Thermal Analysis of Organic and Nanoencapsulated Electrospun Phase Change Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phase Change Materials
2.2. PCM Electrospun Fiber Matrix
3. Results
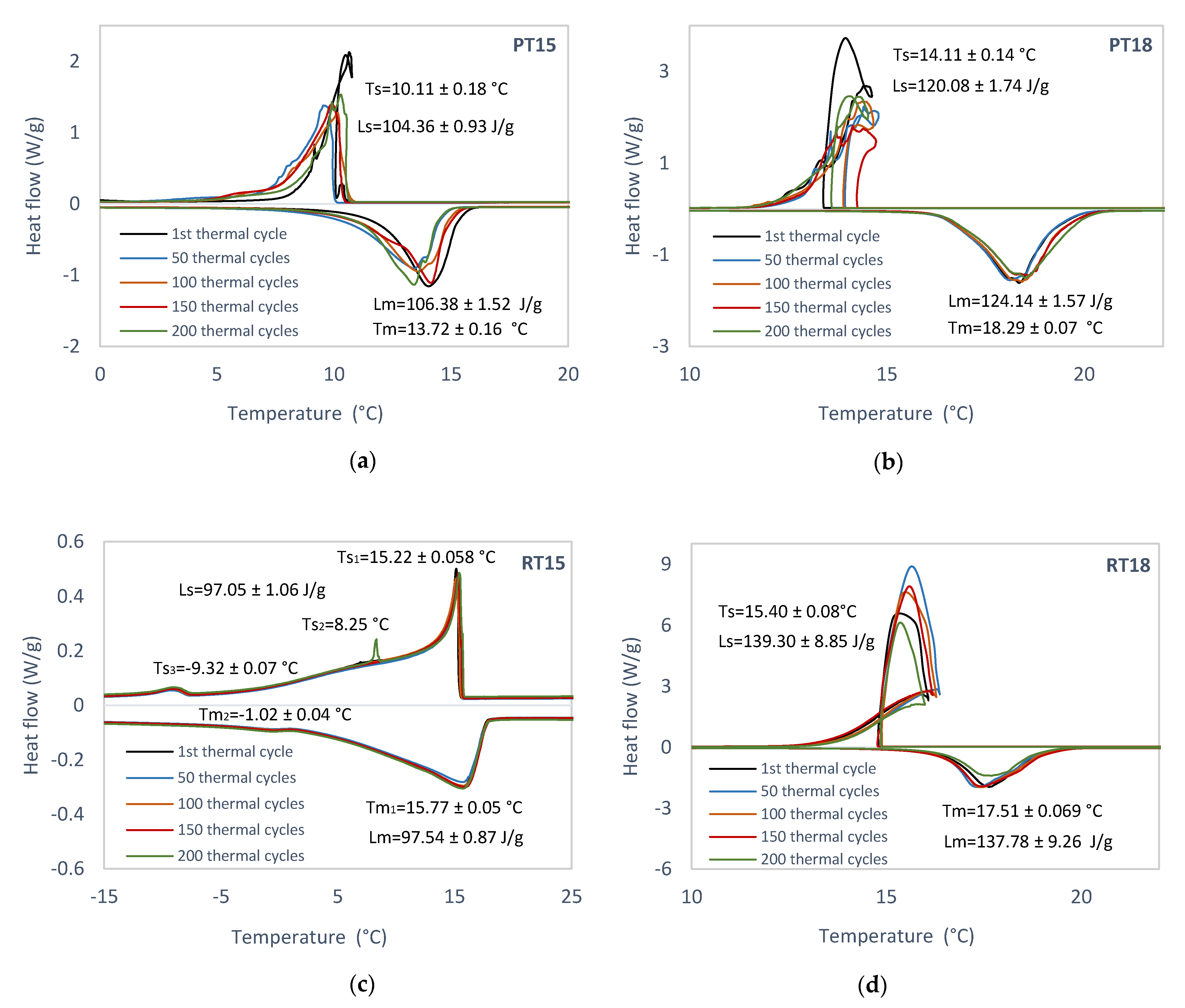
3.1. Experimental Identification of Pure PCM
3.2. Experimental Characterization of PCM Emulsions
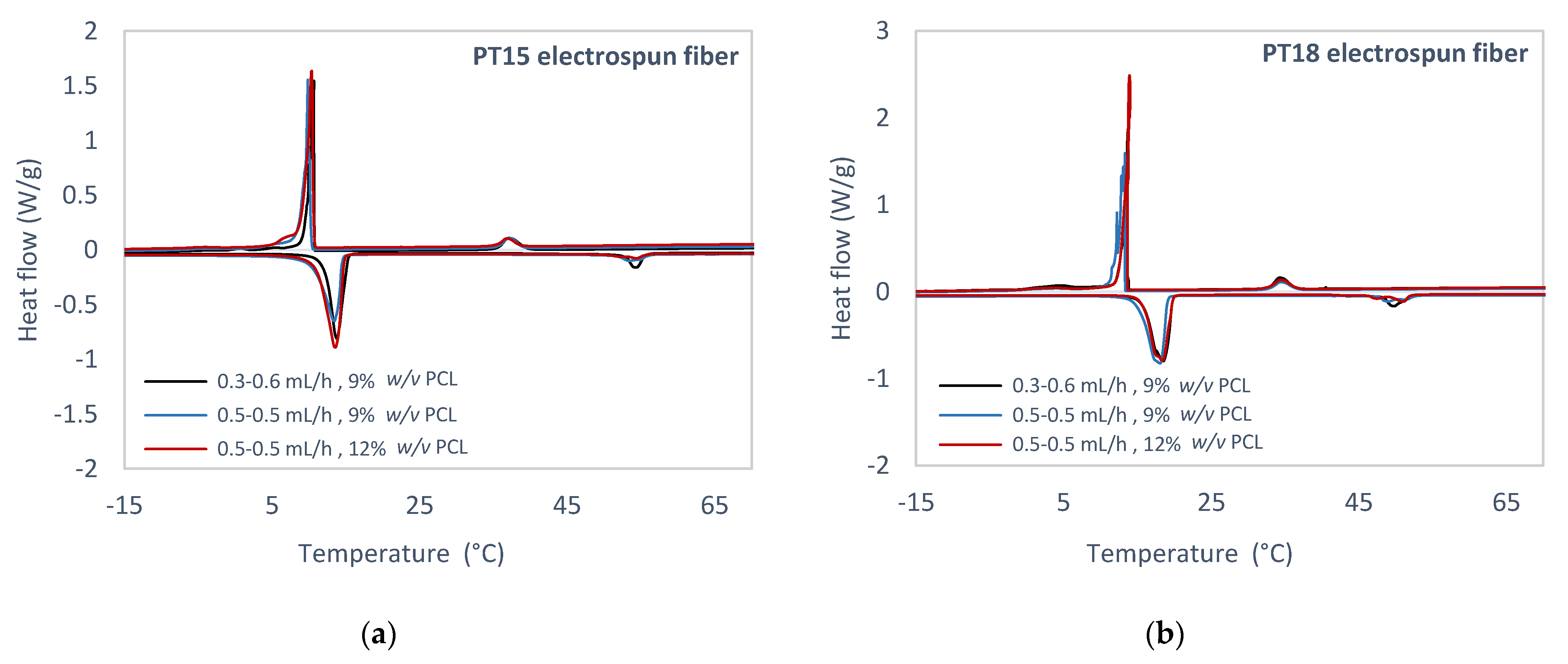
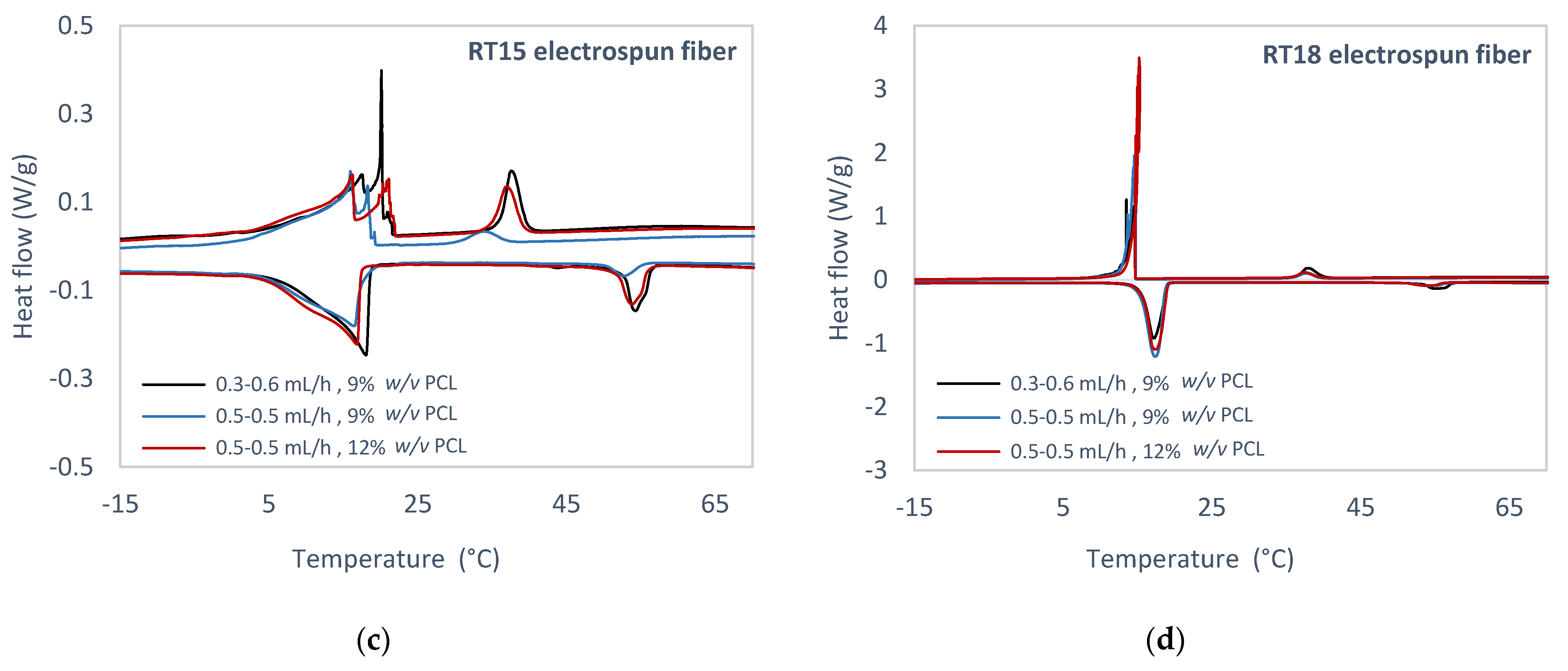
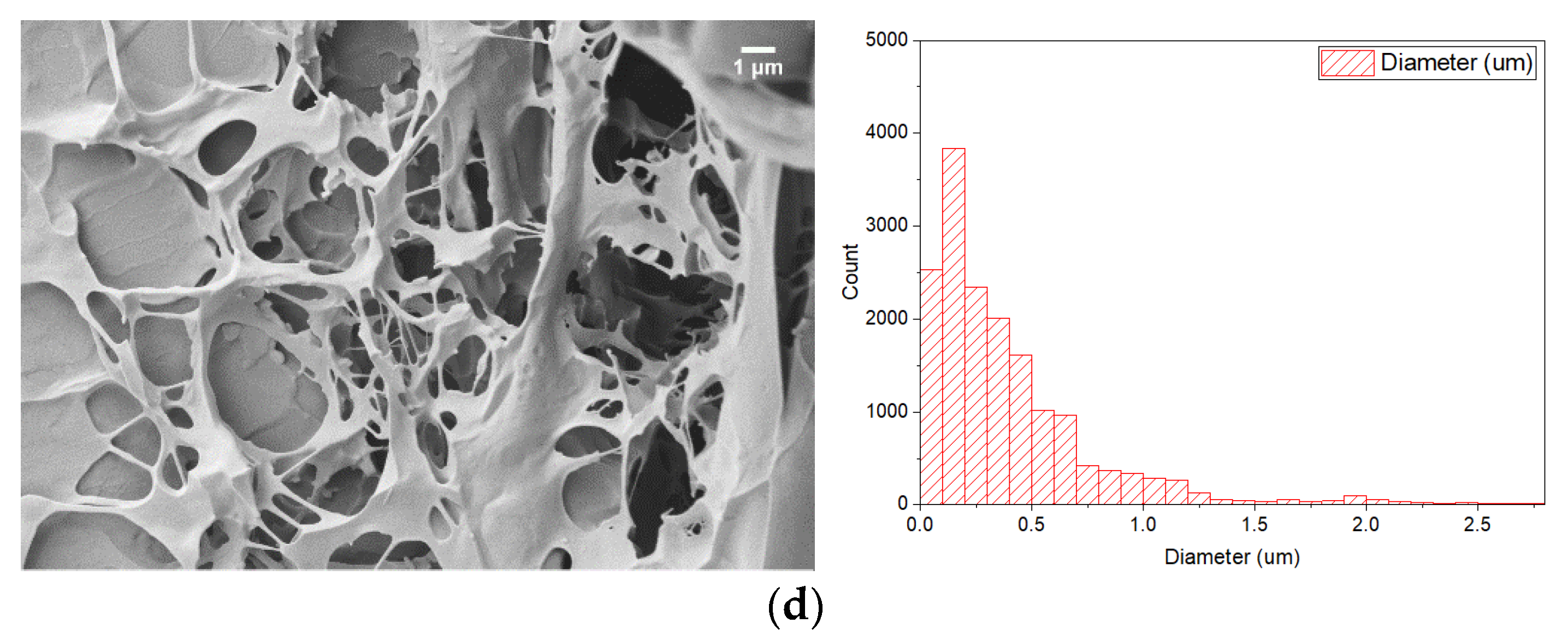
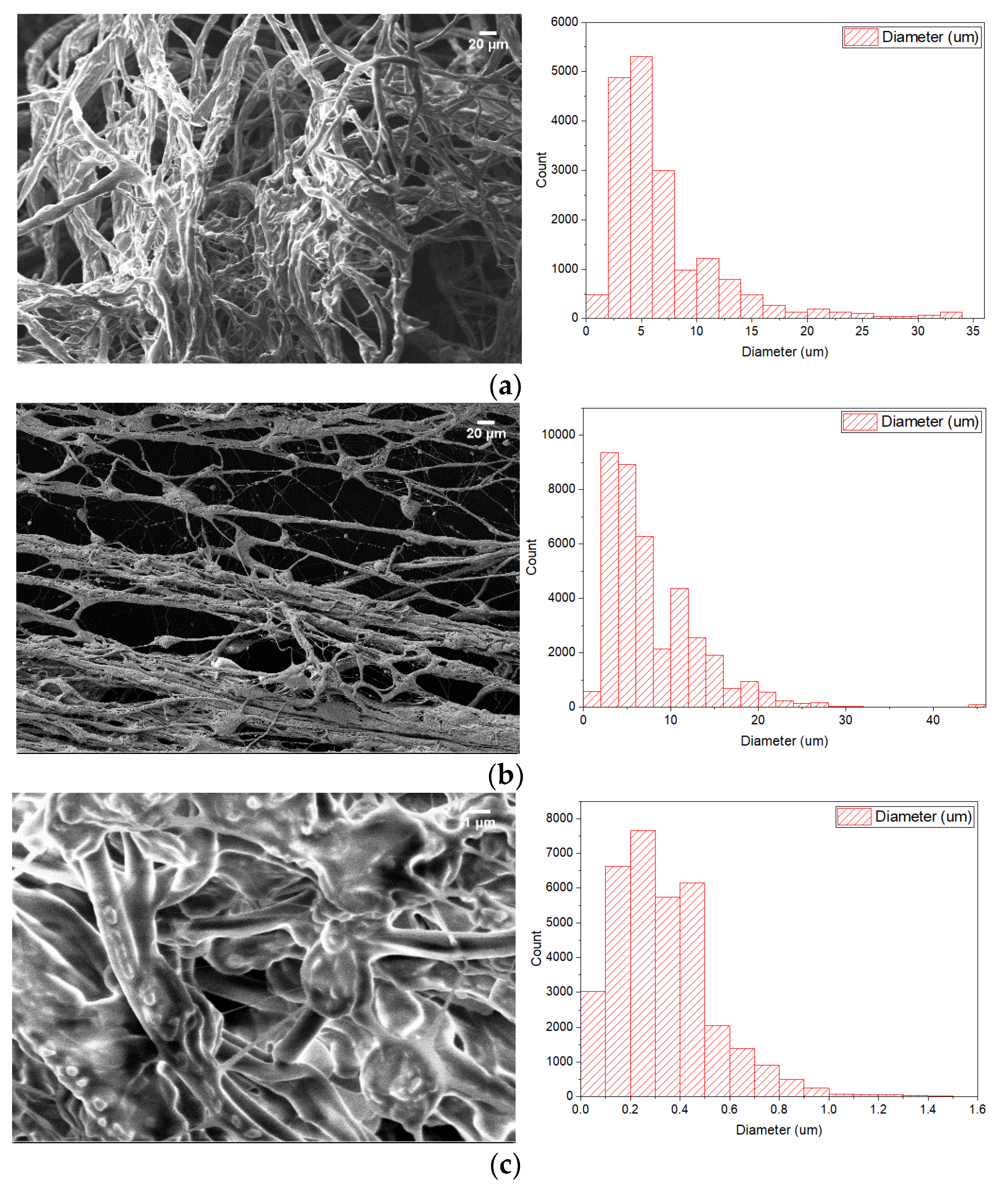
3.3. Experimental Study of PCM Electrospun Fiber Matrix
4. Discussion
4.1. Experimental Identification of Pure PCM
4.2. Experimental Identification of PCM Emulsions
4.3. Experimental Study of PCM Electrospun Fiber Matrix
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbol | Definition | Unit |
| Latent heat of melting for encapsulated PCM | J/g | |
| Latent heat of melting for PCM | J/g | |
| Latent heat of solidification for encapsulated PCM | J/g | |
| Latent heat of solidification for PCM | J/g | |
| n (%) | Encapsulation ratio | - |
| Tm | Melting temperature | °C |
| Ts | Solidification temperature | °C |
| v/v (%) | Volume/Volume | mL/mL |
| w/v (%) | Weight/Volume | g/mL |
| ε(%) | Encapsulation efficiency | - |
| Abbreviations | ||
| DSC | Differential scanning calorimetry | |
| PCL | Polycaprolactone | |
