Evaluating the Potential of Rhodosporidium toruloides-1588 for High Lipid Production Using Undetoxified Wood Hydrolysate as a Carbon Source
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enzyme Production and Extraction
2.2. Enzyme Activity
2.3. Pretreatment and Enzymatic Saccharification
2.4. Microorganism Maintenance and Inoculum Preparation Conditions
2.5. Lipid Production
2.6. Lipid Extraction
2.7. Analytical Methods
2.7.1. Cell Growth Analysis
2.7.2. Inhibitors and Sugar Analysis
2.7.3. Fatty Acid Determination (FAME)
2.7.4. Statistical Analysis
3. Results and Discussion
3.1. Wood Hydrolysate and Its Compositions
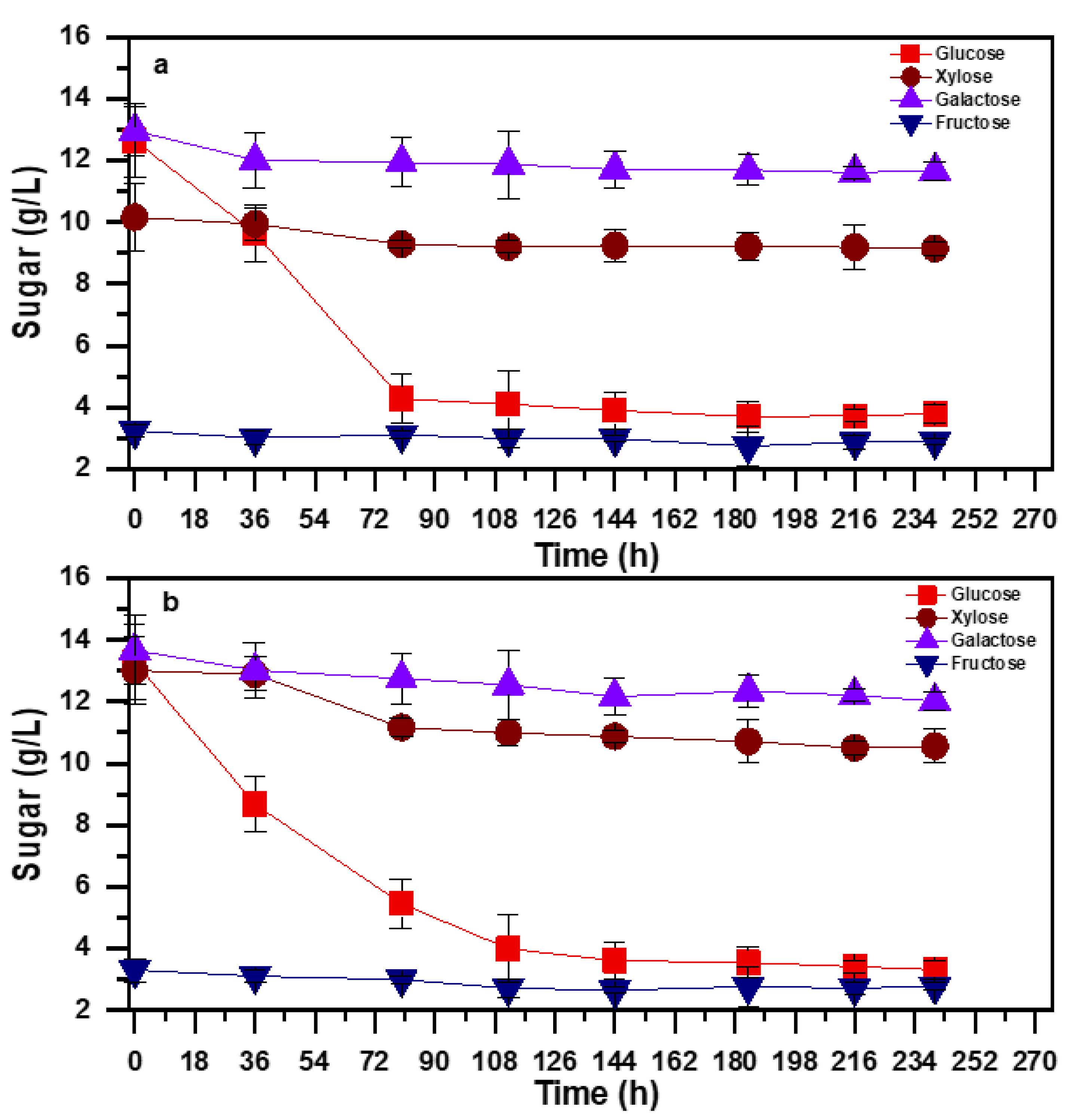
3.2. Biomass Growth and Sugar Consumption in the Wood Hydrolysates
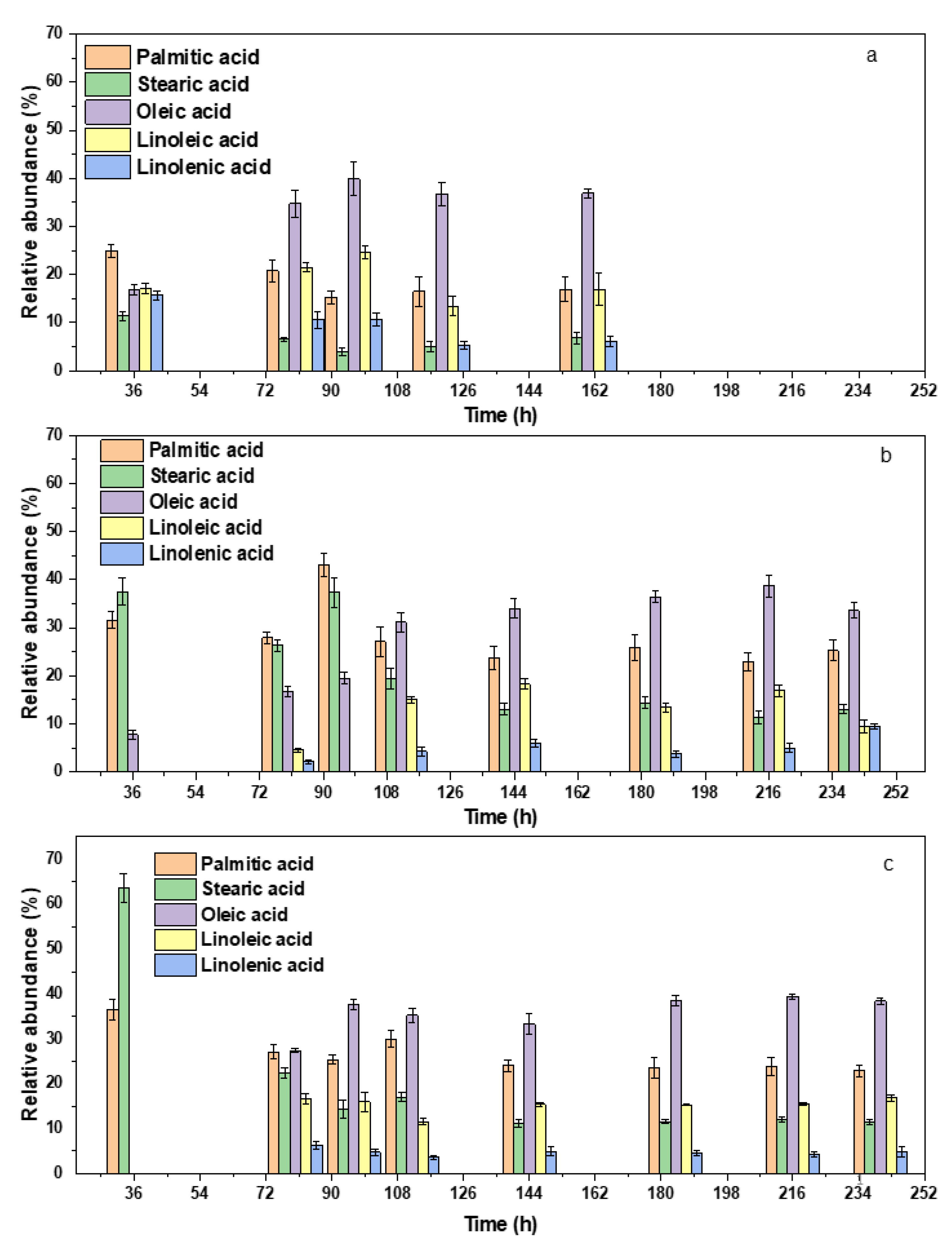
3.3. Lipid accumulation and FAME Composition
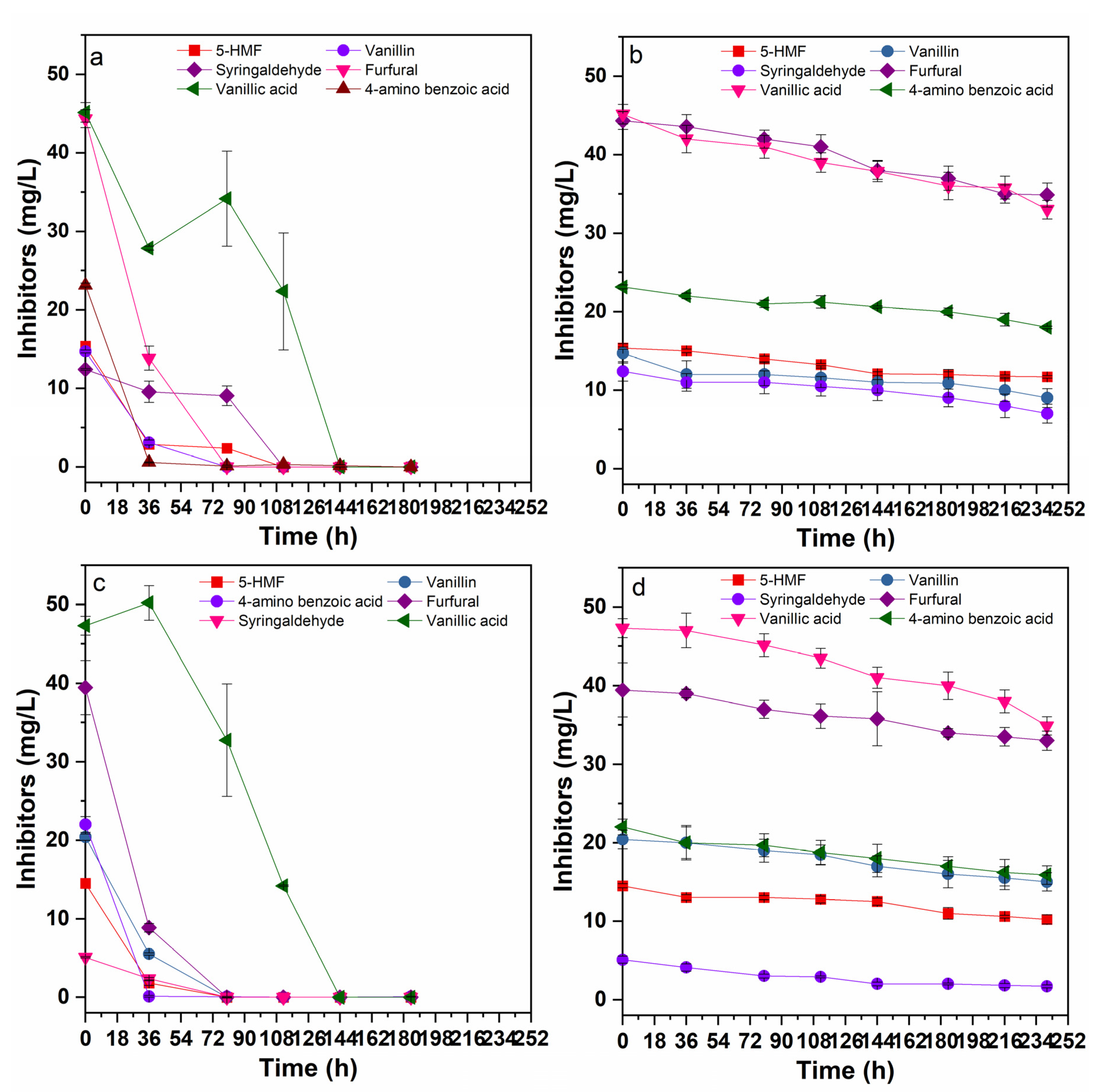
3.4. Effects of Inhibitors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Branduardi, P.; Martani, F.; Porro, D. Improving the stress tolerance of the oleaginous yeast Lipomyces starkeyi for industrial purposes. New Biotechnol. 2018, 44, S90. [Google Scholar] [CrossRef]
- Saini, R.; Hegde, K.; Brar, S.K.; Vezina, P. Advanced biofuel production and road to commercialization: An insight into bioconversion potential of Rhodosporidium sp. Biomass Bioenergy 2020, 132, 105439. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Erten, H. Production of oils and fats by oleaginous microorganisms with an emphasis given to the potential of the nonconventional yeast Yarrowia lipolytica. Crit. Rev. Biotechnol. 2018, 38, 1230–1243. [Google Scholar] [CrossRef] [PubMed]
- Littlejohns, J.; Rehmann, L.; Murdy, R.; Oo, A.; Neill, S. Current state and future prospects for liquid biofuels in Canada. Biofuel Res. J. 2018, 5, 759–779. [Google Scholar] [CrossRef] [Green Version]
