Medical Peat Waste Upcycling to Carbonized Solid Fuel in the Torrefaction Process
Abstract
:1. Introduction
1.1. Peat Applications
1.2. Peat Thermal-Upgrading Methods
1.3. Aim of the Study
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Torrefaction Process—CSF Production
2.2.2. Proximate Analysis and High Heating Value Determination
2.2.3. Statistical Analyses
2.2.4. Thermal Decomposition Analysis
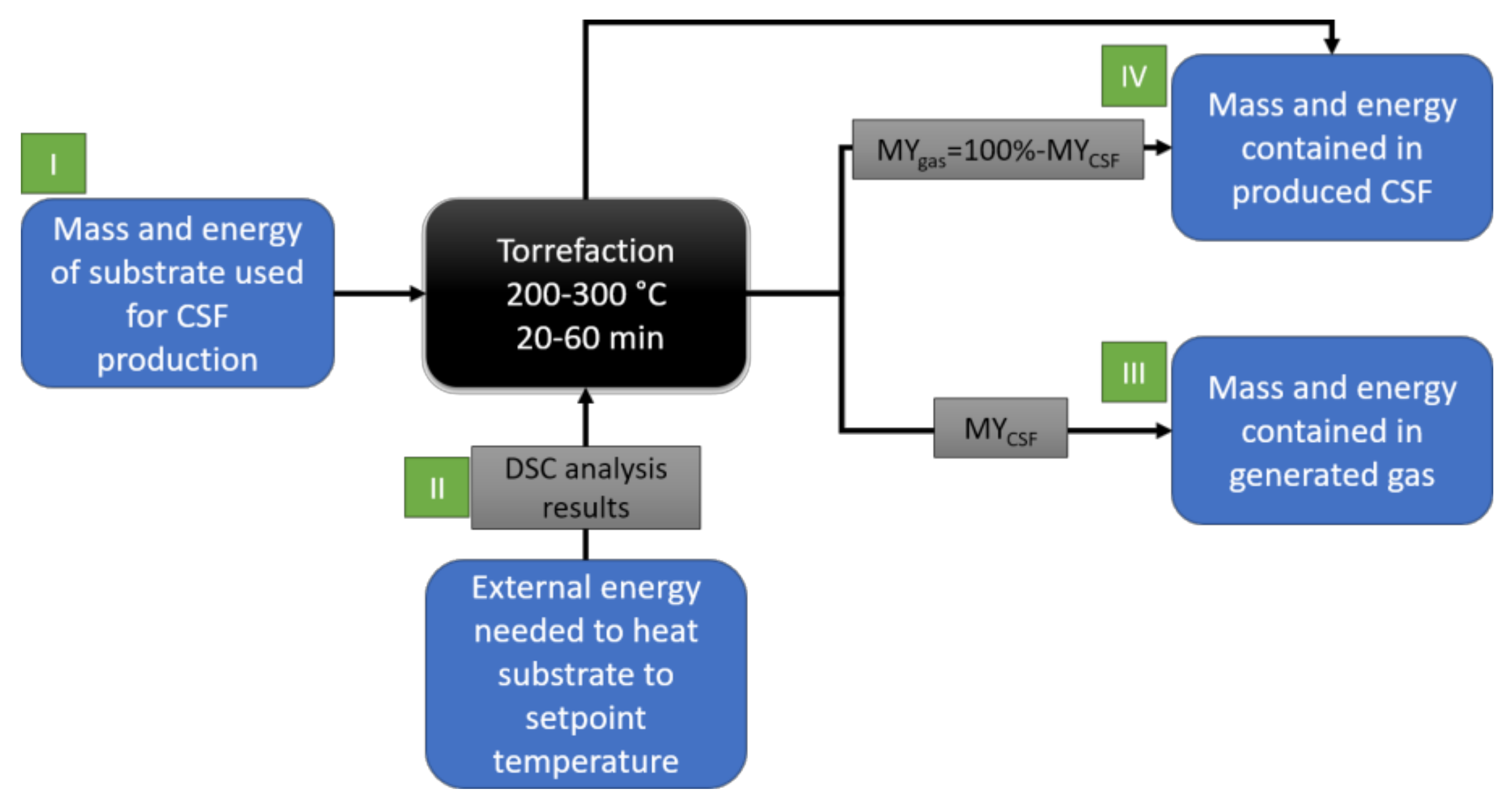
2.2.5. Theoretical Mass and Energy Balance of the Torrefaction Process
- (i)
- Processed material is completely dry, MC = 0%;
- (ii)
- Only external energy is used to provide heat for the process;
- (iii)
- The process has 100% efficiency, no heat losses;
- (iv)
- All external energy after the process goes to gaseous products.