| PCM | Phase change material | |
| SDS | Sodium dodecyl sulfate | |
| TES | Thermal energy storage | |
References
- De Gracia, A.; Cabeza, L.F. Phase Change Materials and Thermal Energy Storage for Buildings. Energy Build. 2015, 103, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Gerislioglu, B.; Bakan, G.; Ahuja, R.; Adam, J.; Mishra, Y.K.; Ahmadivand, A. The Role of Ge 2 Sb 2 Te 5 in Enhancing the Performance of Functional Plasmonic Devices. Mater. Today Phys. 2020, 12. [Google Scholar] [CrossRef]
- Gerislioglu, B.; Dirisaglik, F.; Jurado, Z.; Sullivan, L.; Dana, A. Extracting the Temperature Distribution on a Phase-Change Memory Cell during Crystallization. J. Appl. Phys. 2016, 120, 164504. [Google Scholar] [CrossRef] [Green Version]
- Fleischer, A.S. Thermal Energy Storage Using Phase Change Materials Fundamentals and Applications; Springer International Publishing AG: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Kośny, J. PCM-Enhanced Building Components; Springer International Publishing AG: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of Solid-Liquid Phase Change Materials and Their Encapsulation Technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Letcher, T.M. Storing Energy: With Special Reference to Renewable Energy Sources. Chem. Int. 2016, 38. [Google Scholar] [CrossRef]
