Ultrasonic Delignification and Microstructural Characterization of Switchgrass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Sample Collection and Preparation
2.2. Measurement of Physical Properties of the Native Switchgrass
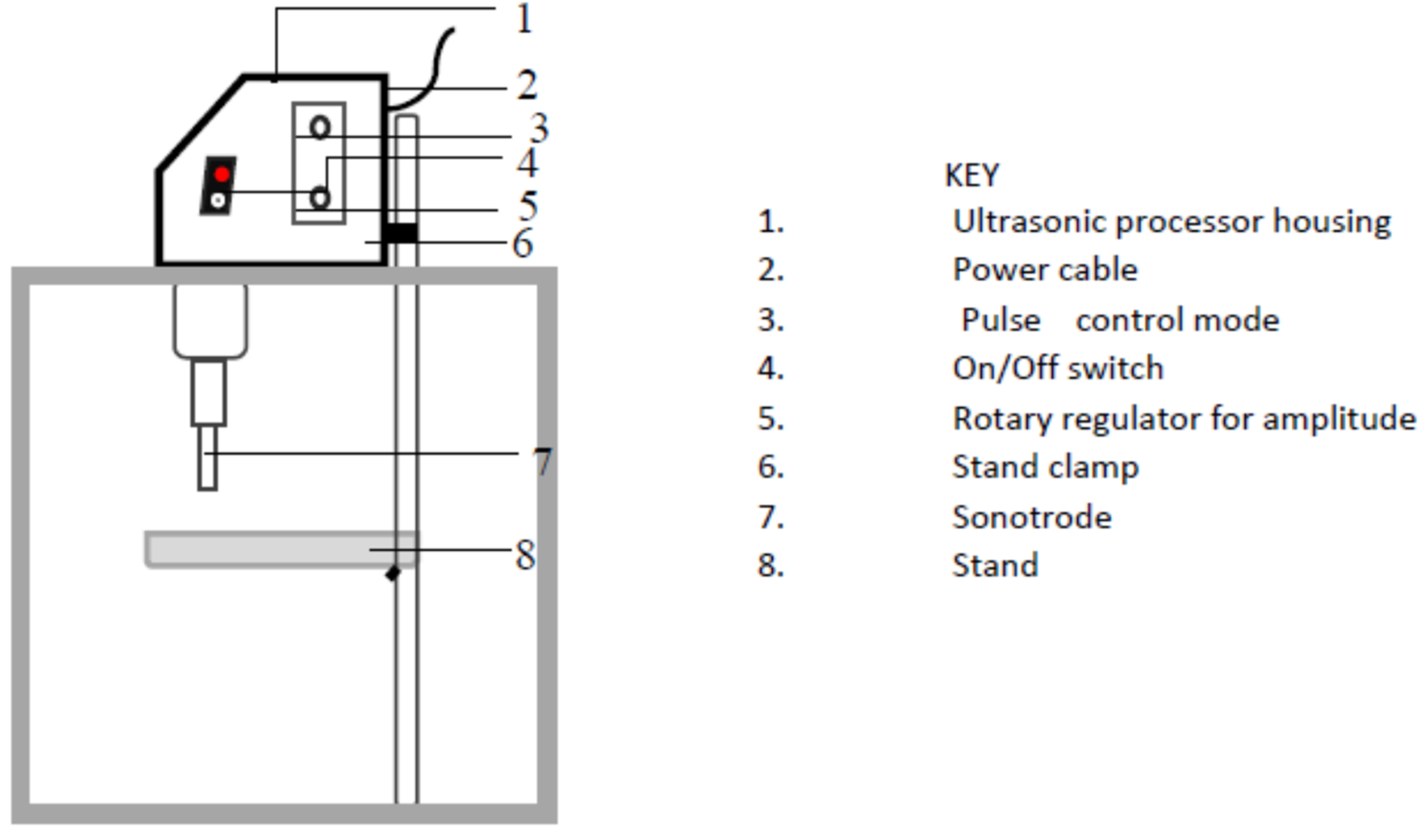
2.3. Ultrasonic Pretreatment
2.4. Design of Experiment
2.5. Characterization
2.5.1. Lignin and Ash Composition Analysis
2.5.2. Scanning Electron Microscopy (SEM)
2.5.3. Transmission Electron Microscopy (TEM)
2.5.4. X-ray Diffraction (XRD) Measurement
3. Results and Discussion
3.1. Physical Properties of the Native Switchgrass Grinds
3.2. Lignin Content and Delignification
3.3. Effect of Independent Variables on Delignification
3.3.1. Effects of Sonication Time and Solid-Solvent Ratio (SSR)
3.3.2. Effects of Acoustic Power, Hammer Mill Screen Size (HMSS) and their Interaction
3.4. Scanning Electron Microscopy
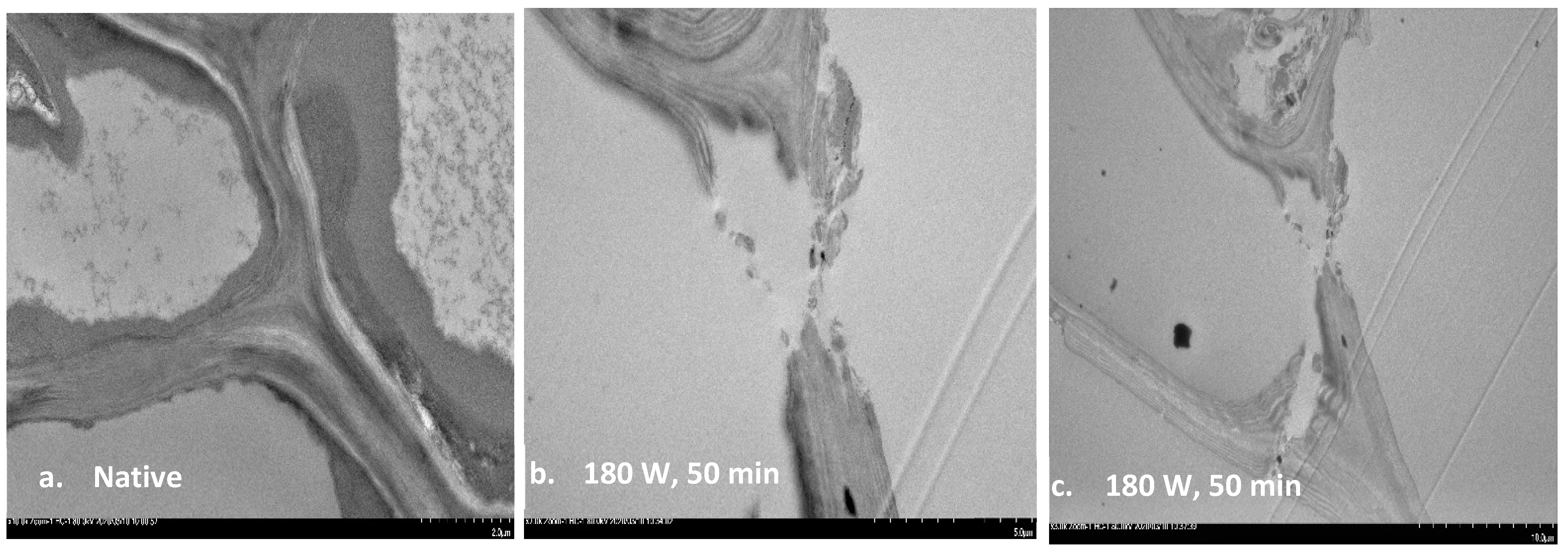
3.5. Transmission Electron Microscopy (TEM)
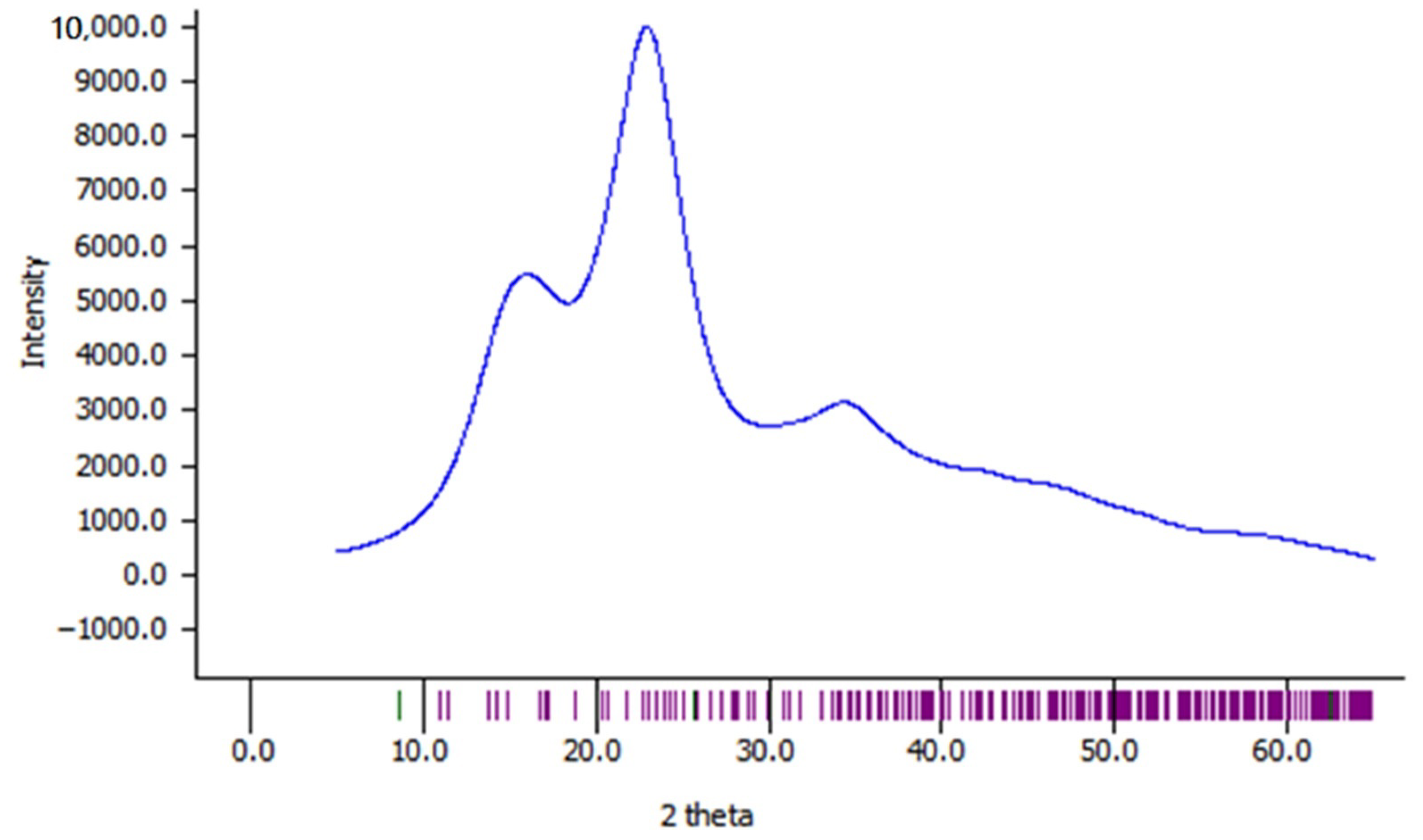
3.6. XRD Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wright, L.L. Historical Perspective on How and Why Switchgrass Was Selected as a “Model” High-Potential Energy Crop; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2007. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, A.; Knoll, J.E. Conventional and Molecular Breeding for Improvement of Biofuel Crops: Past, Present, and Future. In Handbook of Bioenergy Crop Plants; Kole, C., Josh, C.P., Shonnard, D.R., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 3–21. [Google Scholar]