3. Results and Discussion
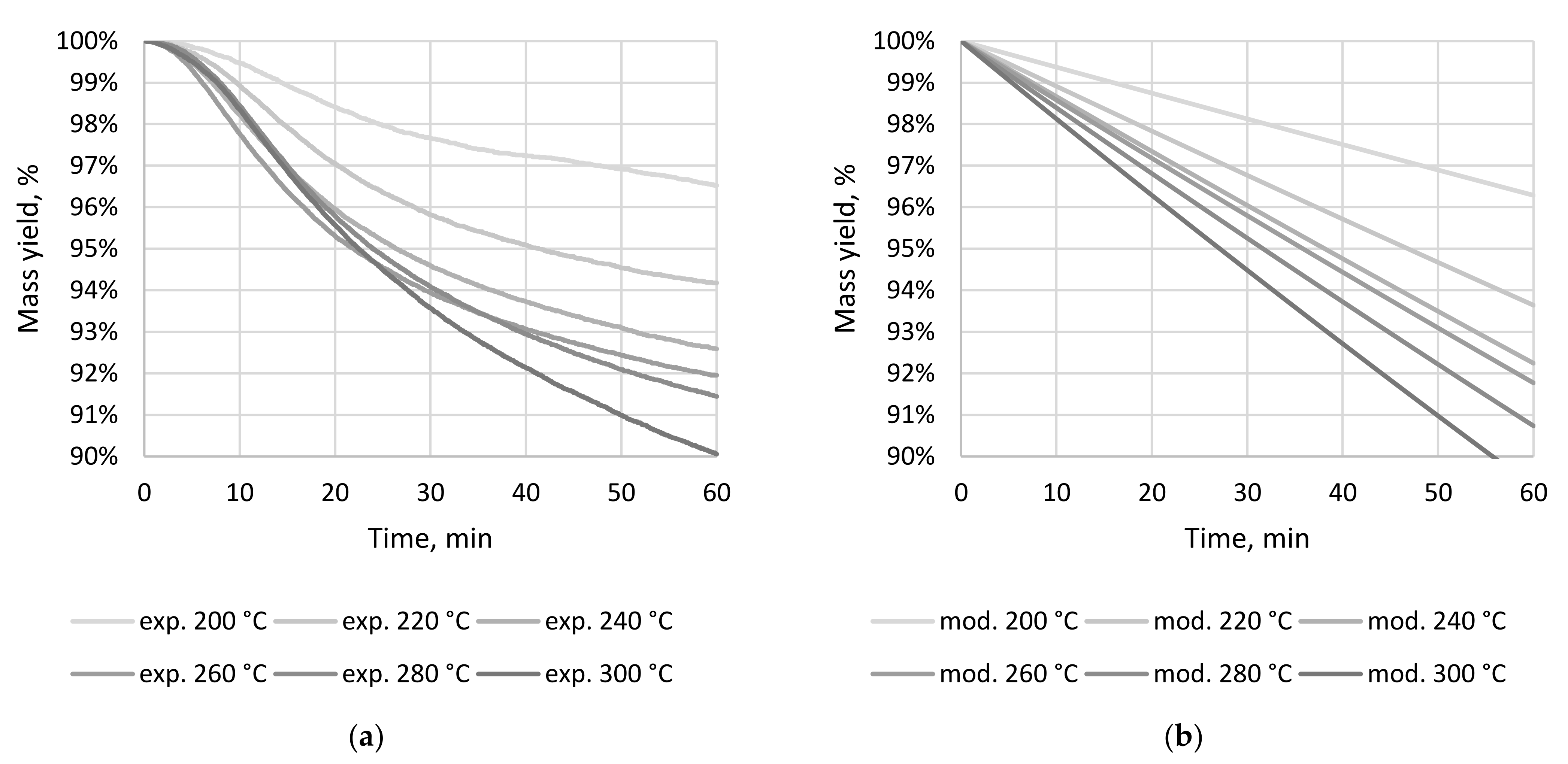
3.1. CSF Production and Proximate Analysis
3.2. Process Kinetics
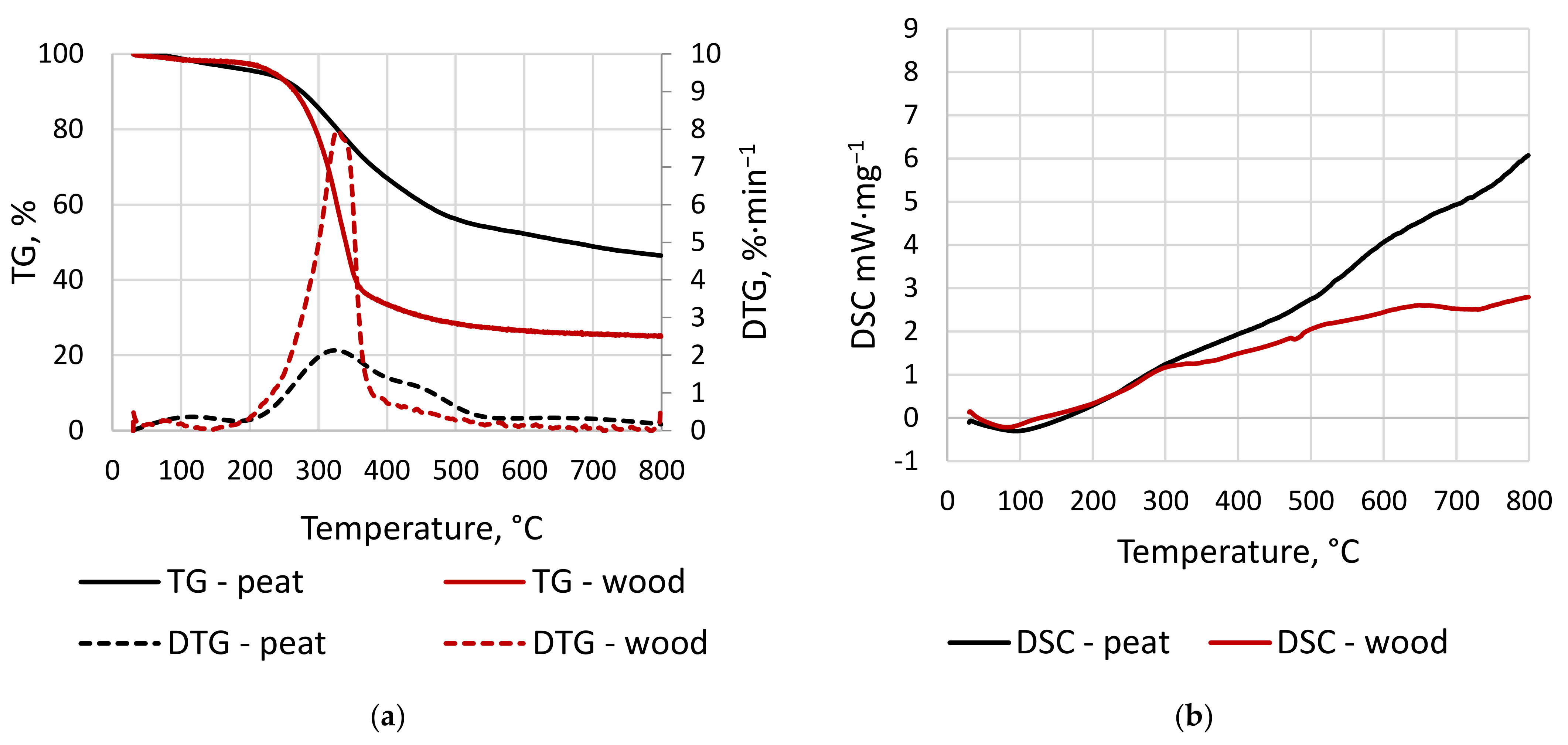
3.2.1. TG/DTG and DSC Results
3.2.2. Peat Thermal Decomposition Kinetics by Coats–Redfern
3.2.3. Peat Torrefaction Kinetics by an Isothermal Method
3.3. Theoretical Mass and Energy Balance
4. Conclusions
- Torrefaction improves fuel properties of peat, HHV increase from 19.0 to 21.3 MJ × kg−1;
- Peat main decomposition takes place at 200–550 °C following second reaction order (n = 2), with an activation energy of 33.34 kJ × mol−1 and pre-exponential factor of 4.40 × 10−1 s−1;
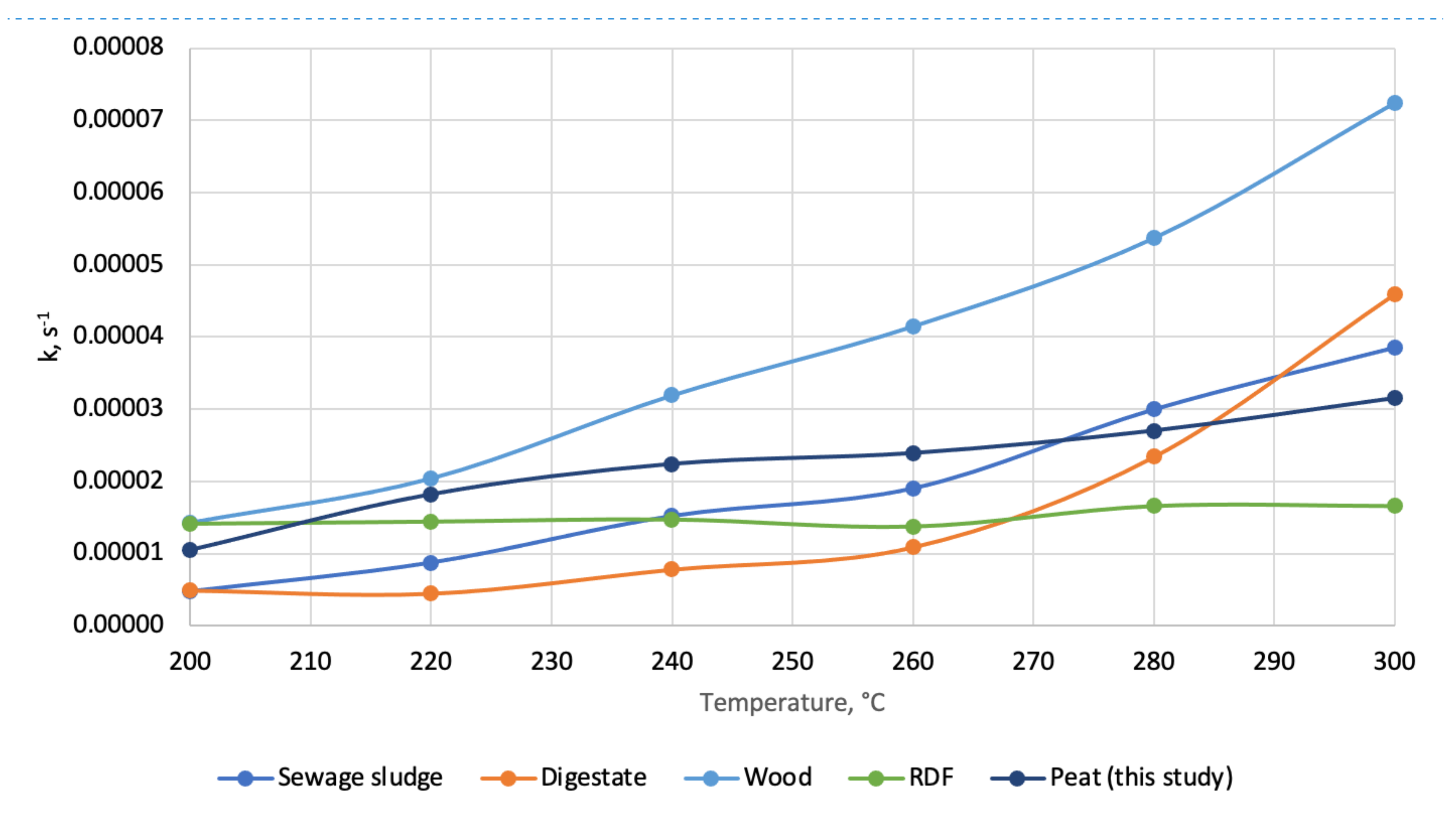
- Macro-TGA shows that peat decomposition at torrefaction temperature is comparable with other waste materials but differs from wood, for which the constant reaction rates (k) are much lower;
- Theoretically, torrefaction of dry peat can be self-sufficient at temperatures higher than 240 °C, but future calculations with specific technology need to be performed to find real conditions of self-sufficient peat processing.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | Pre-exponential factor |
| AC | Ash content |
| AIC | Akaike information criterion |
| ASTM | American Society for Testing and Materials |
| CSF | Carbonized solid fuel |
| CP | Combustible parts |
| CR | Coats–Redfern method |
| DSC | Differential scanning calorimetry |
| Ea | Activation energy |
