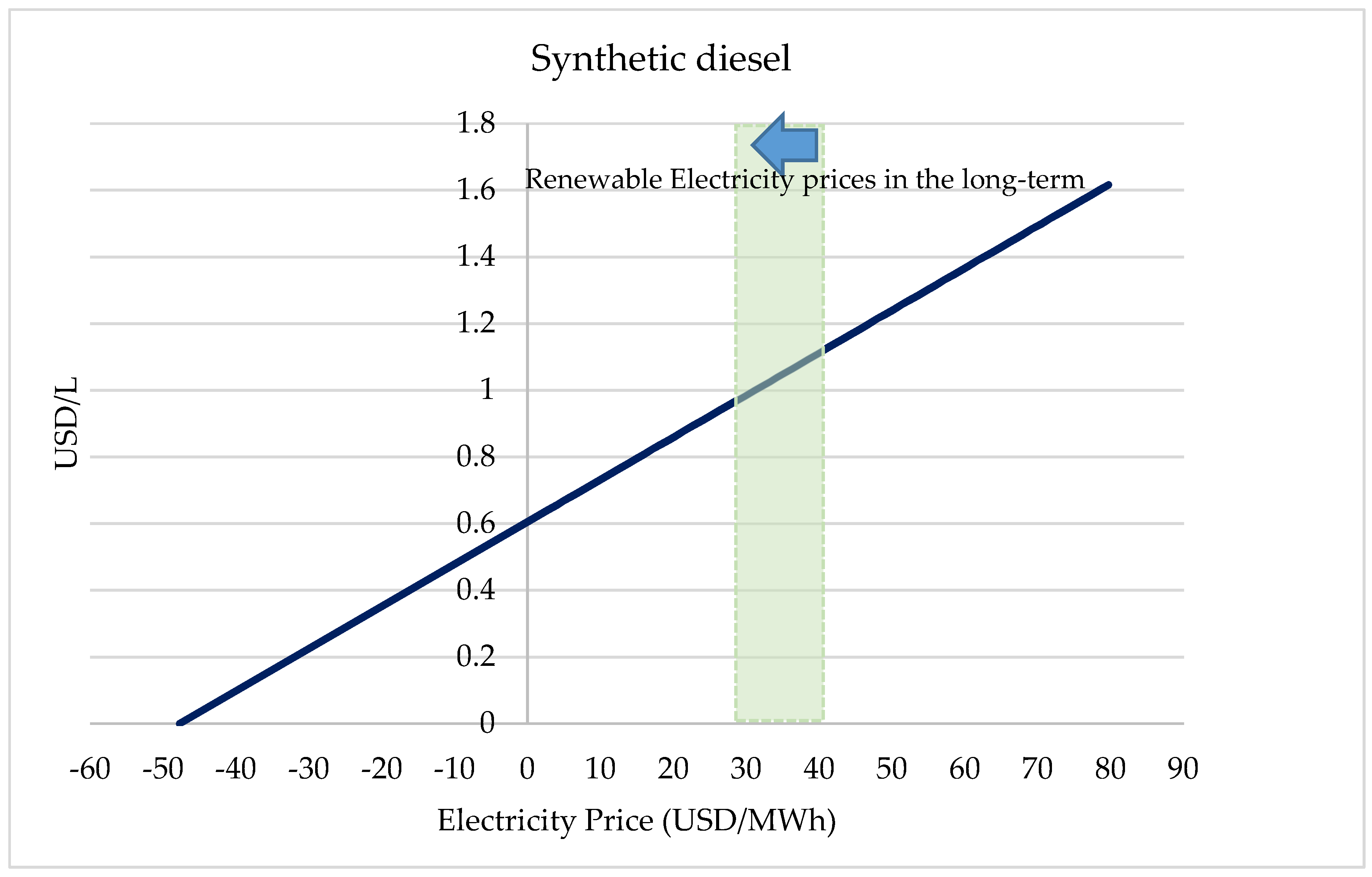
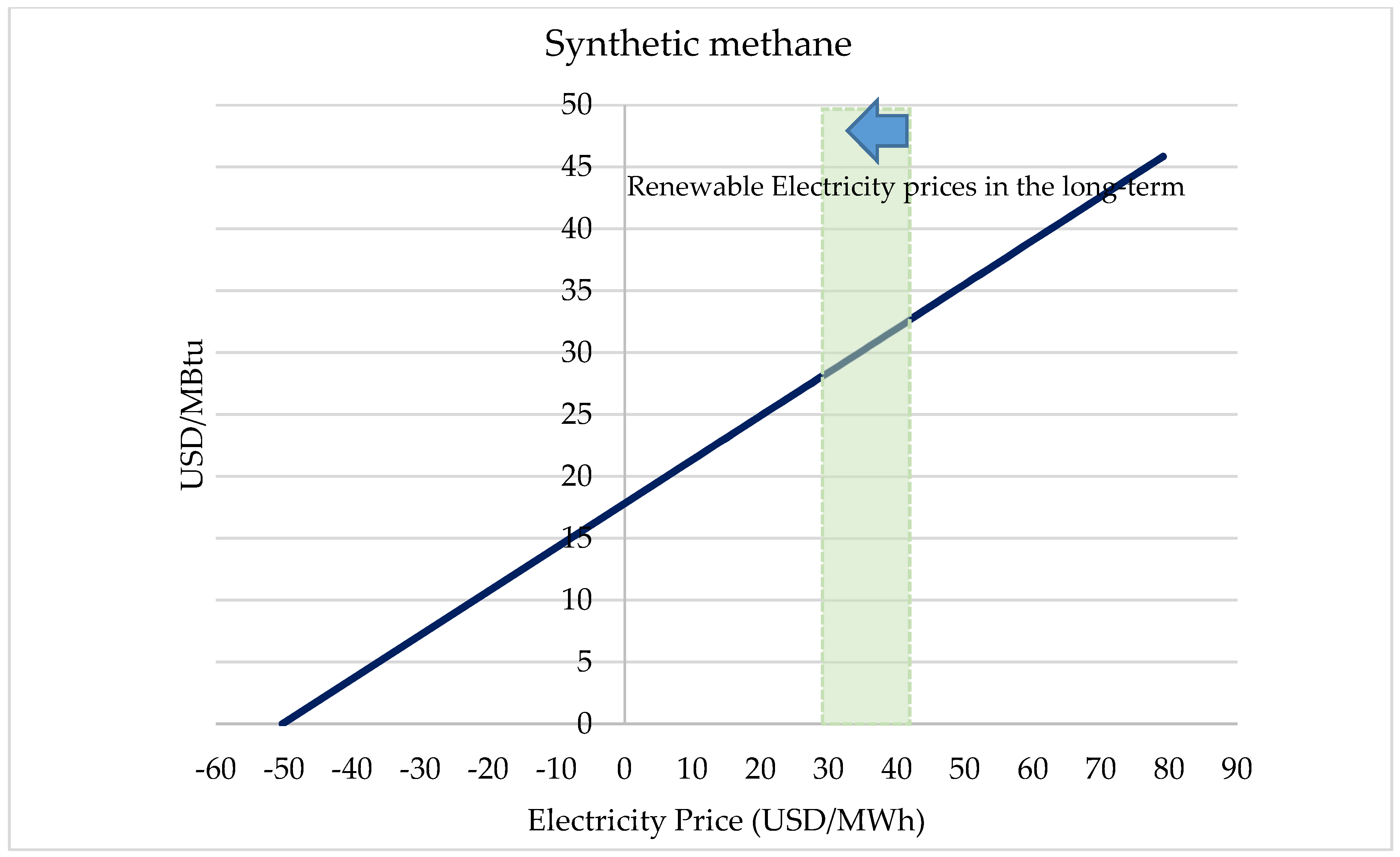
2.3. CO2 Conversion Economics
3. Process Integration Framework
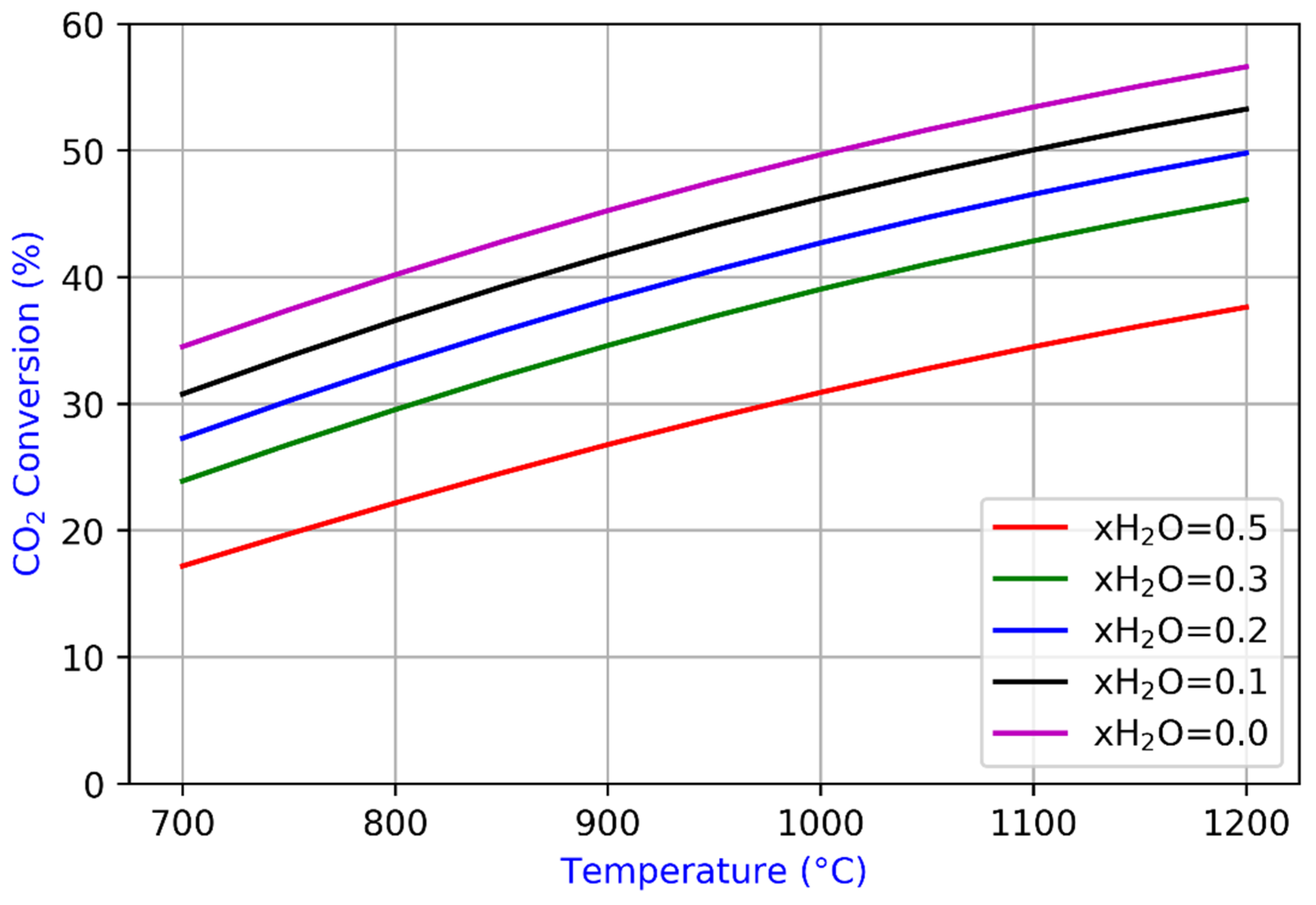
3.1. Reverse Water Gas Shift (RWGS)
3.2. Example Pathway: Biogas to Liquid Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ASU | Air Separation Unit |
| ATR | Autothermal Reformer |
| BNEF | Bloomberg New Energy Finance |
| CapEx | Capital Expenditure |
| DRI | Direct Reduction of Iron |
| FLH | Full Load Hours |
| IEA | International Energy Agency |
| LCOE | Levelized Cost of Electricity |
| LHV | Lower Heating Value |
| MBtu | Million British thermal units |
