An Innovative 500 W Alkaline Water Electrolyser System for the Production of Ultra-Pure Hydrogen and Oxygen Gases
Abstract
:1. Introduction
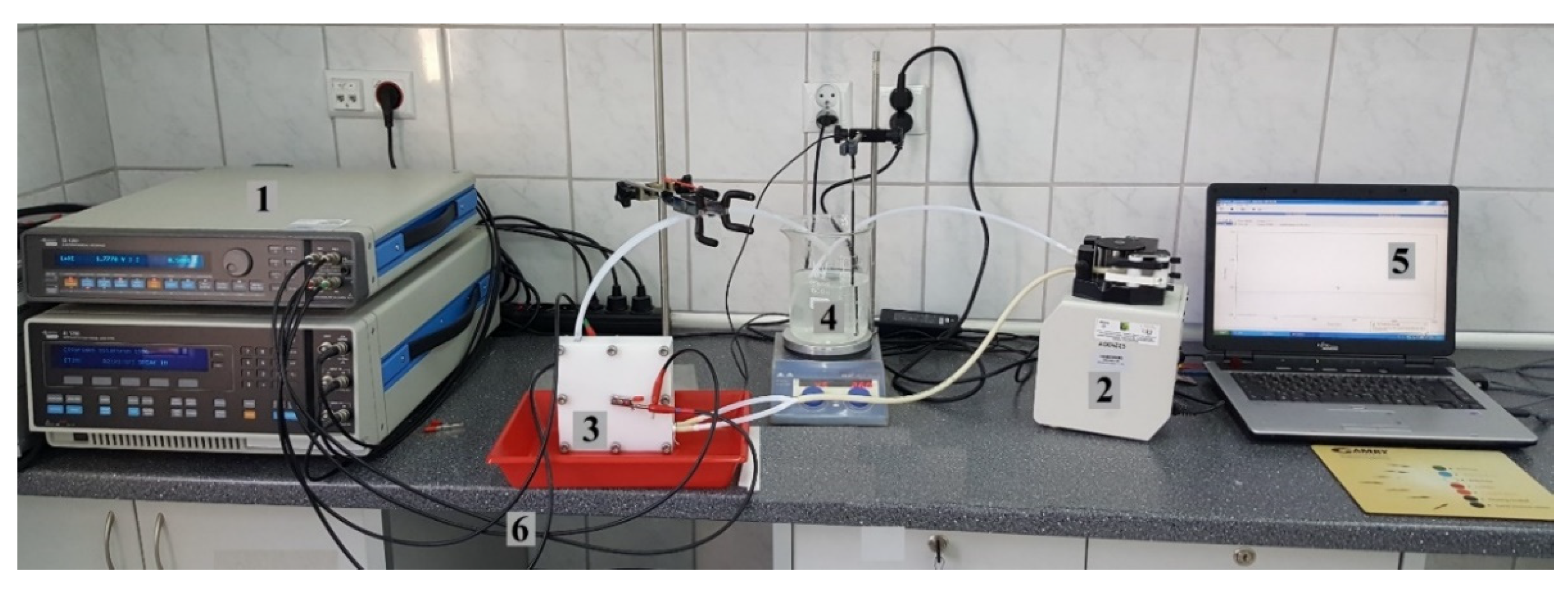
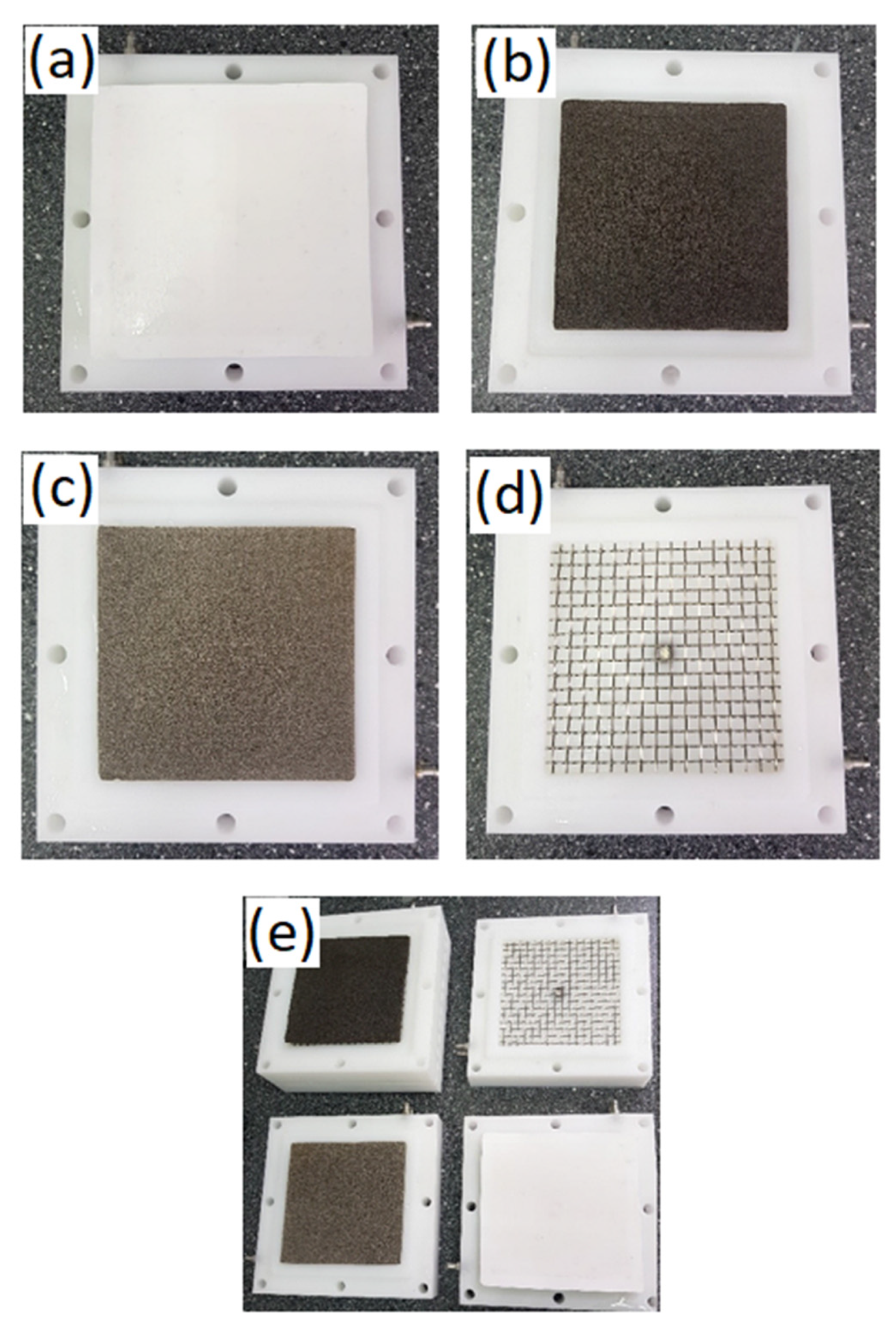
2. Materials and Methods
3. Results and Discussion
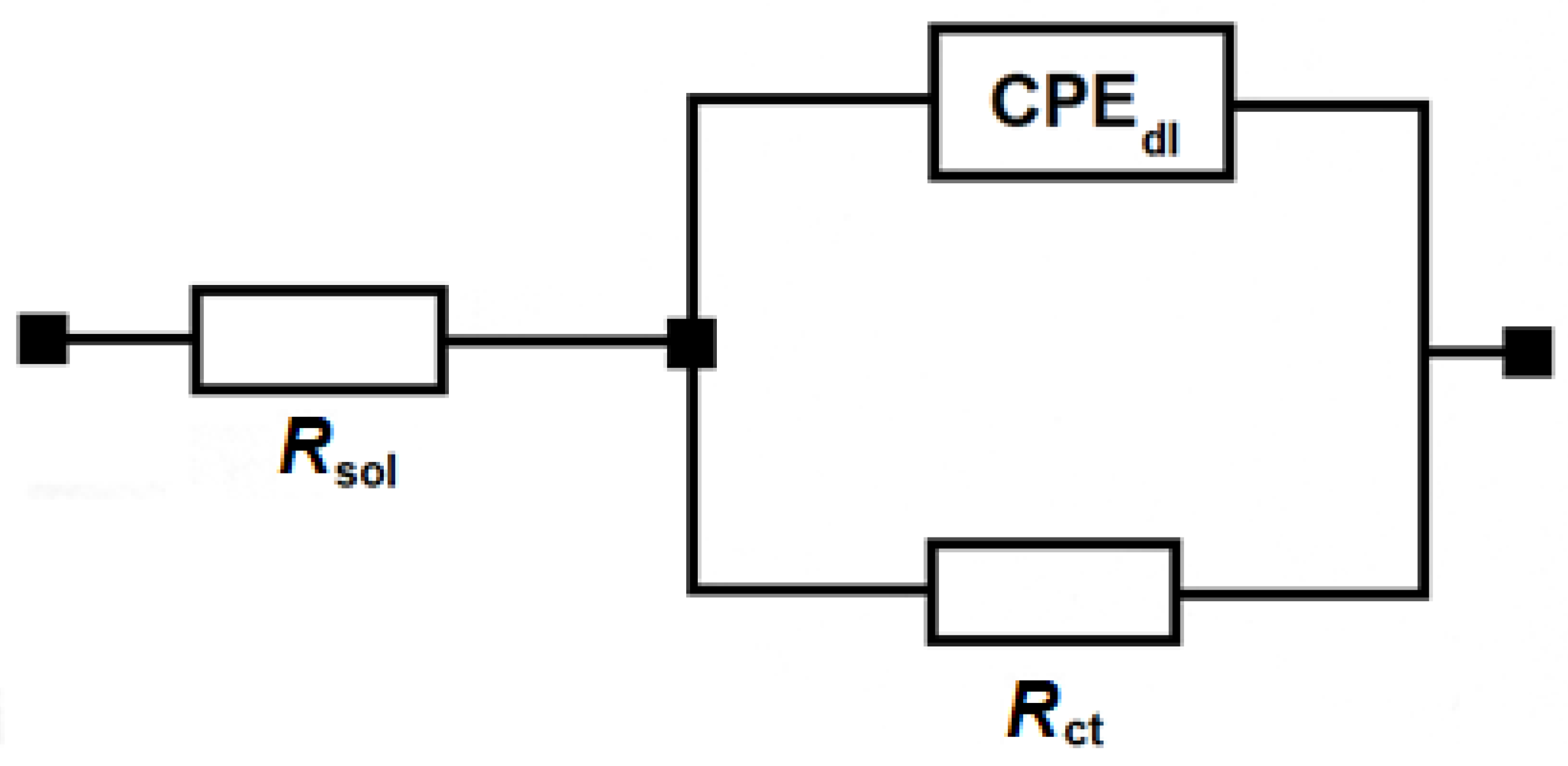
3.1. Single-Cell Electrochemical AWE Tests
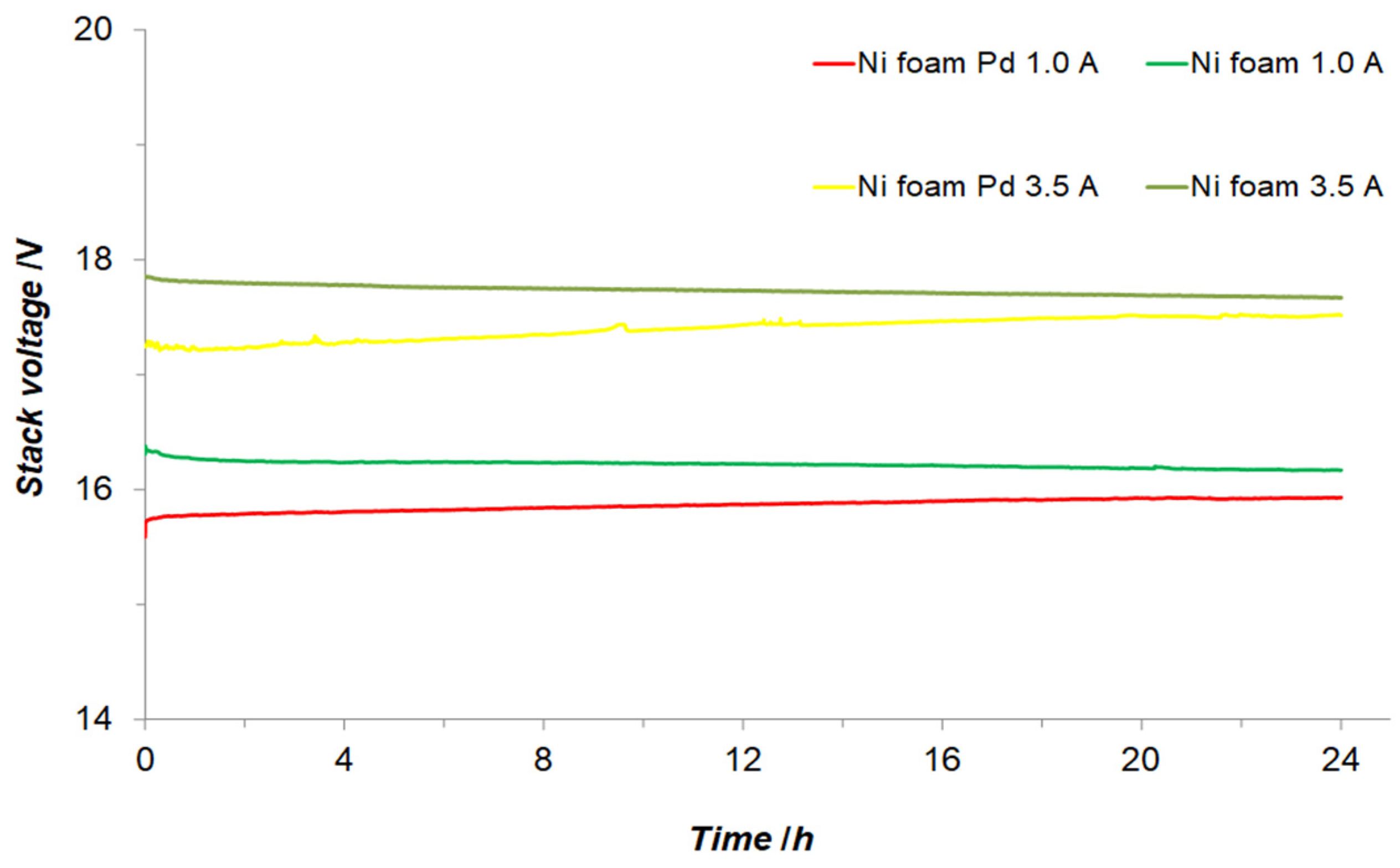
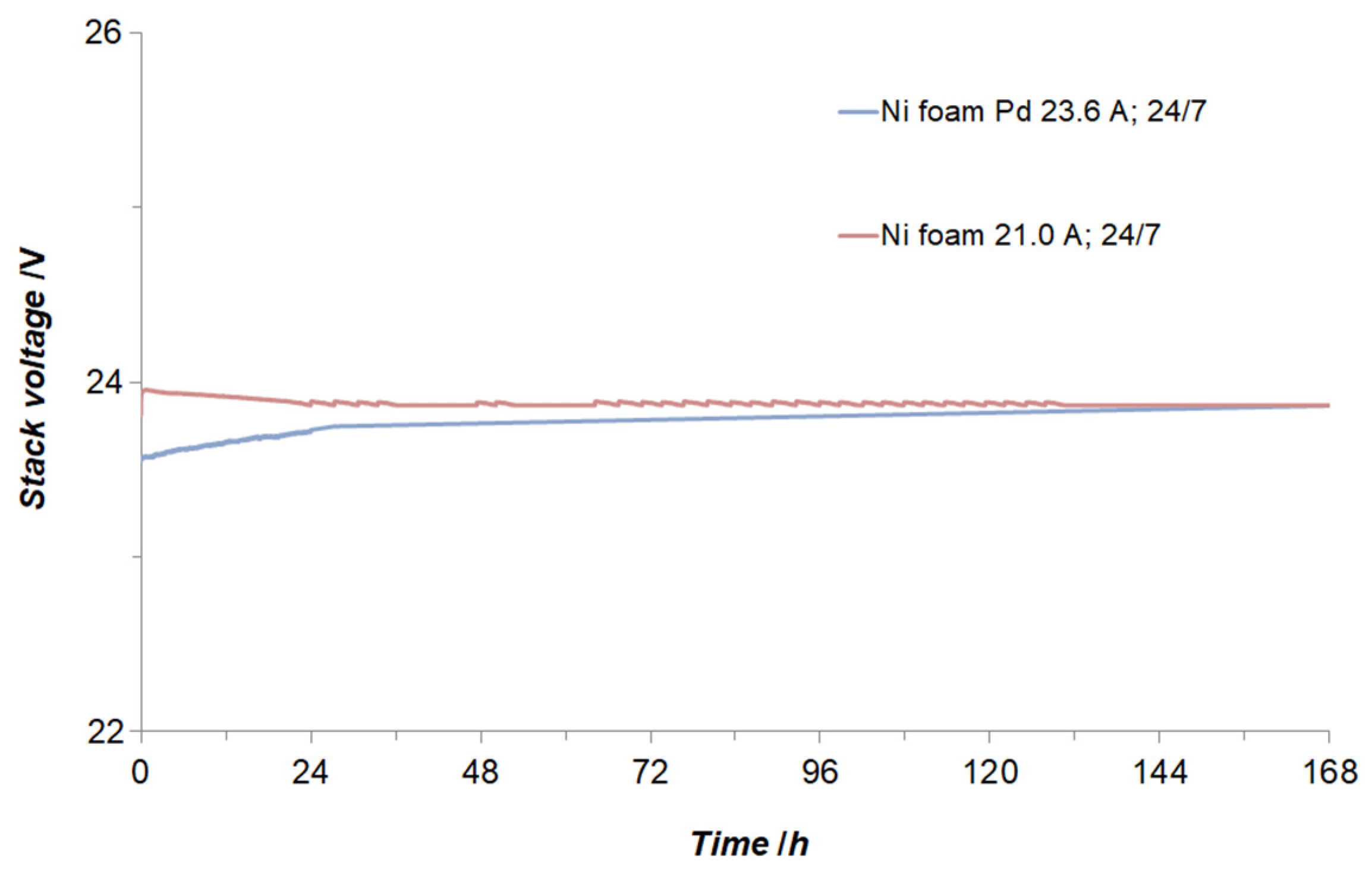
3.2. Electrochemical Examination of Laboratory Size 500 W AWE System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ogawa, T.; Takeuchi, M.; Kajikawa, Y. Analysis of Trends and Emerging Technologies in Water Electrolysis Research Based on a Computational Method: A Comparison with Fuel Cell Research. Sustainability 2018, 10, 478. [Google Scholar] [CrossRef] [Green Version]
- Koj, J.C.; Wulf, C.; Schreiber, A.; Zapp, P. Site-Dependent Environmental Impacts of Industrial Hydrogen Production by Alkaline Water Electrolysis. Energies 2017, 10, 860. [Google Scholar]