| EDr | Energy densification ratio |
| EU | The European Union |
| EY | Energy yield |
| FC | Fixed carbon |
| HHV | High heating value |
| HTC | Hydrothermal carbonization |
| MC | Moisture content |
| MY | Mass yield |
| n | Reaction order |
| PW | Peat waste |
| R2 | Determination coefficient |
| SPA facility | A place devoted to a renewal of mind, body, and spirit by different methods |
| TGA | Thermogravimetric analysis |
| VM | Volatile matter |
| VS | Volatile solids |
Appendix A
| Properties | Standard/Method | Equipment (Manufacturer, Model, City, Country) | Ref. |
|---|---|---|---|
| Moisture content (MC) | PN-EN 14346:2011 | Dryer (WAMED, model KBC-65W, Warsaw, Poland) | [36] |
| Volatile matter (VM) | TGA method | TGA—tubular furnace (Czylok, RST 40 × 200/100, Jastrzębie-Zdrój, Poland) coupled with a laboratory balance (RADWAG, PS 750.3Y, Warsaw, Poland) | |
| Ash content (AC) | PN-Z-15008-04:1993 | Muffle furnace (Snol 8.1/1100, Utena, Lithuania) | |
| Fixed carbon (FC) | by difference (calculation) | - | |
| Volatile solids (VS) | PN-EN 15169:2011 | Muffle furnace (Snol 8.1/1100, Utena, Lithuania) | |
| Combustible parts (CP) | PN-Z-15008-04:1993 | Muffle furnace (Snol 8.1/1100, Utena, Lithuania) | |
| High heating value (HHV) | PN-Z-15008-04:1993 | Calorimeter (IKA, C200, Staufen, Germany) |



References
- Özay, P.; Karagülle, M.; Kardeş, S.; Karagülle, M.Z. Chemical and mineralogical characteristics of peloids in Turkey. Environ. Monit. Assess. 2020, 192, 805. [Google Scholar] [CrossRef] [PubMed]