| OpEx | Operating Expenditure |
| PEM | Polymer-Electrolyte Membrane or alternatively Proton-Exchange Membrane |
| PV | Photovoltaics |
| RWGS | Reverse Water Gas Shift |
| SOEC | Solid Oxide Electrolyser Cell |
References
- Ray, D. Lazard ’s Levelized Cost of Energy Analysis—Version 13.0; Lazard: New York, NY, USA, 2019. [Google Scholar]
- Mitchell, C. Momentum is increasing towards a flexible electricity system based on renewables. Nat. Energy 2016, 1, 15030. [Google Scholar] [CrossRef]
- Fumagalli, E. Energy investment: The many lives of energy storage. Nat. Energy 2016, 1, 16096. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Jamasb, T. Electricity storage: Friend or foe of the networks? Nat. Energy 2017, 2, 17092. [Google Scholar] [CrossRef]
- Asensio, O.I.; Delmas, M.A. The effectiveness of US energy efficiency building labels. Nat. Energy 2017, 2, 17033. [Google Scholar] [CrossRef]
- Hittinger, E. Distributed generation: Residential storage comes at a cost. Nat. Energy 2017, 2, 17006. [Google Scholar] [CrossRef]
- Needell, Z.A.; McNerney, J.; Chang, M.T.; Trancik, J.E. Potential for widespread electrification of personal vehicle travel in the United States. Nat. Energy 2016, 1, 16112. [Google Scholar] [CrossRef]
- New Energy Outlook 2017; Bloomberg New Energy Finance: New York, NY, USA, 2017.
