Technologies for Deep Biogas Purification and Use in Zero-Emission Fuel Cells Systems
Abstract
:1. Introduction
- -
- Adsorption-based systems (solid sorbents)
- -
- Absorption-based systems (scrubbers)
- -
- Innovative cryogenic separation.
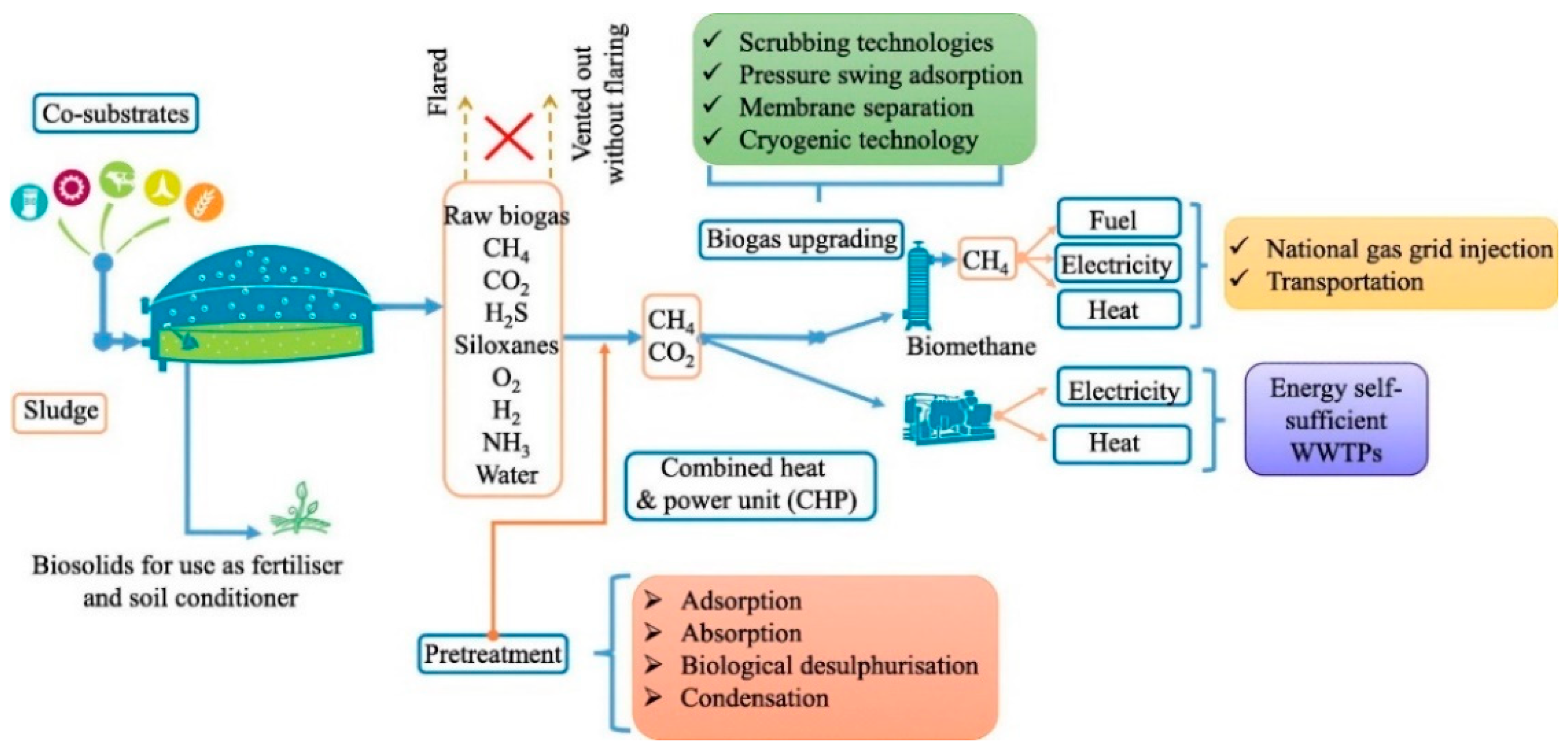
2. Biogas Production and Use
- Hydrolysis allows complex molecules to be cracked into their monomers through water consumption. The reaction can be simplified as shown in Equation (1).
- Acidogenesis. In this second phase, organic monomers are converted into other organic compounds such as organic acids and alcohol by the bacteria metabolism (fermentation). Carbon dioxide is also produced in this phase.
- Acetogenesis. In this third phase, the acetogenic bacteria sustain their metabolism by consuming the acids and alcohols produced by the acidogenic population.
- Methanogenesis. This last phase refers to the methane production as a metabolic leftover of methanogenic bacteria, which feed on the acetic acid produced in the previous step. Additionally, methane is also produced by CO2 reduction using elemental H2, which is a by-product of the previous reactions.
- Temperature optimum is reached if hydrolysis and acidogenesis occur under thermophilic conditions, whilst methanogenesis occurs under mesophilic conditions.
- pH optimum is between 5.5 to 6.5 for acidogenesis and 7.0 for methanogenesis, thus, a two-stage AD to separate hydrolysis and acidogenesis to methanogenesis is preferred.
- C/N ratio is optimized in a range of 25–30:1.
- Hydraulic Retention Time and Organic Loading Rate optimization require a low OLR coupled with a long HRT to maximize the methane yield.