- Leśniak, M. Management of Waste from Cosmetic Industry. Bachelor’s Thesis, Wrocław Univesristy of Envrionmental and Life Sciences, Wroclaw, Poland, 2021. [Google Scholar]
- Dudkiewicz, E. Disposal of the post-treatment mud in Polish spas. Gaz Woda i Technika Sanitarna 2015, 1, 25–28. [Google Scholar] [CrossRef]
- Sutejo, Y.; Saggaff, A.; Rahayu, W. Hanafiah physical and chemical characteristics of fibrous peat. In Proceedings of the 3rd International Conference on Construction and Building Engineering (Iconbuild) 2017: Smart Construction Towards Global Challenges, Palembang, Indonesia, 14–17 August 2017; AIP Conference Proceedings. American Institute of Physics Inc.: College Park, MD, USA, 2017; Volume 1903, p. 090006. [Google Scholar]
- Majcher-Kozieł, M. Borowina-właściwości lecznicze i kosmetologiczne. Chemia w Szkole 2013, 4, 42–44. [Google Scholar]
- Von Post Humification Scale. Available online: https://www.blacklandcentre.org/the-science/von-post-humification-scale/ (accessed on 8 August 2021).
- Drobnik, J.; Stebel, A. Central European ethnomedical and officinal uses of peat, with special emphasis on the Tołpa Peat Preparation (TPP): An historical review. J. Ethnopharmacol. 2020, 246, 112248. [Google Scholar] [CrossRef]
- History of Peat. Available online: https://peatmoss.com/what-is-peat-moss/the-history-of-peat/ (accessed on 11 August 2021).
