Hydrothermal Conversion of Waste Biomass from Greenhouses into Hydrochar for Energy, Soil Amendment, and Wastewater Treatment Applications
Abstract
:1. Introduction
2. Sample Preparation and Experimental Procedures
2.1. Sample Preparation and Analysis
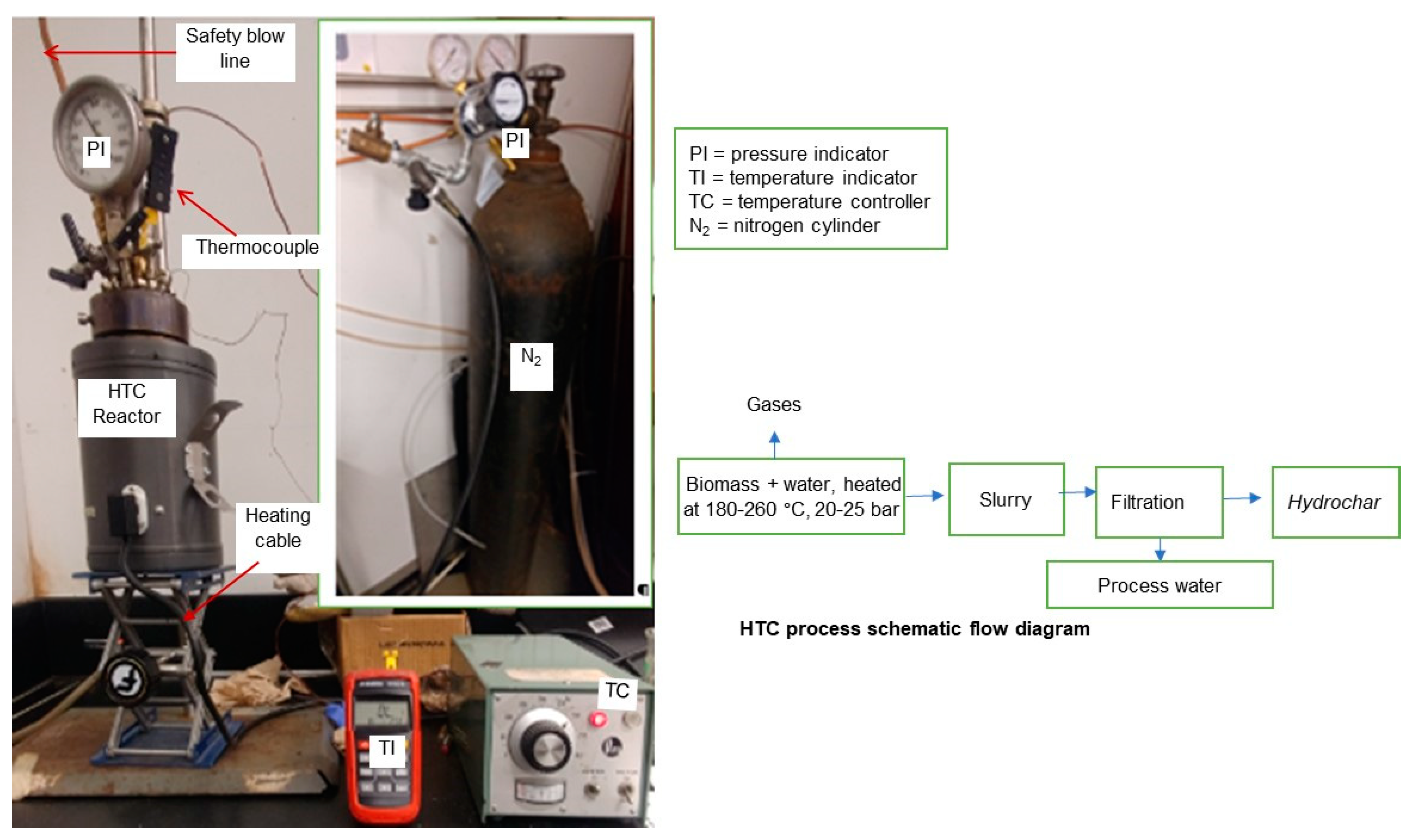
2.2. Carbonization by HTC Process
2.3. Characterization
2.4. Statistical Assessment of Data
3. Results and Discussion
3.1. Experiments for Biomass Fiber Estimation
3.2. Metal Contents in Plant Biomass and HCs
3.3. Quantitative Analysis of Biomass Fibers and HCs
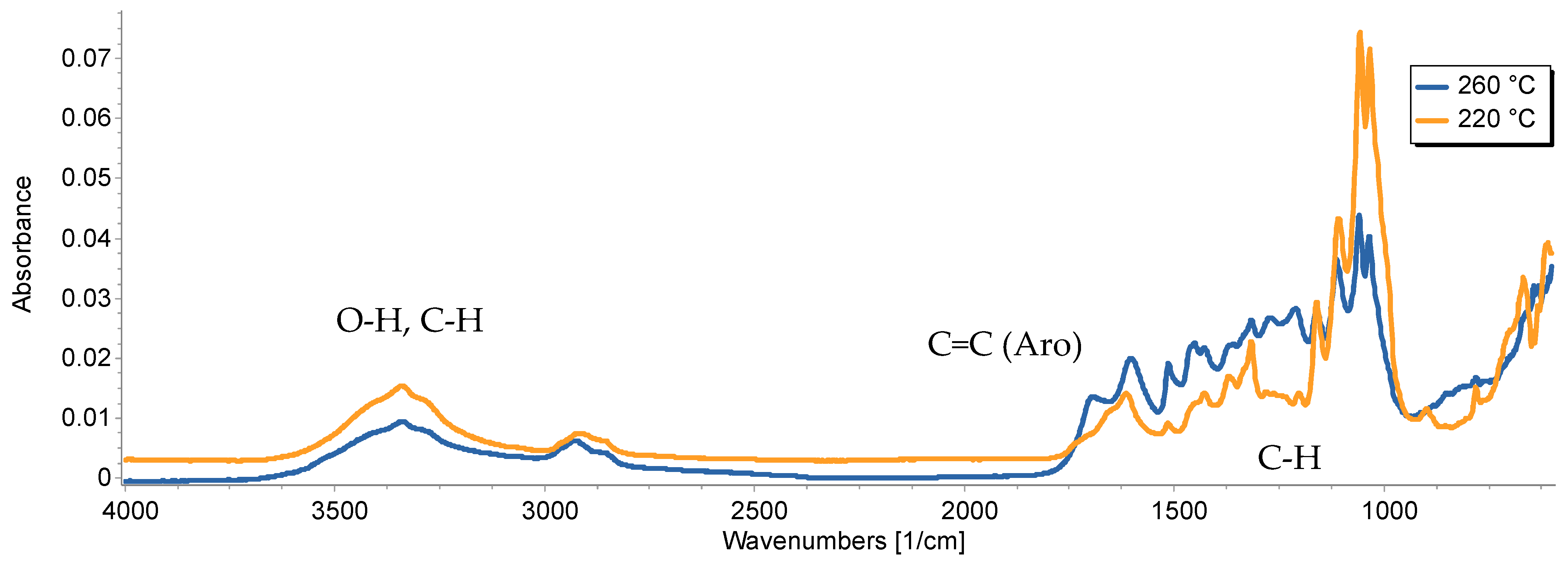
3.4. Functionalization of HC Materials
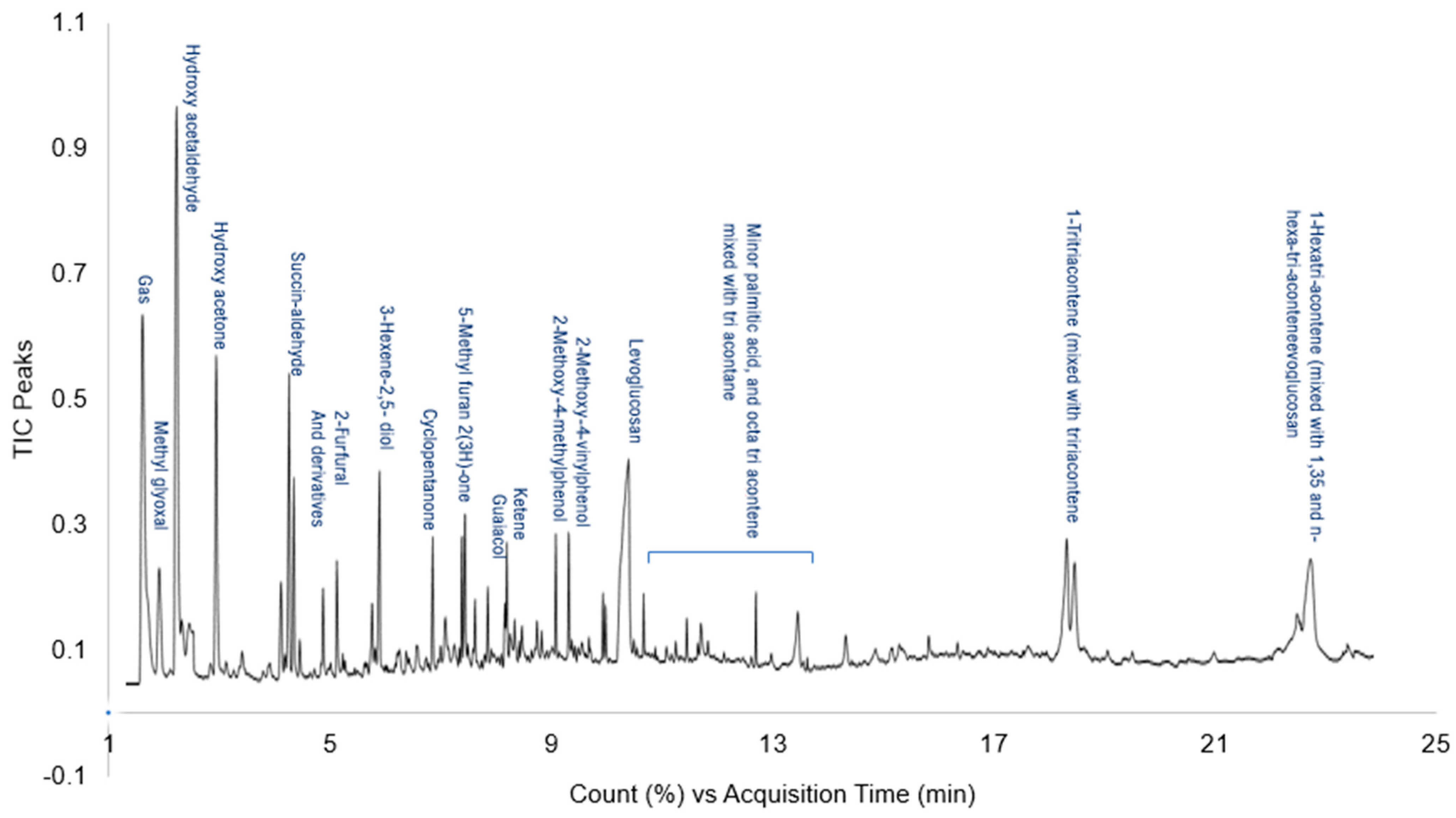
3.5. HC Pyrolites Analysis and Major Components Detection
3.6. Thermogravimetric Analysis (TGA) of HC
4. Applications of HC in GHs
4.1. Experiments on HC Soil Applications
4.2. HC Application in Greenhouse Leached Nutrient Water Treatment
