Mayenite Electrides and Their Doped Forms for Oxygen Reduction Reaction in Solid Oxide Fuel Cells
Abstract
1. Introduction
2. Computational Methods
3. Results
3.1. Structures of C12A7:(e−)4 and S12A7:(e−)4
3.2. Encapsulation of Oxygen Atoms in C12A7:(e−)4 and S12A7:(e−)4
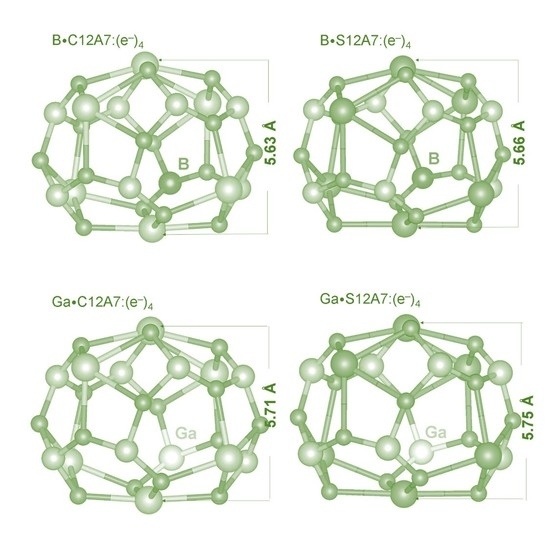
3.3. Encapsulation of Oxygen Atoms in C12A7:(e−)4 and S12A7:(e−)4 Doped with B and Ga
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar]
- Ormerod, R.M. Solid oxide fuel cells. Chem. Soc. Rev. 2003, 32, 17–28. [Google Scholar] [CrossRef]
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Devi, P.S.; Sharma, A.D.; Maiti, H.S. Solid Oxide Fuel Cell Materials: A Review. Trans. Indian Ceram. Soc. 2004, 63, 75–98. [Google Scholar] [CrossRef]
- Sharma, K.; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Wachsman, E.D.; Marlowe, C.A.; Lee, K.T. Role of solid oxide fuel cells in a balanced energy strategy. Energy Environ. Sci. 2012, 5, 5498–5509. [Google Scholar] [CrossRef]
- Sun, C.; Hui, R.; Roller, J. Cathode materials for solid oxide fuel cells: A review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Jacobson, A.J. Materials for Solid Oxide Fuel Cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Sunarso, J.; Hashim, S.S.; Zhu, N.; Zhou, W. Perovskite oxides applications in high temperature oxygen separation, solid oxide fuel cell and membrane reactor: A review. Progress in Energy and Combustion. Science 2017, 61, 57–77. [Google Scholar] [CrossRef]
- Tian, R.; Fan, J.; Liu, Y.; Xia, C. Low-temperature solid oxide fuel cells with La1−xSrxMnO3 as the cathodes. J. Power Sources 2008, 185, 1247–1251. [Google Scholar] [CrossRef]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Yang, G.; Su, C.; Ran, R.; Tade, M.O.; Shao, Z. Advanced Symmetric Solid Oxide Fuel Cell with an Infiltrated K2NiF4-Type La2NiO4 Electrode. Energy Fuels 2014, 28, 356–362. [Google Scholar] [CrossRef]
- Heap, R.; Islam, M.S.; Slater, P.R. Synthesis and structural characterisation of the new K2NiF4-type phases, A2In0.5Sb0.5O4 (A = Sr, Ba). Dalton Trans. 2005, 460–463. [Google Scholar] [CrossRef]
- Berry, F.J.; Moore, E.; Mortimer, M.; Ren, X.; Heap, R.; Slater, P.; Thomas, M.F. Synthesis and structural investigation of a new oxide fluoride of composition Ba2SnO2.5F3·xH2O (x≈0.5). J. Solid State Chem. 2008, 181, 2185–2190. [Google Scholar] [CrossRef]
- Téllez Lozano, H.; Druce, J.; Cooper, S.J.; Kilner, J.A. Double perovskite cathodes for proton-conducting ceramic fuel cells: Are they triple mixed ionic electronic conductors? Sci. Technol. Adv. Mater. 2017, 18, 977–986. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Dass, R.I.; Xing, Z.-L.; Goodenough, J.B. Double Perovskites as Anode Materials for Solid-Oxide Fuel Cells. Science 2006, 312, 254–257. [Google Scholar] [CrossRef]