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- Babic, U.; Suermann, M.; Büchi, F.N.; Gubler, L.; Schmidt, T.J. Critical Review—Identifying Critical Gaps for Polymer Electrolyte Water Electrolysis Development. J. Electrochem. Soc. 2017, 164, 387–399. [Google Scholar] [CrossRef] [Green Version]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Christopher, K.; Dimitrios, R. A review on exergy comparison of hydrogen production methods from renewable energy sources. Energy Environ. Sci. 2012, 5, 6640–6651. [Google Scholar] [CrossRef]
- Vengatesan, S.; Santhi, S.; Jeevanantham, S.; Sozhan, G. Quaternized poly (styrene-co-vinyl benzyl chloride) anion exchange membranes for alkaline water electrolysers. J. Power Sources 2015, 284, 361–368. [Google Scholar] [CrossRef]
- Solmaz, R. Electrochemical preparation and characterization of C/Ni–NiIr composite electrodes as novel cathode materials for alkaline water electrolysis. Int. J. Hydrogen Energy 2013, 38, 2251–2256. [Google Scholar] [CrossRef]
- Manabe, A.; Kashiwase, M.; Hashimoto, T.; Hayashida, T.; Kato, A.; Hirao, K.; Shimomura, I.; Nagashima, I. Basic study of alkaline water electrolysis. Electrochim. Acta 2013, 100, 249–256. [Google Scholar] [CrossRef]
- Rashid, M.M.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen Production by Water Electrolysis: A Review of Alkaline Water Electrolysis, PEM Water Electrolysis and High Temperature Water Electrolysis. Int. J. Eng. Adv. Technol. 2015, 4, 80–93. [Google Scholar]
- Pierozynski, B.; Mikolajczyk, T.; Kowalski, I.M. Hydrogen evolution at catalytically-modified nickel foam in alkaline solution. J. Power Sources 2014, 271, 231–238. [Google Scholar] [CrossRef]
- Pierozynski, B.; Mikolajczyk, T. On the Temperature Dependence of Hydrogen Evolution Reaction at Nickel Foam and Pd-Modified Nickel Foam Catalysts. Electrocatalysis 2015, 6, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Pierozynski, B.; Mikolajczyk, T. Cathodic Evolution of Hydrogen on Platinum-Modified Nickel Foam Catalyst. Electrocatalysis 2016, 7, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Pierozynski, B.; Mikolajczyk, T.; Luba, M.; Zolfaghari, A. Kinetics