- Kapetaki, Z.; Moya, J. Recent Trends in EU Coal, Peat and Oil Shale Regions; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Kapetaki, Z.; Alves Dias, P.; Conte, A.; Kanellopoulos, K.; Mandras, G.; Medarac, H.; Nijs, W.; Ruiz, P.; Somers, J.; Tarvydas, D. Recent Trends in EU Coal, Peat and Oil Shale Regions; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Tcvetkov, P.S. The history, present status and future prospects of the Russian fuel peat industry. Mires and Peat 2017, 19, 1–12. [Google Scholar] [CrossRef]
- Krysanova, K.O.; Krylova, A.Y.; Zaichenko, V.M. Application of hydrothermal carbonization to improve the energy properties of peat. Solid Fuel Chem. 2021, 55, 123–128. [Google Scholar] [CrossRef]
- Misnikov, O. Basic technologies and equipment used for peat deposits development in foreign countries. In E3S Web of Conferences, Proceedings of the 3rd International Innovative Mining Symposium, Kemerovo, Russia, 3–5 October 2018; EDP Sciences: Les Ulis, France, 2018; Volume 41. [Google Scholar]
- Dalias, P.; Prasad, M.; Mumme, J.; Kern, J.; Stylianou, M.; Christou, A. Low-cost post-treatments improve the efficacy of hydrochar as peat replacement in growing media. J. Environ. Chem. Eng. 2018, 6, 6647–6652. [Google Scholar] [CrossRef] [Green Version]
- Chrysargyris, A.; Prasad, M.; Kavanagh, A.; Tzortzakis, N. Biochar type and ratio as a peat additive/partial peat replacement in growing media for cabbage seedling production. Agronomy 2019, 9, 693. [Google Scholar] [CrossRef] [Green Version]
- Neumaier, D.; Lohr, D.; Voßeler, R.; Girmann, S.; Kolbinger, S.; Meinken, E. Hydrochars as peat substitute in growing media for organically grown potted herbs. Acta Hortic. 2017, 1168, 377–386. [Google Scholar] [CrossRef]
- Chen, B.; Koziel, J.A.; Lee, M.; O’Brien, S.C.; Li, P.; Brown, R.C. Mitigation of acute hydrogen sulfide and ammonia emissions from swine manure during three-hour agitation using pelletized biochar. Atmosphere 2021, 12, 825. [Google Scholar] [CrossRef]
- Kosov, V.V.; Sinelshchikov, V.A.; Sytchev, G.A.; Zaichenko, V.M. Effect of torrefaction on properties of solid granulated fuel of different biomass types. High Temp. 2014, 52, 907–912. [Google Scholar] [CrossRef]
- Tabakaev, R.; Astafev, A.; Dubinin, Y.; Yazykov, N.; Yakovlev, V. Evaluation of autothermal peat pyrolysis realization for fuel processing technologies. Waste Biomass Valorization 2019, 10, 1021–1027. [Google Scholar] [CrossRef]
- Zaitsev, A.S.; Taburchinov, R.I.; Ozerova, I.P.; Pereira, A.O.; Egorov, R.I. Allothermal gasification of peat and lignite by a focused light flow. Appl. Sci. 2020, 10, 2640. [Google Scholar] [CrossRef]
- Krysanova, K.; Krylova, A.; Zaichenko, V. Properties of biochar obtained by hydrothermal carbonization and torrefaction of peat. Fuel 2019, 256, 115929. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T. Alternative fuels from forestry biomass residue: Torrefaction process of horse chestnuts, oak acorns, and spruce cones. Energies 2020, 13, 2468. [Google Scholar] [CrossRef]
- Chen, W.H.; Wang, C.W.; Ong, H.C.; Show, P.L.; Hsieh, T.H. Torrefaction, pyrolysis and two-stage thermodegradation of hemicellulose, cellulose and lignin. Fuel 2019, 258, 116168. [Google Scholar] [CrossRef]
- Wei, R.; Li, H.; Chen, Y.; Hu, Y.; Long, H.; Li, J.; Xu, C.C. Environmental issues related to bioenergy. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123964885. [Google Scholar]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An overview of effect of process parameters on hydrothermal carbonization of biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Zaitchenko, V.M.; Krysanova, K.O.; Krysanov, O.N.; Sychev, G.A.; Krylova, A.Y. Effect of temperature on the torrefaction of peat. Solid Fuel Chem. 2018, 52, 70–72. [Google Scholar] [CrossRef]
- Nunes, L.J.R. A case study about biomass torrefaction on an industrial scale: Solutions to problems related to self-heating, difficulties in pelletizing, and excessive wear of production equipment. Appl. Sci. 2020, 10, 2546. [Google Scholar] [CrossRef] [Green Version]
- Shoulaifar, T.K. Chemical Changes in Biomass during Torrefaction; Åbo Akademi University: Turku, Finland, 2016. [Google Scholar]
- Rudolfsson, M.; Borén, E.; Pommer, L.; Nordin, A.; Lestander, T.A. Combined effects of torrefaction and pelletization parameters on the quality of pellets produced from torrefied biomass. Appl. Energy 2017, 191, 414–424. [Google Scholar] [CrossRef]
- Wang, L.; Riva, L.; Skreiberg, Ø.; Khalil, R.; Bartocci, P.; Yang, Q.; Yang, H.; Wang, X.; Chen, D.; Rudolfsson, M.; et al. Effect of torrefaction on properties of pellets produced from woody biomass. Energy Fuels 2020, 34, 15343–15354. [Google Scholar] [CrossRef]
- Agar, D.; DeMartini, N.; Hupa, M. Influence of elevated pressure on the torrefaction of wood. Energy Fuels 2016, 30, 2127–2136. [Google Scholar] [CrossRef]
- Ilnicki, P. Torfowiska i Torf. Recenzje 2004, 50, 203–207. [Google Scholar]
- Uzdrowisko Kołobrzeg, S.A. Medical Peat Brochure. Available online: https://spa.uzdrowisko.kolobrzeg.pl/borowina-lecznicza-1-kg-p-7.html (accessed on 26 August 2021).