- Hu, Y.; Hernandez, O.; Broux, T.; Bahout, M.; Hermet, J.; Ottochian, A.; Ritter, C.; Genestec, G.; Dezanneau, G. Oxygen diffusion mechanism in the mixed ion-electron conductor NdBaCo2O5+x. J. Mater. Chem. 2012, 22, 18744–18747. [Google Scholar] [CrossRef]
- Rupasov, D.; Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W.; Istomin, S.Y.; Antipov, E.V. Oxygen diffusion in Sr0.75Y0.25CoO2.625: A molecular dynamics study. Phys. Rev. B 2009, 79, 172102. [Google Scholar] [CrossRef]
- Parfitt, D.; Kordatos, A.; Filippatos, P.P.; Chroneos, A. Diffusion in energy materials: Governing dynamics from atomistic modelling. Appl. Phys. Rev. 2017, 4, 031305. [Google Scholar] [CrossRef]
- Imlach, J.A.; Dent Glasser, L.S.; Glasser, F.P. Excess oxygen and the stability of “12CaO.7A12O3”. Cem. Concr. Res. 1971, 1, 57–61. [Google Scholar] [CrossRef]
- Watauchi, S.; Tanaka, I.; Hayashi, K.; Hirano, M.; Hosono, H. Crystal growth of Ca12Al14O33 by the floating zone method. J. Cryst. Growth 2002, 237–239, 801–805. [Google Scholar] [CrossRef]
- Kim, S.W.; Matsuishi, S.; Nomura, T.; Kubota, Y.; Takata, M.; Hayashi, K.; Kamiya, T.; Hirano, M.; Hosono, H. Metallic State in a Lime−Alumina Compound with Nanoporous Structure. Nano Lett. 2007, 7, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, O.; Narai, A.; Shimizu, K. New Compound in the System SrO-Al2O3. J. Am. Ceram. Soc. 1986, 69, C-36. [Google Scholar] [CrossRef]
- Kuganathan, N.; Gkanas, E.; Chroneos, A. Encapsulation and substitution of Fe in C12A7 (12CaO⋅7Al2O3). AIP Adv. 2020, 10, 015242. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A.; Grimes, R.W. The encapsulation selectivity for anionic fission products imparted by an electride. Sci. Rep. 2019, 9, 13612. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A. Technetium Encapsulation by A Nanoporous Complex Oxide 12CaO•7Al2O3 (C12A7). Nanomaterials 2019, 9, 816. [Google Scholar] [CrossRef]
- Kuganathan, N.; Grimes, R.W.; Chroneos, A. Encapsulation of heavy metals by a nanoporous complex oxide 12CaO · 7Al2O3. J. Appl. Phys. 2019, 125, 165103. [Google Scholar] [CrossRef]
- Hayashi, F.; Tomota, Y.; Kitano, M.; Toda, Y.; Yokoyama, T.; Hosono, H. NH2− Dianion Entrapped in a Nanoporous 12CaO·7Al2O3 Crystal by Ammonothermal Treatment: Reaction Pathways, Dynamics, and Chemical Stability. J. Am. Chem. Soc. 2014, 136, 11698–11706. [Google Scholar] [CrossRef]
- Hayashi, K.; Hirano, M.; Hosono, H. Thermodynamics and Kinetics of Hydroxide Ion Formation in 12CaO·7Al2O3. J. Phys. Chem. B 2005, 109, 11900–11906. [Google Scholar] [CrossRef]
- Jeevaratnam, J.; Glasser, F.P.; Glasser, L.S.D. Anion Substitution and Structure of 12CaO·7A12O3. J. Am. Ceram. Soc. 1964, 47, 105–106. [Google Scholar] [CrossRef]
- Miyakawa, M.; Kamioka, H.; Hirano, M.; Kamiya, T.; Sushko, P.V.; Shluger, A.L.; Matsunami, N.; Hosono, H. Photoluminescence from Au ion-implanted nanoporous single-crystal 12CaO∙7Al2O3. Phys. Rev. B 2006, 73, 205108. [Google Scholar] [CrossRef]
