Influence of Thermal Aging on the Combustion Characteristics of Cables in Nuclear Power Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Accelerated Aging Method
2.3. Cone Calorimeter FTIR
2.4. SEM-EDS
3. Results and Discussions
3.1. TG Analysis
3.2. Combustion Characteristics in the Early Period
3.3. Combustion Characteristics in the Mid to Late Period
3.4. Emission Characteristics of Toxic Gases
3.5. SEM-EDS Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chi, X.; Li, J.; Ji, M.; Liu, W.; Li, S. Thermal-oxidative aging effects on the dielectric properties of nuclear cable insulation. Materials 2020, 13, 2215. [Google Scholar] [CrossRef]
- Gillen, K.T.; Bernstein, R. Review of Nuclear Power Plant Safety Cable Aging Studies with Recommendations for Improved Approaches and for Future Work; Sandia Report; SAND 2010-7266; Sandia National Laboratories: Albuquerque, NM, USA, 2010. [Google Scholar]
- Meinier, R.; Sonnier, R.; Zavaleta, P.; Suard, S.; Ferry, L. Fire behavior of halogen-free flame retardant electrical cables with the cone calorimeter. J. Hazard. Mater. 2018, 342, 306–316. [Google Scholar] [CrossRef]
- Verardi, L.; Fabiani, D.; Montanari, G.C. Electrical aging markers for EPR-based low-voltage cable insulation wiring of nuclear power plants. Radiat. Phys. Chem. 2014, 94, 166–170. [Google Scholar] [CrossRef]
- Santhosh, T.V.; Gopika, V.; Ghosh, A.K.; Fernandes, B.G. An approach for reliability prediction of instrumentation & control cables by artificial neural networks and Weibull theory for probabilistic safety assessment of NPPs. Reliab. Eng. Syst. Saf. 2018, 170, 31–44. [Google Scholar] [CrossRef]
- Hashemian, H.M.; McConkey, B.; Harmon, G.; Sexton, C. Methods for testing nuclear power plant cables. IEEE Instrum. Meas. Mag. 2013, 16, 31–36. [Google Scholar] [CrossRef]
- Bouguedad, D.; Mekhaldi, A.; Jbara, O.; Rondot, S.; Hadjadj, A.; Douglade, J.; Dony, P. Physico-chemical study of thermally aged EPDM used in power cables insulation. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3207–3215. [Google Scholar] [CrossRef]
- Boukezzi, L.; Rondot, S.; Jbara, O.; Boubakeur, A. Study of thermal aging effects on the conduction and trapping of charges in XLPE cable insulations under electron beam irradiation. Radiat. Phys. Chem. 2018, 149, 110–117. [Google Scholar] [CrossRef]
- Bowler, N.; Liu, S. Aging mechanisms and monitoring of cable polymers. Int. J. Progn. Heal. Manag. 2015, 6. [Google Scholar] [CrossRef]
- Pfaendner, R. (Photo)oxidative degradation and stabilization of flame retarded polymers. Polym. Degrad. Stab. 2013, 98, 2430–2435. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, Z.; Wu, K.; Wang, S.; Li, J.; Li, S. Influence of Oxygen Diffusion on Thermal Ageing of Cross-Linked Polyethylene Cable Insulation. Materials 2020, 13, 2056. [Google Scholar] [CrossRef]
- Šarac, T.; Quiévy, N.; Gusarov, A.; Konstantinović, M.J. Influence of γ-irradiation and temperature on the mechanical properties of EPDM cable insulation. Radiat. Phys. Chem. 2016, 125, 151–155. [Google Scholar] [CrossRef]
- Min, D.; Yan, C.; Huang, Y.; Li, S.; Ohki, Y. Dielectric and carrier transport properties of silicone rubber degraded by gamma irradiation. Polymers 2017, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhao, Y.; Han, Z.; Wang, X.; Wang, Y. Thermal Effect of Different Laying Modes on Cross-Linked Polyethylene (XLPE) Insulation and a New Estimation on Cable Ampacity. Energies 2019, 12, 2994. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.D.; Kim, J.H. Investigation on the insulation resistance characteristics of low voltage cable. Energies 2020, 13, 3611. [Google Scholar] [CrossRef]
- Zhang, B.S.; Zhang, J.Q.; Li, Q.; Wang, L.F.; Xie, H.; Fan, M.H. Effects of Insulating Material Ageing on Ignition Time and Heat Release Rate of the Flame Retardant Cables. Procedia Eng. 2018, 211, 972–978. [Google Scholar] [CrossRef]
- IEEE Std. 383-1974. IEEE Standard for Type Test of Class 1E Electric Cables, Field Splices, and Connections for Nuclear Power Generating Stations; IEEE: New York, NY, USA, 1974.