- Ottmar Edenhofer, E.A. Climate Change 2014: Mitigation of Climate Change Technical Summary; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Clémençon, R. The Two Sides of the Paris Climate Agreement:Dismal Failure or Historic Breakthrough? J. Environ. Dev. 2016, 25, 3–24. [Google Scholar] [CrossRef] [Green Version]
- Tymoczko, J.; Calle-Vallejo, F.; Schuhmann, W.; Bandarenka, A.S. Making the hydrogen evolution reaction in polymer electrolyte membrane electrolysers even faster. Nat. Commun. 2016, 7, 10990. [Google Scholar]
- Bertuccioli, L.; Chan, A.; Hart, D.; Lehner, F.; Madden, B.; Standen, E. Study on Development of Water Electrolysis in the EU; Fuel Cells and Hydrogen Joint Undertaking; E4tech Sàrl: Lausanne, Switzerland, 2014. [Google Scholar]
- Bustamante, F.; Enick, R.M.; Cugini, A.; Killmeyer, R.P.; Howard, B.H.; Rothenberger, K.S.; Ciocco, M.V.; Morreale, B.D.; Chattopadhyay, S.; Shi, S. High-temperature kinetics of the homogeneous reverse water–gas shift reaction. AIChE J. 2004, 50, 1028–1041. [Google Scholar] [CrossRef]
- Correa, A.; Martín, R. Metal-Catalyzed Carboxylation of Organometallic Reagents with Carbon Dioxide. Angew. Chem. Int. Ed. 2009, 48, 6201–6204. [Google Scholar]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic Conversion of Carbon Dioxide to Methane and Methanol on Transition Metal Surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef] [PubMed]
- Rego de Vasconcelos, B.; Lavoie, J.-M. Recent Advances in Power-to-X Technology for the Production of Fuels and Chemicals. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, P.; Batteiger, V.; Roth, A.; Weindorf, W.; Raksha, T. Power-to-Liquids as Renewable Fuel Option for Aviation: A Review. Chem. Ing. Tech. 2018, 90, 127–140. [Google Scholar]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquid via synthesis of methanol, DME or Fischer–Tropsch-fuels: A review. Energy Environ. Sci. 2020. [Google Scholar] [CrossRef]
- Rafiee, A.; Khalilpour, K.R.; Milani, D.; Panahi, M. Trends in CO2 conversion and utilization: A review from process systems perspective. J. Environ. Chem. Eng. 2018, 6, 5771–5794. [Google Scholar] [CrossRef]
- New Energy Outlook 2019; Bloomberg New Energy Finance: New York, NY, USA, 2019.
- Green, M.A. Commercial progress and challenges for photovoltaics. Nat. Energy 2016, 1, 15015. [Google Scholar]
- Svarc, J. Most Efficient Solar Panels 2020; Clean Energy Reviews: Dulwich Hill, Australia, 2020. [Google Scholar]
- Most Efficient Solar Panels. 2020. Available online: https://www.cleanenergyreviews.info/blog/most-efficient-solar-panels (accessed on 3 September 2020).
- IEA. The Future of Hydrogen; IEA: Paris, France, 2019. [Google Scholar]
- Blanco, H.; Faaij, A. A review at the role of storage in energy systems with a focus on Power to Gas and long-term storage. Renew. Sustain. Energy Rev. 2018, 81, 1049–1086. [Google Scholar] [CrossRef]
- Al-Musleh, E.I.; Mallapragada, D.S.; Agrawal, R. Continuous power supply from a baseload renewable power plant. Appl. Energy 2014, 122, 83–93. [Google Scholar] [CrossRef]
- Gençer, E.; Al-Musleh, E.; Mallapragada, D.S.; Agrawal, R. Uninterrupted renewable power through chemical storage cycles. Curr. Opin. Chem. Eng. 2014, 5, 29–36. [Google Scholar] [CrossRef]
- Graves, C.R.; Ebbesen, S.D.; Mogensen, M.B.; Lackner, K.S. Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. Renew. Sustain. Energy Rev. 2011, 15, 1–23. [Google Scholar] [CrossRef]
- Nordic Blue Crude. 2020. Available online: https://nordicbluecrude.no/ (accessed on 3 August 2020).
- Sunfire. 2020. Available online: https://www.sunfire.de/ (accessed on 3 August 2020).
- Retail Prices or Gasoline. Available online: https://www.eia.gov/dnav/pet/hist/LeafHandler.ashx?n=pet&s=emm_epm0_pte_nus_dpg&f=m (accessed on 16 September 2020).
- Henry Hub Natural Gas Spot Price. Available online: https://www.eia.gov/dnav/ng/hist/rngwhhdM.htm (accessed on 3 August 2020).