5. Conclusions
- ➢
- Compared to the 12 other biomasses reviewed, TPB was revealed to be in the lower grade, in terms of HHV (MJ/kg) and moisture %. It may be in the rank of hay and poplar. TPB HHV was higher than poplar.
- ➢
- Numerous beneficial uses of waste biomass were established: (a) HC for energy production, as high HHV values (25.9 MJ/kg) are comparable to peat and can be a fuel source for greenhouses or be a commercial commodity, (b) HC as fertilizer and soil ameliorant, (c) HC for GNF nutrient treatment.
- ➢
- HTC substantially reduced inorganics from HC and lowered the slagging/fouling index of produced fuels; another benefit over other carbonizations.
- ➢
- The uses of tomato plant HC in soil application and leached GNF nutrient reduction are new successes opening a door for biomass valorization and profit opportunity for greenhouses, along with providing environmental sustainability.
- ➢
- More valuable advanced products, such as activated carbon and nanocomposites, seem to be possible from HC. Thus, HTC can play a vital role in production of nanostructured carbon; for example, N dopants may confer electronic as well as catalytic properties. Such an initiation for more advanced HTC materials development in future research would be a highly encouraging area.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunch, K. Binational Plans Call for 40 Percent Reduction to Algal-Fueling Nutrients. 2018. Available online: https://www.ijc.org/en/binational-plans-call-40-percent-reduction-algal-fueling-nutrients (accessed on 22 April 2022).
- Statistics Canada. Canadian Tomatoes, from Farm to Fork. 2022. Available online: https://www150.statcan.gc.ca/n1/pub/11-627-m/11-627-m2021038-eng.htm (accessed on 29 April 2022).