- Salunkhe, P.B.; Shembekar, P.S. A Review on Effect of Phase Change Material Encapsulation on the Thermal Performance of a System. Renew. Sustain. Energy Rev. 2012, 16, 5603–5616. [Google Scholar] [CrossRef]
- Park, J.S. Electrospinning and Its Applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1. [Google Scholar] [CrossRef] [Green Version]
- Perez-Masia, R.; Lopez-Rubio, A.; Fabra, M.J.; Lagaron, J.M. Biodegradable Polyester-Based Heat Management Materials of Interest in Refrigeration and Smart Packaging Coatings. J. Appl. Polym. Sci. 2013, 130, 3251–3262. [Google Scholar] [CrossRef]
- Chalco-Sandoval, W.; Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Development of an Encapsulated Phase Change Material via Emulsion and Coaxial Electrospinning. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Maccarini, A.; Hultmark, G.; Bergsøe, N.C.; Afshari, A. Free Cooling Potential of a PCM-Based Heat Exchanger Coupled with a Novel HVAC System for Simultaneous Heating and Cooling of Buildings. Sustain. Cities Soc. 2018, 42, 384–395. [Google Scholar] [CrossRef]
- Paroutoglou, E.; Afshari, A.; Bergsøe, N.C.; Fojan, P.; Hultmark, G. A PCM Based Cooling System for Office Buildings: A State of the Art Review. E3S Web Conf. 2019, 111, 01026. [Google Scholar] [CrossRef] [Green Version]