- Osorio-González, C.S.; Hegde, K.; Brar, S.K.; Kermanshahipour, A.; Avalos-Ramírez, A. Challenges in lipid production from lignocellulosic biomass using Rhodosporidium sp.; A look at the role of lignocellulosic inhibitors. Biofuels Bioprod. Biorefin. 2019, 13, 740–759. [Google Scholar] [CrossRef]
- Guo, M.; Cheng, S.; Chen, G.; Chen, J. Improvement of lipid production in oleaginous yeast Rhodosporidium toruloides by ultraviolet mutagenesis. Eng. Life Sci. 2019, 19, 548–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osorio-González, C.S.; Hegde, K.; Ferreira, P.; Brar, S.K.; Kermanshahipour, A.; Soccol, C.R.; Avalos-Ramírez, A. Lipid production in Rhodosporidium toruloides using C-6 and C-5 wood hydrolysate: A comparative study. Biomass Bioenergy 2019, 130, 105355. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Potential of apple pomace as a solid substrate for fungal cellulase and hemicellulase bioproduction through solid-state fermentation. Ind. Crops Prod. 2012, 38, 6–13. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Hegde, K.; Brar, S.K.; Kermanshahipour, A.; Avalos-Ramírez, A. Data set of green extraction of valuable chemicals from lignocellulosic biomass using microwave method. Data Brief 2019, 26, 104347. [Google Scholar] [CrossRef]
- Ma, X.; Gao, Z.; Gao, M.; Ma, Y.; Ma, H.; Zhang, M.; Liu, Y.; Wang, Q. Microbial lipid production from food waste saccharified liquid and the effects of compositions. Energy Convers. Manag. 2018, 172, 306–315. [Google Scholar] [CrossRef]