- IRENA. Innovation Landscape Brief: Renewable Power-to-Hydrogen; International Renewable Energy Agency: Abu Dhabi, UAE, 2019. [Google Scholar]
- Ostadi, M.; Hillestad, M. Enriched Air or Pure Oxygen as Oxidant for Gas-to-Liquid Process with Microchannel Reactors. Chem. Eng. Technol. 2017, 40, 1946–1951. [Google Scholar] [CrossRef]
- Bilika, J.; Pustejovskab, P.; Brozovac, S.; Jursova, S. Efficiency of hydrogen utilization in reduction processes in ferrous metallurgy. Sci. Iran. 2013, 20, 337–342. [Google Scholar]
- Lawoko, M.; Berggren, R.; Berthold, F.; Henriksson, G.; Gellerstedt, G. Changes in the lignin-carbohydrate complex in softwood kraft pulp during kraft and oxygen delignification. Holzforschung 2004, 58, 603. [Google Scholar] [CrossRef]
- Xu, Y.; Isom, L.; Hanna, M.A. Adding value to carbon dioxide from ethanol fermentations. Bioresour. Technol. 2010, 101, 3311–3319. [Google Scholar] [CrossRef]
- Hillestad, M.; Ostadi, M.; Del Alamo, G.; Rytter, E.; Austbø, B.; Pharoah, J.G.; Burheim, O. Improving carbon efficiency and profitability of the biomass to liquid process with hydrogen from renewable power. Fuel 2018, 234, 1431–1451. [Google Scholar] [CrossRef]
- Amsterchem. 2020. Available online: https://www.amsterchem.com/ (accessed on 3 August 2020).
- Peters, R.; Decker, M.; Eggemann, L.; Schemme, S.; Schorn, F.; Breuer, J.L.; Weiske, S.; Pasel, J.; Samsun, R.C.; Stolten, D. Thermodynamic and ecological preselection of synthetic fuel intermediates from biogas at farm sites. Energy Sustain. Soc. 2020, 10, 4. [Google Scholar] [CrossRef]
