1. Introduction
Oxygen is widely used in chemical industry including steel and semi-conductor production, food processing, and healthcare [
1,
2]. Nitrogen is used mainly as a reactant for ammonia production and as an inert gas in various industrial processes [
3,
4]. On commercial scale, these gases are produced from the separation of air. The operation of air separation units (ASU) can be broadly classified into two categories, namely, non-cryogenic and cryogenic air separation processes. Non-cryogenic techniques for air separation include pressure swing adsorption (PSA) and membrane-based systems. These processes are relatively simple in operation; however, their major drawbacks are scalability and relatively lower quality of product gases [
5,
6,
7]. Cryogenic processes, though complex in nature, are more efficient and cost-effective. They are widely applied on industrial scale for production of high-purity oxygen and nitrogen gases from air [
5,
6,
8,
9,
10].
Many alternative designs of the cryogenic air separation process have been reported in literature. These include process designs based on 1, 2, and 3 distillation columns. The one-column process is the simplest design to produce high-purity oxygen and nitrogen from air. However, it offers somewhat limited opportunities for heat integration in the process and is rarely used in industrial practice [
11]. The three-column air separation process is a relatively complex design and usually employed only when in addition to high-purity oxygen and nitrogen, separation of argon is also required [
12]. The most widely used process for industrial-scale air separation is based on a two-column design. These columns are operated at different pressures, and are thermally coupled through additional compressors, expanders, and/or heat exchangers in order to reduce the overall utility requirements for the process [
13].
The major drawback of cryogenic air separation processes is their significantly higher energy requirement. To overcome this issue, the cryogenic process is generally highly heat-integrated to reduce and possibly eliminate the use of external utilities. This is generally achieved by (1) adjusting the pressures of the distillation columns such that there is a positive temperature difference driving force for heat transfer from the condenser of the high-pressure column to the reboiler of the low-pressure column, (2) adjusting the feed rates to the distillation columns such that the duty of the condenser of the high-pressure column is balanced with the duty of the reboiler of the low-pressure column, and (3) using cold products from the process to precool and liquify the incoming feed air in multi-stream heat exchangers before feeding the distillation columns. The heat-integrated air separation process requires up to 40% less energy than the non-heat-integrated process [
6,
14]. The cryogenic air separation process can also be heat-integrated with other processes. Examples of such externally coupled processes include integrated gasification combined cycle (IGCC) [
1], oxy-fuel combustion [
15], and liquified natural gas (LNG) regasification [
16].
Castle [
6] reviewed recent advancements in air separation and liquefaction processes and identified capital cost, technology selection and upgradation, and process integration as important factors for future design. Smith and Klosek [
8] reviewed various air-separation processes based on startup time, economic range, % purity limit (that is, the maximum purity which can be economically produced using the specific technology), and the ability to simultaneously produce byproducts and concluded that the cryogenic process is generally the most suitable choice for large-scale applications. They have also reported various schemes for heat integration within ASU for improving overall efficiency and reducing the operational costs of the system.