- Fei, Q.; O’Brien, M.; Nelson, R.; Chen, X.; Lowell, A.; Dowe, N. Enhanced lipid production by Rhodosporidium toruloides using different fed-batch feeding strategies with lignocellulosic hydrolysate as the sole carbon source. Biotechnol. Biofuels 2016, 9, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, J.; Kirby, J.; Ito, M.; Sun, J.; Dutta, T.; Mirsiaghi, M.; Sundstrom, E.R.; Rodriguez, A.; Baidoo, E.; Tanjore, D.; et al. Rhodosporidium toruloides: A new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts. Biotechnol. Biofuels 2017, 10, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Braunwald, T.; Schwemmlein, L.; Graeff-Hönninger, S.; French, W.T.; Hernandez, R.; Holmes, W.E.; Claupein, W. Effect of different C/N ratios on carotenoid and lipid production by Rhodotorula glutinis. Appl. Microbiol. Biotechnol. 2013, 97, 6581–6588. [Google Scholar] [CrossRef] [PubMed]
- Tehlivets, O.; Scheuringer, K.; Kohlwein, S.D. Fatty acid synthesis and elongation in yeast. Biochim. Biophys. Acta 2007, 1771, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Matsakas, L.; Novak, K.; Enman, J.; Christakopoulos, P.; Rova, U. Acetate-detoxification of wood hydrolysates with alkali tolerant Bacillus sp. as a strategy to enhance the lipid production from Rhodosporidium toruloides. Bioresour. Technol. 2017, 242, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Peng, F.; Du, W.; Liu, C.; Liu, D. Effects of some inhibitors on the growth and lipid accumulation of oleaginous yeast Rhodosporidium toruloides and preparation of biodiesel by enzymatic transesterification of the lipid. Bioprocess Biosyst. Eng. 2012, 35, 993–1004. [Google Scholar] [CrossRef]
- Tsigie, Y.A.; Wang, C.-Y.; Truong, C.-T.; Ju, Y.-H. Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate. Bioresour. Technol. 2011, 102, 9216–9222. [Google Scholar] [CrossRef]