2.1. Biogas Utilization Paths
2.2. Biogas Pollutants
2.2.1. Hydrogen Sulfide
2.2.2. Siloxanes
2.2.3. Volatile Organic Compounds and Halogens
3. Biogas Cleaning and Upgrading Technologies
3.1. Adsorption-Based Systems
3.1.1. Activated Carbons
3.1.2. Metal Oxides
Zinc Oxides
Copper Oxides
3.1.3. Zeolites
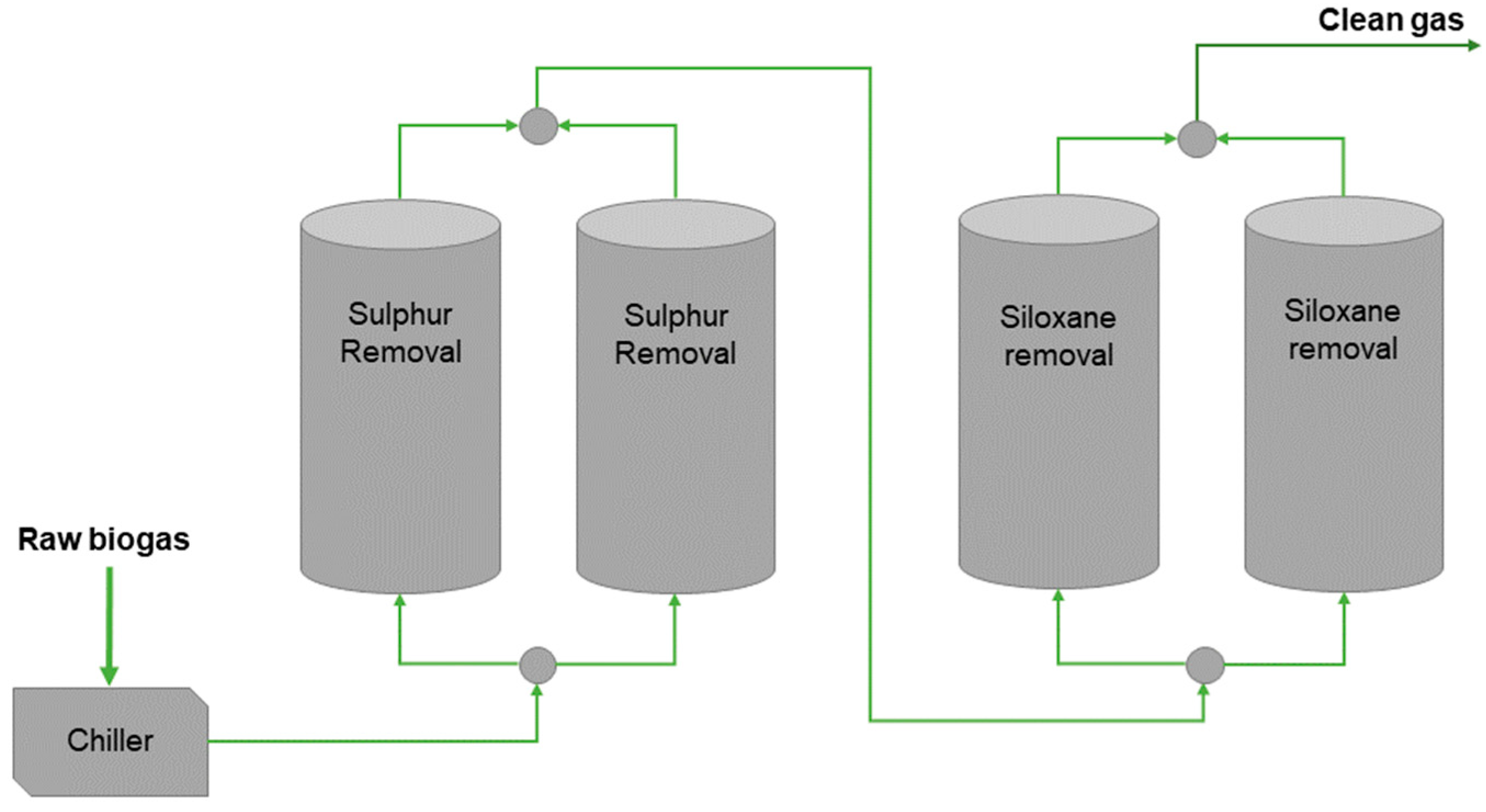
3.1.4. Adsorption-Based Systems Plant Layout
3.1.5. Benefits and Criticalities of Adsorption-Based Systems
- ACs can effectively remove sulfur compounds and siloxanes, and in literature, ACs are often defined as the best performing materials in terms of adsorption.
- AC regeneration is problematic. Therefore, industrial plants prefer to dispose of the exhausted ACs and substitute them with fresh ones [57].
- Metal oxides are regenerated easily, but the presence of solid sulfur deposits limits the recovery of adsorption capacity.
- High-quality commercial sorbents produced by the SulfaTrap company are metal oxides [64].
- Zeolites are well-known at the commercial level for their use in natural gas cleaning applications and have therefore gained interest in biogas cleaning from H2S and VOCs.
- COS formation from H2S and CO2 has been observed in zeolite cleaning reactors, leading to the risk of the roll-up effect.
3.2. Scrubbers: Absorption-Based Systems and Biological Desulfurization
3.2.1. Water Scrubbing
3.2.2. Scrubbing with Organic Solvents
3.2.3. Chemical Scrubbing
3.2.4. Biological Scrubbing and Filtering
3.2.5. Absorption-Based Systems Comparison
3.2.6. Absorption-Based Systems Plant Layout Analysis
3.3. Cryogenic Technology
3.3.1. Cryogenic Plant Design Analysis
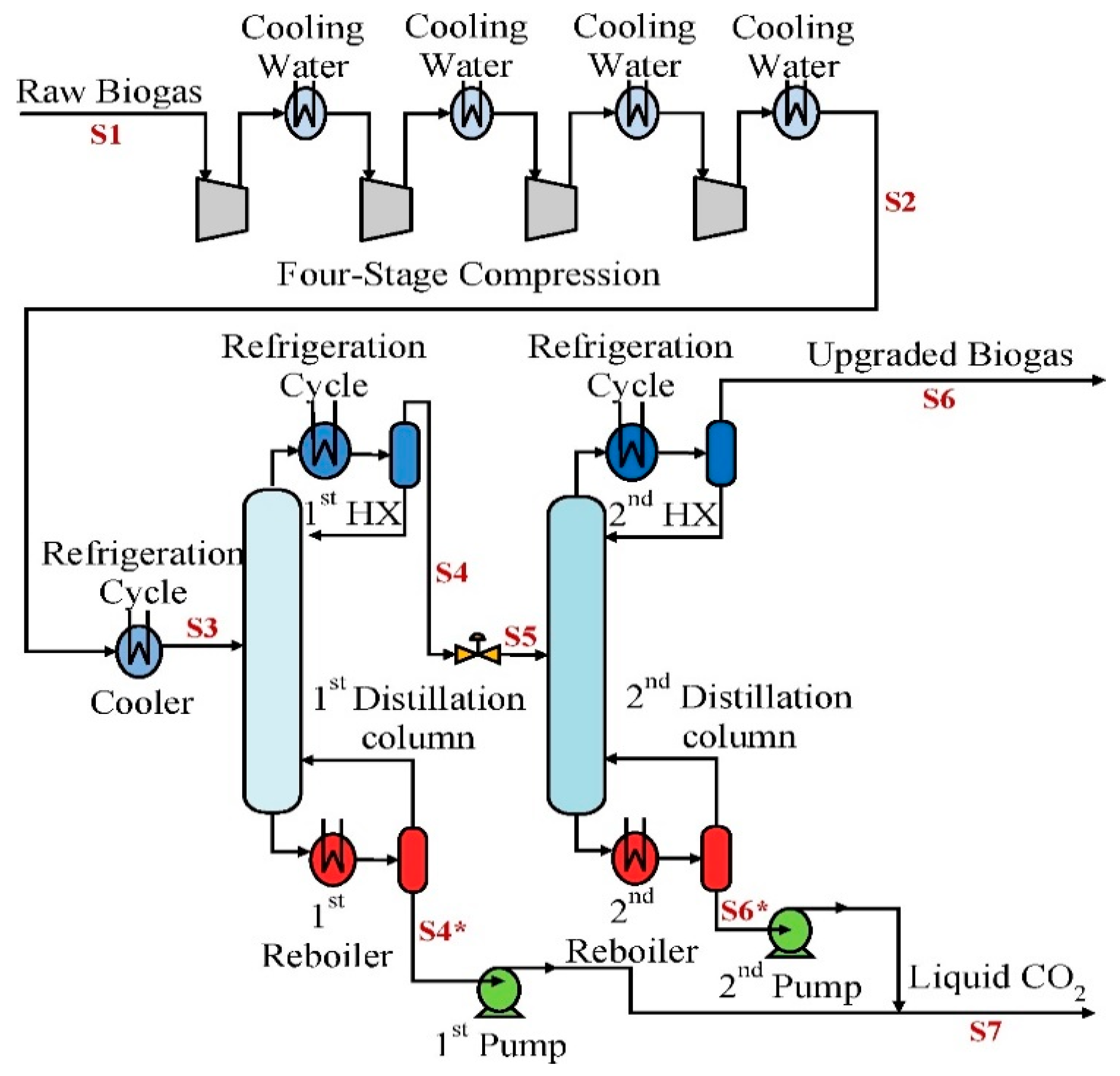
3.3.2. Carbon Dioxide Freeze-Out
3.3.3. Comparing Innovative Cryogenic Systems to Other Upgrading Technologies
- Biomethane with high methane purity.