- Syguła, E.; Świechowski, K.; Hejna, M.; Kunaszyk, I.; Białowiec, A. Municipal solid waste thermal analysis—Pyrolysis kinetics and decomposition reactions. Energies 2021, 14, 4510. [Google Scholar] [CrossRef]
- Świechowski, K.; Stępień, P.; Syguła, E.; Koziel, J.; Białowiec, A. Lab-scale study of temperature and duration effects on carbonized solid fuels properties produced from municipal solid waste components. Materials 2021, 14, 1191. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.; Ferrari, A.; Lita, A.; Crosswhite, M.; Ashley, B.; Stiegman, A.E. Microwave-specific enhancement of the carbon−carbon dioxide (Boudouard) reaction. J. Phys. Chem. 2013, 117, 26871–26880. [Google Scholar] [CrossRef]
- Świechowski, K.; Hnat, M.; Stępień, P.; Stegenta-Dąbrowska, S.; Kugler, S.; Koziel, J.A.; Białowiec, A. Waste to energy: Solid fuel production from biogas plant digestate and sewage sludge by torrefaction-process kinetics, fuel properties, and energy balance. Energies 2020, 13, 3161. [Google Scholar] [CrossRef]
- Noszczyk, T.; Dyjakon, A.; Koziel, J.A. Kinetic parameters of nut shells pyrolysis. Energies 2021, 14, 682. [Google Scholar] [CrossRef]
- Świechowski, K.; Stegenta-Dabrowska, S.; Liszewski, M.; Babelewski, P.; Koziel, J.A.; Białowiec, A. Oxytree pruned biomass torrefaction: Process kinetics. Materials 2019, 12, 3334. [Google Scholar] [CrossRef] [Green Version]
- Badour, C.; Gilbert, A.; Xu, C.; Li, H.; Shao, Y.; Tourigny, G.; Preto, F. Combustion and air emissions from co-firing a wood biomass, a Canadian peat and a Canadian lignite coal in a bubbling fluidised bed combustor. Can. J. Chem. Eng. 2012, 90, 1170–1177. [Google Scholar] [CrossRef]
- Al Tanjil, H.; Akter, S. Characterization of peat for identifying the effectiveness as energy source by proximate analysis. Petronelum Coal 2020, 62, 63–70. [Google Scholar]
- Świechowski, K.; Liszewski, M.; Babelewski, P.; Koziel, J.A.; Białowiec, A. Oxytree pruned biomass torrefaction: Mathematical models of the influence of temperature and residence time on fuel properties improvement. Materials 2019, 12, 2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, L.J.R.; Matias, J.C.O.; Loureiro, L.M.E.F.; Sá, L.C.R.; Silva, H.F.C.; Rodrigues, A.M.; Causer, T.P.; Devallance, D.B.; Ciolkosz, D.E. Evaluation of the potential of agricultural waste recovery: Energy densification as a factor for residual biomass logistics optimization. Appl. Sci. 2021, 11, 20. [Google Scholar] [CrossRef]
- Pulka, J.; Wiśniewski, D.; Gołaszewski, J.; Białowiec, A. Is the biochar produced from sewage sludge a good quality solid fuel? Arch. Environ. Prot. 2016, 42, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Ho, S.-H.; Chen, W.-H.; Xie, Y.; Liu, Z.; Chang, J.-S. Torrefaction performance and energy usage of biomass wastes and their correlations with torrefaction severity index. Appl. Energy 2018, 220, 598–604. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.; Meng, A.; Chen, S.; Li, Q.; Zhang, Y. A novel method for kinetics analysis of pyrolysis of hemicellulose, cellulose, and lignin in TGA and macro-TGA. RSC Adv. 2015, 5, 26509–26516. [Google Scholar] [CrossRef]
- Bhavsar, P.A.; Jagadale, M.H.; Khandetod, Y.P.; Mohod, A.G. Proximate analysis of selected non woody biomass. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2846–2849. [Google Scholar] [CrossRef]
- Słupska, M.; Dyjakon, A.; Stopa, R. Determination of strength properties of energy plants on the example of Miscanthus-Giganteus, Rosa Multiflora and Salix Viminalis. Energies 2019, 12, 3660. [Google Scholar] [CrossRef] [Green Version]
- Telmo, C.; Lousada, J.; Moreira, N. Proximate analysis, backwards stepwise regression between gross calorific value, ultimate and chemical analysis of wood. Bioresour. Technol. 2010, 101, 3808–3815. [Google Scholar] [CrossRef]
- Donahue, C.J.; Rais, E.A. Proximate analysis of coal. J. Chem. Educ. 2009, 86, 222. [Google Scholar] [CrossRef]
- Pettersen, R.C. The chemical composition of wood. In The Chemistry of Solid Wood; American Chemical Society: Washington, DC, USA, 1984; pp. 57–126. [Google Scholar]
- Spedding, P.J. Peat. Fuel 1988, 67, 883–900. [Google Scholar] [CrossRef]
