CFD Simulation of Syngas Combustion in a Two-Pass Oxygen Transport Membrane Reactor for Fire Tube Boiler Application
Abstract
:1. Introduction
2. Model
2.1. Descriptions of the Membrane Reactor
2.2. Governing Equations
2.3. Geometry and Boundary Conditions
2.4. Solution Procedures
3. Validation of the Model
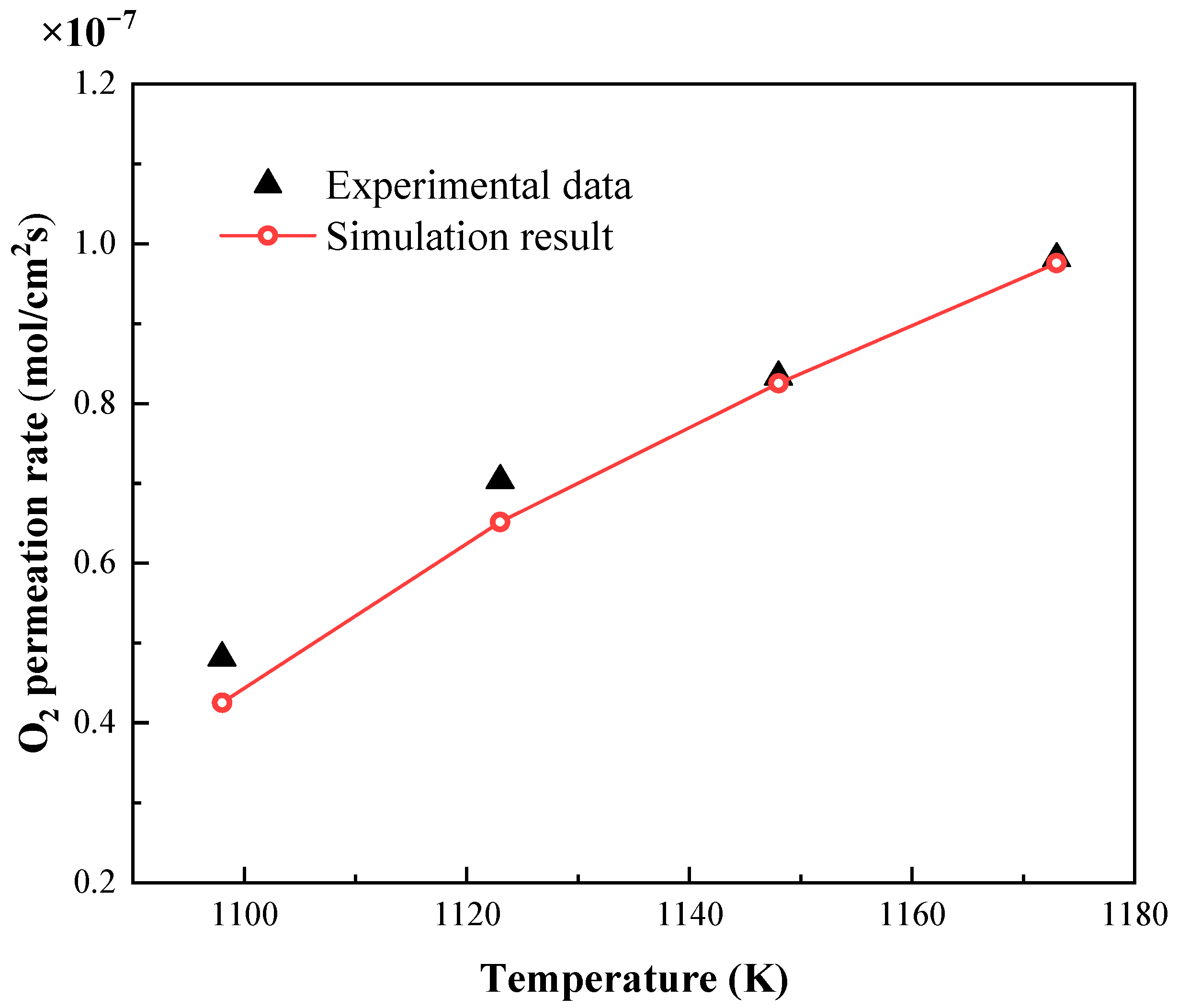
3.1. Validation of Oxygen Permeation Model
3.2. Validation of Syngas Combustion Kinetic Model
4. Results and Discussion
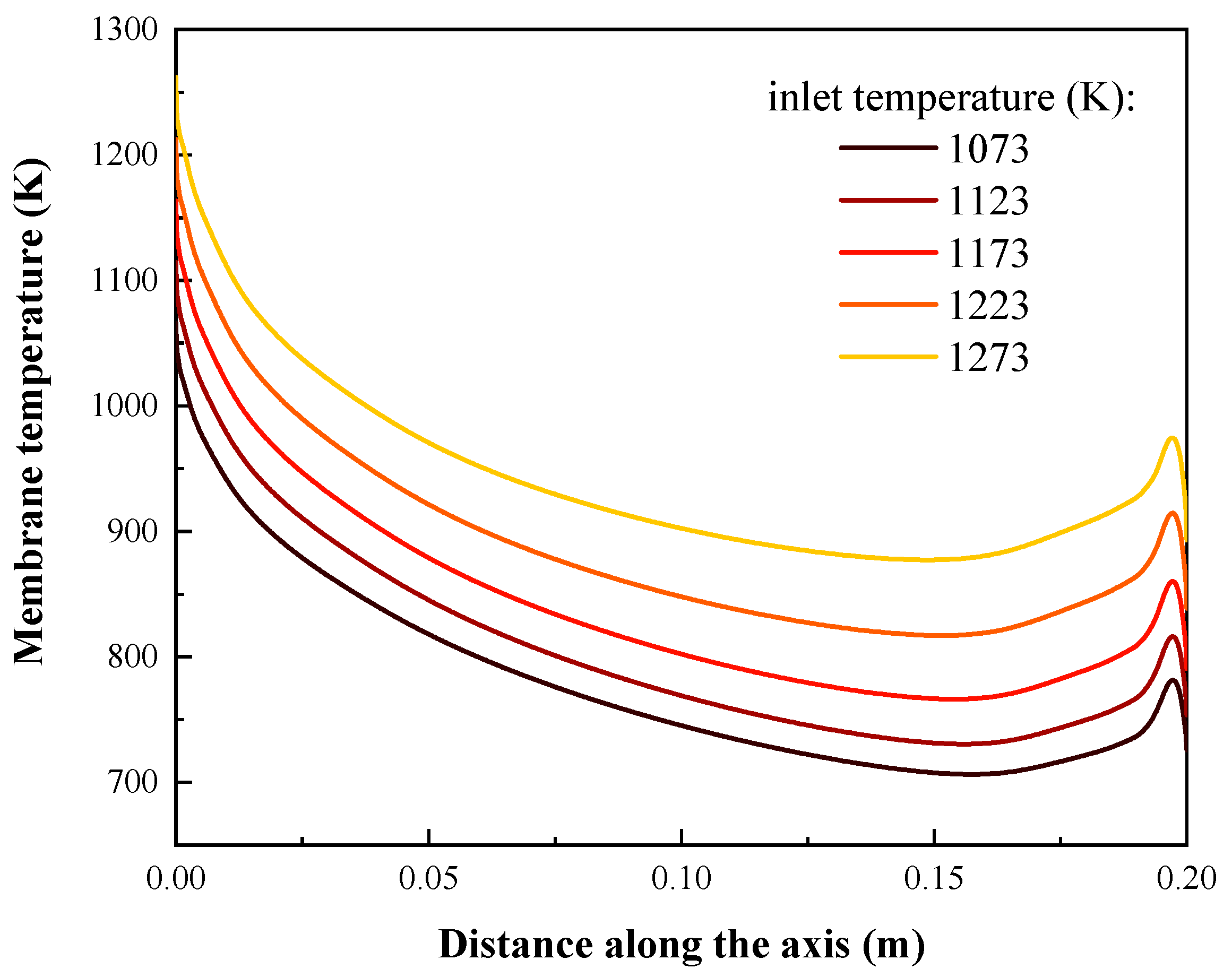
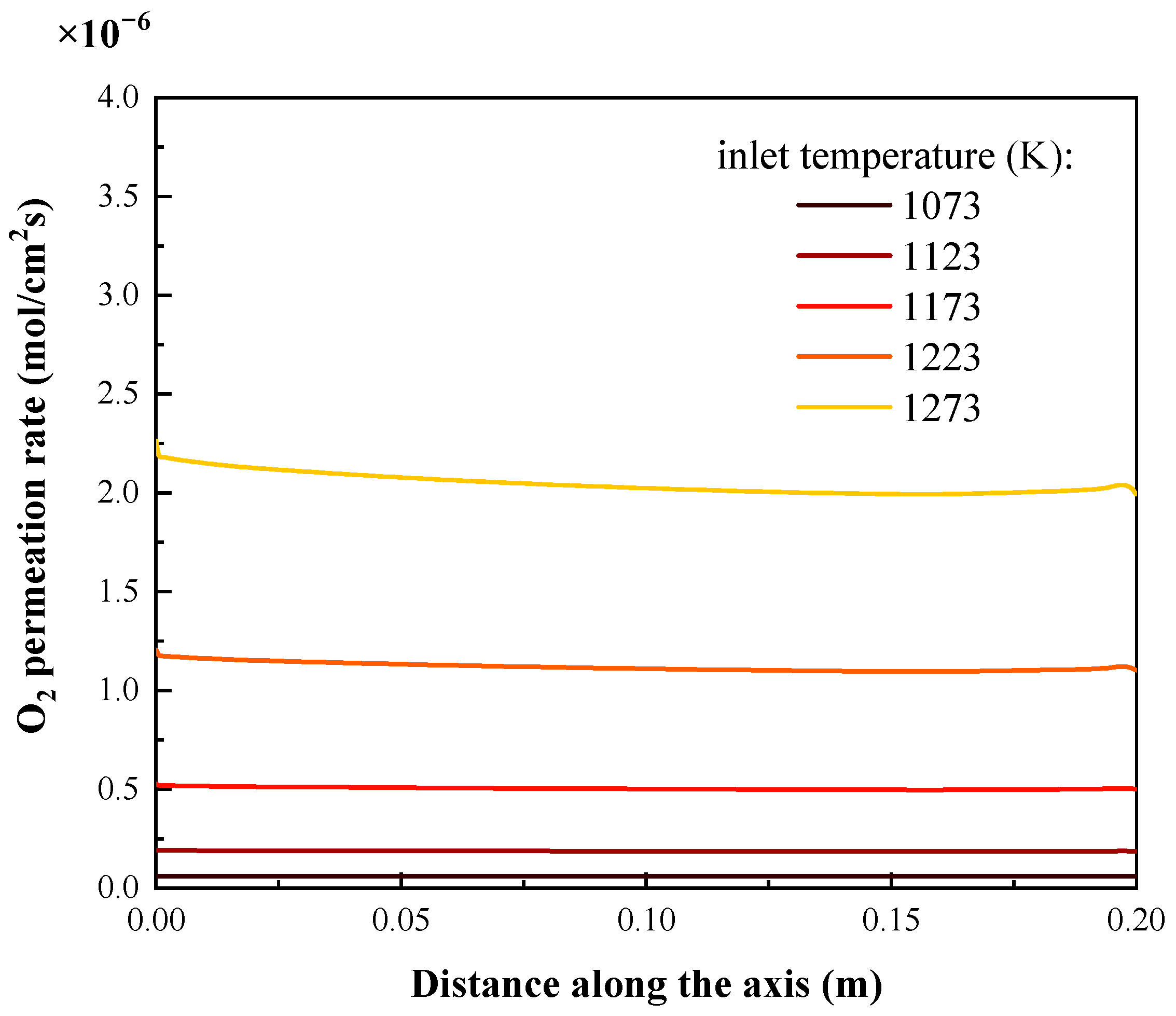
4.1. Effects of Inlet Temperature
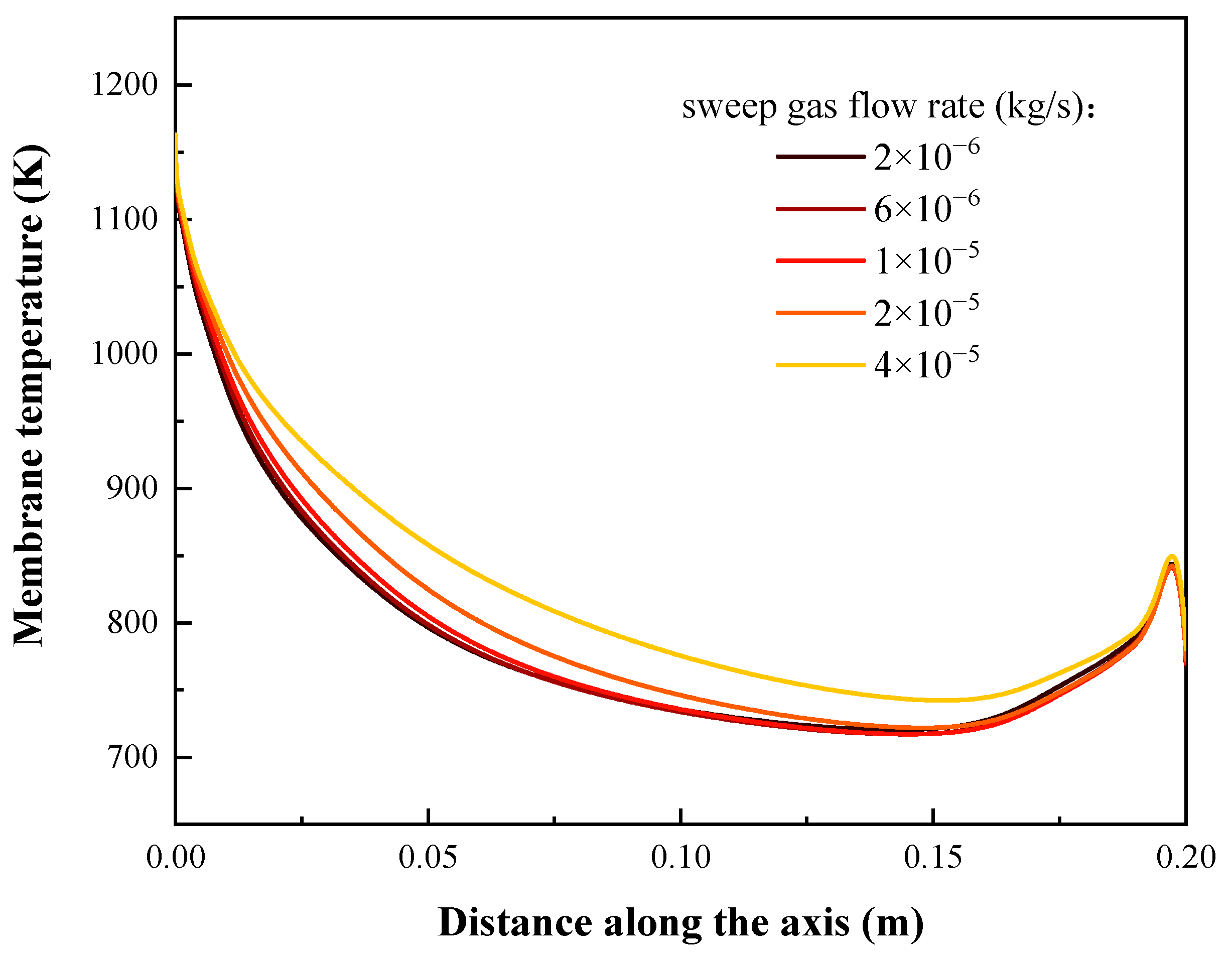
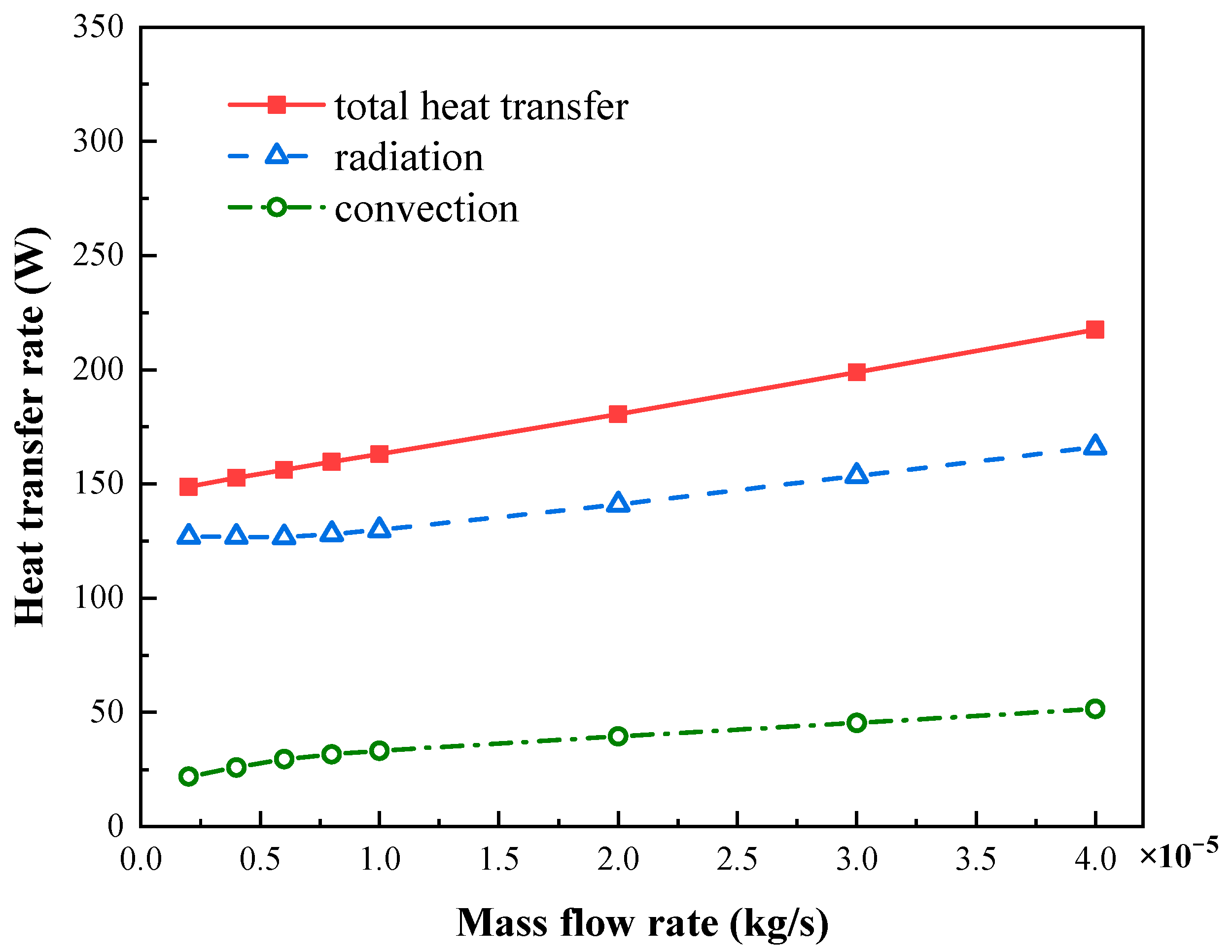
4.2. Effects of Sweep Gas Flow Rate