- Extremely low methane losses.
- High purity carbon dioxide recovery.
- No chemical reactions, no reactants purchasing cost.
- No continuous hazardous waste production (e.g., impregnated ACs).
- Not mature technology, still under development.
- High CAPEX and OPEX due to the high energy consumption.
- Occasional need to use fresh coolant (infrequent hazardous waste production) [92].
4. Technology Comparison and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Green Deal. Available online: https://ec.europa.eu/clima/eu-action/european-green-deal_en (accessed on 27 April 2022).
- EU Climate Action and the European Green Deal|Climate Action. Available online: https://ec.europa.eu/clima/policies/eu-climate-action (accessed on 5 June 2021).
- Tian, W.; Li, J.; Zhu, L.; Li, W.; He, L.; Gu, L.; Deng, R.; Shi, D.; Chai, H.; Gao, M. Insights of Enhancing Methane Production under High-Solid Anaerobic Digestion of Wheat Straw by Calcium Peroxide Pretreatment and Zero Valent Iron Addition. Renew. Energy 2021, 177, 1321–1332. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. Overview of Recent Progress towards In-Situ Biogas Upgradation Techniques. Fuel 2018, 226, 686–697. [Google Scholar] [CrossRef]
- Nilsson, M.; Zamparutti, T.; Petersen, J.E.; Nykvist, B.; Rudberg, P.; Mcguinn, J. Understanding Policy Coherence: Analytical Framework and Examples of Sector-Environment Policy Interactions in the EU. Environ. Policy Gov. 2012, 22, 395–423. [Google Scholar] [CrossRef]
- Riley, D.M.; Tian, J.; Güngör-Demirci, G.; Phelan, P.; Villalobos, J.R.; Milcarek, R.J. Techno-Economic Assessment of CHP Systems in Wastewater Treatment Plants. Environments 2020, 7, 74. [Google Scholar] [CrossRef]
- Gandiglio, M.; Lanzini, A.; Santarelli, M.; Acri, M.; Hakala, T.; Rautanen, M. Results from an Industrial Size Biogas-Fed SOFC Plant (the DEMOSOFC Project). Int. J. Hydrogen Energy 2020, 45, 5449–5464. [Google Scholar] [CrossRef]
- DEMOnstration of Large SOFC System Fed with Biogas from WWTP|DEMOSOFC Project|H2020|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/671470 (accessed on 5 June 2021).
- Deliverable “DEMOnstration of Large SOFC System Fed with Biogas from WWTP”. Available online: http://www.demosofc.eu/wp-content/uploads/2017/10/SOFC-based-CHP-market-potential-analysis.pdf (accessed on 5 June 2021).
- DESCRIPTION|Waste2watts-Project. Available online: https://waste2watts-project.net/description/ (accessed on 5 June 2021).
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and Potential of the Anaerobic Digestion of Waste-Activated Sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- EUR-Lex-32019R1009-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32019R1009 (accessed on 4 June 2021).
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food Waste Biorefinery: Sustainable Strategy for Circular Bioeconomy; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 248. [Google Scholar]
- Lisowyj, M.; Wright, M.M. A Review of Biogas and an Assessment of Its Economic Impact and Future Role as a Renewable Energy Source. Rev. Chem. Eng. 2020, 36, 401–421. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of Anaerobic Digestion on Digestate Nutrient Availability and Crop Growth: A Review Anaerobic Digestion (AD) for Biogas Production Leads to Several Changes in The. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Liedl, B.E.; Cummins, M.; Young, A.; Williams, M.L.; Chatfield, J.M. Hydroponic Lettuce Production Using Liquid Effluent from Poultry Waste Bioremediation as a Nutrient Source. In Proceedings of the VII International Symposium on Protected Cultivation in Mild Winter Climates: Production, Pest Management and Global Competition, Recife, Brazil, 1 January 2004; Volume 659. [Google Scholar]
- Panuccio, M.R.; Mallamaci, C.; Attinà, E.; Muscolo, A. Using Digestate as Fertilizer for a Sustainable Tomato Cultivation. Sustainability 2021, 13, 1574. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on Research Achievements of Biogas from Anaerobic Digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Hofmann, J.; Nachtmann, K.; Falk, O.; Baum, S.; Fuchsz, M. Pressureless Cryogenic Conversion of Biogas into Liquefied Biomethane and Solid Carbon Dioxide. In Proceedings of the European Biomass Conference and Exhibition Proceedings, Amsterdam, The Netherlands, 6–9 June 2016; Volume 2016. [Google Scholar]
- Nachtmann, K.; Baum, S.; Fuchsz, M.; Falk, O.; Hofmann, J. Efficient Storage and Mobile Use of Biogas as Liquid Biomethane. Landtechnik 2017, 72, 179–201. [Google Scholar] [CrossRef]
- Persson, M.; Jonsson, O.; Wellinger, A. Biogas Upgrading to Vehicle Fuel Standards and Grid. IEA Bioenergy 2007, 37, 1–32. [Google Scholar]
- Wellinger, A.; Lindberg, A. Biogas Upgrading and Utilisation. IEA Bioenergy 2000, 24, 3–20. [Google Scholar]
- Nguyen, L.N.; Kumar, J.; Vu, M.T.; Mohammed, J.A.H.; Pathak, N.; Commault, A.S.; Sutherland, D.; Zdarta, J.; Tyagi, V.K.; Nghiem, L.D. Biomethane Production from Anaerobic Co-Digestion at Wastewater Treatment Plants: A Critical Review on Development and Innovations in Biogas Upgrading Techniques. Sci. Total Environ. 2021, 765, 142753. [Google Scholar] [CrossRef] [PubMed]
- Bruce, N.; Pope, D.; Rehfuess, E.; Balakrishnan, K.; Adair-Rohani, H.; Dora, C. WHO Indoor Air Quality Guidelines on Household Fuel Combustion: Strategy Implications of New Evidence on Interventions and Exposure-Risk Functions. Atmos. Environ. 2015, 106, 451–457. [Google Scholar] [CrossRef]
- Allegue, L.B.; Hinge, J. Biogas Upgrading Evaluation of Methods for H2S Removal. Dan. Technol. Inst. 2014, 1–31. Available online: https://www.teknologisk.dk/_/media/60599_Biogas%20upgrading.%20Evaluation%20of%20methods%20for%20H2S%20removal.pdf (accessed on 27 April 2022).
- Allegue, L.B.; Hinge, J. Biogas and Bio-Syngas Upgrading. Dan. Technol. Inst. 2012, 1–97. Available online: http://www.teknologisk.dk/_/media/52679_Report-Biogas%20and%20syngas%20upgrading.pdf (accessed on 27 April 2022).