- Toda, Y.; Hirayama, H.; Kuganathan, N.; Torrisi, A.; Sushko, P.V.; Hosono, H. Activation and splitting of carbon dioxide on the surface of an inorganic electride material. Nat. Commun. 2013, 4, 2378. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Kanbara, S.; Inoue, Y.; Kuganathan, N.; Sushko, P.V.; Yokoyama, T.; Hara, M.; Hosono, H. Electride support boosts nitrogen dissociation over ruthenium catalyst and shifts the bottleneck in ammonia synthesis. Nat. Commun. 2015, 6, 6731. [Google Scholar] [CrossRef] [PubMed]
- Kuganathan, N.; Hosono, H.; Shluger, A.L.; Sushko, P.V. Enhanced N2 Dissociation on Ru-Loaded Inorganic Electride. J. Am. Chem. Soc. 2014, 136, 2216–2219. [Google Scholar] [CrossRef]
- Lacerda, M.; Irvine, J.T.S.; Glasser, F.P.; West, A.R. High oxide ion conductivity in Ca12Al14O33. Nature 1988, 332, 525–526. [Google Scholar] [CrossRef]
- Hosono, H.; Hayashi, K.; Kajihara, K.; Sushko, P.V.; Shluger, A.L. Oxygen ion conduction in 12CaO·7Al2O3: O2− conduction mechanism and possibility of O− fast conduction. Solid State Ion. 2009, 180, 550–555. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes in C (2nd ed.): The Art of Scientific Computing; Cambridge University Press: Cambridge, UK, 1992; p. 994. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Palacios, L.; De La Torre, Á.G.; Bruque, S.; García-Muñoz, J.L.; García-Granda, S.; Sheptyakov, D.; Aranda, M.A. Crystal Structures and in-Situ Formation Study of Mayenite Electrides. Inorg. Chem. 2007, 46, 4167–4176. [Google Scholar] [CrossRef]
- Miyakawa, M.; Ueda, N.; Kamiya, T.; Hirano, M.; Hosono, H. Novel Room Temperature Stable Electride 12SrO•7Al2O3 Thin Films: Fabrication, Optical and Electron Transport Properties. J. Ceram. Soc. Jpn. 2007, 115, 567–570. [Google Scholar] [CrossRef]
- Bader, R.F.W. The zero-flux surface and the topological and quantum definitions of an atom in a molecule. Theor. Chem. Acc. 2001, 105, 276–283. [Google Scholar] [CrossRef]
- Martin, W.C. CRC Handbook of Chemistry and Physics, 86th ed.; CRC: Boca Raton, FL, USA, 2005. [Google Scholar]








| Parameters | Electrides | |
|---|---|---|
| C12A7:(e−)4 | S12A7:(e−)4 | |
| A = b = c (Å) | 12.06 (12.00) [48] | 12.40 |
| α = β = γ (°) | 90.0 | 90.0 |
| V (Å3) | 1752.51 | 1908.59 |
| Parameters | Oxygen Encapsulated Structures | |||
|---|---|---|---|---|
| C12A7:(e−)2O2− | S12A7:(e−)2O2− | C12A7:(O2−)2 | S12A7:(O2−)2 | |
| a (Å) | 12.06 | 12.41 | 12.05 (11.99) [49] | 12.38 (12.33) [49] |
| b (Å) | 12.06 | 12.41 | 12.01(11.99) [49] | 12.35 (12.33) [49] |
| c (Å) | 12.00 | 12.36 | 12.02 (11.99) [49] | 12.36 (12.33) [49] |
| α = β = γ (°) | 90.0 | 90.0 | 90.0 | 90.0 |
| V (Å3) | 1746.59 | 1902.48 | 1739.95 | 1889.90 |
| Encapsulation Process | Encapsulation Energy (eV) | Bader Charge on Encapsulated Oxygen (|e|) |