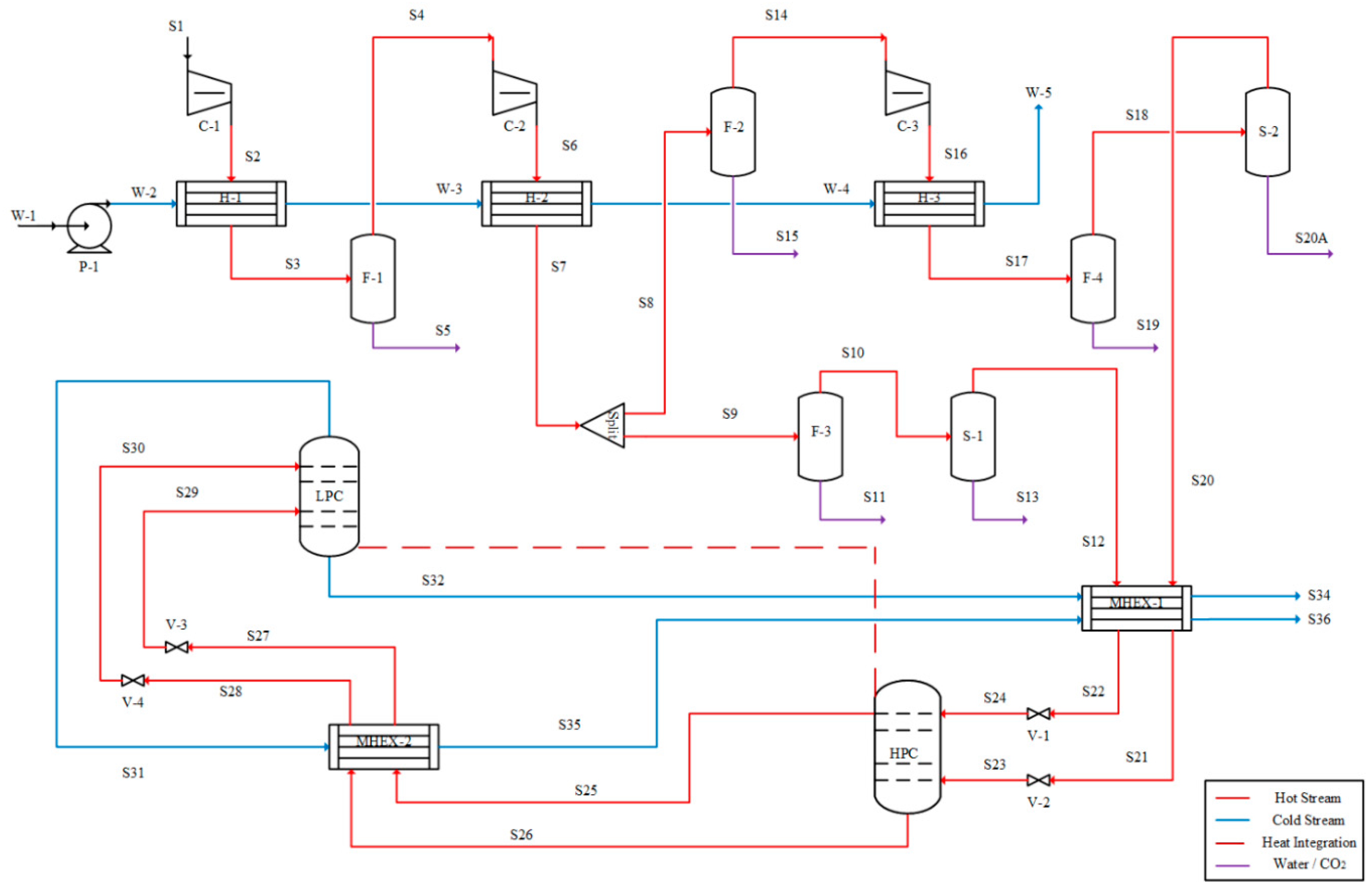
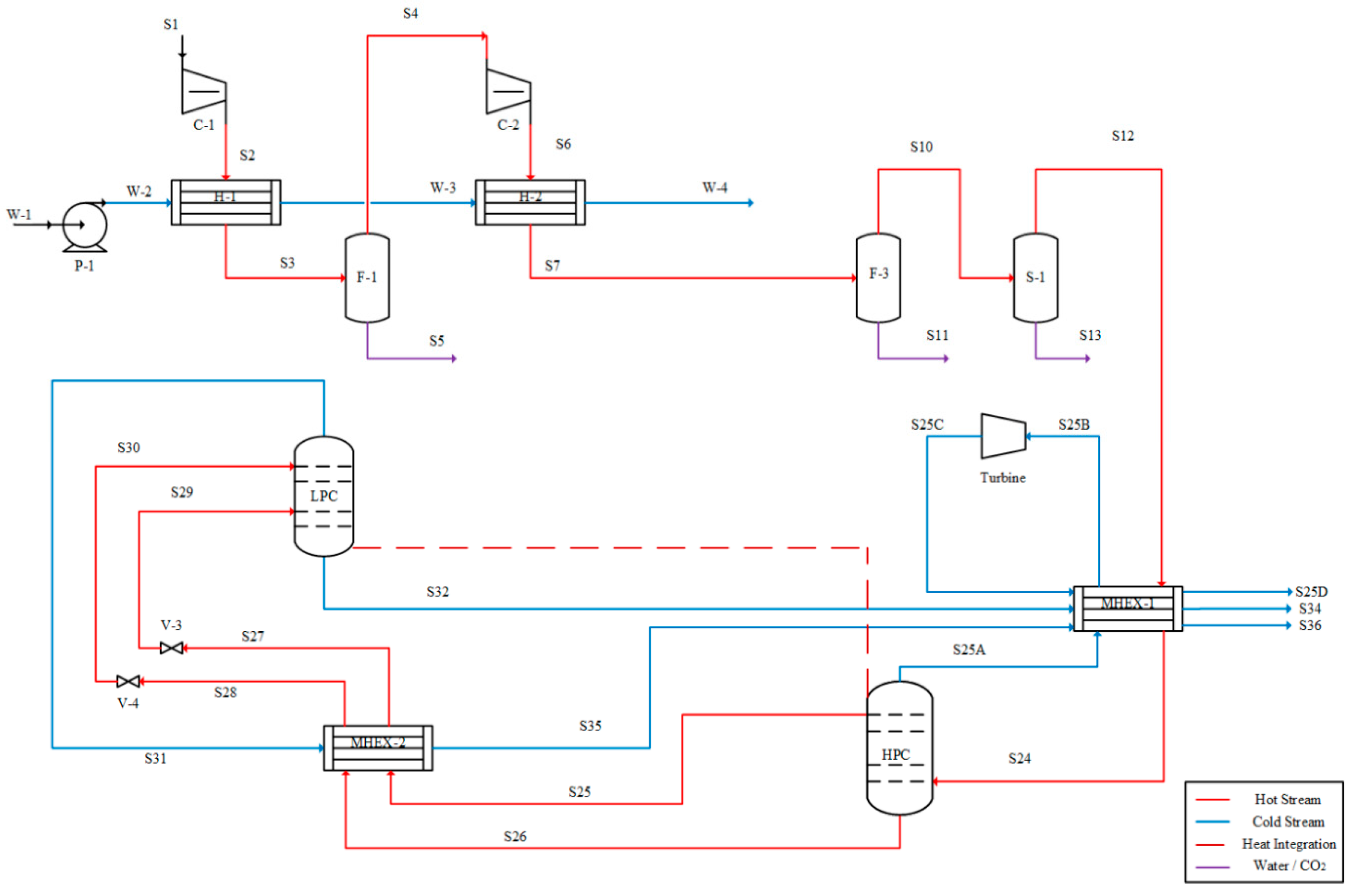
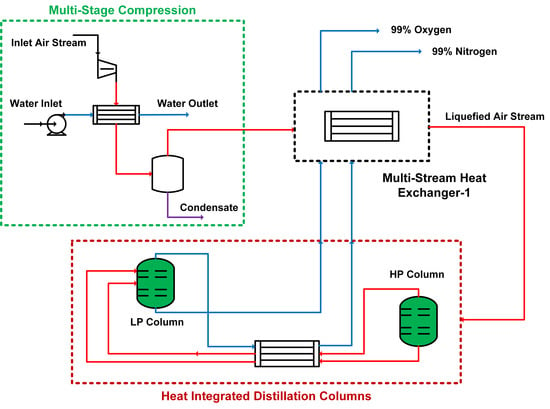
Cryogenic distillation of air is frequently carried out in a two-column process configuration [
17]. The columns operate at different pressures—low-pressure (LP) and high-pressure (HP) columns—and are tightly heat-integrated to minimize the operational cost and to maintain the very low process temperatures. Multi-stage compressors are used to increase the pressure of the feed air, followed by liquefaction using multi-stream heat exchangers (MHEX). The liquefied air is fed to the distillation columns for the separation of nitrogen and oxygen. High-purity oxygen is obtained from the bottom of the LP column, whereas high-purity nitrogen may be obtained either from the top of the LP column or from the top of both the LP and HP columns [
18,
19,
20]. These products are used to liquefy the feed air in the MHEX and then compressed to the next facility for applications.