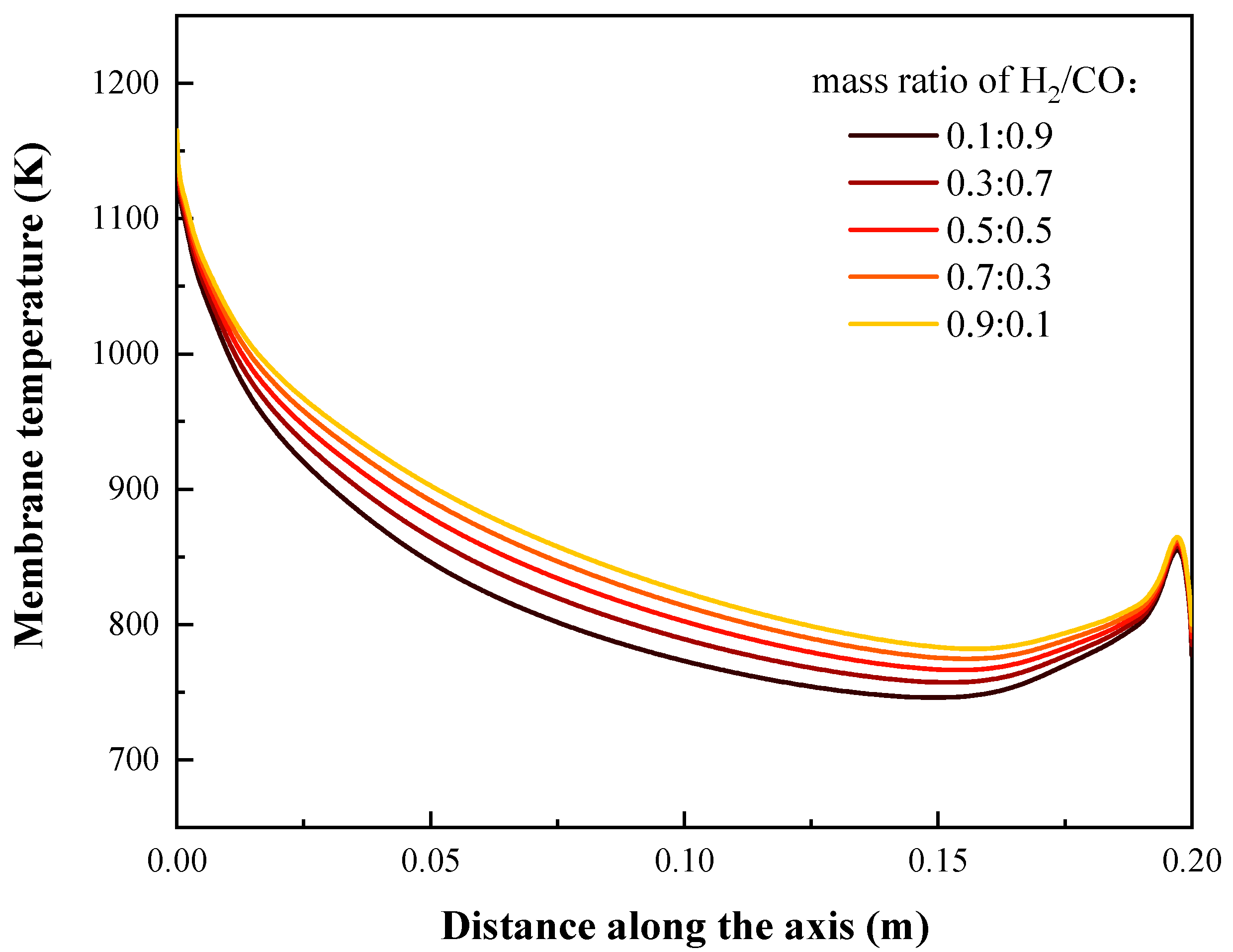
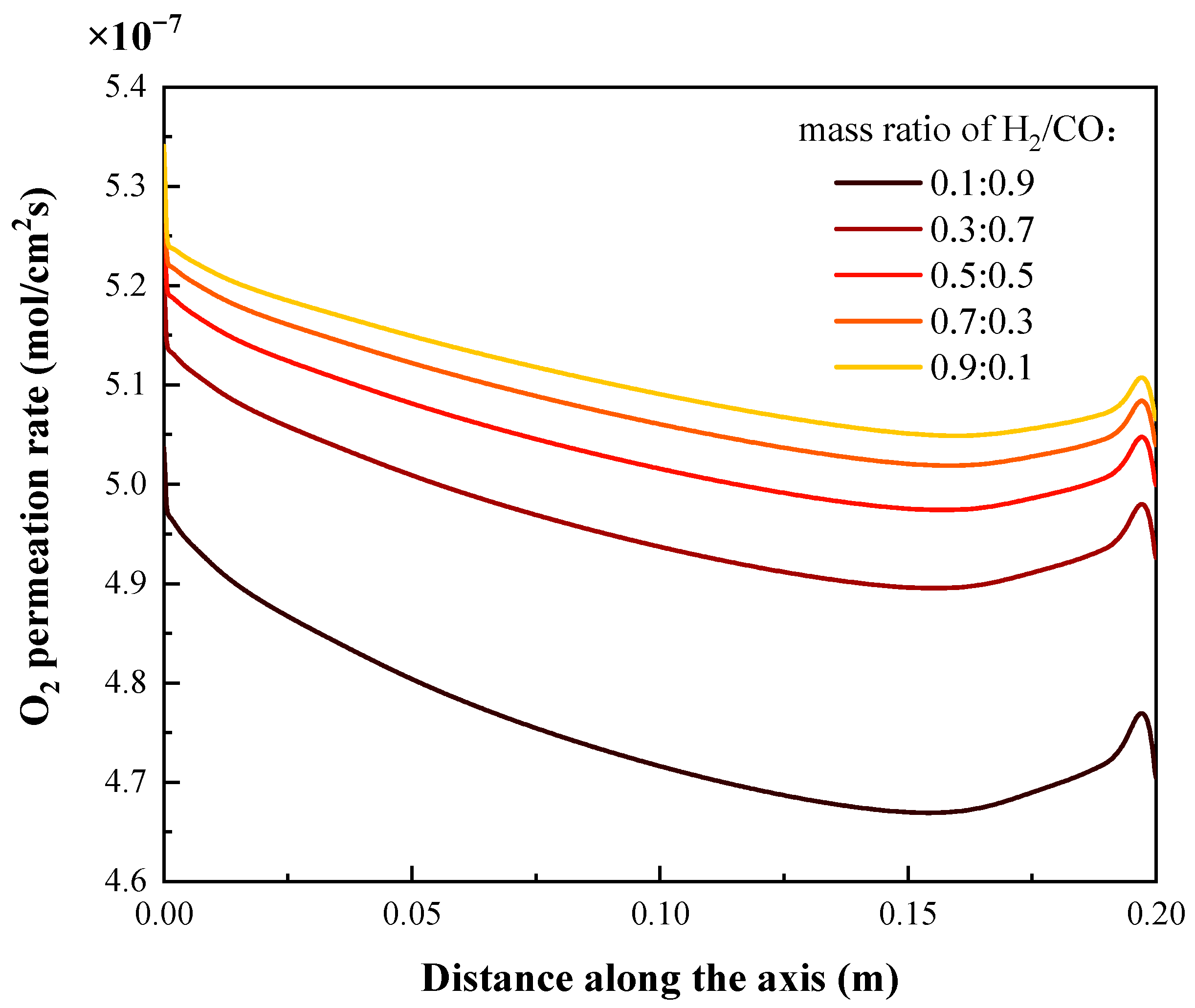
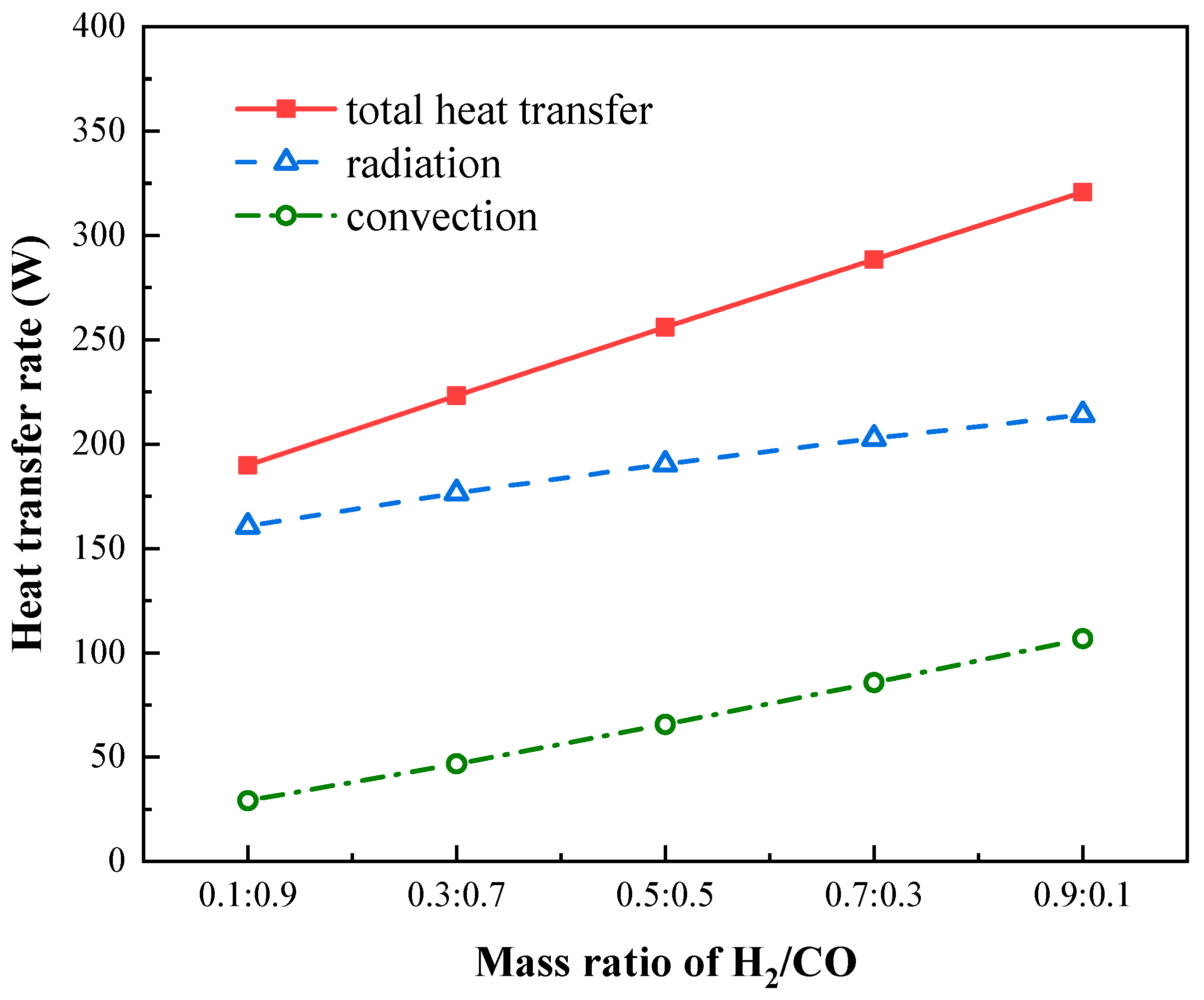
4.3. Effects of Syngas Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Habib, M.A.; Badr, H.M.; Ahmed, S.F.; Ben-Mansour, R.; Mezghani, K.; Imashuku, S.; Jlo, G. A review of recent developments in carbon capture utilizing oxy-fuel combustion in conventional and ion transport membrane systems. Int. J. Energy Res. 2011, 35, 741–764. [Google Scholar] [CrossRef]
- Joshi, M.M.; Lee, S. Integrated gasification combined cycle-A review of IGCC technology. Energy Sour. 1996, 18, 537–568. [Google Scholar] [CrossRef]
