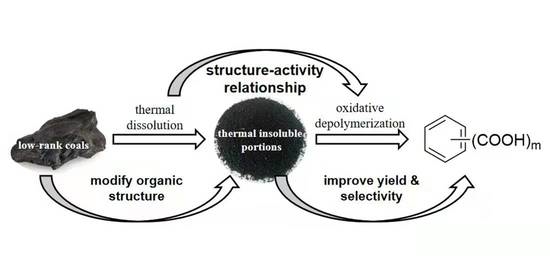
Insight into Relationship between Thermal Dissolution of Low-Rank Coals and Their Subsequent Oxidative Depolymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Method
2.3. Analytical Method
3. Results
3.1. Oxidation of LRCs and its Thermal Insoluble Portions
3.2. Investigation on the Cause of Thermal Dissolution Improving Oxidation of LRCs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rao, Z.; Zhao, Y.; Huang, C.-L.; Duan, C.; He, J. Recent developments in drying and dewatering for low rank coals. Prog. Energy Combust. Sci. 2015, 46, 1–11. [Google Scholar] [CrossRef]
- Liu, F.-J.; Wei, X.-Y.; Fan, M.; Zong, Z.-M. Separation and structural characterization of the value-added chemicals from mild degradation of lignites: A review. Appl. Energy 2016, 170, 415–436. [Google Scholar] [CrossRef]
- Li, C.-Z. Importance of volatile–char interactions during the pyrolysis and gasification of low-rank fuels—A review. Fuel 2013, 112, 609–623. [Google Scholar] [CrossRef]
- Nolan, P.; Shipman, A.; Rui, H. Coal Liquefaction, Shenhua Group, and China’s Energy Security. Eur. Manag. J. 2004, 22, 150–164. [Google Scholar] [CrossRef]
- Gong, L.; Hou, Y.; Wu, W.; Yang, F.; Ren, S. Catalytic O2 oxidation of lignite to carboxylic acids with iron-based catalysts in acidic aqueous solutions. Fuel Process. Technol. 2019, 191, 54–59. [Google Scholar] [CrossRef]
- Wang, Y.-G.; Bo, C.-H.; Shen, J.; Wang, Z.-L.; Niu, Y.-X.; Wei, X.-Y. Effect of pyrolysis on Zhaotong lignite oxidation with aqueous sodium hypochlorite. Carbon Resour. Convers. 2021, 4, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Zong, Z.-M.; Zhang, Y.-Y.; Wei, X.-Y.; Zhu, Y. Thermal treatment of Shengli lignite and subsequent oxidation. J. Anal. Appl. Pyrolysis 2020, 152, 104810. [Google Scholar] [CrossRef]
- Ren, H.; Sun, J.; Zhao, Q.; Zhou, Q.; Ling, Q. Synthesis and characterization of a novel heat resistant epoxy resin based on N,N′-bis(5-hydroxy-1-naphthyl)pyromellitic diimide. Polymer 2008, 49, 5249–5253. [Google Scholar] [CrossRef]
- Wang, X.-L.; Li, J.; Lin, H.-Y.; Hu, H.-L.; Chen, B.-K.; Mu, B. Synthesis, structures and electrochemical properties of two novel metal–organic coordination complexes based on trimesic acid (H3BTC) and 2,5-bis(3-pyridyl)-1,3,4-oxadiazole (BPO). Solid State Sci. 2009, 11, 2118–2124. [Google Scholar] [CrossRef]
- Hazra, S.; Deb, M.; Elias, A.J. Iodine catalyzed oxidation of alcohols and aldehydes to carboxylic acids in water: A metal-free route to the synthesis of furandicarboxylic acid and terephthalic acid. Green Chem. 2017, 19, 5548–5552. [Google Scholar] [CrossRef]
