A Di-Carbazole-Based Dye as a Potential Sensitizer for Greenhouse-Integrated Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Route of the Di-Carbazole-Based Dye
2.3. Fabrication of the Dye-Sensitized Solar Cells
2.4. Characterization Methods
3. Results
3.1. Synthesis of the Di-Carbazole-Based Dye
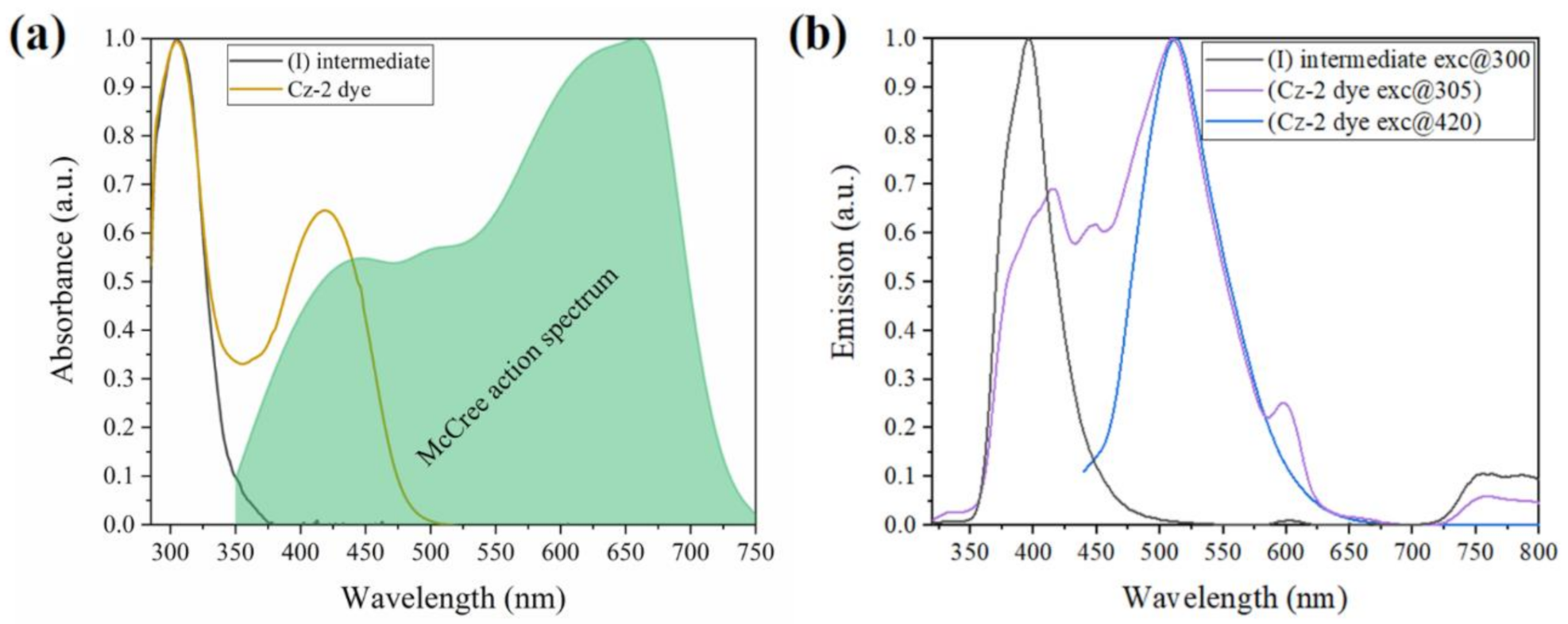