- Casler, M.D. Switchgrass Breeding, Genetics, and Genomics. In Switchgrass: A Valuable Biomass Crop for Energy; Monti, A., Ed.; Springer: London, UK, 2012; pp. 29–54. [Google Scholar] [CrossRef]
- McLaughlin, S.B.; Bouton, J.; Bransby, D.; Conger, B.V.; Ocumpaugh, W.R.; Parrish, D.J.; Taliaferro, C.; Vogel, K.P.; Wullschleger, S.D. Developing Switchgrass as a Bioenergy Crop. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, Egypt, 1999; pp. 282–299. [Google Scholar]
- Vogel, K.P.; Jung, H.-J.G. Genetic Modification of Herbaceous Plants for Feed and Fuel. Crit. Rev. Plant Sci. 2001, 20, 15–49. [Google Scholar] [CrossRef]
- Dien, B.S.; Jung, H.-J.G.; Vogel, K.P.; Casler, M.D.; Lamb, J.F.; Iten, L.; Mitchell, R.B.; Sarath, G. Chemical composition and response to dilute-acid pretreatment and enzymatic saccharification of alfalfa, reed canarygrass, and switchgrass. Biomass Bioenergy 2006, 30, 880–891. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Hu, Z.; Pu, Y.; Brummer, E.C.; Ragauskas, A. Chemical compositions of four switchgrass populations. Biomass Bioenergy 2010, 34, 48–53. [Google Scholar] [CrossRef]
- Arshadi, M.; Grundberg, H. Biochemical production of bioethanol. In Handbook of Biofuels Production: Processes and Technologies; Luque, R., Campelo, J., Clark, J., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2011; pp. 199–220. [Google Scholar] [CrossRef]