- Wang, W.; Hou, Y.; Wu, W.; Niu, M.; Liu, W. Production of Benzene Polycarboxylic Acids from Lignite by Alkali-Oxygen Oxidation. Ind. Eng. Chem. Res. 2012, 51, 14994–15003. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, W.; Liu, M.; Zhao, G.; Cao, Q.; Zhao, B.; Kou, K.; Gao, J. Oxidation of Zhundong subbituminous coal by Fe2+/H2O2 system under mild conditions. Korean J. Chem. Eng. 2020, 37, 597–603. [Google Scholar] [CrossRef]
- Wang, W.; Hou, Y.; Wu, W.; Niu, M.; Wu, T. High-Temperature Alkali-Oxygen Oxidation of Lignite to Produce Benzene Polycarboxylic Acids. Ind. Eng. Chem. Res. 2012, 52, 680–685. [Google Scholar] [CrossRef]
- Yang, F.; Hou, Y.; Wu, W.; Lu, T.; Liu, Z. Oxidation of Xiaolongtan lignite to oxygen-containing chemicals over NaVO3-H2SO4 by introducing methanol to suppress the formation of CO2. Fuel 2019, 236, 75–81. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Jiang, Y.; Yu, J.; Li, X.; Lucas, J. Solvent extraction of Chinese lignite and chemical structure changes of the residue during H2O2 oxidation. Fuel Process. Technol. 2015, 129, 213–221. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Chen, X.-H.; Ding, M.-J.; Li, J.-Z. Oxidation of Coal Pitch by H2O2 under Mild Conditions. Energy Fuels 2018, 32, 796–800. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Shen, J.; Niu, Z.; Niu, Y.; Wei, X. Investigation of organic structures in coal pitch based on separable selective destruction analysis method. J. China Coal Soc. 2021, 46, 1121–1129. [Google Scholar]
- Shi, Q.; Wang, J.; Zhou, X.; Xu, C.; Zhao, S.; Chung, K.H. Ruthenium Ion-Catalyzed Oxidation for Petroleum Molecule Structural Features: A Review. In Structure and Modeling of Complex Petroleum Mixtures; Springer: Singapore, 2015; pp. 71–91. [Google Scholar]
- Lv, J.; Wei, X.; Zhang, Y.; Zong, Z. Mild oxidation of Yanshan petroleum coke with aqueous sodium hypochlorite. Fuel 2018, 226, 658–664. [Google Scholar] [CrossRef]
- Lv, J.-H.; Wei, X.-Y.; Zhang, Y.-Y.; Zong, Z.-M.; Zhang, Y.-L.; Ma, M.-J.; Bai, H.-C. Benzenecarboxylic acid production by oxidation of Shenmu char powders with aqueous sodium hypochlorite. Fuel 2020, 278, 118194. [Google Scholar] [CrossRef]
- Yao, Z.-S.; Wei, X.-Y.; Lv, J.; Liu, F.-J.; Huang, Y.-G.; Xu, J.-J.; Chen, F.-J.; Huang, Y.; Li, Y.; Lu, Y.; et al. Oxidation of Shenfu Coal with RuO4 and NaOCl. Energy Fuels 2010, 24, 1801–1808. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, Y.; Tahmasebi, A.; Han, Y.; Li, X.; Lucas, J.; Wall, T. Coal Oxidation under Mild Conditions: Current Status and Applications. Chem. Eng. Technol. 2014, 37, 1635–1644. [Google Scholar] [CrossRef]
- Liu, F.-J.; Wei, X.-Y.; Zhu, Y.; Wang, Y.-G.; Li, P.; Fan, X.; Zhao, Y.-P.; Zong, Z.-M.; Zhao, W.; Wei, Y.-B. Oxidation of Shengli lignite with aqueous sodium hypochlorite promoted by pretreatment with aqueous hydrogen peroxide. Fuel 2013, 111, 211–215. [Google Scholar] [CrossRef]