3.2. Optical and Electrochemical Characteristics of the Di-Carbazole-Based Dye
3.3. Characteristics of Dye-Sensitized Working Electrodes
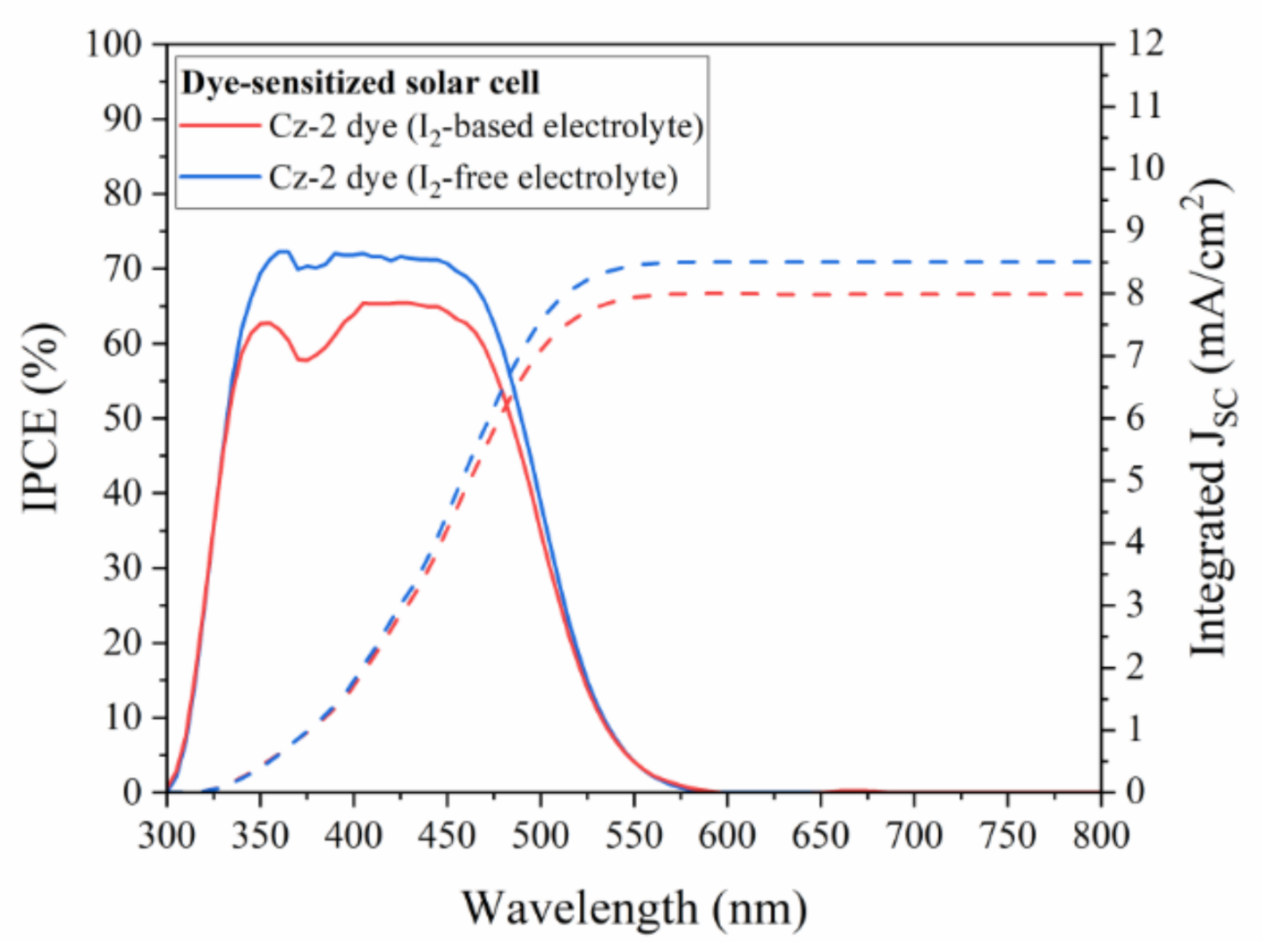
3.4. Characteristics of the Solar Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration. Today in Energy. Available online: https://www.eia.gov/todayinenergy/detail.php?id=41433 (accessed on 15 January 2021).