- Wi, S.G.; Cho, E.J.; Lee, D.-S.; Lee, S.J.; Lee, Y.J.; Bae, H.-J. Lignocellulose conversion for biofuel: A new pretreatment greatly improves downstream biocatalytic hydrolysis of various lignocellulosic materials. Biotechnol. Biofuels 2015, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Boerjan, W.; Ralph, J.; Baucher, M. Ligninbiosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Kishimoto, T.; Chiba, W.; Saito, K.; Fukushima, K.; Uraki, Y.; Ubukata, M. Influence of Syringyl to Guaiacyl Ratio on the Structure of Natural and Synthetic Lignins. J. Agric. Food Chem. 2010, 58, 895–901. [Google Scholar] [CrossRef]
- Wilkerson, C.G.; Mansfield, S.D.; Lu, F.; Withers, S.; Park, J.-Y.; Karlen, S.D.; Gonzales-Vigil, E.; Padmakshan, D.; Unda, F.; Rencoret, J.; et al. Monolignol Ferulate Transferase Introduces Chemically Labile Linkages into the Lignin Backbone. Science 2014, 344, 90–93. [Google Scholar] [CrossRef] [Green Version]
- Cass, C.L.; Lavell, A.A.; Santoro, N.; Foster, C.E.; Karlen, S.D.; Smith, R.A.; Ralph, J.; Garvin, D.F.; Sedbrook, J.C. Cell Wall Composition and Biomass Recalcitrance Differences Within a Genotypically Diverse Set of Brachypodium distachyon Inbred Lines. Front. Plant Sci. 2016, 7, 708. [Google Scholar] [CrossRef] [Green Version]
- DeMartini, J.D.; Pattathil, S.; Miller, J.S.; Li, H.; Hahn, M.G.; Wyman, C.E. Investigating plant cell wall components that affect biomass recalcitrance in poplar and switchgrass. Energy Environ. Sci. 2013, 6, 898–909. [Google Scholar] [CrossRef]
- Lionetti, V.; Francocci, F.; Ferrari, S.; Volpi, C.; Bellincampi, D.; Galletti, R.; D’Ovidio, R.; De Lorenzo, G.; Cervone, F. Engineering the cell wall by reducing de-methyl-esterified homogalacturonan improves saccharification of plant