- Rubitherm Technologies GmbH. Available online: https://www.rubitherm.eu/en/about-us.html (accessed on 12 February 2021).
- Pure Temp LLC. Available online: https://www.puretemp.com/ (accessed on 12 February 2021).
- Imagej Image Processing Program. Available online: http://imagej.nih.gov/ij/ (accessed on 12 February 2021).
- Christiansen, L.; Fojan, P. Solution Electrospinning of Particle-Polymer Composite Fibres. Manuf. Rev. 2016, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wang, L.; Huang, Y. A Novel Shape-Stabilized PCM: Electrospun Ultrafine Fibers Based on Lauric Acid/Polyethylene Terephthalate Composite. Mater. Lett. 2008, 62, 3515–3517. [Google Scholar] [CrossRef]
- Hu, W.; Yu, X. Encapsulation of Bio-Based PCM with Coaxial Electrospun Ultrafine Fibers. RSC Adv. 2012, 2, 5580–5584. [Google Scholar] [CrossRef]
- Zdraveva, E.; Fang, J.; Mijovic, B.; Lin, T. Electrospun Poly(Vinyl Alcohol)/Phase Change Material Fibers: Morphology, Heat Properties, and Stability. Ind. Eng. Chem. Res. 2015, 54, 8706–8712. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Innovative Design of Microencapsulated Phase Change Materials for Thermal Energy Storage and Versatile Applications: A Review. Sustain. Energy Fuels 2019, 3, 1091–1149. [Google Scholar] [CrossRef]