- Hassanien, R.H.E.; Li, M.; Dong Lin, W. Advanced applications of solar energy in agricultural greenhouses. Renew. Sustain. Energy Rev. 2016, 54, 989–1001. [Google Scholar] [CrossRef]
- Ravishankar, E.; Booth, R.E.; Saravitz, C.; Sederoff, H.; Ade, H.W.; O’Connor, B.T. Achieving net zero energy greenhouses by integrating semitransparent organic solar cells. Joule 2020, 4, 490–506. [Google Scholar] [CrossRef]
- Cossu, M.; Murgia, L.; Ledda, L.; Deligios, P.A.; Sirigu, A.; Chessa, F.; Pazzona, A. Solar radiation distribution inside a greenhouse with south-oriented photovoltaic roofs and effects on crop productivity. Appl. Energy 2014, 133, 89–100. [Google Scholar] [CrossRef]
- Roslan, N.; Ya’acob, M.E.; Radzi, M.A.M.; Hashimoto, Y.; Jamaludin, D.; Chen, G. Dye sensitized solar cell (DSSC) greenhouse shading: New insights for solar radiation manipulation. Renew. Sustain. Energy Rev. 2018, 92, 171–186. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Dessì, A.; Chalkias, D.A.; Bilancia, S.; Sinicropi, A.; Calamante, M.; Mordini, A.; Karavioti, A.; Stathatos, E.; Zani, L.; Reginato, G. D-A-π-A organic dyes with tailored green light absorption for potential application in greenhouse-integrated Dye-Sensitized Solar Cells. Sustain. Energy Fuels 2021. [Google Scholar] [CrossRef]
- Dessì, A.; Calamante, M.; Sinicropi, A.; Parisi, M.L.; Vesce, L.; Mariani, P.; Taheri, B.; Ciocca, M.; Di Carlo, A.; Zani, L.; et al. Thiazolo [5,4-d] thiazole-based organic sensitizers with improved spectral properties for application in greenhouse-integrated dye-sensitized solar cells. Sustain. Energy Fuels 2020, 4, 2309–2321. [Google Scholar] [CrossRef]
- Duvva, N.; Raptis, D.; Kumar, C.V.; Koukaras, E.N.; Giribabu, L.; Lianos, P. Design of diketopyrrolopyrrole chromophores applicable as sensitizers in dye-sensitized photovoltaic windows for green houses. Dye. Pigment. 2016, 134, 472–479. [Google Scholar] [CrossRef]
- Kim, J.-J.; Kang, M.; Kwak, O.K.; Yoon, Y.-J.; Min, K.S.; Chu, M.J. Fabrication and characterization of dye-sensitized solar cells for greenhouse application. Int. J. Photoenergy 2014, 2014. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-free organic dyes for dye-sensitized solar cells: From structure: Property relationships to design rules. Angew. Chemie Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-C.; Sahu, D.; Hsu, Y.-C.; Padhy, H.; Patra, D.; Lin, J.-T.; Bhattacharya, D.; Lu, K.-L.; Wei, K.-H.; Lin, H.-C. Structural planarity and conjugation effects of novel symmetrical acceptor-donor-acceptor organic sensitizers on dye-sensitized solar cells. Dye. Pigment. 2012, 93, 1488–1497. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yun, H.J.; Choi, S.K.; Yang, Y.S.; Park, T.; Ahn, K.-S.; Suresh, T.; Kim, J.H. Triphenylamine-based tri-anchoring organic dye with enhanced electron lifetime and long-term stability for dye sensitized solar cells. Synth. Met. 2016, 217, 248–255. [Google Scholar] [CrossRef]
- Huang, Z.-S.; Meier, H.; Cao, D. Phenothiazine-based dyes for efficient dye-sensitized solar cells. J. Mater. Chem. C 2016, 4, 2404–2426. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Li, W.; Wang, Z.-S.; Tian, H.; Zhu, W. Hexylthiophene-featured D-A-π-A structural indoline chromophores for coadsorbent-free and panchromatic dye-sensitized solar cells. Adv. Energy Mater. 2012, 2, 149–156. [Google Scholar] [CrossRef]
- Xie, X.; Sun, D.; Wei, Y.; Yuan, Y.; Zhang, J.; Ren, Y.; Wang, P. Thienochrysenocarbazole based organic dyes for transparent solar cells with over 10% efficiency. J. Mater. Chem. A 2019, 7, 11338–11346. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Wang, W.; Gurzadyan, G.G.; Li, J.; Li, X.; An, J.; Yu, Z.; Wang, H.; Cai, B.; et al. 13.6% efficient organic dye-sensitized solar cells by minimizing energy losses of the excited state. ACS Energy Lett. 2019, 4, 943–951. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef]