- Marchese, M.; Giglio, E.; Santarelli, M.; Lanzini, A. Energy performance of Power-to-Liquid applications integrating biogas upgrading, reverse water gas shift, solid oxide electrolysis and Fischer-Tropsch technologies. Energy Convers. Manag. X 2020, 6, 100041. [Google Scholar] [CrossRef]
- Verbeeck, K.; Buelens, L.C.; Galvita, V.; Marin, G.B.; Van Geem, K.M.; Rabaey, K. Upgrading the value of anaerobic digestion via chemical production from grid injected biomethane. Energy Environ. Sci. 2018, 11, 1788–1802. [Google Scholar] [CrossRef]
- Ostadi, M.; Rytter, E.; Hillestad, M. Boosting carbon efficiency of the biomass to liquid process with hydrogen from power: The effect of H2/CO ratio to the Fischer-Tropsch reactors on the production and power consumption. Biomass Bioenergy 2019, 127, 105282. [Google Scholar] [CrossRef]
- Ostadi, M.; Dalane, K.; Rytter, E.; Hillestad, M. Conceptual design of an autonomous once-through gas-to-liquid process—Comparison between fixed bed and microchannel reactors. Fuel Process. Technol. 2015, 139, 186–195. [Google Scholar] [CrossRef]
- Ostadi, M.; Rytter, E.; Hillestad, M. Evaluation of kinetic models for Fischer–Tropsch cobalt catalysts in a plug flow reactor. Chem. Eng. Res. Des. 2016, 114, 236–246. [Google Scholar] [CrossRef]
- Ostadi, M.; Austbø, B.; Hillestad, M. Parametric Optimization of a Power and Biomass to Liquid Process. In Computer Aided Chemical Engineering; Muñoz, S.G., Laird, C.D., Realff, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 287–292. [Google Scholar]