of oxygen evolution reaction on nickel foam and platinum-modified nickel foam materials in alkaline solution. J. Electroanal. Chem. 2019, 847, 113194–113201. [Google Scholar] [CrossRef]
- Martin, M.H.; Lasia, A. Influence of Experimental Factors on the Constant Phase Element Behavior of Pt Electrodes. Electrochim. Acta 2011, 56, 8058–8068. [Google Scholar] [CrossRef]
- Marini, S.; Salvi, P.; Nelli, P.; Pesenti, R.; Villa, M.; Berrettoni, M.; Zangari, G.; Kiros, Y. Advanced Alkaline Water Electrolysis. Electrochim. Acta 2012, 82, 384–391. [Google Scholar] [CrossRef]
- Mergel, J.; Carmo, M.; Fritz, D. Status on Technologies for Hydrogen Production by Water Electrolysis. In Transition to Renewable Energy Systems; Stolten, D., Scherer, V., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 423–450. ISBN 978-3-527-67387-2. [Google Scholar]
- Elayappan, V.; Shanmugam, R.; Chinnusamy, S.; Yoo, D.J.; Mayakrishnan, G.; Kim, K.; Noh, H.S.; Kim, M.K.; Lee, H. Three-Dimensional Bimetal TMO Supported Carbon Based Electrocatalyst Developed via Dry Synthesis for Hydrogen and Oxygen Evolution. Appl. Surf. Sci. 2020, 505, 144642. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Balamurugan, J.; Vinothkannan, M.; Kim, A.R.; Sengodan, S.; Yoo, D.J. Nitrogen-Doped Graphene Encapsulated FeCoMoS Nanoparticles as Advanced Trifunctional Catalyst for Water Splitting Devices and Zinc–Air Batteries. Appl. Catal. B Environ. 2020, 279, 119381. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, W.; Lu, H.; Huang, Y.; Zuo, L.; Fan, W.; Liu, T. Carbon-Nanotube-Incorporated Graphene Scroll-Sheet Conjoined Aerogels for Efficient Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2017, 5, 6994–7002. [Google Scholar] [CrossRef]





| Element | Sample | Content |
|---|---|---|
| Ni | 1 | 94.53 |
| 2 | 93.05 | |
| C | 1 | 3.16 |
| 2 | 4.35 | |
| O | 1 | 2.12 |
| 2 | 2.35 | |
| Pd | 1 | 0.19 |
| 2 | 0.25 |
| Sample | Element | Content |
|---|---|---|
| Ni foam 1 | C | 2.18 |
| O | 1.28 | |
| Ni | 96.54 | |
| Ni foam 2 | C | 1.88 |