- IEEE Std. 1202-1991. IEEE Standard for Flame Testing of Cable Tray in Industrial and Commercial Occupancies; IEEE: New York, NY, USA, 1991.
- NUREG-0800. Standard Review Plan for the Review of Safety Analysis Reports for Nuclear Power Plants: LWR Edition; Nuclear Regulatory Commission (NRC): Rockville, MD, USA, 1987.
- Jiang, Z.; Liu, Z.; Fei, B.; Cai, Z.; Yu, Y.; Liu, X. The pyrolysis characteristics of moso bamboo. J. Anal. Appl. Pyrolysis 2012, 94, 48–52. [Google Scholar] [CrossRef]
- Farag, S.; Chaouki, J. A modified microwave thermo-gravimetric-analyzer for kinetic purposes. Appl. Therm. Eng. 2015, 75, 65–72. [Google Scholar] [CrossRef]
- Seo, H.J.; Kim, N.K.; Lee, M.C.; Lee, S.K.; Moon, Y.S. Investigation into the toxicity of combustion products for CR/EPR cables based on aging period. J. Mech. Sci. Technol. 2020, 34, 1785–1794. [Google Scholar] [CrossRef]
- Xu, L.; Li, S.; Sun, W.; Ma, X.; Cao, S. Combustion behaviors and characteristic parameters determination of sassafras wood under different heating conditions. Energy 2020, 203. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Apaydin-Varol, E.; Polat, S.; Putun, A.E. Pyrolysis kinetics and thermal decomposition behavior of polycarbonate—A TGA-FTIR study. Therm. Sci. 2014, 18, 833–842. [Google Scholar] [CrossRef]
- ISO 5660-1. Reaction to Fire tests—Heat release, Smoke Production and Mass Loss Rate—Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement); ISO: Geneva, Switzerland, 2015.
- ISO 19702. Guidance for Sampling and Analysis of Toxic Gases and Vapours in Fire Effluents Using Fourier Transform Infrared (FTIR) Spectroscopy; ISO: Geneva, Switzerland, 2015.
- Fateh, T.; Rogaume, T.; Luche, J.; Richard, F.; Jabouille, F. Characterization of the thermal decomposition of two kinds of plywood with a cone calorimeter—FTIR apparatus. J. Anal. Appl. Pyrolysis 2014, 107, 87–100. [Google Scholar] [CrossRef]
- Puente, E.; Lázaro, D.; Alvear, D. Study of tunnel pavements behaviour in fire by using coupled cone calorimeter—FTIR analysis. Fire Saf. J. 2016, 81, 1–7. [Google Scholar] [CrossRef]
- Luche, J.; Rogaume, T.; Richard, F.; Guillaume, E. Characterization of thermal properties and analysis of combustion behavior of PMMA in a cone calorimeter. Fire Saf. J. 2011, 46, 451–461. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM-EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Genestar, C.; Pons, C. Earth pigments in painting: Characterisation and differentiation by means of FTIR spectroscopy and SEM-EDS microanalysis. Anal. Bioanal. Chem. 2005, 382, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Damartzis, T.; Vamvuka, D.; Sfakiotakis, S.; Zabaniotou, A. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour. Technol. 2011, 102, 6230–6238. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, D.H. Thermal analysis and statistical evaluation of EPR used in nuclear power plants. In Proceedings of the 2015 IEEE Electrical Insulation Conference (EIC), Seattle, WA, USA, 7–10 June 2015; pp. 5–8. [Google Scholar] [CrossRef]
- Kim, J.; Yang, J.; Lee, G.; Seong, B.; Bang, J.; Park, D. Mechanical Properties and Statistical Evaluation of EPR According to the Accelerated Degradation. J. Korean Inst. Electr. Electron. Mater. Eng. 2015, 28, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Marquis, D.; Guillaume, E.; Lesenechal, D. Accuracy (trueness and precision) of cone calorimeter tests with and without a vitiated air enclosure. Procedia Eng. 2013, 62, 103–119. [Google Scholar] [CrossRef] [Green Version]
- Jiao, C.; Chen, X.; Zhang, J. Synergistic effects of Fe2O3 with layered double hydroxides in EVA/LDH composites. J. Fire Sci. 2009, 27, 465–479. [Google Scholar] [CrossRef]
- Wang, B.; Tang, Q.; Hong, N.; Song, L.; Wang, L.; Shi, Y.; Hu, Y. Effect of cellulose acetate butyrate microencapsulated ammonium polyphosphate on the flame retardancy, mechanical, electrical, and thermal properties of intumescent flame-retardant ethylene-vinyl acetate copolymer/microencapsulated ammonium polyphosphate/. ACS Appl. Mater. Interfaces 2011, 3, 3754–3761. [Google Scholar] [CrossRef] [PubMed]
- Clough, R.L. Aging Effects on Fire-Retardant Additives in Organic Materials for Nuclear Plant Applications. In NUREG/CR-2868 SAND82-0485; Nuclear Regulatory Commission (NRC): Rockville, MD, USA, 1982. [Google Scholar]
- Nowlen, S.P. An Investigation of the Effects of Thermal Aging on, the Fire Damageability of Electric Cables. In NUREG/CR-5619 SAND90-2121; Nuclear Regulatory Commission (NRC): Rockville, MD, USA, 1991. [Google Scholar]
- Lee, S.K.; Moon, Y.S.; Yoo, S.Y. A Study on Validation Methodology of Fire Retardant Performance for Cables in Nuclear Power Plants. J. Korean Soc. Saf. 2017, 32, 140–144. [Google Scholar] [CrossRef]
- Seo, H.J.; Kim, N.K.; Lee, M.C.; Lee, S.K.; Moon, Y.S. An Experimental Study on the Combustion Characteristics of Non Class 1E Cables. J. Korean Soc. Combust. 2019, 24, 15–24. [Google Scholar] [CrossRef]
- Gallina, G.; Bravin, E.; Badalucco, C.; Audisio, G.; Armanini, M.; De Chirico, A.; Provasoli, F. Application of cone calorimeter for the assessment of class of flame retardants for polypropylene. Fire Mater. 1998, 22, 15–18. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, X.; Fan, W.; Hu, Y.; Qu, B.; Gui, Z. Effects of poly(ethylene-co-propylene) elastomer on mechanical properties and combustion behaviour of flame retarded polyethylene/magnesium hydroxide composites. Polym. Int. 2002, 51, 653–657. [Google Scholar] [CrossRef]