tissues for bioconversion. Proc. Natl. Acad. Sci. USA 2009, 107, 616–621. [Google Scholar] [CrossRef] [Green Version]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, D.G.J.; Labbé, N.; Sykes, R.W.; Gracom, K.; Kline, L.; Swamidoss, I.M.; Burris, J.N.; Davis, M.; Stewart, C.N. Rapid Assessment of Lignin Content and Structure in Switchgrass (Panicum virgatum L.) Grown Under Different Environmental Conditions. Bioenergy Res. 2009, 2, 246–256. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.; Holtzapple, M.; Ladisch, M.R. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Kardos, N.; Luche, J.-L. Sonochemistry of carbohydrate compounds. Carbohydr. Res. 2001, 332, 115–131. [Google Scholar] [CrossRef]
- Bundhoo, M.Z.; Mudhoo, A.; Mohee, R. Promising Unconventional Pretreatments for Lignocellulosic Biomass. Crit. Rev. Environ. Sci. Technol. 2012, 43, 2140–2211. [Google Scholar] [CrossRef]
- Saini, A.; Aggarwal, N.K.; Sharma, A.; Yadav, A. Prospects for Irradiation in Cellulosic Ethanol Production. Biotechnol. Res. Int. 2015, 2015, 157139. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Ur Rehman, M.S.; Kim, I.; Chisti, Y.; Han, J.I. Use of Ultrasound in the Production of Bioethanol from Lignocellulosic Biomass. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2013, 30, 1391–1410. [Google Scholar] [CrossRef]
- Bussemaker, M.J.; Zhang, D. Effect of Ultrasound on Lignocellulosic Biomass as a Pretreatment for Biorefinery and Biofuel Applications. Ind. Eng. Chem. Res. 2013, 52, 3563–3580. [Google Scholar] [CrossRef]