- Ostadi, M.; Austbø, B.; Hillestad, M. Exergy analysis of a process converting power and biomass to a liquid fuel. Chem. Eng. Trans. 2019, 76, 205–210. [Google Scholar]



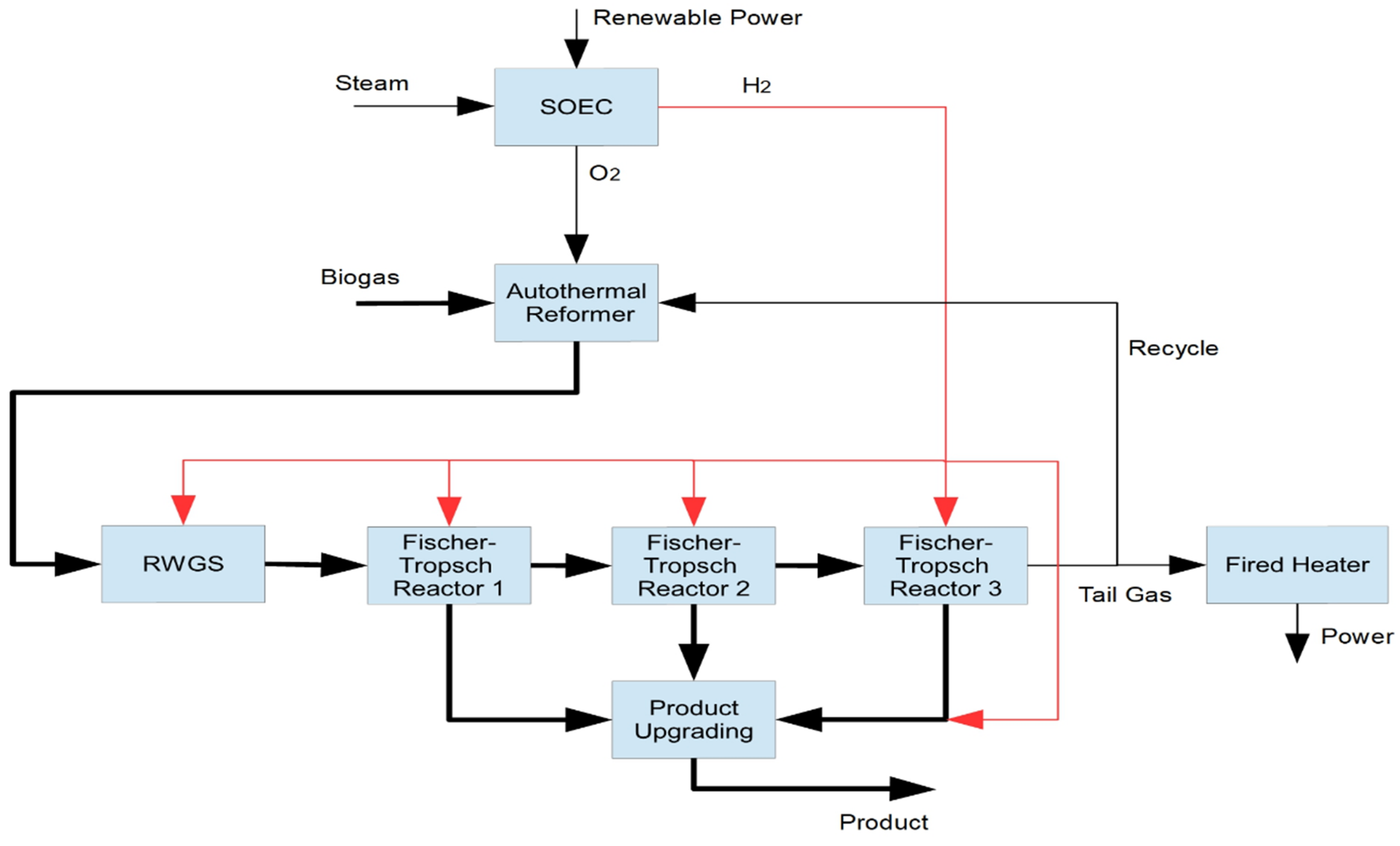
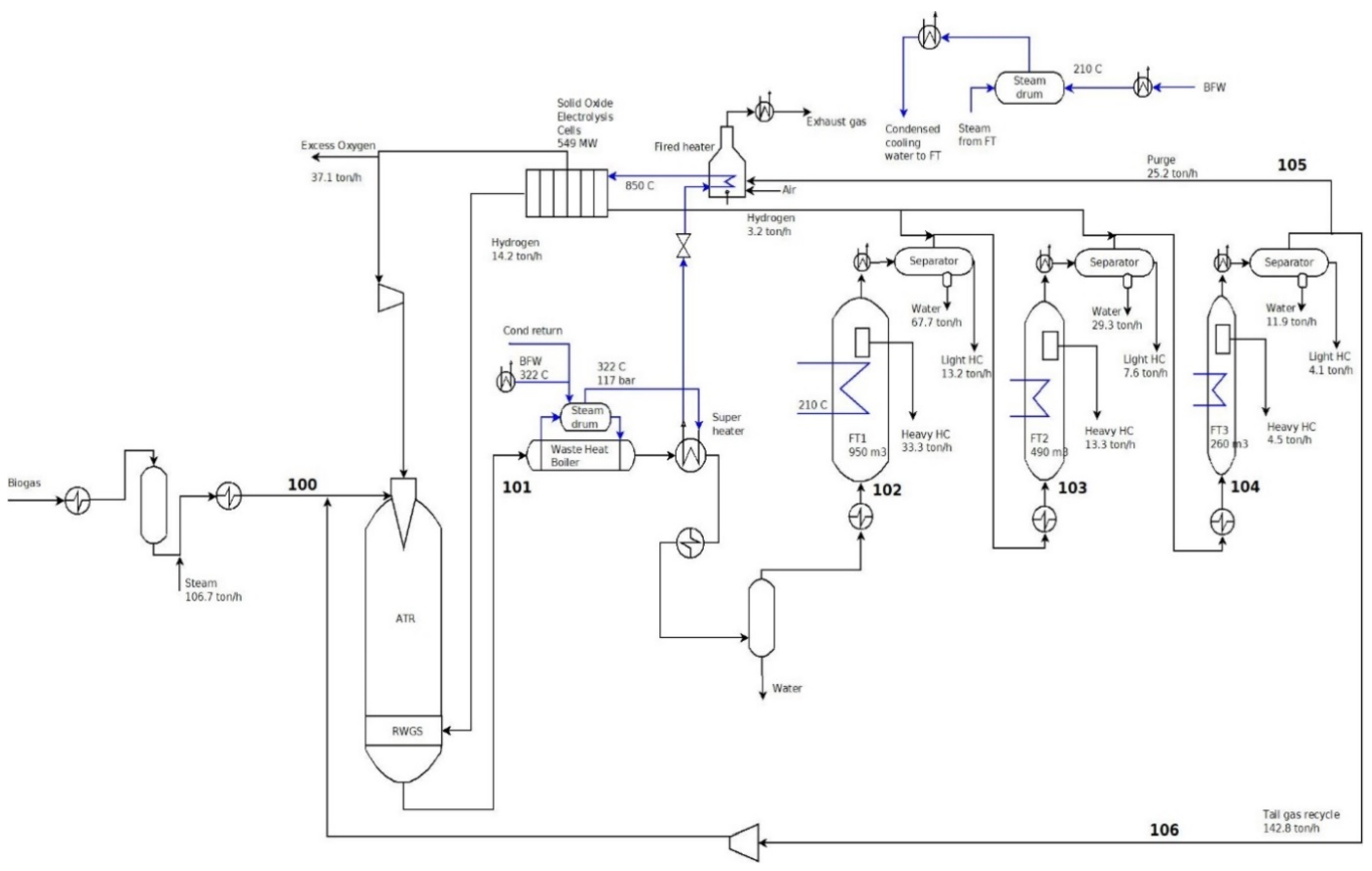
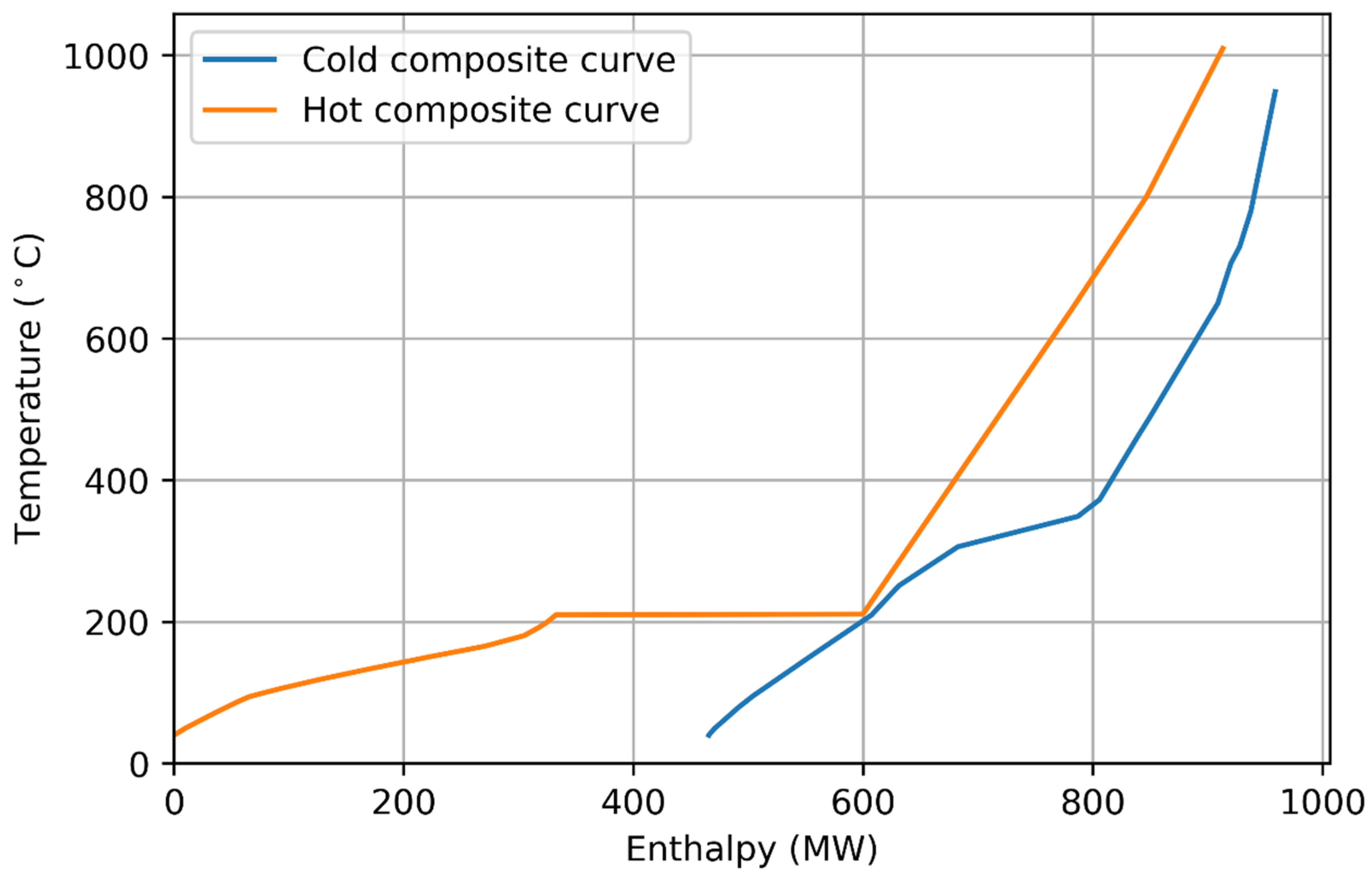
| Parameter | Methanation | Fischer–Tropsch |
|---|---|---|
| CO2 transport and storage costs for CCUS (USD/t CO2) | 20 | 20 |
| CapEx (USD/kWliquid) | 565 | 565 |
| Efficiency % (LHV) | 77 | 73 |
| Annual OPEX (% of CapEx) | 4 | 4 |
| Lifetime (years) | 30 | 30 |
| Electricity consumption (GJe/GJprod) | 0.013 | 0.018 |
| Stage 1 | Stage 2 | Stage 3 | |
|---|---|---|---|
| Volume [m3] | 950 | 490 | 260 |
| Inlet H2/CO | 1.8 | 1.8 | 1.8 |
| H2 addition between stage [kmol/h] | 0 | 1085 | 486 |
| CH4 selectivity [%] | 4 | 4.4 | 5 |
| CO conversion [%] | 56 | 55 | 50 |
| Syncrude production [t/h] | 46.6 | 20.9 | 8.7 |
| Stream | 100 | 101 | 102 | 103 | 104 | 105 | 106 |
|---|---|---|---|---|---|---|---|
| Temperature (°C) | 650 | 1010 | 210 | 210 | 210 | 40 | 40 |
| Pressure (bar) | 40 | 38.5 | 36.5 | 34.5 | 32.5 | 30.5 | 30.5 |
| Mass flow (t/h) | 270 | 523.6 | 349.8 | 237.8 | 188.5 | 25.2 | 142.8 |
| Molar flow (kmol/h) | 11,916 | 31,566 | 21,919 | 11,668 | 7231 | 786 | 4454 |
| Mole fractions | |||||||
| CO | 0 | 0.210 | 0.303 | 0.249 | 0.179 | 0.123 | 0.123 |
| H2 | 0 | 0.379 | 0.545 | 0.448 | 0.322 | 0.183 | 0.183 |
| H2O | 0.496 | 0.306 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 |