- Wang, C.; Wu, Y.; Liu, Q.; Yang, H.; Wang, F. Analysis of the behaviour of pollutant gas emissions during wheat straw/coal cofiring by TG-FTIR. Fuel Process. Technol. 2011, 92, 1037–1041. [Google Scholar] [CrossRef]
- Beneš, M.; Milanov, N.; Matuschek, G.; Kettrup, A.; Plaček, V.; Balek, V. Thermal degradation of PVC cable insulation studied by simultaneous TG-FTIR and TG-EGA methods. J. Therm. Anal. Calorim. 2004, 78, 621–630. [Google Scholar] [CrossRef]
- Zhu, H.M.; Jiang, X.G.; Yan, J.H.; Chi, Y.; Cen, K.F. TG-FTIR analysis of PVC thermal degradation and HCl removal. J. Anal. Appl. Pyrolysis 2008, 82, 1–9. [Google Scholar] [CrossRef]
- Subramaniam, K.; Das, A.; Häußler, L.; Harnisch, C.; Stöckelhuber, K.W.; Heinrich, G. Enhanced thermal stability of polychloroprene rubber composites with ionic liquid modified MWCNTs. Polym. Degrad. Stab. 2012, 97, 776–785. [Google Scholar] [CrossRef]
- Wang, B.; Sheng, H.; Shi, Y.; Song, L.; Zhang, Y.; Hu, Y.; Hu, W. The influence of zinc hydroxystannate on reducing toxic gases (CO, NOx and HCN) generation and fire hazards of thermoplastic polyurethane composites. J. Hazard. Mater. 2016, 314, 260–269. [Google Scholar] [CrossRef]
- Chen, S.I.; Sheu, Y.L.; Sheu, J.L.; Lee, C.T.; Lin, J.S. Morphology of perfluoroalkylacrylate/stearyl methacrylate polymers and their effect on water/oil repellency. J. Appl. Polym. Sci. 1997, 63, 903–909. [Google Scholar] [CrossRef]
- Errifai, I.; Jama, C.; Le Bras, M.; Delobel, R.; Gengembre, L.; Mazzah, A.; De Jaeger, R. Elaboration of a fire retardant coating for polyamide-6 using cold plasma polymerization of a fluorinated acrylate. Surf. Coatings Technol. 2004, 180–181, 297–301. [Google Scholar] [CrossRef]
- Iezzi, R.A.; Gaboury, S.; Wood, K. Acrylic-fluoropolymer mixtures and their use in coatings.pdf. Prog. Org. Coat. 2000, 40, 55–60. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Chang-Liao, K.S.; Wang, T.K.; Kuo, C.T. Monitoring the moisture-related degradation of ethylene propylene rubber cable by electrical and SEM methods. Polym. Degrad. Stab. 2006, 91, 2357–2364. [Google Scholar] [CrossRef]
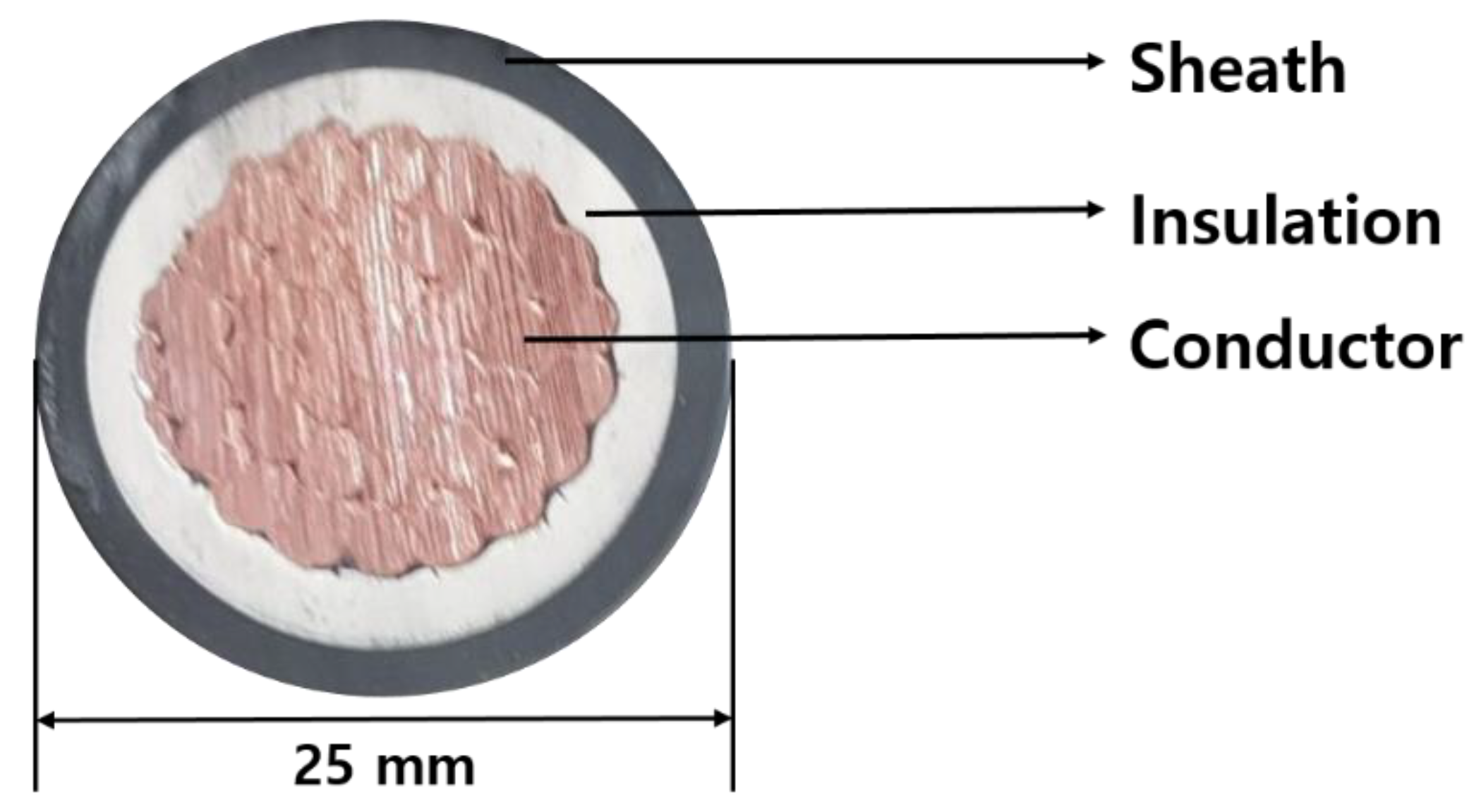












| Division | Detail | |
|---|---|---|
| Application | Power/Control | |
| Voltage [V] | 600 | |
| Outer diameter [mm] | 25 | |
| Material properties | Sheath | Polychloroprene rubber (CR) |
| Insulation | Ethylene propylene rubber (EPR) | |