- Wakim, S.; Beaupré, S.; Blouin, N.; Aich, B.-R.; Rodman, S.; Gaudiana, R.; Tao, Y.; Leclerc, M. Highly efficient organic solar cells based on a poly(2,7-carbazole) derivative. J. Mater. Chem. 2009, 19, 5351–5358. [Google Scholar] [CrossRef]
- Tsutsumi, N. Molecular design of photorefractive polymers. Polym. J. 2016, 48, 571–588. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Yang, X.; Cai, B.; Zhang, L.; Yang, K.; Yu, Z.; Wang, X.; Hagfeldt, A.; Sun, L. Fine-tuning by triple bond of carbazole derivative dyes to obtain high efficiency for dye-sensitized solar cells with copper electrolyte. ACS Appl. Mater. Interfaces 2020. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Fei, X.; Liao, L.; Fan, J. 9,9′-bicarbazole: New molecular skeleton for organic light-emitting diodes. Chem. A Eur. J. 2019, 25, 4501–4508. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, H.; Chen, L.; Chen, Y. N,N′-bicarbazole: A versatile building block toward the construction of conjugated porous polymers for CO2 capture and dyes adsorption. Macromolecules 2017, 50, 4993–5003. [Google Scholar] [CrossRef]
- Feng, S.; Xu, H.; Zhang, C.; Chen, Y.; Zeng, J.; Jiang, D.; Jiang, J.X. Bicarbazole-based redox-active covalent organic frameworks for ultrahigh-performance energy storage. Chem. Commun. 2017, 53, 11334–11337. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Otsuka, T.; Kyomen, T.; Unno, M.; Hanaya, M. An achievement of over 12 percent efficiency in an organic dye-sensitized solar cell. Chem. Commun. 2014, 50, 6379–6381. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, K.; Kondratowicz, M.; Lis, T.; Chmielewski, P.J.; Cybińska, J.; Zafra, J.L.; Casado, J.; Vives, T.; Crassous, J.; Favereau, L.; et al. Lemniscular [16] cycloparaphenylene: A radially conjugated figure-eight aromatic molecule. J. Am. Chem. Soc. 2019, 141, 7421–7427. [Google Scholar] [CrossRef]
- Coulson, D.R.; Satek, L.C.; Grim, S.O. Tetrakis(triphenylphosphine)palladium(0). In Inorganic Syntheses; Cotton, F.A., Ed.; Wiley Online Library: Hoboken, NJ, USA, 1972; Volume 13, pp. 121–124. [Google Scholar]
- Chalkias, D.A.; Laios, A.I.; Petala, A.; Papanicolaou, G.C. Evaluation of the limiting factors affecting large-sized, flexible, platinum-free dye-sensitized solar cells performance: A combined experimental and equivalent circuit analysis. J. Mater. Sci. Mater. Electron. 2018, 29, 9621–9634. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, J.; Chang, Q.; Babikier, M.; Wang, H.; Li, H.; Yu, Q.; Gao, S.; Jiao, S. Fabrication of mesoporous TiO2 electrodes by chemical technique for dye-sensitized solar cells. Electrochim. Acta 2013, 94, 277–284. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Kawauchi, H.; Kashima, T.; Arakawa, H. Significant influence of TiO2 photoelectrode morphology on the energy conversion efficiency of N719 dye-sensitized solar cell. Coord. Chem. Rev. 2004, 248, 1381–1389. [Google Scholar] [CrossRef]
- Chalkias, D.A.; Giannopoulos, D.I.; Kollia, E.; Petala, A.; Kostopoulos, V.; Papanicolaou, G.C. Preparation of polyvinylpyrrolidone-based polymer electrolytes and their application by in-situ gelation in dye-sensitized solar cells. Electrochim. Acta 2018, 271, 632–640. [Google Scholar] [CrossRef]
- Ito, S.; Nazeeruddin, M.K.; Liska, P.; Comte, P.; Charvet, R.; Péchy, P.; Jirousek, M.; Kay, A.; Zakeeruddin, S.M.; Grätzel, M. Photovoltaic characterization of dye-sensitized solar cells: Effect of device masking on conversion efficiency. Prog. Photovolt. Res. Appl. 2006, 14, 589–601. [Google Scholar] [CrossRef]