- Liu, F.-J.; Wei, X.-Y.; Lu, Y.; Qing, Y.; Zhu, Y.; Li, L.; Lv, J.; Sun, B.; Yue, X.-M.; Zong, Z.-M.; et al. The Effect of Pretreatment with H2O2on the Oxidation of Shenfu Subbituminous Coal with NaOCl. Energy Sources Part A Recover. Util. Environ. Eff. 2013, 35, 1967–1974. [Google Scholar] [CrossRef]
- Yang, F.; Hou, Y.; Ren, S.; Wu, W. Selective oxidation of lignite to carboxyl chemicals. Sci. Sin. Chim. 2018, 48, 574–589. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.-X.; Wei, X.-Y.; Shui, H.-F.; Wang, Z.-C.; Gao, J.; Wei, C.; Cao, X.-Z.; Zong, Z.-M. Investigation on the macromolecular network structure of Xianfeng lignite by a new two-step depolymerization. Fuel 2013, 109, 49–53. [Google Scholar] [CrossRef]
- Kozliak, E.I.; Kubátová, A.; Artemyeva, A.A.; Nagel, E.; Zhang, C.; Rajappagowda, R.B.; Smirnova, A.L. Thermal Liquefaction of Lignin to Aromatics: Efficiency, Selectivity, and Product Analysis. ACS Sustain. Chem. Eng. 2016, 4, 5106–5122. [Google Scholar] [CrossRef]
- Lu, H.-Y.; Wei, X.-Y.; Yu, R.; Peng, Y.-L.; Qi, X.-Z.; Qie, L.-M.; Wei, Q.; Lv, J.; Zong, Z.-M.; Zhao, W.; et al. Sequential Thermal Dissolution of Huolinguole Lignite in Methanol and Ethanol. Energy Fuels 2011, 25, 2741–2745. [Google Scholar] [CrossRef]
- Chen, B.; Wei, X.-Y.; Zong, Z.-M.; Yang, Z.-S.; Qing, Y.; Liu, C. Difference in chemical composition of supercritical methanolysis products between two lignites. Appl. Energy 2011, 88, 4570–4576. [Google Scholar] [CrossRef]
- Li, S.; Zong, Z.-M.; Li, Z.-K.; Wang, S.-K.; Yang, Z.; Xu, M.-L.; Shi, C.; Wei, X.-Y.; Wang, Y.-G. Sequential thermal dissolution and alkanolyses of extraction residue from Xinghe lignite. Fuel Process. Technol. 2017, 167, 425–430. [Google Scholar] [CrossRef]
- Li, S.; Zong, Z.-M.; Liu, J.; Liu, F.-J.; Wei, X.-Y.; Liu, G.-H.; Wang, S.-K. Changes in oxygen-functional moieties during sequential thermal dissolution and methanolysis of the extraction residue from Zhaotong lignite. J. Anal. Appl. Pyrolysis 2019, 139, 40–47. [Google Scholar] [CrossRef]
- Liu, G.-H.; Zong, Z.-M.; Ma, Z.-H.; Liu, F.-J.; Wei, X.-Y.; Kang, Y.-H.; Fan, X.; Ma, F.Y.; Liu, J.M.; Mo, W.L. Observing the structural variation of Dahuangshan lignite and four derived residues by non-destructive techniques and flash pyrolysis. Fuel 2020, 269, 117335. [Google Scholar] [CrossRef]
- Nie, B.; Liu, X.; Yang, L.; Meng, J.; Li, X. Pore structure characterization of different rank coals using gas adsorption and scanning electron microscopy. Fuel 2015, 158, 908–917. [Google Scholar] [CrossRef]
- Pan, C.-X.; Liu, H.-L.; Liu, Q.; Shui, H.-F.; Wang, Z.-C.; Lei, Z.-P.; Kang, S.-G.; Wei, X.-Y. Oxidative depolymerization of Shenfu subbituminous coal and its thermal dissolution insoluble fraction. Fuel Process. Technol. 2017, 155, 168–173. [Google Scholar] [CrossRef]