Because of the complex heat integration, many configurations of the two-column air separation process have been reported in literature. For example, the liquefied air may be directly fed to the HP column [
21], or the liquefied air may be split and both fractions fed to the HP column [
3], or the liquefied air may be split and split-fed to both columns [
22]. These configurations not only have different flowsheet topologies, but they also differ in terms of the factors already described above [
8,
23]. For example, Mehrpooya and Zonouz [
24] demonstrated that installing phase separators before introducing feed into the LP column results in up to 16% decrease is power consumption without compromising the purity of the products. Khalel et al. [
25] compared two alternative designs for the two-column ASU. In one configuration, a turbine was used to reduce the pressure of air, whereas in the alternative configuration, the turbine was replaced by a flash vessel. The reported power consumption and the specific power consumption of the latter configuration were 8% and 20% lower, respectively. Furthermore, the flash-vessel-based design produced 5.5% less waste nitrogen, 5.4% more high-purity nitrogen, and 14.3% more high-purity oxygen.
Exergy is defined as the maximum available work, which can be obtained by bringing a system to equilibrium with its environment [
26]. Exergy analysis based on the second law of thermodynamics is a useful technique for identifying the causes and effects of irreversibility in a process and ultimately leads to improved process design through better utilization of energy resources. In recent years, exergy analysis has been increasingly used to evaluate the performance of various chemical processes and to compare alternative process designs [
27,
28,
29,
30].
Agrawal and Woodward [
31] performed exergy analysis of a two-column ASU and reported that LP column and MHEX contribute significantly toward total exergy destruction. The reported exergy destruction rate was 2.2 kJ/mol(N
2). The exergy destruction rate in the LP column was reduced by 9% by installation of an intermediate heat exchanger in the LP column. As a result, the overall exergy efficiency of the process increased from 36% to 38%. Cornelissen and Hirs [
32,
33] reported an overall exergy efficiency of 28% and an exergy destruction rate of 15.57 kJ/mol(air) for the two-column ASU. They reported that the liquefaction (that is, the MHEX) and the compression sections are largely responsible for exergy destruction in the process. Yong et al. [
34] reported an overall exergy destruction rate of 2.03 kJ/mol(air) for an improved two-column ASU and identified the MHEX and the distillation columns as major sources of exergy destruction in the process. Van der Ham and Kjelstrup [
1] reported an overall exergy destruction rate of 4.76 kJ/mol(air) and an overall exergy efficiency of 35% for the two-column ASU. Almost one-third of the total exergy destruction (1.6–2.0 kJ/mol(air)) occurred in the cold section (that is, the MHEX). The compression and distillation sections were identified as other major sources of exergy destruction in the process. Fu and Gundersen [
21] compared two, two-column ASU configurations based on energy and exergy analysis, and reported that up to 10% less energy was required for the configuration with dual reboilers for the LP column, which permitted operation at lower compression ratio. The compression and distillation sections were identified as the most important sources of exergy destruction. Rizk et al. [
35] showed that up to 23% less exergy destruction occurred in a diabatic two-column ASU compared to an adiabatic two-column ASU. This improvement was attributed to a lower pressure of the HP column and hence lower power requirement in the compression section. Khalel et al. [
36] reported that the two-column ASU configuration with a flash separator had up to 4% higher exergy efficiency and 8% lower energy consumption than the alternative configuration with a turbine. Using exergy analysis, Ebrahimi et al. [
3] reported that the two-column ASU becomes economically feasible for oxygen production capacities greater than 9 kg/s.
Because of the high refrigeration costs involved in the cryogenic process, it is important to identify the most suitable configuration for a given separation target. While many alternative two-column ASU configurations have been reported in literature, a fair comparison between the reported studies is not possible because of the use of different basis (that is, feed and product flowrates), different operating conditions (that is, pressures of the LP and HP columns), and different separation targets (that is, purities of the oxygen and nitrogen products).
Through a closer look at the two-column ASU configurations reported in literature, we were able to identify seven unique designs. The main objective of this work is to identify the most suitable two-column ASU configuration by evaluating these seven alternatives based on exergy analysis. To allow a fair comparison, the feed conditions, product purities, and operational conditions are kept same in all designs. The process flowsheets are developed and simulated using Aspen Plus® V10. The steady-state simulation results are used to calculate the exergy efficiency (%) of individual components as well as its contribution toward total exergy destruction rate.