- IRENA. Biogas for Road Vehicles Technology Brief; IRENA: Abu Dhabi, United Arab Emirates, 2017; ISBN 9789292600020. [Google Scholar]
- Lanzini, A.; Madi, H.; Chiodo, V.; Papurello, D.; Maisano, S.; Santarelli, M.; Van Herle, J. Dealing with Fuel Contaminants in Biogas-Fed Solid Oxide Fuel Cell (SOFC) and Molten Carbonate Fuel Cell (MCFC) Plants: Degradation of Catalytic and Electro-Catalytic Active Surfaces and Related Gas Purification Methods. Prog. Energy Combust. Sci. 2017, 61, 150–188. [Google Scholar] [CrossRef] [Green Version]
- Papurello, D.; Lanzini, A.; Drago, D.; Leone, P.; Santarelli, M. Limiting Factors for Planar Solid Oxide Fuel Cells under Different Trace Compound Concentrations. Energy 2016, 95, 67–78. [Google Scholar] [CrossRef]
- Wasajja, H.; Lindeboom, R.E.F.; van Lier, J.B.; Aravind, P.V. Techno-Economic Review of Biogas Cleaning Technologies for Small Scale off-Grid Solid Oxide Fuel Cell Applications. Fuel Process. Technol. 2020, 197, 106215. [Google Scholar] [CrossRef]
- Yeong, Y.; Nancy, G.; Ryan, B.; National Research Council Canada. Review of Hydrogen Tolerance of Key Power-to-Gas (P2G) Components and Systems in Canada: Final Report; 2017; ISBN 9780660241302. Available online: https://nrc-publications.canada.ca/eng/view/object/?id=94a036f4-0e60-4433-add5-9479350f74de (accessed on 27 April 2022).
- Du, W.; Parker, W. Modeling Volatile Organic Sulfur Compounds in Mesophilic and Thermophilic Anaerobic Digestion of Methionine. Water Res. 2012, 46, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Niakolas, D.K. Sulfur Poisoning of Ni-Based Anodes for Solid Oxide Fuel Cells in H/C-Based Fuels. Appl. Catal. A Gen. 2014, 486, 123–142. [Google Scholar] [CrossRef]
- Munawer, M.E. Human Health and Environmental Impacts of Coal Combustion and Post-Combustion Wastes. J. Sustain. Min. 2018, 17, 87–96. [Google Scholar] [CrossRef]
- Khan, M.A.H.; Rao, M.V.; Li, Q. Recent Advances in Electrochemical Sensors for Detecting Toxic Gases: NO2, SO2 and H2S. Sensors 2019, 19, 905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Arespacochaga, N.; Valderrama, C.; Raich-Montiu, J.; Crest, M.; Mehta, S.; Cortina, J.L. Understanding the Effects of the Origin, Occurrence, Monitoring, Control, Fate and Removal of Siloxanes on the Energetic Valorization of Sewage Biogas—A Review. Renew. Sustain. Energy Rev. 2015, 52, 366–381. [Google Scholar] [CrossRef] [Green Version]
- Papadias, D.D.; Ahmed, S.; Kumar, R. Fuel Quality Issues with Biogas Energy—An Economic Analysis for a Stationary Fuel Cell System. Energy 2012, 44, 257–277. [Google Scholar] [CrossRef]
- Madi, H.; Lanzini, A.; Diethelm, S.; Papurello, D.; Van Herle, J.; Lualdi, M.; Gutzon Larsen, J.; Santarelli, M. Solid Oxide Fuel Cell Anode Degradation by the Effect of Siloxanes. J. Power Sources 2015, 279, 460–471. [Google Scholar] [CrossRef]
- Persson, M.; Jönsson, O.; Wellinger, A. Biogas Upgrading to Vehicle Fuel Standards and Grid Injection. 2006. Available online: https://www.ieabioenergy.com/blog/publications/biogas-upgrading-to-vehicle-fuel-standards-and-grid-injection/ (accessed on 27 April 2022).
- Spiegel, R.J.; Preston, J.L. Test Results for Fuel Cell Operation on Anaerobic Digester Gas. J. Power Sources 2000, 86, 283–288. [Google Scholar] [CrossRef]
- Papurello, D.; Lanzini, A. SOFC Single Cells Fed by Biogas: Experimental Tests with Trace Contaminants. Waste Manag. 2018, 72, 306–312. [Google Scholar] [CrossRef]
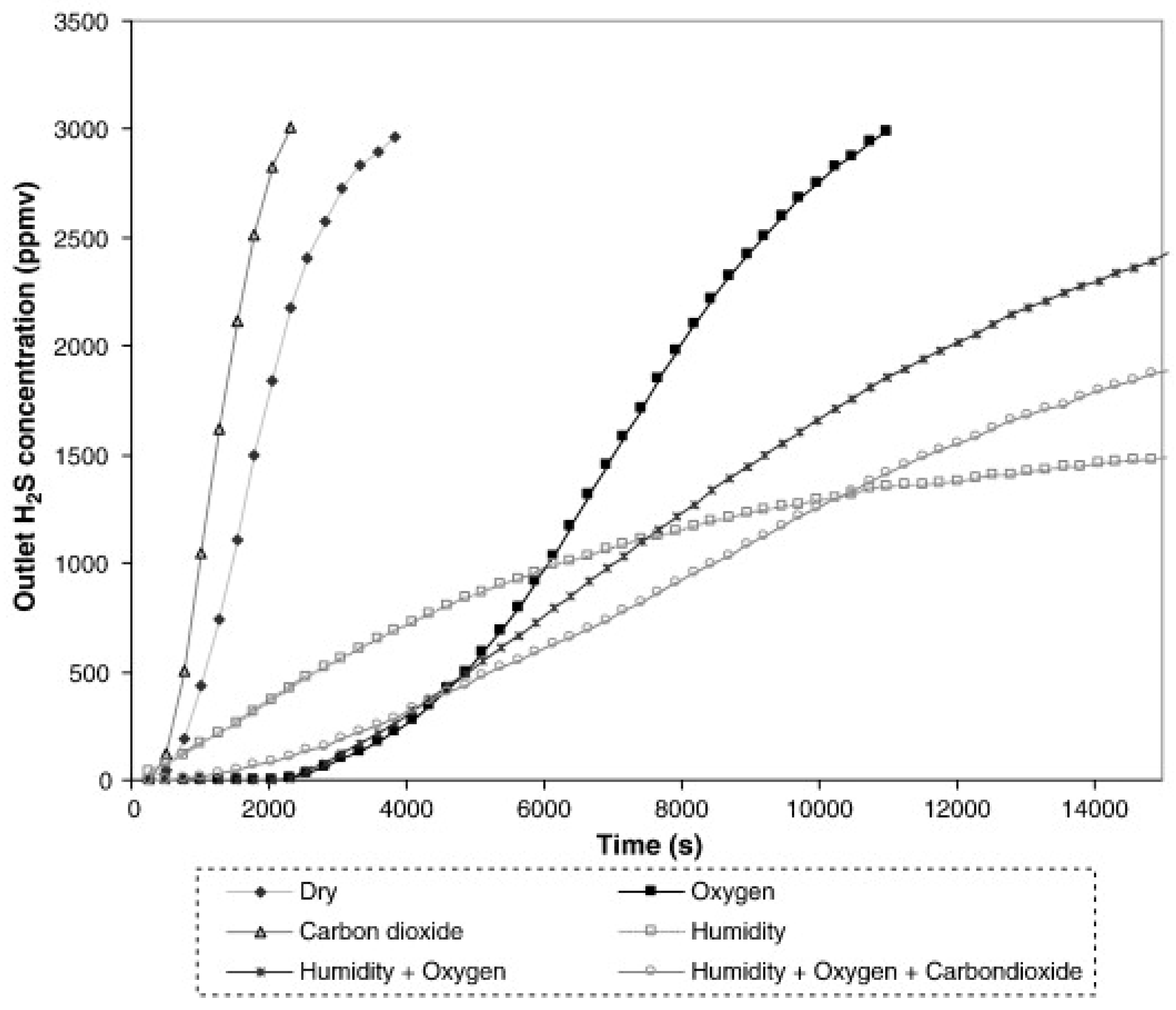
- Sitthikhankaew, R.; Chadwick, D.; Assabumrungrat, S.; Laosiripojana, N. Effects of Humidity, O2, and CO2 on H2S Adsorption onto Upgraded and KOH Impregnated Activated Carbons. Fuel Process. Technol. 2014, 124, 249–257. [Google Scholar] [CrossRef]
- Bona, D.; Beggio, G.; Weil, T.; Scholz, M.; Bertolini, S.; Grandi, L.; Baratieri, M.; Schievano, A.; Silvestri, S.; Pivato, A. Effects of Woody Biochar on Dry Thermophilic Anaerobic Digestion of Organic Fraction of Municipal Solid Waste. J. Environ. Manag. 2020, 267, 110633. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment Technologies for Emerging Contaminants in Wastewater Treatment Plants: A Review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Marchelli, F.; Cordioli, E.; Patuzzi, F.; Sisani, E.; Barelli, L.; Baratieri, M.; Arato, E.; Bosio, B. Experimental Study on H2S Adsorption on Gasification Char under Different Operative Conditions. Biomass Bioenergy 2019, 126, 106–116. [Google Scholar] [CrossRef]