| Conductor | Copper | |
| Test Condition | Value |
|---|---|
| Size of specimens (mm3) | 100 × 100 × 25 |
| The number of tests for each cable | 3 |
| Test running time (min) | 20 |
| Heat flux (kW/m2) | 50 |
| Exhaust flow (m3/s) | 0.024 ± 0.002 |
| Aging Period [y] | Required Time [h] | ||
|---|---|---|---|
| Ea = 1.69 eV (The Present Study) | Ea = 1.23 eV (Ref. [34]) | Ea = 1.69 eV (The Present Study) | |
| 10 | 157 | 887 | 2256 |
| 20 | 315 | 1774 | 4511 |
| 30 | 472 | 2660 | 6767 |
| 40 | 630 | 3547 | 9022 |
| Parameter | Number of Repeated Tests | Aging Period [y] | Mean | SD | SD/Mean (%) |
|---|---|---|---|---|---|
| PHRR [kW/m2] | 3 | Non-aged | 146.19 | 3.08 | 2.11% |
| 10 | 131.14 | 2.32 | 1.77% | ||
| 20 | 125.23 | 1.77 | 1.41% | ||
| 30 | 92.05 | 3.70 | 4.02% | ||
| 40 | 99.32 | 1.96 | 1.97% | ||
| TTI [s] | 3 | Non-aged | 38 | 0.58 | 1.52% |
| 10 | 44 | 12.66 | 28.77% | ||
| 20 | 31 | 3.46 | 11.16% | ||
| 30 | 73 | 49.10 | 67.26% | ||
| 40 | 56 | 42.20 | 75.36% | ||
| tPHRR [s] | 3 | Non-aged | 72 | 2.65 | 3.68% |
| 10 | 88 | 4.93 | 5.60% | ||
| 20 | 80 | 3.79 | 4.74% | ||
| 30 | 88 | 11.93 | 13.56% | ||
| 40 | 83 | 9.50 | 11.45% | ||
| FPI [s-m2/kW] | 3 | Non-aged | 0.262 | 0.005 | 1.88% |
| 10 | 0.333 | 0.094 | 28.26% | ||
| 20 | 0.248 | 0.027 | 10.73% | ||
| 30 | 0.789 | 0.505 | 64.01% | ||
| 40 | 0.560 | 0.396 | 70.71% | ||
| FGI [kW/m2-s] | 3 | Non-aged | 2.030 | 0.096 | 4.71% |
| 10 | 1.490 | 0.073 | 4.92% | ||
| 20 | 1.565 | 0.037 | 2.33% | ||
| 30 | 1.046 | 0.244 | 23.33% | ||
| 40 | 1.197 | 0.113 | 9.42% | ||
| Initial mass [g] | 3 | Non-aged | 719 | 10.15 | 1.41% |
| 10 | 667 | 2.94 | 0.44% | ||
| 20 | 645 | 6.31 | 0.98% | ||
| 30 | 650 | 5.11 | 0.79% | ||
| 40 | 619 | 7.63 | 1.23% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.H.; Seo, H.J.; Lee, S.K.; Lee, M.C. Influence of Thermal Aging on the Combustion Characteristics of Cables in Nuclear Power Plants. Energies 2021, 14, 2003. https://doi.org/10.3390/en14072003
Kim MH, Seo HJ, Lee SK, Lee MC. Influence of Thermal Aging on the Combustion Characteristics of Cables in Nuclear Power Plants. Energies. 2021; 14(7):2003. https://doi.org/10.3390/en14072003
Chicago/Turabian StyleKim, Min Ho, Hyun Jeong Seo, Sang Kyu Lee, and Min Chul Lee. 2021. "Influence of Thermal Aging on the Combustion Characteristics of Cables in Nuclear Power Plants" Energies 14, no. 7: 2003. https://doi.org/10.3390/en14072003
APA StyleKim, M. H., Seo, H. J., Lee, S. K., & Lee, M. C. (2021). Influence of Thermal Aging on the Combustion Characteristics of Cables in Nuclear Power Plants. Energies, 14(7), 2003. https://doi.org/10.3390/en14072003