- Huang, C.; Wu, H.; Li, R.-f.; Zong, M.-H. Improving lipid production from bagasse hydrolysate with Trichosporon fermentans by response surface methodology. New Biotechnol. 2012, 29, 372–378. [Google Scholar] [CrossRef]
- Bommareddy, R.R.; Sabra, W.; Maheshwari, G.; Zeng, A.-P. Metabolic network analysis and experimental study of lipid production in Rhodosporidium toruloides grown on single and mixed substrates. Microb. Cell Fact. 2015, 14, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanikolaou, S.; Sarantou, S.; Komaitis, M.; Aggelis, G. Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiple-limited media. J. Appl. Microbiol. 2004, 97, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An Overview of Potential Oleaginous Microorganisms and Their Role in Biodiesel and Omega-3 Fatty Acid-Based Industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonturi, N.; Crucello, A.; Viana, A.J.C.; Miranda, E.A. Microbial oil production in sugarcane bagasse hemicellulosic hydrolysate without nutrient supplementation by a Rhodosporidium toruloides adapted strain. Process Biochem. 2017, 57, 16–25. [Google Scholar] [CrossRef]
- Gorsich, S.W.; Dien, B.S.; Nichols, N.N.; Slininger, P.J.; Liu, Z.L.; Skory, C.D. Tolerance to furfural-induced stress is associated with pentose phosphate pathway genes ZWF1, GND1, RPE1, and TKL1 in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2006, 71, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-E.; Koo, H.M.; Park, Y.K.; Park, S.M.; Park, J.C.; Lee, O.-K.; Park, Y.-C.; Seo, J.-H. Expression of aldehyde dehydrogenase 6 reduces inhibitory effect of furan derivatives on cell growth and ethanol production in Saccharomyces cerevisiae. Bioresour. Technol. 2011, 102, 6033–6038. [Google Scholar] [CrossRef]