- Li, M.-F.; Sun, S.-N.; Xu, F.; Sun, R.-C. Ultrasound-enhanced extraction of lignin from bamboo (Neosinocalamus affinis): Characterization of the ethanol-soluble fractions. Ultrason. Sonochem. 2012, 19, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-X.; Sun, R.-C.; Sun, X.-F.; Su, Y. Fractional and physico-chemical characterization of hemicelluloses from ultrasonic irradiated sugarcane bagasse. Carbohydr. Res. 2004, 339, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, R.; Muthukumar, K. Ultrasound-assisted alkaline pretreatment of sugarcane bagasse for fermentable sugar production: Optimization through response surface methodology. Bioresour. Technol. 2012, 112, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Easson, M.W.; Condon, B.; Dien, B.S.; Iten, L.; Slopek, R.; Yoshioka-Tarver, M.; Lambert, A.; Smith, J. The Application of Ultrasound in the Enzymatic Hydrolysis of Switchgrass. Appl. Biochem. Biotechnol. 2011, 165, 1322–1331. [Google Scholar] [CrossRef]
- Song, X.; Zhang, M.; Pei, Z.J. Effects of ultrasonic vibration-assisted pelleting of cellulosic biomass on sugar yield for biofuel manufacturing. Biomass-Convers. Biorefin. 2013, 3, 231–238. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, J.; He, J.; Liu, Z.; Yu, Z. Combinations of mild physical or chemical pretreatment with biological pretreatment for enzymatic hydrolysis of rice hull. Bioresour. Technol. 2009, 100, 903–908. [Google Scholar] [CrossRef]
- ANSI/ASAE S358.3. In Standard Test for Moisture Content Measurement of Forages; American Society of Agricultural Engineers: St. Joseph, MI, USA, 2012.