- Iglovikov, A.; Motorin, A. Composition of organic matter in peat soils of the northern trans-Urals depending on groundwater level. In E3S Web of Conferences, Proceedings of the Innovative Technologies in Environmental Science and Education (ITESE-2019), Divnomorskoe Village, Russia, 9–14 September 2019; EDP Sciences: Les Ulis, France, 2019; Volume 135, p. 01004. [Google Scholar] [CrossRef]
- Brown, A.; Mathur, S.P.; Kauri, T.I.; Kushner, D.J. Measurement and significance of cellulose in peat soils. Can. J. Soil Science 1988, 68, 681–685. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, T.; Wu, J.; Wu, J. A novel gaussian-DAEM-reaction model for the pyrolysis of cellulose, hemicellulose and lignin. RSC Adv. 2014, 4, 17513. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Murugan, P.; Mani, T.; Mahinpey, N.; Dong, M. Pyrolysis kinetics of athabasca bitumen using a TGA under the influence of reservoir sand. Can. J. Chem. Eng. 2012, 90, 315–319. [Google Scholar] [CrossRef]
- Santos, A.; Bertoli, A.; Borges, A.C.; Gomes, R.; Garcia, J.; Trevisan, M. New organomineral complex from humic substances extracted from poultry wastes: Synthesis, characterization and controlled release study. J. Braz. Chem. Soc. 2017, 29, 140–150. [Google Scholar] [CrossRef]
- Łyczko, J.; Masztalerz, K.; Lipan, L.; Iwiński, H.; Lech, K.; Carbonell-Barrachina, Á.A.; Szumny, A. Coriandrum Sativum L.—Effect of multiple drying techniques on volatile and sensory profile. Foods 2021, 10, 403. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, W.; Liu, N. Thermal analysis and decomposition kinetics of Chinese forest peat under nitrogen and air atmospheres. Energy Fuels 2011, 25, 797–803. [Google Scholar] [CrossRef]
- Wen, Y.; Wang, S.; Mu, W.; Yang, W.; Jönsson, P.G. Pyrolysis performance of peat moss: A simultaneous in-situ thermal analysis and bench-scale experimental study. Fuel 2020, 277, 118173. [Google Scholar] [CrossRef]
- Fraga, L.G.; Silva, J.; Teixeira, S.; Soares, D.; Ferreira, M.; Teixeira, J. Thermal conversion of pine wood and kinetic analysis under oxidative and non-oxidative environments at low heating rate. Proceedings 2020, 58, 23. [Google Scholar] [CrossRef]
- Grishin, A.M.; Golovanov, A.N.; Sukov, Y.V. Experimental determination of thermophysical, thermokinetic, and filtration characteristics of peat. J. Eng. Phys. Thermophys. 2006, 79, 557–562. [Google Scholar] [CrossRef]
- Anusha, S.; Samarasekara, L.; Coorey, R.V. Thermal capacity as a function of moisture content of Sri Lankan wood species: Wheatstone bridge method. Proc. Tech. Sess. 2011, 27, 9–16. [Google Scholar]
- Nopens, M.; Sazama, U.; Krause, A.; Fröba, M. Specific heat capacity of wood between −140 and 50 °C in dry and wet state. Holzforschung 2021, 75, 779–785. [Google Scholar] [CrossRef]
| Temp. | Time | MY, % | EDr, - | EY, % | VM, % | AC, % | FC, % | VS, % | CP, % | HHV, J × g−1 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | - | - | - | 59.3 | 14.4 | 26.3 | 83.0 | 85.6 | 19,013 |
| 200 | 20 | 97.6 | 1.03 | 100.6 | 56.0 | 14.1 | 29.9 | 83.1 | 85.9 | 19,598 |
| 40 | 97.3 | 1.05 | 102.1 | 55.8 | 14.2 | 30.0 | 82.9 | 85.8 | 19,941 | |
| 60 | 97.1 | 1.04 | 101.4 | 57.2 | 14.6 | 28.2 | 82.7 | 85.4 | 19,841 | |
| 220 | 20 | 98.4 | 1.03 | 101.5 | 56.8 | 14.1 | 29.0 | 83.2 | 85.9 | 19,619 |
| 40 | 95.4 | 1.07 | 101.7 | 56.3 | 14.3 | 29.4 | 82.8 | 85.7 | 20,272 | |
| 60 | 91.2 | 1.06 | 97.0 | 55.0 | 15.2 | 29.7 | 81.9 | 84.8 | 20,217 | |
| 240 | 20 | 96.7 | 1.02 | 98.9 | 55.3 | 14.2 | 30.5 | 83.0 | 85.8 | 19,444 |
| 40 | 90.7 | 1.07 | 97.0 | 54.0 | 14.8 | 31.2 | 82.2 | 85.2 | 20,341 | |
| 60 | 87.7 | 1.07 | 93.7 | 53.0 | 15.1 | 31.9 | 81.9 | 84.9 | 20,318 | |
| 260 | 20 | 93.1 | 1.05 | 97.5 | 54.3 | 14.7 | 31.0 | 82.4 | 85.3 | 19,922 |
| 40 | 83.4 | 1.10 | 91.3 | 49.3 | 16.9 | 33.8 | 80.0 | 83.1 | 20,836 | |
| 60 | 83.4 | 1.13 | 93.9 | 49.1 | 17.0 | 33.9 | 79.8 | 83.0 | 21,419 | |
| 280 | 20 | 81.1 | 1.08 | 87.4 | 47.5 | 16.8 | 35.7 | 79.9 | 83.2 | 20,495 |
| 40 | 79.9 | 1.12 | 89.6 | 44.6 | 17.7 | 37.7 | 78.8 | 82.3 | 21,324 | |
| 60 | 77.6 | 1.11 | 86.2 | 45.6 | 17.6 | 36.8 | 79.0 | 82.4 | 21,124 | |
| 300 | 20 | 81.8 | 1.11 | 91.0 | 44.7 | 17.3 | 38.0 | 79.4 | 82.7 | 21,163 |
| 40 | 73.4 | 1.12 | 82.2 | 40.3 | 18.5 | 41.2 | 77.7 | 81.5 | 21,315 | |
| 60 | 69.1 | 1.10 | 75.9 | 38.1 | 20.3 | 41.6 | 75.8 | 79.7 | 20,873 |