3. Results and Discussion
For analysis of exergy efficiency and exergy destruction, the process is divided into the following sections:
Compression: compressors, inter-stage coolers, water pump, flash separators, and adsorber for water and carbon dioxide removal
MHEX: multi-stream heat exchanger
LP column: low-pressure column
HP column: high-pressure column
Others: turbines, flash separators (excluding those in compression section), valves, and oxygen pump (when applicable)
Steady-state simulation results have been used to calculate the exergy flows and exergy destruction rates for each configuration. Exergy efficiencies of individual equipment in configurations C1–C7 are presented in
Table 10. A summary of section-wise exergy efficiency of the system is presented in
Figure 8. The analysis shows that MHEXs have relatively higher exergy efficiencies, because they operate with minimal temperature difference driving force between cold and hot streams. Similarly, the HP column in all configurations has higher exergy efficiency than the respective LP column. This is because the HP column provides only crude separation whereas the LP column is responsible for final separation causing extensive exergy destruction. In all configurations, the compression section is the least exergy efficient. This is because compression followed by inter-stage cooling with finite temperature difference driving force causes significant irreversibility and hence higher exergy destruction rate.
Some anomalies are also observed in
Table 10, especially for configuration C7. Here, the exergy efficiencies of compressors (C-1 and C-2) are relatively low because the feed air being compressed is at cryogenic temperature. Similarly, the exergy efficiency of heat exchanger H-2 is relatively low because of the large temperature change required for the air being cooled. Moreover, the exergy efficiency of MHEX-1 is relatively low because it operates with considerably larger temperature difference driving forces than all other configurations. Finally, the relatively high exergy efficiency of the turbine/expander in configuration C5 can be attributed to the absolute temperatures of the inlet and outlet streams being higher than encountered in other configurations.
Figure 9 shows a summary of the percent contribution of each section to the overall exergy destruction in each configuration. In all configurations, the compression section is responsible for most exergy destruction, ranging from 30.8% in configuration C7 to 45.5% in configuration C1. In configurations C1–C5 and C7, the LP column is the second largest source of exergy destruction and is responsible for 29.0% (C7) to 38.7% (C1) of the total exergy destruction in the process. Only in configuration C6, MHEX has a higher exergy destruction rate (22.6%) than the LP column (17.0%). In configurations C2–C5 and C7, the MHEX is the third largest source of exergy destruction and is responsible for 11.7% (C2) to 24.3% (C7) of the total exergy destruction in the process. Only in configuration C1, the HP column has a higher exergy destruction rate (7.1%) than the MHEX (6.8%). In configurations C2–C5, the HP column ranks 4th in terms of exergy destruction rate and is responsible for 7.5% (C3) to 8.3% (C2) of the total exergy destruction in the process. In configurations C1–C5, the contribution of the “Others” section to the total exergy destruction is 3% or less. Only in configurations C6 and C7, these equipment cause more exergy destruction (ca. 9.6–9.8%) than the HP column and rank 4th.
A deeper look into the exergy destruction rates of individual equipment reveals important information for future process improvement. For example, in configuration C1, the total exergy destruction in the compression section is 14.82 MW (45.49% of the total exergy destruction in the process). The three compressors and the corresponding inter- and aftercoolers are collectively responsible for exergy destruction of 7.48 MW and 7.05 MW, respectively. Since none of these equipment causes any change in chemical composition, this exergy destruction is primarily because of the physical exergy component, and its origin can be traced back to the practical limitations of equipment efficiency and driving forces. For example, if the compressor efficiency were 90%, instead of the presently assumed 72%, the combined exergy destruction rate in compressors would be reduced to 2.52 MW (66.3% reduction). Similarly, if the inter- and aftercoolers could be designed and operated with a minimum approach temperature of 5 °C, instead of the presently assumed 10 °C, the combined exergy destruction rate in these coolers would be reduced to 3.34 MW (52.6% reduction). The same principle can also be extended to the MHEX. For example, in configuration C7, the MHEX is moved before the compression section. As a result, heat exchange in this exchanger takes place under large temperature difference driving force, leading to larger exergy destruction than other configurations.
Based on the total exergy destruction rate (MW), the configuration C1 has been identified as the most efficient process design. The overall ranking of all configurations is as follows:
We reiterate that the equipment and the process configurations that operate with smaller driving forces are more exergy efficient. However, this attribute is generally in conflict with the economic considerations, e.g., the need to reduce the number and the size of the equipment. A prudent approach to process design and optimization should aim at striking a balance between these two conflicting objectives.