- ANSI/ASAE S319.4. In Standard Method of Determining and Expressing Fineness of Feed Materials by Sieving; American Society of Agricultural Engineers: St. Joseph, MI, USA, 2017.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. NREL/TP-510-42618 Analytical Procedure—Determination of Structural Carbohydrates and Lignin in Biomass; Revised August 2012; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. NREL/TP-510-42618 Analytical Procedure—Determination of Ash in Biomass; Issue Date: 7/17/2005; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. Opportunity for New Developments in All Phases of Textile Manufacturing. Literature Cited an Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-ray Diffractometer; Sage Publications: Thousand Oaks, CA, USA, 1952; Volume 43. [Google Scholar]
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. Crystal Structure and Hydrogen Bonding System in Cellulose Iαfrom Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef]
- Kim, U.-J.; Eom, S.H.; Wada, M. Thermal decomposition of native cellulose: Influence on crystallite size. Polym. Degrad. Stab. 2010, 95, 778–781. [Google Scholar] [CrossRef]
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Grinding performance and physical properties of wheat and barley straws, corn stover and switchgrass. Biomass Bioenergy 2004, 27, 339–352. [Google Scholar] [CrossRef]
- Karunanithy, C.; Muthukumarappan, K. Optimization of switchgrass and extruder parameters for enzymatic hydrolysis using response surface methodology. Ind. Crop. Prod. 2011, 33, 188–199. [Google Scholar] [CrossRef]
- Yat, S.C.; Berger, A.; Shonnard, D.R. Kinetic characterization for dilute sulfuric acid hydrolysis of timber varieties and switchgrass. Bioresour. Technol. 2008, 99, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wen, Z. Enhancing enzymatic digestibility of switchgrass by microwave-assisted alkali pretreatment. Biochem. Eng. J. 2008, 38, 369–378. [Google Scholar] [CrossRef]
- Hu, Z.-H.; Wang, Y.; Wen, Z. Alkali (NaOH) Pretreatment of Switchgrass by Radio Frequency-based Dielectric Heating. Appl. Biochem. Biotechnol. 2007, 148, 71–81. [Google Scholar] [CrossRef]
- Kim, Y.; Mosier, N.S.; Ladisch, M.R.; Pallapolu, V.R.; Lee, Y.; Garlock, R.; Balan, V.; Dale, B.E.; Donohoe, B.S.; Vinzant, T.B.; et al. Comparative study on enzymatic digestibility of switchgrass varieties and harvests processed by leading pretreatment technologies. Bioresour. Technol. 2011, 102, 11089–11096. [Google Scholar] [CrossRef] [PubMed]
- Bals, B.; Rogers, C.; Jin, M.; Balan, V.; Dale, B.E. Evaluation of ammonia fibre expansion (AFEX) pretreatment for enzymatic hydrolysis of switchgrass harvested in different seasons and locations. Biotechnol. Biofuels 2010, 3, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Jianguo, W.; Chunfai, L.; Feng, G. A Multi-Parameter Optimization Model for the Evaluation of Shale Gas Recovery Enhancement Title. Energies 2018, 11, 654. [Google Scholar] [CrossRef] [Green Version]
- Olughu, O.O.; Tabil, L.G.; Dumonceaux, T. Effect of Ultrasonic Pretreatment on the Chemical Composition and Pellet Quality of Camelina Straw. In Proceedings of the 2019 ASABE Annual International Meeting, Boston, MA, USA, 7–10 July 2019; p. 1. [Google Scholar] [CrossRef]