|---|---|---|
| C12A7:(e−)4 + ½ O2 → C12A7:(e−)2O2− | −4.81 | −1.36 |
| C12A7:(e−)2O2− + ½ O2 → C12A7:(O2−)2 | −4.30 | −1.39 (2) |
| S12A7:(e−)4 + ½ O2 → S12A7:(e−)2O2− | −4.18 | −1.42 |
| S12A7:(e−)2O2− + ½ O2 → S12A7:(O2−)2 | −4.04 | −1.43 (2) |
| Parameters | Doped Electrides | |||
|---|---|---|---|---|
| B·C12A7:(e−)4 | Ga·C12A7:(e−)4 | B·S12A7:(e−)4 | Ga·S12A7:(e−)4 | |
| a = b = c (Å) | 12.02 | 12.07 | 12.36 | 12.42 |
| α = β = γ (°) | 90.0 | 90.0 | 90.0 | 90.0 |
| V (Å3) | 1735.69 | 1757.16 | 1890.11 | 1915.53 |
| Parameters | Oxygen Encapsulated Doped Structures | |||
|---|---|---|---|---|
| B·C12A7:(e−)2O2− | B·S12A7:(e−)2O2− | B·C12A7:(O2−)2 | B·S12A7:(O2−)2 | |
| a (Å) | 12.02 | 12.36 | 11.98 | 12.32 |
| b (Å) | 12.02 | 12.36 | 11.98 | 12.32 |
| c (Å) | 11.97 | 12.33 | 11.97 | 12.32 |
| α = β = γ (°) | 90.0 | 90.0 | 90.0 | 90.0 |
| V (Å3) | 1730.13 | 1883.53 | 1719.30 | 1870.62 |
| Ga·C12A7:(e−)2O2− | Ga·S12A7:(e−)2O2− | G·C12A7:(O2−)2 | Ga·S12A7:(O2−)2 | |
| a (Å) | 12.08 | 12.42 | 12.07 | 12.40 |
| b (Å) | 12.07 | 12.41 | 12.02 | 12.36 |
| c (Å) | 12.02 | 12.37 | 12.03 | 12.38 |
| α = β = γ (°) | 90.0 | 90.0 | 90.0 | 90.0 |
| V (Å3) | 1751.07 | 1907.55 | 1744.47 | 1896.43 |
| Encapsulation Process | Encapsulation Energy (eV) | Bader Charge (|e|) | |
|---|---|---|---|
| B or Ga | O | ||
| B·C12A7:(e−)4 + ½ O2 → B·C12A7:(e−)2O2− | −4.72 | +3.00 | −1.39 |
| B·C12A7:(e−)2O2− + ½ O2 → B·C12A7:(O2−)2 | −4.62 | +3.00 | −1.40, −1.44 |
| Ga·C12A7:(e−)4 + ½ O2 → Ga·C12A7:(e−)2O2− | −4.56 | +3.00 | −1.37 |
| Ga·C12A7:(e−)2O2− + ½ O2 → Ga·C12A7:(O2−)2 | −4.25 | +3.00 | −1.38, −1.40 |
| B·S12A7:(e−)4 + ½ O2 → B·S12A7:(e−)2O2− | −4.31 | +3.00 | −1.42 |
| B·S12A7:(e−)2O2− + ½ O2 → B·S12A7:(O2−)2 | −4.29 | +3.00 | −1.43 (2) |
| Ga·S12A7:(e−)4 + ½ O2 → Ga·S12A7:(e−)2O2− | −4.17 | +3.00 | −1.40 |
| Ga·S12A7:(e−)2O2− + ½ O2 → Ga·S12A7:(O2−)2 | −3.94 | +3.00 | −1.43 (2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuganathan, N.; Vovk, R.V.; Chroneos, A. Mayenite Electrides and Their Doped Forms for Oxygen Reduction Reaction in Solid Oxide Fuel Cells. Energies 2020, 13, 4978. https://doi.org/10.3390/en13184978
Kuganathan N, Vovk RV, Chroneos A. Mayenite Electrides and Their Doped Forms for Oxygen Reduction Reaction in Solid Oxide Fuel Cells. Energies. 2020; 13(18):4978. https://doi.org/10.3390/en13184978
Chicago/Turabian StyleKuganathan, Navaratnarajah, Ruslan V. Vovk, and Alexander Chroneos. 2020. "Mayenite Electrides and Their Doped Forms for Oxygen Reduction Reaction in Solid Oxide Fuel Cells" Energies 13, no. 18: 4978. https://doi.org/10.3390/en13184978
APA StyleKuganathan, N., Vovk, R. V., & Chroneos, A. (2020). Mayenite Electrides and Their Doped Forms for Oxygen Reduction Reaction in Solid Oxide Fuel Cells. Energies, 13(18), 4978. https://doi.org/10.3390/en13184978