- Qian, Q.; Machida, M.; Tatsumoto, H. Textural and Surface Chemical Characteristics of Activated Carbons Prepared from Cattle Manure Compost. Waste Manag. 2008, 28, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Hervy, M.; Pham Minh, D.; Gérente, C.; Weiss-Hortala, E.; Nzihou, A.; Villot, A.; Le Coq, L. H2S Removal from Syngas Using Wastes Pyrolysis Chars. Chem. Eng. J. 2018, 334, 2179–2189. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Luo, A.; Zhao, Y. Preparation and Characterisation of Activated Carbon from Waste Tea by Physical Activation Using Steam. J. Air Waste Manag. Assoc. 2018, 68, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, P.; Mackey, H.R.; Mariyam, S.; Zuhara, S.; Al-Ansari, T.; McKay, G. Char Products from Bamboo Waste Pyrolysis and Acid Activation. Front. Mater. 2021, 7, 624791. [Google Scholar] [CrossRef]
- Dolder-NORIT®. Available online: https://www.dolder.com/it/filtration-purification/noritr/ (accessed on 29 September 2021).
- Purification Du Biogaz Pour Sa Valorisation Énergétique: Adsorption de Siloxanes Sur Charbons Actifs. Available online: https://tel.archives-ouvertes.fr/tel-02887086/document (accessed on 5 June 2021).
- Gas Processing-Chemviron. Available online: https://www.chemviron.eu/applications/industrial-processes/gas-processing/ (accessed on 29 September 2021).
- Final Report Summary-MCFC-CONTEX (MCFC Catalyst and Stack Component Degradation and Lifetime: Fuel Gas CONTaminant Effects and EXtraction Strategies)|FP7|CORDIS|European Commission. Available online: https://cordis.europa.eu/project/id/245171/reporting/it (accessed on 1 October 2021).
- Home|Desotec. Available online: https://www.desotec.com/en (accessed on 19 November 2021).
- Calbry-Muzyka, A.S.; Gantenbein, A.; Schneebeli, J.; Frei, A.; Knorpp, A.J.; Schildhauer, T.J.; Biollaz, S.M.A. Deep Removal of Sulfur and Trace Organic Compounds from Biogas to Protect a Catalytic Methanation Reactor. Chem. Eng. J. 2019, 360, 577–590. [Google Scholar] [CrossRef]
- Activated Carbon Leader in Air and Water Purification. Available online: https://www.calgoncarbon.com/ (accessed on 19 November 2021).
- Georgiadis, A.G.; Charisiou, N.D.; Goula, M.A. Removal of Hydrogen Sulfide from Various Industrial Gases: A Review of The Most Promising Adsorbing Materials. Catalysts 2020, 10, 521. [Google Scholar] [CrossRef]
- Novochinskii, I.I.; Song, C.; Ma, X.; Liu, X.; Shore, L.; Lampert, J.; Farrauto, R.J. Low-Temperature H2S Removal from Steam-Containing Gas Mixtures with ZnO for Fuel Cell Application. 1. ZnO Particles and Extrudates. Energy Fuels 2004, 18, 576–583. [Google Scholar] [CrossRef]
- Neveux, L.; Chiche, D.; Bazer-Bachi, D.; Favergeon, L.; Pijolat, M. New Insight on the ZnO Sulfidation Reaction: Evidences for an Outward Growth Process of the ZnS Phase. Chem. Eng. J. 2012, 181–182, 508–515. [Google Scholar] [CrossRef]
- Sadegh-Vaziri, R.; Babler, M.U. Numerical Investigation of the Outward Growth of ZnS in the Removal of H2S in a Packed Bed of ZnO. Chem. Eng. Sci. 2017, 158, 328–339. [Google Scholar] [CrossRef]
- Liu, D.; Chen, S.; Fei, X.; Huang, C.; Zhang, Y. Regenerable CuO-Based Adsorbents for Low Temperature Desulfurization Application. Ind. Eng. Chem. Res. 2015, 54, 3556–3562. [Google Scholar] [CrossRef]
- Frilund, C.; Simell, P.; Kaisalo, N.; Kurkela, E.; Koskinen-Soivi, M.L. Desulfurization of Biomass Syngas Using ZnO-Based Adsorbents: Long-Term Hydrogen Sulfide Breakthrough Experiments. Energy Fuels 2020, 34, 3316–3325. [Google Scholar] [CrossRef]
- C&CS: Products. Available online: https://www.candcs.eu/en/products.html (accessed on 26 October 2021).
- Sorbents|Desulfurization—SulfaTrap. Available online: https://sulfatrap.com/sulfatrap-products/sorbents/ (accessed on 18 September 2021).
- Biocustom|Additivi per Biogas—Miglioramento Digestione e Desolforazione. Available online: https://www.biocustom.it/prodotti-tecnici-materie-prime/additivi-biogas/ (accessed on 29 September 2021).