| Material, - | Note, - | Temperature Range, °C | n, - | Ea, kJ × mol−1 | A, s−1 | R2, - |
|---|---|---|---|---|---|---|
| Peat | Whole process | 30–800 | 1.33 | 21.10 | 1.97 × 10−2 | 0.97 |
| Peat | First peak | 30–200 | 3.00 | 27.11 | 1.92 × 10−1 | 0.89 |
| Peat | Main decomposition peak | 200–550 | 2.00 | 33.34 | 4.40 × 10−1 | 0.99 |
| Wood | Whole process | 30–800 | 1.54 | 27.91 | 2.08 × 10−1 | 0.91 |
| Wood | First peak | 30–200 | 3.00 | 6.67 | 6.86 × 10−5 | 0.99 |
| Wood | Main decomposition peak | 215–430 | 1.68 | 65.47 | 9.05 × 102 | 0.98 |
| Temperature, °C | k, s−1 | Ea, kJ × mol−1 | A, s−1 | R2, - | Reference | |
|---|---|---|---|---|---|---|
| Peat | 200 | 1.05 × 10−5 | 22.02 | 3.41 × 10−3 | 0.894 | This study |
| 220 | 1.82 × 10−5 | |||||
| 240 | 2.24 × 10−5 | |||||
| 260 | 2.39 × 10−5 | |||||
| 280 | 2.70 × 10−5 | |||||
| 300 | 3.15 × 10−5 | |||||
| Wood | 200 | 1.43 × 10−5 | 36.44 | 1.53 × 10−1 | 0.996 | [41] |
| 220 | 2.04 × 10−5 | |||||
| 240 | 3.19 × 10−5 | |||||
| 260 | 4.15 × 10−5 | |||||
| 280 | 5.37 × 10−5 | |||||
| 300 | 7.25 × 10−5 |
| Temp. °C | Time, min | Mass of Substrate Used to Produce 1 g of CSF, g | Energy Contained in the Raw Material Used to Produce 1 g of CSF, J | External Energy Needed to Produce 1 g of CSF, J * | Energy Contained in 1 g of CSF, J ** | Mass Of Gas Generated during the Production of 1 g of CSF, g | Energy Contained in Gas after Production of 1 g of CSF, J *** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peat | Wood | Peat | Wood | Peat | Wood | Peat | Wood | Peat | Wood | Peat | Wood | ||
| 200 | 20 | 1.025 | 1.042 | 19,491 | 19,068 | 179 | 136 | 19,598 | 18,551 | 0.025 | 0.042 | 71 | 654 |
| 40 | 1.027 | 1.071 | 19,533 | 19,596 | 179 | 136 | 19,941 | 18,960 | 0.027 | 0.071 | −229 | 773 | |
| 60 | 1.030 | 1.088 | 19,576 | 19,898 | 179 | 136 | 19,841 | 19,178 | 0.030 | 0.088 | −87 | 856 | |
| 220 | 20 | 1.016 | 1.063 | 19,326 | 19,440 | 224 | 185 | 19,619 | 18,753 | 0.016 | 0.063 | −70 | 871 |
| 40 | 1.049 | 1.125 | 19,939 | 20,589 | 224 | 185 | 20,272 | 19,312 | 0.049 | 0.125 | −109 | 1462 | |
| 60 | 1.097 | 1.139 | 20,849 | 20,830 | 224 | 185 | 20,217 | 19,440 | 0.097 | 0.139 | 857 | 1575 | |
| 240 | 20 | 1.034 | 1.144 | 19,666 | 20,931 | 289 | 251 | 19,444 | 19,498 | 0.034 | 0.144 | 511 | 1684 |
| 40 | 1.103 | 1.256 | 20,965 | 22,981 | 289 | 251 | 20,341 | 20,077 | 0.103 | 0.256 | 913 | 3155 | |
| 60 | 1.141 | 1.290 | 21,685 | 23,604 | 289 | 251 | 20,318 | 20,371 | 0.141 | 0.290 | 1657 | 3484 | |
| 260 | 20 | 1.074 | 1.133 | 20,425 | 20,720 | 379 | 336 | 19,922 | 19,456 | 0.074 | 0.133 | 883 | 1600 |
| 40 | 1.199 | 1.391 | 22,804 | 25,442 | 379 | 336 | 20,836 | 20,767 | 0.199 | 0.391 | 2347 | 5011 | |
| 60 | 1.200 | 1.554 | 22,809 | 28,428 | 379 | 336 | 21,419 | 21,136 | 0.200 | 0.554 | 1770 | 7627 | |
| 280 | 20 | 1.233 | 1.308 | 23,447 | 23,932 | 495 | 446 | 20,495 | 20,467 | 0.233 | 0.308 | 3447 | 3911 |
| 40 | 1.252 | 1.531 | 23,809 | 28,012 | 495 | 446 | 21,324 | 21,432 | 0.252 | 0.531 | 2980 | 7026 | |
| 60 | 1.288 | 1.759 | 24,497 | 32,177 | 495 | 446 | 21,124 | 21,646 | 0.288 | 0.759 | 3868 | 10,976 | |
| 300 | 20 | 1.223 | 1.621 | 23,248 | 29,653 | 633 | 578 | 21,163 | 21,457 | 0.223 | 0.621 | 2718 | 8773 |
| 40 | 1.363 | 1.829 | 25,918 | 33,454 | 633 | 578 | 21,315 | 22,034 | 0.363 | 0.829 | 5236 | 11,998 | |
| 60 | 1.446 | 1.831 | 27,498 | 33,493 | 633 | 578 | 20,873 | 22,002 | 0.446 | 0.831 | 7257 | 12,069 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świechowski, K.; Leśniak, M.; Białowiec, A. Medical Peat Waste Upcycling to Carbonized Solid Fuel in the Torrefaction Process. Energies 2021, 14, 6053. https://doi.org/10.3390/en14196053
Świechowski K, Leśniak M, Białowiec A. Medical Peat Waste Upcycling to Carbonized Solid Fuel in the Torrefaction Process. Energies. 2021; 14(19):6053. https://doi.org/10.3390/en14196053
Chicago/Turabian StyleŚwiechowski, Kacper, Małgorzata Leśniak, and Andrzej Białowiec. 2021. "Medical Peat Waste Upcycling to Carbonized Solid Fuel in the Torrefaction Process" Energies 14, no. 19: 6053. https://doi.org/10.3390/en14196053
APA StyleŚwiechowski, K., Leśniak, M., & Białowiec, A. (2021). Medical Peat Waste Upcycling to Carbonized Solid Fuel in the Torrefaction Process. Energies, 14(19), 6053. https://doi.org/10.3390/en14196053