| O | 1.20 | |
| Ni | 96.92 | |
| Ni foam 3 | C | 2.24 |
| O | 1.24 | |
| Ni | 96.52 | |
| Ni foam oxidized 1 | C | 2.24 |
| O | 5.94 | |
| Ni | 91.83 | |
| Ni foam oxidized 2 | C | 2.21 |
| O | 4.25 | |
| Ni | 93.55 | |
| Ni foam oxidized 3 | C | 1.82 |
| O | 3.82 | |
| Ni | 94.36 |
| U/V | Rct/Ω | Cdl/mF |
|---|---|---|
| Cathode: Ni foam | ||
| 1.50 | 0.94 | 140 |
| 1.60 | 0.58 | 121 |
| 1.65 | 0.38 | 113 |
| 1.70 | 0.18 | 103 |
| 1.80 | 0.09 | 96 |
| Cathode: Pd-modified Ni foam | ||
| 1.50 | 0.12 | 669 |
| 1.60 | 0.12 | 654 |
| 1.65 | 0.11 | 486 |
| 1.70 | 0.08 | 450 |
| 1.80 | 0.06 | 399 |
| Sample | Constituents | Content/% |
|---|---|---|
| Oxygen 1 | O2 | 99.83 |
| H2O | 0.17 | |
| Oxygen 2 | O2 | 98.71 |
| H2 | 1.11 | |
| H2O | 0.18 | |
| Oxygen 3 | O2 | 99.96 |
| H2O | 0.04 | |
| Oxygen 4 | O2 | 99.87 |
| H2O | 0.13 | |
| Hydrogen 1 | H2 | 99.86 |
| H2O | 0.14 | |
| Hydrogen 2 | H2 | 99.85 |
| H2O | 0.15 | |
| Hydrogen 3 | H2 | 99.94 |
| H2O | 0.06 | |
| Hydrogen 4 | H2 | 99.89 |