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Separation of CH4/CO2/N2 Mixtures by Layered Pressure Swing Adsorption for Upgrade of Natural Gas. Chem. Eng. Sci. 2006, 61, 3893–3906. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.S.; Fisher, E.P. Adsorption of CO2, N2, and O2 on Natural Zeolites. Energy Fuels 2003, 17, 571–576. [Google Scholar] [CrossRef]
- Cosoli, P.; Ferrone, M.; Pricl, S.; Fermeglia, M. Hydrogen Sulphide Removal from Biogas by Zeolite Adsorption. Part I. GCMC Molecular Simulations. Chem. Eng. J. 2008, 145, 86–92. [Google Scholar] [CrossRef]
- Thompson, J.A. Acid Gas Adsorption on Zeolite SSZ-13: Equilibrium and Dynamic Behavior for Natural Gas Applications. AIChE J. 2020, 66, e16549. [Google Scholar] [CrossRef]
- Langnickel, H.; Rautanen, M.; Gandiglio, M.; Santarelli, M.; Hakala, T.; Acri, M.; Kiviaho, J. Efficiency Analysis of 50 KWe SOFC Systems Fueled with Biogas from Waste Water. J. Power Sources Adv. 2020, 2, 100009. [Google Scholar] [CrossRef]
- DEMOSOFC Deliverables. Available online: http://www.demosofc.eu/?p=861 (accessed on 27 August 2020).
- Gandiglio, M. EU Waste2Watts Project, Deliverable D2.3—Sorbents Testing Intermediate Report; Cordis Europe: Brussels, Belgium, 2019. [Google Scholar]
- De Arespacochaga, N.; Valderrama, C.; Mesa, C.; Bouchy, L.; Cortina, J.L. Biogas Deep Clean-up Based on Adsorption Technologies for Solid Oxide Fuel Cell Applications. Chem. Eng. J. 2014, 255, 593–603. [Google Scholar] [CrossRef]
- Boulinguiez, B.; Le Cloirec, P. Adsorption on Activated Carbons of Five Selected Volatile Organic Compounds Present in Biogas: Comparison of Granular and Fiber Cloth Materials. Energy Fuels 2010, 24, 4756–4765. [Google Scholar] [CrossRef]
- Nguyen-Thanh, D.; Bandosz, T.J. Activated Carbons with Metal Containing Bentonite Binders as Adsorbents of Hydrogen Sulfide. Carbon 2005, 43, 359–367. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J.; Aguilera, P.G.; Ollero, P. Biogas Desulfurization by Adsorption on Thermally Treated Sewage-Sludge. Sep. Purif. Technol. 2014, 123, 200–213. [Google Scholar] [CrossRef]
- Papurello, D.; Gandiglio, M.; Lanzini, A. Experimental Analysis and Model Validation on the Performance of Impregnated Activated Carbons for the Removal of Hydrogen Sulfide (H2S) from Sewage Biogas. Processes 2019, 7, 548. [Google Scholar] [CrossRef] [Green Version]
- Pineda, M.; Palacios, J.M.; Alonso, L.; García, E.; Moliner, R. Performance of Zinc Oxide Based Sorbents for Hot Coal Gas Desulfurization in Multicycle Tests in a Fixed-Bed Reactor. Fuel 2000, 79, 885–895. [Google Scholar] [CrossRef]
- Sigot, L.; Ducom, G.; Germain, P. Adsorption of Hydrogen Sulfide (H2S) on Zeolite (Z): Retention Mechanism. Chem. Eng. J. 2016, 287, 47–53. [Google Scholar] [CrossRef]
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of Appropriate Biogas Upgrading Technology-a Review of Biogas Cleaning, Upgrading and Utilisation. Renew. Sustain. Energy Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas Upgrading and Utilization: Current Status and Perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [Green Version]
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for Transformation of Biogas to Biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Bauer, F.; Hulteberg, C.; Persson, T.; Tamm, D. Biogas Upgrading—Review of Commercial Technologies. 2013. Available online: http://www.sgc.se/ckfinder/userfiles/files/SGC270.pdf (accessed on 27 April 2022).
- Miltner, M.; Makaruk, A.; Harasek, M. Review on Available Biogas Upgrading Technologies and Innovations towards Advanced Solutions. J. Clean. Prod. 2017, 161, 1329–1337. [Google Scholar] [CrossRef]
- Rafiee, A.; Khalilpour, K.R.; Prest, J.; Skryabin, I. Biogas as an Energy Vector. Biomass Bioenergy 2021, 144, 105935. [Google Scholar] [CrossRef]
- Couvert, A.; Sanchez, C.; Laplanche, A.; Renner, C. Scrubbing Intensification for Sulphur and Ammonia Compounds Removal. Chemosphere 2008, 70, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Cano, P.I.; Colón, J.; Ramírez, M.; Lafuente, J.; Gabriel, D.; Cantero, D. Life Cycle Assessment of Different Physical-Chemical and Biological Technologies for Biogas Desulfurization in Sewage Treatment Plants. J. Clean. Prod. 2018, 181, 663–674. [Google Scholar] [CrossRef]
- Soreanu, G.; Béland, M.; Falletta, P.; Ventresca, B.; Seto, P. Evaluation of Different Packing Media for Anoxic H2S Control in Biogas. Environ. Technol. 2009, 30, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Ramírez, M.; Pérez, R.M.; Gómez, J.M.; Cantero, D. Hydrogen Sulphide Removal from Biogas by an Anoxic Biotrickling Filter Packed with Pall Rings. Chem. Eng. J. 2013, 225, 456–463. [Google Scholar] [CrossRef]
- San-Valero, P.; Penya-roja, J.M.; Javier Álvarez-Hornos, F.; Buitrón, G.; Gabaldón, C.; Quijano, G. Fully Aerobic Bioscrubber for the Desulfurization of H2S-Rich Biogas. Fuel 2019, 241, 884–891. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. Upgrading Biogas to Biomethane and Liquid CO2: A Novel Cryogenic Process. Fuel 2019, 251, 611–628. [Google Scholar] [CrossRef]
- Adnan, A.I.; Ong, M.Y.; Nomanbhay, S.; Chew, K.W.; Show, P.L. Technologies for Biogas Upgrading to Biomethane: A Review. Bioengineering 2019, 6, 92. [Google Scholar] [CrossRef] [Green Version]
- Waste2watts-Project. Available online: https://waste2watts-project.net/ (accessed on 1 October 2019).
- Falco, F. Application of the Yokozeki Thermodynamic Model for the Design of an Innovative Cryogenic System for Biogas Purification—Webthesis. Ph.D. Thesis, Politecnico di Torino, Turin, Italy, 2020. [Google Scholar]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New Approach for Biogas Purification Using Cryogenic Separation and Distillation Process for CO2 Capture. Energy 2018, 156, 328–351. [Google Scholar] [CrossRef]
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O. A Review of Biochar Properties and Their Roles in Mitigating Challenges with Anaerobic Digestion. Renew. Sustain. Energy Rev. 2019, 103, 291–307. [Google Scholar] [CrossRef]
- Weiland, P. Biogas Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Håkansson, A. Preventing Microbial Growth on Pall-Rings When Upgrading Biogas Using Absorption with Water Wash; 2006; Available online: http://www.sgc.se/ckfinder/userfiles/files/SGC166.pdf (accessed on 27 April 2022).