- Paroutoglou, E.; Fojan, P.; Hultmark, G.; Afshari, A. Investigation of Thermal Behaviour of Paraffins, Fatty Acids, Salt Hydrates and Renewable Based Oils as PCM, Procceedings of International Renewable Energy Storage Conference, Webinar, 2020. In Atlantis Highlights in Engineering; Atlantis Press: Paris, France, 2021; p. 7, accepted. [Google Scholar]
- Behzadi, S.; Farid, M.M. Long Term Thermal Stability of Organic PCMs. Appl. Energy 2014, 122, 11–16. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.; Tyagi, V.V. Long-Term Thermal and Chemical Reliability Study of Different Organic Phase Change Materials for Thermal Energy Storage Applications. J. Therm. Anal. Calorim. 2016, 124, 1357–1366. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal Stability of Phase Change Materials Used in Latent Heat Energy Storage Systems: A Review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Shukla, A.; Buddhi, D.; Sawhney, R.L. Thermal Cycling Test of Few Selected Inorganic and Organic Phase Change Materials. Renew. Energy 2008, 33, 2606–2614. [Google Scholar] [CrossRef]
- Hasan, A.; Sayigh, A. Some Fatty Acids as PCM Energy Storage Materials. Renew. Energy 1994, 4, 69–76. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, J.; Wu, J.Y. Development and Characterization of Novel and Stable Silicon Nanoparticles-Embedded PCM-in-Water Emulsions for Thermal Energy Storage. Appl. Energy 2019, 238, 1407–1416. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J.Y.; Niu, J. PCM-in-Water Emulsion for Solar Thermal Applications: The Effects of Emulsifiers and Emulsification Conditions on Thermal Performance, Stability and Rheology Characteristics. Sol. Energy Mater. Sol. Cells 2016, 147, 211–224. [Google Scholar] [CrossRef]
- Günther, E.; Schmid, T.; Mehling, H.; Hiebler, S.; Huang, L. Subcooling in Hexadecane Emulsions. Int. J. Refrig. 2010, 33, 1605–1611. [Google Scholar] [CrossRef]








| Classification | Material | Phase Change Temperature (°C) | Latent Heat (J/g) |
|---|---|---|---|
| Organic non-paraffins | PT 15 15 | 15 | 182 |
| PT 18 15 | 18 | 192 | |
| Organic paraffins | RT 15 14 | 10–17 | 155 |
| RT 18 14 | 17–19 | 260 |
| Cases | Sheath Solution Concentration (% w/v) | Flow Rate (mL/h) Sheath Solution | Flow Rate (mL/h) Core Solution |
|---|---|---|---|
| 1st | 9% | 0.6 | 0.3 |
| 2nd | 9% | 0.5 | 0.5 |
| 3rd | 12% | 0.5 | 0.5 |
| Emulsions | PT15 | PT18 | RT15 | RT18 |
|---|---|---|---|---|
| Average emulsion size (um) | 5.23 ± 0.20 | 11.00 ± 0.42 | 8.95 ± 1.33 | 6.61 ± 0.46 |
| PCM | Case | Melting Temperature (°C) | Enthalpy (J/g) | Solidification Temperature (°C) | Enthalpy (J/g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCM | PCL | PCM | PCL | PCM | PCL | PCM | PCL | |||