- Qin, Z.-H.; Chen, H.; Yan, Y.-J.; Li, C.-S.; Rong, L.-M.; Yang, X.-Q. FTIR quantitative analysis upon solubility of carbon disulfide/N-methyl-2-pyrrolidinone mixed solvent to coal petrographic constituents. Fuel Process. Technol. 2015, 133, 14–19. [Google Scholar] [CrossRef]
- Li, Z.; Ni, G.; Wang, H.; Sun, Q.; Wang, G.; Jiang, B.; Zhang, C. Molecular structure characterization of lignite treated with ionic liquid via FTIR and XRD spectroscopy. Fuel 2020, 272, 117705. [Google Scholar]
- Zhao, J.; Xu, H.; Tang, D.; Mathews, J.P.; Li, S.; Tao, S. A comparative evaluation of coal specific surface area by CO2 and N2 adsorption and its influence on CH4 adsorption capacity at different pore sizes. Fuel 2016, 183, 420–431. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Choi, S.K.; Lu, Y. Surface properties and pore structure of anthracite, bituminous coal and lignite. Energies 2018, 11, 1502. [Google Scholar] [CrossRef] [Green Version]
- Ren, L.-F.; Deng, J.; Li, Q.-W.; Ma, L.; Zou, L.; Laiwang, B.; Shu, C.-M. Low-temperature exothermic oxidation characteristics and spontaneous combustion risk of pulverised coal. Fuel 2019, 252, 238–245. [Google Scholar] [CrossRef]







| Samples | Ultimate Analysis (wt%, daf) | C/H | fa | ||||
|---|---|---|---|---|---|---|---|
| C | H | N | Odiff | S | |||
| ZT | 57.57 | 5.71 | 1.73 | 34.30 | 0.69 | 0.84 | 0.47 |
| RZT | 65.76 | 5.94 | 2.16 | 25.53 | 0.61 | 0.92 | 0.52 |
| YN | 71.31 | 4.21 | 0.73 | 23.38 | 0.37 | 1.41 | 0.74 |
| RYN | 81.01 | 4.26 | 0.86 | 13.25 | 0.62 | 1.59 | 0.78 |
| Samples | Oxidative Weightlessness Rate (wt%) | Yield of BCAs (mg/g) | Yield of Alphatic Acids (mg/g) | Selectivity of BCAs (wt%) |
|---|---|---|---|---|
| ZT | 54.88 | 10.86 | 286.10 | 3.66 |
| RZT | 58.98 | 13.44 | 266.39 | 4.80 |
| YN | 70.65 | 64.46 | 369.12 | 14.86 |
| RYN | 77.80 | 76.71 | 320.54 | 19.31 |
| Samples | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|
| ZT | 2.48 | 0.015 |
| RZT | 6.46 | 0.029 |
| YN | 2.13 | 0.0089 |
| RYN | 2.35 | 0.0086 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, X.; Wang, Z.; Dong, C.; Shen, J.; Fan, X. Insight into Relationship between Thermal Dissolution of Low-Rank Coals and Their Subsequent Oxidative Depolymerization. Energies 2022, 15, 32. https://doi.org/10.3390/en15010032
Wang Y, Liu X, Wang Z, Dong C, Shen J, Fan X. Insight into Relationship between Thermal Dissolution of Low-Rank Coals and Their Subsequent Oxidative Depolymerization. Energies. 2022; 15(1):32. https://doi.org/10.3390/en15010032
Chicago/Turabian StyleWang, Yugao, Xiaochen Liu, Zhilei Wang, Chuan Dong, Jun Shen, and Xing Fan. 2022. "Insight into Relationship between Thermal Dissolution of Low-Rank Coals and Their Subsequent Oxidative Depolymerization" Energies 15, no. 1: 32. https://doi.org/10.3390/en15010032