- Qi, F.; Zhao, X.; Kitahara, Y.; Li, T.; Ou, X.; Wei, D.; Dehua, L.; Huang, J. Integrative transcriptomic and proteomic analysis of the mutant lignocellulosic hydrolyzate-tolerant Rhodosporidium toruloides. Eng. Life Sci. 2017, 17, 249–261. [Google Scholar] [CrossRef]
- Wierckx, N.; Koopman, F.; Ruijssenaars, H.J.; de Winde, J.H. Microbial degradation of furanic compounds: Biochemistry, genetics, and impact. Appl. Microbiol. Biotechnol. 2011, 92, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Zhang, S.; Liu, H.; Shen, H.; Lin, X.; Yang, F.; Zhou, Y.J.; Jin, G.; Ye, M.; Zou, H.; et al. A multi-omic map of the lipid-producing yeast Rhodosporidium toruloides. Nat. Commun. 2012, 3, 1112. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Xia, J.; Mehmood, M.A.; Zhao, X.-Q.; Liu, C.-G.; Bai, F.-W. Extracellular redox potential regulation improves yeast tolerance to furfural. Chem. Eng. Sci. 2019, 196, 54–63. [Google Scholar] [CrossRef]


| Components | Hardwood Hydrolysate (g/L) | Softwood Hydrolysate (g/L) |
|---|---|---|
| Glucose | 12.64 ± 1.52 | 13.27 ± 1.23 |
| Xylose | 10.16 ± 0.95 | 13 ± 0.89 |
| Galactose | 12.96 ± 0.81 | 13.66 ± 1.12 |
| Fructose | 3.24 ± 0.21 | 3.30 ± 0.37 |
| Trehalose | 0.03 ± 0.00 | 2.4 ± 0.07 |
| Furfural | 0.067 ± 0.01 | 0.057 ± 0.01 |
| 5-hydroxymehtyl furfural | 0.025 ± 0.002 | 0.035 ± 0.001 |
| Vanillic acid | 0.034 ± 0.003 | 0.042 ± 0.001 |
| Vanillin | 0.007 ± 0.0005 | 0.01 ± 0.002 |
| Levulinic acid | 0.007 ± 0.0003 | 0.007 ± 0.0006 |
| Polyunsaturated Fatty Acids (%) | SM | HW | HW-AS | SW | SW-AS |
|---|---|---|---|---|---|
| Alpha linolenic acid | 5.21 | 9.48 | 2.73 | 6.29 | 3.74 |
| Stearidonic acid | 1.26 | ND | 0.68 | 0.22 | 0.43 |