| H2O | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierozynski, B.; Mikolajczyk, T.; Wojciechowski, B.; Luba, M. An Innovative 500 W Alkaline Water Electrolyser System for the Production of Ultra-Pure Hydrogen and Oxygen Gases. Energies 2021, 14, 526. https://doi.org/10.3390/en14030526
Pierozynski B, Mikolajczyk T, Wojciechowski B, Luba M. An Innovative 500 W Alkaline Water Electrolyser System for the Production of Ultra-Pure Hydrogen and Oxygen Gases. Energies. 2021; 14(3):526. https://doi.org/10.3390/en14030526
Chicago/Turabian StylePierozynski, Boguslaw, Tomasz Mikolajczyk, Boguslaw Wojciechowski, and Mateusz Luba. 2021. "An Innovative 500 W Alkaline Water Electrolyser System for the Production of Ultra-Pure Hydrogen and Oxygen Gases" Energies 14, no. 3: 526. https://doi.org/10.3390/en14030526
APA StylePierozynski, B., Mikolajczyk, T., Wojciechowski, B., & Luba, M. (2021). An Innovative 500 W Alkaline Water Electrolyser System for the Production of Ultra-Pure Hydrogen and Oxygen Gases. Energies, 14(3), 526. https://doi.org/10.3390/en14030526