- Starr, K.; Gabarrell, X.; Villalba, G.; Talens, L.; Lombardi, L. Life Cycle Assessment of Biogas Upgrading Technologies. Waste Manag. 2012, 32, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, J.; Broekhuis, A.A.; van Gemert, W.J.T. Optimisation of a Green Gas Supply Chain—A Review. Bioresour. Technol. 2010, 101, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.; Abbas, S. Exploring CO2 Sources and Sinks Nexus through Integrated Approach: Insight from Pakistan. J. Environ. Inform. 2013, 22, 112–122. [Google Scholar] [CrossRef] [Green Version]



| Application | H2S Tolerance | Unit | Reference |
|---|---|---|---|
| Boiler | <1000 | ppm(v) | [22] |
| ICE | <50–500 | ppm(v) | [25] |
| Stationary Engine | <1000 | ppm(v) | [22] |
| Turbine | <1000 | ppm(v) | [25] |
| Micro-Turbines | <70,000 | ppm(v) | [26] |
| Vehicle Fuel | <5 | ppm(v) | [27] |
| SOFC | <1 | ppm(v) | [28,29] |
| <2 | [30] | ||
| NG grid injection | <5 | mg/m3 | [31] |
| Class | Chemical Name | Formula | Max [ppm] | Average [ppm] |
|---|---|---|---|---|
| Siloxanes | (D4) Octamethylcyclotetrasiloxane | C8H24O4Si4 | 20.144 | 0.825 |
| (D5) Decamethylcyclopentasiloxane | C10H30O5Si5 | 18.129 | 1.689 | |
| (L2) Hexamethyldisiloxane | C6H18OSi2 | 2.26 | 0.115 | |
| (L3) Octamethyltrisiloxane | C8H24O2Si3 | 0.465 | 0.061 | |
| Sulfur Compound | Hydrogen sulfide | H2S | 2897 | 400 |
| Methanethiol (Methyl mercaptan) | CH4S | 1.07 | 0.08 | |
| Carbon disulfide | CS2 | 0.05 | 0.05 | |
| Dimethyl sulfide (DMS) | C2H6S | 0.04 | 0.04 | |
| Halocarbons | Methylene chloride (Dichloromethane) | CH2Cl2 | 0.052 | 0.052 |
| Chloroform (Trichloromethane) | CHCl3 | 0.009 | excl. | |
| Carbon tetrachloride | CCl4 | 0.005 | excl. | |
| Chloroethene (Vinyl chloride) | C2H3Cl | 0.119 | 0.119 | |
| 1,2-Dichloroethane | C2H4Cl2 | 0.308 | 0.157 | |
| Trichloroethylene | C2HCl3 | excl. | excl. | |
| 1,1,2-Trichloroethane | C2H3Cl3 | N.A. | N.A. | |
| Tetrachloroethylene | C2Cl4 | 0.1 | 0.1 | |
| Chlorobenzene | C6H5Cl | 0.693 | 0.255 | |
| Dichlorobenzene (all isomers) | C6H4Cl2 | 0.61 | 0.254 | |
| Trichlorofluoromethane (R-11) | CCl3F | 0.004 | 0.004 | |
| Chlorodifluoromethane (R-22) | CHClF2 | N.A. | N.A. | |
| Alkanes | Ethane | C2H6 | 51 | 40 |
| Propane | C3H8 | 2 | 1 | |
| Butane | C4H10 | 1.3 | 0.7 | |
| Pentane | C5H12 | 15 | 7 | |
| Hexane | C6H14 | 108 | 25.144 | |
| Heptane | C7H16 | 0.358 | 0.358 | |
| Octane | C8H18 | 0.275 | 0.221 | |
| Nonane | C9H20 | 6.2 | 1.246 | |
| Aromatic | Benzene | C6H6 | 0.85 | 0.168 |
| Toluene (Methylbenzene) | C7H8 | 2.274 | 1.037 | |
| Ethylbenzene | C8H10 | 5.911 | 1.251 | |
| Xylenes (o-, m-, p-, mixtures) | C8H10 | 4.095 | 0.784 | |
| 1,3,5-Trimethylbenzene | C9H12 | 1.859 | 1.859 | |
| 1-Methyl-4-propylbenzene (p-cymene) | C10H14 | 3.072 | 1.157 | |
| Cyclic | Cyclohexane | C6H12 | excl. | excl. |
| Methylcyclohexane | C7H14 | 0.13 | 0.13 | |
| Dimethylcyclohexane (all isomers) | C8H16 | 0.39 | 0.39 | |
| Limonene | C10H16 | 48.9 | 9.729 | |
| Alcohol | Ethanol | C2H6O | N.A. | N.A. |
| 2-Propanol (Isopropyl alcohol) | C3H8O | N.A. | N.A. | |
| Ester | Ethyl acetate | C4H8O2 | N.A. | N.A. |
| Ethyl butanoate (Butanoic acid) | C6H12O2 | N.A. | N.A. | |
| Ether | Dimethyl ether | C2H6O | N.A. | N.A. |
| 2-methoxy-2-methyl-propane (MTBE) | C5H12O | N.A. | N.A. | |
| Acetone | C3H6O | N.A. | N.A. | |
| Ketone | 2-Butanone (Methyl ethyl ketone) | C4H8O | N.A. | N.A. |
| 4-Methyl-2-pentanone (MIBK) | C6H12O | N.A. | N.A. |
| Sorbent Name | Supplier | BET Surface Area [m2/g] | Key Features (Supplier) |
|---|---|---|---|
| Norit RST | Dolder | 1117 [45] | The company offers reactivation treatments as well as waste disposal operations [50] |
| Norit RGM3 | 1031 (Politecnico di Torino experimental campaign) | ||
| Envirocarb | Chemviron | 622 (Purification Du Biogaz Pour Sa Valorisation Énergétique: Adsorption de Siloxanes Sur Charbons Actifs, n.d. [51]) | The company offers thermal reactivation processes in conjunction with fresh sorbent supplies [52] |
| Airpel Ultra | Arol Energy, Desotec | 781 [53] | Desotec offer several recycling or treatment options (recycling, energy valorization, or elimination) [54] |
| SulfaTrap R8 | SulfaTrap | 640 [55] | Specific for non-H2S sulfur pollutants (COS and C2S) [54] |
| 687 (Politecnico di Torino experimental campaign) | |||
| Solcarb KS3 | Chemviron | 691.6 (Politecnico di Torino experimental campaign), | Can remove thiols and some organic sulfides as well as for H2S [56] |
| Sorbent Name | Supplier | BET Surface Area [m2/g] | Key Features |
|---|---|---|---|
| Actisorb S2 | C&CS | 42.6 [62] | Specific for H2S removal from gases [63] |
| SulfaTrap R7H | SulfaTrap | 79.5 [55] | Several sorbents optimized for various biogas pollutants [64] |
| SulfaTrap R2F | 432 [55] | ||
| SulfaTrap R5E | 160.74 (Politecnico di Torino experimental campaign) | ||
| SulfaTrap R2DB | 432 [55] | ||
| BioClean Gas Granular | BioCustom | Not Found (new on the market) | Easy regeneration (direct air contact), disposed of by inserting it in the digester where it performs in situ desulfurization [65] |
| Producer | Products | Specific for: | Industrial Price |
|---|---|---|---|
| Calgon Carbon/Chemviron | Solcarb KS3 Impregnated AC | H2S, mercaptans and some organic sulfides | 5 €/kg |
| Envirocarb STIX Impregnated AC | H2S in presence of a 1.8 stoichiometric ratio of oxygen | 5 €/kg | |
| Envirocarb AP-60 non-impregnated AC | VOC, siloxanes | 3.5 €/kg | |
| Desotec | Airpel Ultra DS-6 impregnated | H2S, SO2, mercaptans and acid compounds, and CS2 (no experience with COS and CH4S) | 3.8 €/kg for both products |
| Organosorb 10-CO coconut-based, non-impregnated | DMS (from customer experience) and siloxanes | ||
| Sulfatrap | R7 | H2S removal | 10.17 $/kg at multiple tons scale |
| R2F | Mercaptans (methyl mercaptan, ethyl mercaptan) and complex sulfides (DMS, methyl ethyl sulfide), as well as polishing of H2S | 25.75 $/kg at multiple tons scale | |