| Max Peak | Min Peak | |||||||||
| PT15 | 1st | 13.79 | 54.32 | 64.32 | 10.32 | 10.67 | 37.14 | 55.85 | 11.43 | |
| 2nd | 13.42 | 53.42 | 64.42 | 6.90 | 9.83 | 37.06 | 61.68 | 8.25 | ||
| 3rd | 13.52 | 54.38 | 84.71 | 4.50 | 10.36 | 36.82 | 81.37 | 5.83 | ||
| PT18 | 1st | 18.53 | 49.80 | 82.41 | 11.54 | 13.74 | 34.29 | 54.55 | 11.47 | |
| 2nd | 18.08 | 49.00 | 85.30 | 8.08 | 13.31 | 34.47 | 66.49 | 9.40 | ||
| 3rd | 18.46 | 51.06 | 82.24 | 8.48 | 13.93 | 34.47 | 74.64 | 11.42 | ||
| RT15 | 1st | 18.04 | 54.32 | 49.75 | 11.40 | 20.11 | 17.45 | 37.62 | 44.79 | 12.96 |
| 2nd | 16.43 | 52.73 | 40.08 | 3.72 | 15.95 | 18.25 | 34 | 44.21 | 4.49 | |
| 3rd | 16.82 | 53.81 | 47.11 | 10.68 | 16.18 | 21.29 | 36.95 | 42.34 | 11.89 | |
| RT18 | 1st | 17.19 | 55.20 | 80.26 | 12.07 | 14.85 | 37.88 | 56.24 | 13.79 | |
| 2nd | 17.40 | 53.99 | 109.00 | 5.67 | 14.57 | 37.60 | 88.12 | 7.61 | ||
| 3rd | 17.33 | 54.63 | 102.10 | 6.11 | 15.21 | 37.42 | 82.21 | 7.30 | ||
| PCM | PCL | Core Flow Rate (mL/h) | Shell Flow Rate (mL/h) | Encapsulation Ratio n (%) | Encapsulation Efficiency ε (%) | Mean Fiber Diameter (um) | Fiber Diameter SD (um) |
|---|---|---|---|---|---|---|---|
| PT15 | 9% | 0.3 | 0.6 | 60.98 | 57.45 | 0.1926 ± 0.0016 | 0.1641 |
| 0.5 | 0.5 | 61.08 | 60.29 | 2.2934 ± 0.0057 | 0.5422 | ||
| 12% | 0.5 | 0.5 | 80.31 | 79.40 | 4.5659 ± 0.0175 | 2.3694 | |