- Zabetta, E.C.; Xilpinen, P.; Hupa, M. Kinetic modeling study on the potential of staged combustion in gas turbines for the reduction of nitrogen oxide emissions from biomass IGCC plants. Energy Fuel 2000, 14, 751–761. [Google Scholar] [CrossRef]
- Casleton, K.H.; Breault, R.W.; Richards, G.A. System issues and tradeoffs associated with syngas production and combustion. Combust. Sci. Technol. 2008, 180, 1013–1052. [Google Scholar] [CrossRef]
- Yuan, R.H.; He, Z.; Zhang, Y.; Wang, W.D.; Chen, C.S.; Wu, H.; Zhan, Z.L. Partial oxidation of methane to syngas in a packed bed catalyst membrane reactor. AIChE J. 2016, 62, 2170–2176. [Google Scholar] [CrossRef]
- Nemitallah, M.A. Characteristics of oxygen permeation and partial oxidation of methane in a catalytic membrane reactor for syngas production. Energy Fuels 2020, 34, 7522–7532. [Google Scholar] [CrossRef]
- Parente, M.; Soria, M.; Madeira, L.M. Hydrogen and/or syngas production through combined dry and steam reforming of biogas in a membrane reactor: A thermodynamic study. Renew. Energy 2020, 157, 1254–1264. [Google Scholar] [CrossRef]
- Hu, T.; Liang, W.; Xia, X.; Peng, H.; Jiang, H. Simultaneous production of pure nitrogen and syngas in BaCe0.5Fe0.5O3-δ membrane reactor. Catal. Today 2021, 364, 125–131. [Google Scholar] [CrossRef]
- Li, C.; He, Z.; Ban, X.; Li, N.; Chen, C.; Zhan, Z. Membrane-based catalytic partial oxidation of ethanol coupled with steam reforming for solid oxide fuel cells. J. Membr. Sci. 2021, 622, 119032. [Google Scholar] [CrossRef]