| R8C | COS (chemically modified carbon sorbents) | 9.75 $/kg at multiple tons scale | |
| R8HB | CS2 (chemically modified carbon sorbents) | 7.00 $/kg at multiple tons scale |
| Sorbent Type | Key Features | Material Origin | Waste Disposal |
|---|---|---|---|
| Activated Carbons | Can perform chemisorption if impregnated Non-impregnated ACs usually have poor performances for deep desulfurization requirements. | Fossil carbon, conventional biomass, or biowastes Can be produced in the circular economy framework | Difficult to regenerate, impregnation often results in a ‘special waste’ disposal. Disposal cost is affected by national laws, impregnation often results in high waste management costs |
| Metal Oxides and Hydroxides | Always perform chemisorption (higher adsorption capacity and longest breakthrough time) | Often iron-based. Other metals of interest can be copper and zinc | Easy to regenerate, some can be disposed of directly in the digester |
| Zeolites | Can be treated to perform VOC removal. Risk of roll-up effect when working con H2S in CO2 presence | Can be natural or synthesized. Zeolites for VOCs may require chemical treatments to properly adsorb biogas pollutants | Zeolites used for Oil & Gas fields (e.g., N2-capture) can be regenerated. Zeolite regeneration for biogas applications is limited by the formation of elemental sulfur within the zeolite structure [79]. |
| Scrubber | Material Quantity | External Resources | Target Gases | Biogas Applications |
|---|---|---|---|---|
| Water Scrubbing (WS) | Water is not an optimized solvent. Higher volumes are needed compared to other scrubbers. | If regeneration is not performed, it results in a high-water consumption. In WWTPs, this solution can benefit from the continuous production of freshwater to avoid regeneration. | CO2 (biogas upgrading). H2S: possible target, but it can lead to solid sulfur deposition. | Biogas upgrading requires a sulfur-free stream. Sulfur-free applications (e.g., SOFC): preliminary assistance to adsorption systems if H2S content is high. |
| Physical Scrubbing (PS) | The sorbent is selective. Lower methane losses than WS. Lower volumes than WS are required. | The sorbent must be regenerated. Regeneration energies are higher than WS. This solution can benefit from recovered heat if available on-site. | CO2 (biogas upgrading). H2S can be removed but regeneration energies are too high (higher temperatures) | Biogas upgrading requires a sulfur-free stream to mitigate the high regeneration temperature needs. Biogas desulfurization is usually avoided due to high regeneration temperature. |
| Chemical Scrubbing (CS) | Reactants target acid gases. Lower methane losses than W.S. a P.S. Lower volumes are required. | Reactants must be regenerated. Regeneration energies are higher than W.S. | CO2 (biogas upgrading). H2S can be removed using oxidizing species, resulting in high regeneration energies. | Biogas upgrading requires a sulfur-free stream to mitigate the high regeneration temperature needs. Biogas desulfurization is usually avoided due to high regeneration temperature. |
| Biological Scrubbing | Water and substrate allow the microbial film to grow. | Regenerative air flow completes the metabolic processes. | H2S: high removal efficiency. | Biogas desulfurization occurs without the need for high regeneration energies and pressure differences typical of other scrubbers. |
| Upgrading Technologies | Methane Content [% vol.] | Ref. |
|---|---|---|
| Water Scrubbing | 96–99% | [21] |
| 93–98% | [96] | |
| Organic Solvents | 95–98% | [22] |
| 97% | [97] | |
| 93–97% | [98] | |
| 95–99% | [83] | |
| Chemical Scrubbing | 99% | [83] |
| 98% | [99] | |
| Cryogenic Separation | 98% | [83] |
| 98% | [98] | |
| 99% | [91] | |
| 99.4–99.9% | [20] |
| Upgrading Technology | Methane Losses [%] | Ref. |
|---|---|---|
| Water Scrubbing | 2% | [21] |
| Water Scrubbing + Regeneration | 1% | [97,100] |
| 1–2% | [98] | |
| 2% | [21,83] | |
| 6% | [99] | |
| Physisorption | 2% | [98] |
| 2–4% | [101] | |
| 4% | [83,97] | |
| 8–13% | [21] | |
| Chemisorption | 0.1% | [83,98] |
| 1% | [97] | |
| 8–10% | [21] | |
| 10% | [99] | |
| Cryogenic Separation | 0.5% | [98] |
| 0.65% | [97] | |
| 1% | [99] |
| Technology | H2S | Siloxanes | Others | Quality Target | Notes |
|---|---|---|---|---|---|
| Water Scrubbing |
| Not designed for siloxane removal |
| CH4 content from 80 to 99% (depending on the amount of non-condensable gasses) |
|
| Organic Solvents |
| Not designed for siloxane removal |
| CH4 content up to 98% |
|
| Chemical Scrubbing |
| Not designed for siloxane removal |
| CH4 content up to 99% |
|
| Biological Scrubbers and Filters |
| Not designed for siloxane removal |
|
| |
| Activated Carbons |
| Some ACs can be designed to reach low siloxane levels |
| Able to reach sulfur-free biogas (<1 ppm(v)) |
|
| Metal Oxides |
| Some commercial metal oxides are designed specifically for deep siloxane removal |
| Able to reach sulfur-free biogas (<1 ppm(v)) | |
| Zeolites |
|
|
| ||
| Cryogenic Separation |
| High-purity bio CH4 and CO2 |
| ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paglini, R.; Gandiglio, M.; Lanzini, A. Technologies for Deep Biogas Purification and Use in Zero-Emission Fuel Cells Systems. Energies 2022, 15, 3551. https://doi.org/10.3390/en15103551
Paglini R, Gandiglio M, Lanzini A. Technologies for Deep Biogas Purification and Use in Zero-Emission Fuel Cells Systems. Energies. 2022; 15(10):3551. https://doi.org/10.3390/en15103551
Chicago/Turabian StylePaglini, Roberto, Marta Gandiglio, and Andrea Lanzini. 2022. "Technologies for Deep Biogas Purification and Use in Zero-Emission Fuel Cells Systems" Energies 15, no. 10: 3551. https://doi.org/10.3390/en15103551
APA StylePaglini, R., Gandiglio, M., & Lanzini, A. (2022). Technologies for Deep Biogas Purification and Use in Zero-Emission Fuel Cells Systems. Energies, 15(10), 3551. https://doi.org/10.3390/en15103551