| PT18 | 9% | 0.3 | 0.6 | 66.03 | 55.77 | 0.2314 ± 0.0008 | 0.0919 |
| 0.5 | 0.5 | 68.35 | 72.57 | 5.4409 ± 0.0478 | 2.8615 | ||
| 12% | 0.5 | 0.5 | 65.90 | 75 | 5.5630 ± 0.0200 | 3.9536 | |
| RT15 | 9% | 0.3 | 0.6 | 50.98 | 48.70 | 0.1176 ± 0.0003 | 0.0401 |
| 0.5 | 0.5 | 41.07 | 43.42 | 8.1486 ± 0.0764 | 7.1189 | ||
| 12% | 0.5 | 0.5 | 48.28 | 46.08 | 0.2714 ± 0.0009 | 0.1593 | |
| RT18 | 9% | 0.3 | 0.6 | 58.68 | 49.65 | 0.1482 ± 0.0003 | 0.0521 |
| 0.5 | 0.5 | 79.69 | 71.71 | 0.2174 ± 0.0013 | 0.1663 | ||
| 12% | 0.5 | 0.5 | 74.65 | 67.05 | 0.8022 ± 0.0017 | 0.3261 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paroutoglou, E.; Fojan, P.; Gurevich, L.; Hultmark, G.; Afshari, A. Thermal Analysis of Organic and Nanoencapsulated Electrospun Phase Change Materials. Energies 2021, 14, 995. https://doi.org/10.3390/en14040995
Paroutoglou E, Fojan P, Gurevich L, Hultmark G, Afshari A. Thermal Analysis of Organic and Nanoencapsulated Electrospun Phase Change Materials. Energies. 2021; 14(4):995. https://doi.org/10.3390/en14040995
Chicago/Turabian StyleParoutoglou, Evdoxia, Peter Fojan, Leonid Gurevich, Göran Hultmark, and Alireza Afshari. 2021. "Thermal Analysis of Organic and Nanoencapsulated Electrospun Phase Change Materials" Energies 14, no. 4: 995. https://doi.org/10.3390/en14040995