- Habib, M.A.; Salaudeen, S.A.; Nemitallah, M.A.; Ben-Mansour, R.; Mokheimer, E. Numerical investigation of syngas oxy-combustion inside a LSCF-6428 oxygen transport membrane reactor. Energy 2016, 96, 654–665. [Google Scholar] [CrossRef]
- Habib, M.A.; Nemitallah, M.A. Design of an ion transport membrane reactor for application in fire tube boilers. Energy 2015, 81, 787–801. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Adebiyi, A.A.; Habib, M.A. Investigation of performance of fire-tube boilers integrated with ion transport membrane for oxy-fuel combustion. Int. J. Energy Res. 2016, 40, 1673–1687. [Google Scholar] [CrossRef]
- Mansir, I.B.; Ben-Mansour, R.; Habib, M.A. Oxy-fuel combustion in a two-pass oxygen transport reactor for fire tube boiler application. Appl. Energy 2018, 229, 828–840. [Google Scholar] [CrossRef]
- Mansir, I.B.; Ben-Mansour, R.; Habib, M.A. Numerical modeling of heat transfer characteristics in a two-pass oxygen transport reactor for fire tube boilers under oxy-fuel combustion. Appl. Therm. Eng. 2021, 195, 117248. [Google Scholar] [CrossRef]
- Coltrin, M.E.; Glarborg, P. Chemically Reacting Flow: Theory and Practice; Wiley-Interscience: New York, NY, USA, 2003. [Google Scholar]
- Fluent, I. FLUENT 6.3 user’s guide. In Fluent Documentation; Fluent Inc.: Lebanon, NH, USA, 2006. [Google Scholar]
- McGee, H.A. Molecular Engineering; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Xu, S.J.; Thomson, W.J. Oxygen permeation rates through ion-conducting perovskite membranes. Chem. Eng. Sci. 1999, 54, 3839–3850. [Google Scholar] [CrossRef]
- Rui, Z.B.; Zhang, K.; Li, Y.; Lin, Y. Simulation of methane conversion to syngas in a membrane reactor: Part I A model including product oxidation. Int. J. Hydrogen Energy 2008, 33, 2246–2253. [Google Scholar] [CrossRef]
- Cuoci, A.; Frassoldati, A.; Faravelli, T.; Ranzi, E. Accuracy and flexibility of simplified kinetic models for CFD applications. Combust. Colloq. 2009, 1–6. Available online: https://www.researchgate.net/publication/266176558_Accuracy_and_Flexibility_of_Simplified_Kinetic_Models_for_CFD_applications (accessed on 8 July 2021).