| CH4 | 0.302 | 0.006 | 0.009 | 0.029 | 0.057 | 0.085 | 0.085 |
| C2-C4 | 0 | 0 | 0 | 0.006 | 0.013 | 0.021 | 0.021 |
| CO2 | 0.201 | 0.098 | 0.141 | 0.262 | 0.421 | 0.578 | 0.578 |
| N2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Overall Results of Biogas to Liquid Process | |
|---|---|
| Conversion of CO in the Fischer–Tropsch section, defined in terms of each single pass (%) | 90 |
| Carbon efficiency (overall) (%) | 88 |
| Fischer–Tropsch production (overall) (t/h) | 76 |
| Fischer–Tropsch production (overall) (L/h) | 95,000 |
| Fischer–Tropsch Reactor volume (m3) | 1700 |
| Required power to the SOEC (MW) | 549 |
| Steam to SOEC (t/h) | 188 |
| Recycle flow to the ATR (t/h) | 142 |
| Tail gas compositions (mol%) | |
| H2 | 18.3 |
| CO | 12.3 |
| CH4 | 8.5 |
| CO2 | 57.8 |
| N2 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostadi, M.; Paso, K.G.; Rodriguez-Fabia, S.; Øi, L.E.; Manenti, F.; Hillestad, M. Process Integration of Green Hydrogen: Decarbonization of Chemical Industries. Energies 2020, 13, 4859. https://doi.org/10.3390/en13184859
Ostadi M, Paso KG, Rodriguez-Fabia S, Øi LE, Manenti F, Hillestad M. Process Integration of Green Hydrogen: Decarbonization of Chemical Industries. Energies. 2020; 13(18):4859. https://doi.org/10.3390/en13184859
Chicago/Turabian StyleOstadi, Mohammad, Kristofer Gunnar Paso, Sandra Rodriguez-Fabia, Lars Erik Øi, Flavio Manenti, and Magne Hillestad. 2020. "Process Integration of Green Hydrogen: Decarbonization of Chemical Industries" Energies 13, no. 18: 4859. https://doi.org/10.3390/en13184859