- García, A.; Alriols, M.G.; Llano-Ponte, R.; Labidi, J. Ultrasound-assisted fractionation of the lignocellulosic material. Bioresour. Technol. 2011, 102, 6326–6330. [Google Scholar] [CrossRef]
- Sun, R.-C.; Tomkinson, J. Comparative study of lignins isolated by alkali and ultrasound-assisted alkali extractions from wheat straw. Ultrason. Sonochem. 2002, 9, 85–93. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Azin, M. Pretreatment of sugarcane bagasse by ultrasound energy and dilute acid. Asia-Pac. J. Chem. Eng. 2011, 7, 274–278. [Google Scholar] [CrossRef]
- Karp, E.M.; Resch, M.G.; Donohoe, B.S.; Ciesielski, P.N.; O’Brien, M.H.; Nill, J.E.; Mittal, A.; Biddy, M.J.; Beckham, G.T. Alkaline Pretreatment of Switchgrass. ACS Sustain. Chem. Eng. 2015, 3, 1479–1491. [Google Scholar] [CrossRef]
- Pérez-Pimienta, J.A.; Lopez-Ortega, M.G.; Varanasi, P.; Stavila, V.; Cheng, G.; Singh, S.; Simmons, B.A. Comparison of the impact of ionic liquid pretreatment on recalcitrance of agave bagasse and switchgrass. Bioresour. Technol. 2013, 127, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Varanasi, P.; Li, C.; Liu, H.; Melnichenko, Y.B.; Simmons, B.A.; Kent, M.S.; Singh, S. Transition of Cellulose Crystalline Structure and Surface Morphology of Biomass as a Function of Ionic Liquid Pretreatment and Its Relation to Enzymatic Hydrolysis. Biomacromolecules 2011, 12, 933–941. [Google Scholar] [CrossRef] [Green Version]
- Perez-Maqueda, L.A.; Franco, F.; Avilés, M.A.; Poyato, J.; Perez-Rodriguez, J.L. Effect of Sonication on Particle-size Distribution in Natural Muscovite and Biotite. Clays Clay Miner. 2003, 51, 701–708. [Google Scholar] [CrossRef]
- Sumari, S.; Roesyadi, A.; Sumarno, S. Effects of Ultrasound on the Morphology, Particle Size, Crystallinity, and Crystallite Size of Cellulose. Sci. Study Res. 2013, 14, 229–239. [Google Scholar]
- Wada, M.; Sugiyama, J.; Okano, T. Native celluloses on the basis of two crystalline phase (Iα/Iβ) system. J. Appl. Polym. Sci. 1993, 49, 1491–1496. [Google Scholar] [CrossRef]





| Code | Actual Value | |||
|---|---|---|---|---|
| Solid-Solvent Ratio, (g/mL) | Sonication Time, (min) | Acoustic Power, (W) | Hammer Mill Screen Size, (mm) | |
| 1 | 1/15 | 50 | 240 | 6.4 |
| 0 | 1/20 | 30 | 180 | 3.2 |
| −1 | 1/25 | 10 | 120 | 1.6 |
| Hammer Mill Screen Size (mm) | Geometric Mean Diameter (mm) | Geometric Standard Deviation (mm) | Bulk Density (kg m−3) | Particle Density (kg m−3) | Porosity (%) |
|---|---|---|---|---|---|
| 6.4 | 0.59 | 0.497 | 90 ± 5.3 | 846 | 89.37 |
| 3.2 | 0.50 | 0.489 | 116 ± 6.1 | 903 | 87.15 |
| 1.6 | 0.42 | 0.429 | 136 ± 4.3 | 1059 | 87.16 |
| Hammer Mill Screen Size (mm) | Shapiro-Wilk’s Test | Skewness | Kurtosis | |
|---|---|---|---|---|
| Statistical Value | p-Value | |||
| 6.4 | 0.970 | 0.900 | 0.124 | −1.206 |
| 3.2 | 0.897 | 0.316 | 0.787 | −0.432 |
| 1.6 | 0.896 | 0.310 | 1.321 | 1.805 |
| Runs | Factors | Response | ||||||
|---|---|---|---|---|---|---|---|---|
(g/mL) | (s) | (W) | (mm) | Acid Insoluble Lignin (% Dry Matter) | Acid Soluble Lignin (% Dry Matter) | Total Lignin (% Dry Matter) | Delignification (%) | |