- Neugebauer, F.A.; Fischer, H.; Bamberger, S.; Smith, H.O. Aminyle, 6. tert.-butyl-substituierte 9-carbazolyl-radikale, carbazol-radikalkationen und carbazol-9-oxyl-radikale. Chem. Ber. 1972, 105, 2694–2713. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Meteorol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Gupta, K.S.V.; Suresh, T.; Singh, S.P.; Islam, A.; Han, L.; Chandrasekharam, M. Carbazole based A-π-D-π-A dyes with double electron acceptor for dye-sensitized solar cell. Org. Electron. 2014, 15, 266–275. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Dye-sensitized solar cells, 1st ed.; Taylor and Francis Group LLC: Lausanne, Switzerland, 2010. [Google Scholar]
- Inada, K. Action spectra for photosynthesis in higher plants. Plant Cell Physiol. 1976, 17, 355–365. [Google Scholar]
- Ri, J.H.; Jin, J.; Xu, J.; Peng, T.; Ryu, K. Il Preparation of iodine-free ionic liquid gel electrolyte using polyethylene oxide (PEO)-polyethylene glycol (PEG) and its application in Ti-foil-based dye-sensitized solar cells. Electrochim. Acta 2016, 201, 251–259. [Google Scholar] [CrossRef]
- Sacco, A. Electrochemical impedance spectroscopy: Fundamentals and application in dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2017, 79, 814–829. [Google Scholar] [CrossRef]
- Dessì, A.; Calamante, M.; Mordini, A.; Peruzzini, M.; Sinicropi, A.; Basosi, R.; Fabrizi De Biani, F.; Taddei, M.; Colonna, D.; di Carlo, A.; et al. Thiazolo[5,4-d]thiazole-based organic sensitizers with strong visible light absorption for transparent, efficient and stable dye-sensitized solar cells. RSC Adv. 2015, 5, 32657–32668. [Google Scholar] [CrossRef]
- Yu, W.-C.; Lin, L.-Y.; Chang, W.-C.; Zhong, S.-H.; Su, C.-C. Iodine-free nanocomposite gel electrolytes for quasi-solid-state dye-sensitized solar cells. J. Power Sources 2018, 403, 157–166. [Google Scholar] [CrossRef]
- Shi, H.; Xia, R.; Zhang, G.; Yip, H.-L.; Cao, Y. Spectral engineering of semitransparent polymer solar cells for greenhouse applications. Adv. Energy Mater. 2019, 9, 1803438. [Google Scholar] [CrossRef]
- Emmott, C.J.M.; Röhr, J.A.; Campoy-Quiles, M.; Kirchartz, T.; Urbina, A.; Ekins-Daukes, N.J.; Nelson, J. Organic photovoltaic greenhouses: A unique application for semi-transparent PV? Energy Environ. Sci. 2015, 8, 1317–1328. [Google Scholar] [CrossRef]







| Dye | (Ag vs. AgCl ) | (Ag vs. AgCl) | HOMO (eV) | LUMO (eV) | |
|---|---|---|---|---|---|
| Cz-2 | 2.56 | 1.24 | −1.10 | −5.89 | −3.33 |
| Solar Cell | JSC (mA/cm2) | VOC (mV) | FF (−) | ECE (%) | |
|---|---|---|---|---|---|
| Dye | Electrolyte | ||||
| Cz-2 | I2-based | 8.02 ± 0.05 | 648 ± 5 | 0.68 ± 0.02 | 3.51 ± 0.05 |
| Cz-2 | I2-free | 8.50 ± 0.32 | 685 ± 5 | 0.54 ± 0.03 | 3.13 ± 0.06 |
| Solar Cell | Rs (Ohm) | RPt (Ohm) | Rrec (Ohm) | τe (ms) | |
|---|---|---|---|---|---|
| Dye | Electrolyte | ||||
| Cz-2 | I2-based | 8.7 | 8 | 65 | 2.9 |
| Cz-2 | I2-free | 10.8 | 20 | 268 | 19.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalkias, D.A.; Charalampopoulos, C.; Aivali, S.; Andreopoulou, A.K.; Karavioti, A.; Stathatos, E. A Di-Carbazole-Based Dye as a Potential Sensitizer for Greenhouse-Integrated Dye-Sensitized Solar Cells. Energies 2021, 14, 1159. https://doi.org/10.3390/en14041159
Chalkias DA, Charalampopoulos C, Aivali S, Andreopoulou AK, Karavioti A, Stathatos E. A Di-Carbazole-Based Dye as a Potential Sensitizer for Greenhouse-Integrated Dye-Sensitized Solar Cells. Energies. 2021; 14(4):1159. https://doi.org/10.3390/en14041159
Chicago/Turabian StyleChalkias, Dimitris A., Christos Charalampopoulos, Stefania Aivali, Aikaterini K. Andreopoulou, Aggeliki Karavioti, and Elias Stathatos. 2021. "A Di-Carbazole-Based Dye as a Potential Sensitizer for Greenhouse-Integrated Dye-Sensitized Solar Cells" Energies 14, no. 4: 1159. https://doi.org/10.3390/en14041159