| Eicosatrienoic acid | 0.71 | 3.86 | ND | 2.82 | 3.76 |
| Docosapentaenoic acid | 5.31 | 5.9 | ND | 1.98 | 1.12 |
| Linoleic acid | 0.67 | 4.51 | 10.05 | 16.65 | 9.86 |
| Trans-linoleic acid | ND | 0.49 | 1.12 | 0.06 | 0.62 |
| Gamma linoleic acid | ND | 0.22 | 0.29 | 0.07 | 0.15 |
| Eicosadienoic acid | 0.18 | 1.06 | 0.17 | 0.46 | 0.36 |
| Dihomogamma linolenic | ND | 0.10 | 0.06 | 0.09 | 0.11 |
| Elaidic acid | ND | 0.88 | 1.66 | 1.09 | 0.71 |
| Erucic acid | 0.72 | ND | 0.03 | 0.54 | 0.24 |
| Eicosenoate | ND | 0.93 | ND | 0.83 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saini, R.; Hegde, K.; Osorio-Gonzalez, C.S.; Brar, S.K.; Vezina, P. Evaluating the Potential of Rhodosporidium toruloides-1588 for High Lipid Production Using Undetoxified Wood Hydrolysate as a Carbon Source. Energies 2020, 13, 5960. https://doi.org/10.3390/en13225960
Saini R, Hegde K, Osorio-Gonzalez CS, Brar SK, Vezina P. Evaluating the Potential of Rhodosporidium toruloides-1588 for High Lipid Production Using Undetoxified Wood Hydrolysate as a Carbon Source. Energies. 2020; 13(22):5960. https://doi.org/10.3390/en13225960
Chicago/Turabian StyleSaini, Rahul, Krishnamoorthy Hegde, Carlos Saul Osorio-Gonzalez, Satinder Kaur Brar, and Pierre Vezina. 2020. "Evaluating the Potential of Rhodosporidium toruloides-1588 for High Lipid Production Using Undetoxified Wood Hydrolysate as a Carbon Source" Energies 13, no. 22: 5960. https://doi.org/10.3390/en13225960
APA StyleSaini, R., Hegde, K., Osorio-Gonzalez, C. S., Brar, S. K., & Vezina, P. (2020). Evaluating the Potential of Rhodosporidium toruloides-1588 for High Lipid Production Using Undetoxified Wood Hydrolysate as a Carbon Source. Energies, 13(22), 5960. https://doi.org/10.3390/en13225960