| 1 | −1 | 0 | −1 | 0 | 21.45 | 1.1 | 22.55 | 16.01 |
| 2 | −1 | −1 | 0 | 0 | 24.45 | 1.2 | 25.65 | 4.47 |
| 3 | 0 | 1 | −1 | 0 | 23.05 | 1.1 | 24.15 | 10.06 |
| 4 | 0 | 0 | 1 | −1 | 23.15 | 0.7 | 23.85 | 11.17 |
| 5 | 1 | 0 | 0 | 1 | 24.25 | 1.1 | 25.35 | 5.59 |
| 6 | −1 | 0 | 0 | 1 | 24.45 | 1.1 | 25.55 | 4.84 |
| 7 | 0 | −1 | 0 | −1 | 21.55 | 1.1 | 22.65 | 15.64 |
| 8 | −1 | 1 | 0 | 0 | 20.35 | 1.1 | 21.45 | 20.11 |
| 9 | 0 | −1 | 0 | 1 | 22.65 | 1.1 | 23.75 | 11.55 |
| 10 | 0 | 0 | 0 | 0 | 24.65 | 1.0 | 25.65 | 4.47 |
| 11 | 0 | 0 | 0 | 0 | 22.75 | 1 | 23.75 | 11.55 |
| 12 | 1 | 0 | 0 | −1 | 22.85 | 1.2 | 24.05 | 10.43 |
| 13 | 0 | 0 | 0 | 0 | 25.15 | 1.1 | 26.25 | 2.23 |
| 14 | 0 | 1 | 0 | 1 | 20.55 | 1.8 | 22.35 | 16.76 |
| 15 | 0 | 0 | 0 | 0 | 25.05 | 1 | 26.05 | 2.98 |
| 16 | 0 | 1 | 0 | −1 | 22.55 | 1 | 23.55 | 12.29 |
| 17 | −1 | 0 | 1 | 0 | 21.05 | 0.8 | 21.85 | 18.62 |
| 18 | 0 | 0 | −1 | 1 | 22.45 | 0.9 | 23.35 | 13.04 |
| 19 | 1 | 1 | 0 | 0 | 21.95 | 1.3 | 23.25 | 13.41 |
| 20 | 0 | 1 | 1 | 0 | 20.75 | 1 | 21.75 | 18.99 |
| 21 | 1 | −1 | 0 | 0 | 25.05 | 1 | 26.05 | 2.98 |
| 22 | 1 | 0 | −1 | 0 | 23.05 | 1 | 24.05 | 10.43 |
| 23 | 0 | −1 | −1 | 0 | 24.35 | 1.1 | 25.45 | 5.21 |
| 24 | 0 | 0 | 0 | 0 | 25.25 | 1 | 26.25 | 2.23 |
| 25 | 1 | 0 | 1 | 0 | 22.65 | 0.6 | 23.25 | 13.41 |
| 26 | 0 | 0 | 1 | 1 | 23.85 | 0.7 | 24.55 | 8.57 |
| 27 | 0 | −1 | 1 | 0 | 22.75 | 1.1 | 23.85 | 11.17 |
| 28 | 0 | 0 | −1 | −1 | 25.25 | 1.1 | 26.35 | 1.86 |
| 29 | −1 | 0 | 0 | −1 | 22.45 | 0.8 | 23.25 | 13.41 |
| Source | Delignification | ||||
|---|---|---|---|---|---|
| Coefficient | F Value | p Value | Level of Impact | ||
| Coded Factors | Actual Factors | ||||
| Model | 2.84 | 0.0280 * | |||
| b | −1.77 | −1733.526 | 1.89 | 0.1841 | 4 |
| a | 3.38 | −0.408 | 6.93 | 0.0159 * | 1 |
| 2.11 | −0.202 | 2.70 | 0.1162 | 2 | |
| −0.37 | 4.152 | 0.08 | 0.7758 | 3 | |
| −3.44 | −0.024 | 2.40 | 0.1373 | ||
| 2.73 | 14,977.570 | 2.53 | 0.1274 | ||
| 3.85 | 0.010 | 5.02 | 0.0365 * | ||
| 3.33 | 0.001 | 3.77 | 0.0663 | ||
| Lack of fit | 1.34 | 0.4240 | |||
| Treatment Condition | Crystallinity Index (%) | d-Spacings (Å) | Crystallite Size (Å) | Mean | ||||
|---|---|---|---|---|---|---|---|---|
| (1 1 0) | (2 0 0) | (0 0 4) | (1 1 0) | (2 0 0) | (0 0 4) | |||
| Untreated | 48.86 | 5.40 | 4.03 | 2.58 | 12.31 | 23.36 | 10.51 | 15.39 |
| 50 min, 180 W | 47.49 | 5.48 | 4.03 | 2.59 | 12.34 | 23.81 | 3.23 | 13.13 |
| 50 min, 240 W | 48.76 | 5.51 | 4.07 | 2.58 | 12.13 | 24.25 | 10.61 | 15.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onu Olughu, O.; Tabil, L.G.; Dumonceaux, T. Ultrasonic Delignification and Microstructural Characterization of Switchgrass. Energies 2021, 14, 263. https://doi.org/10.3390/en14020263
Onu Olughu O, Tabil LG, Dumonceaux T. Ultrasonic Delignification and Microstructural Characterization of Switchgrass. Energies. 2021; 14(2):263. https://doi.org/10.3390/en14020263
Chicago/Turabian StyleOnu Olughu, Onu, Lope G. Tabil, and Tim Dumonceaux. 2021. "Ultrasonic Delignification and Microstructural Characterization of Switchgrass" Energies 14, no. 2: 263. https://doi.org/10.3390/en14020263