- Nemitallah, M.A.; Habib, M.A.; Salaudeen, S.A.; Mansir, I. Hydrogen production, oxygen separation and syngas oxy-combustion inside a water splitting membrane reactor. Renew. Energy 2017, 113, 221–234. [Google Scholar] [CrossRef]
- Jin, W.Q.; Li, S.G.; Huang, P.; Xu, N.P.; Shi, J.; Lin, Y.S. Tubular lanthanum cobaltite perovskite-type membrane reactors for partial oxidation of methane to syngas. J. Membr. Sci. 2000, 166, 13–22. [Google Scholar] [CrossRef]
- Jin, W.Q.; Gu, X.; Li, S.G.; Huang, P.; Xu, N.P.; Shi, J. Experimental and simulation study on a catalyst packed tubular dense membrane reactor for partial oxidation of methane to syngas. Chem. Eng. Sci. 2000, 55, 2617–2625. [Google Scholar] [CrossRef]
- Barlow, R.S.; Fiechtner, G.J.; Carter, D.C. Experiments on the scalar structure of turbulent CO/H2/N2 jet flames. Combust. Flame 2000, 120, 549–569. [Google Scholar] [CrossRef]
- Zheng, H.J.; Bai, T.; Wang, Q.; Cao, F. Infrared radiation characteristics of high-temperature H2O and CO2 gas mixture jet flows. Acta Opt. Sin. 2017, 37, 262–272. [Google Scholar]




| Oxygen Transport Membrane | La0.6Sr0.4Co0.2Fe0.8O3-δ (LSCoF-6428) |
|---|---|
| Effective length of membrane reactor | 20 cm |
| Effective length of baffle | 19.5 cm |
| Internal radii of quartz tube | 20 mm |
| Internal radii of baffle | 15 mm |
| Internal radii of membrane tube | 10 mm |
| Thickness of baffle | 1 mm |
| Thickness of membrane | 1 mm |
| Density of membrane | 6000 kg/m3 |
| Thermal conductivity of membrane | 4 W/(m·K) |
| Emissivity of membrane and quartz tube | 0.8 |
| Thermal conductivity of baffle | 0.1 W/(m·K) |
| Emissivity of baffle | 0.95 |
| Inlet Temperature of Two Sides (K) | Mass Flow Rate of Sweep Gas (kg/s) | H2/CO Mass Ratio |
|---|---|---|
| 1073 | 2 × 10−6 | 0.1:0.9 |
| 1123 | 6 × 10−6 | 0.3:0.7 |
| 1173 | 1 × 10−5 | 0.5:0.5 |
| 1223 | 2 × 10−5 | 0.7:0.3 |
| 1273 | 4 × 10−5 | 0.9:0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Chen, C.; Ye, H. CFD Simulation of Syngas Combustion in a Two-Pass Oxygen Transport Membrane Reactor for Fire Tube Boiler Application. Energies 2021, 14, 7348. https://doi.org/10.3390/en14217348
Zhao T, Chen C, Ye H. CFD Simulation of Syngas Combustion in a Two-Pass Oxygen Transport Membrane Reactor for Fire Tube Boiler Application. Energies. 2021; 14(21):7348. https://doi.org/10.3390/en14217348
Chicago/Turabian StyleZhao, Te, Chusheng Chen, and Hong Ye. 2021. "CFD Simulation of Syngas Combustion in a Two-Pass Oxygen Transport Membrane Reactor for Fire Tube Boiler Application" Energies 14, no. 21: 7348. https://doi.org/10.3390/en14217348