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An overview of effect of process parameters on hydrothermal carbonization of biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Kambo, H.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Wang, R.; Wang, C.Z.; Zhao, Z.; Jia, J.; Jin, Q. Energy recovery from high-ash municipal sewage sludge by hydrothermal carbonization: Fuel characteristics of biosolid products. Energy 2019, 186, 115848. [Google Scholar] [CrossRef]
- Shrestha, A.; Acharya, B.; Farooque, A.A. Study of hydrochar and process water from hydrothermal carbonization of sea lettuce, Renew. Energy 2021, 163, 589–598. [Google Scholar] [CrossRef]
- Nanda, S.; Pant, K.; Naik, S. Characterization of North American lignocellulosic biomass and biochars in terms of their candidacy for alternate renewable fuels. Bioenergy Res. 2013, 6, 663–677. [Google Scholar] [CrossRef]
- Yu, Y.; Lou, X.; Wu, H. Some recent advances in hydrolysis of biomass in hot-compressed, water and its comparisons with other hydrolysis methods. Energy Fuels 2008, 22, 46–60. [Google Scholar] [CrossRef]
- Bobleter, O. Hydrothermal Degradation of Polymers Derived From Plants. Prog. Polym. Sci. 1994, 19, 797–841. [Google Scholar] [CrossRef]
- Wang, D.; Czernik, S.; Chornet, E. Production of hydrogen from biomass by catalytic steam reforming of fast pyrolysis oils. Energy Fuels 1998, 12, 19–24. [Google Scholar] [CrossRef]
- Isahak, W.N.R.W.; Mohd, H.; Yarmo, M.A.; Hin, T. A review on bio-oil production from biomass by using pyrolysis method. Renew. Sustain. Energy Rev. 2012, 16, 5910–5923. [Google Scholar] [CrossRef]
- Kyoto Protocol Under the umbrella of “United Nations Climate Change”, Kyoto Protocol is a commitment of industrialized countries. 1997, 11, 1097. Available online: https://unfccc.int/resource/docs/convkp/kpeng.pdf (accessed on 29 June 2019).
- Azarghor, D.; Nanda, S.; Dalal, A. Densification of Agricultural Wastes and Forest Residues: A Review on Influential Parameters and Treatments. In Recent Advancements in Biofuels and Bioenergy Utilization; Sarangi, P.K., Nanda, S., Mohanty, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-981-13-1307-3. [Google Scholar] [CrossRef]
- Roman, K.; Barwicki, J.; Hryniewicz, M.; Szadkowska, D.; Szadkowski, J. Production of Electricity and Heat from Biomass Wastes Using a Converted Aircraft Turbine AI-20. Processes 2021, 9, 364. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Production of high surface area mesoporous activated carbons from waste biomass using hydrogen peroxide-mediated hydrothermal treatment for adsorption applications. Chem. Eng. J. 2015, 273, 622–629. [Google Scholar] [CrossRef]
- Jiang, L.; Sheng, L.; Fan, Z. Biomass-derived carbon materials with structural diversities and their applications in energy storage. Sci. China Mater. 2018, 61, 133–158. [Google Scholar] [CrossRef] [Green Version]
- Bargmann, I.; Martens, R.; Rillig, M.C.; Kruse, A.; Kücke, M. Hydrochar amendment promotes microbial immobilization of mineral nitrogen. J. Plant Nutr. Soil Sci. 2014, 177, 59–67. [Google Scholar] [CrossRef]
- Chen, W.H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 126–1140. [Google Scholar] [CrossRef]
- Nicolae, S.A.; Au, H.; Modugno, P.; Luo, H.; Szego, A.E.; Qiao, M.; Li, L.; Yin, W.; Heeres, H.J.; Berged, N.; et al. Recent advances in hydrothermal carbonsation: From tailored carbon materials and biochemicals to applications and bioenergy. Green Chem. 2020, 22, 4747–4800. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bio-Prod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Jamal-Uddin, A.T.; Zytner, P.; Zytner, R.G. Hybrid Treatment System to Remove Micromolecular SMPs from Fruit Wastewater Treated with an MBR. Can. J. Civ. Eng. 2022, 49, 548–557. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Christopher, T.; Wright, C.T.; Hess, J.R.; Kenney, K.L. A review of biomass densification systems to develop uniform feedstock commodities for bioenergy application. Biofuels Bioprod. Biorefin. 2011, 5, 683–707. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Chemicals from Biomass; US Department of Energy, Energy Efficiency and Renewable Energy, NREL: Washington, DC, USA, 2004; Volume 1, pp. 1–76. Available online: http://www.ntis.gov/ordering.htm (accessed on 13 April 2022).
- Titirici, M.M.; Thomas, A.; Yu, S.-H.; Müller, J.-O.; Antonietti, M. A Direct Synthesis of Mesoporous Carbons with Bicontinuous Pore Morphology from Crude Plant Material by Hydrothermal Carbonization. Chem. Mater. 2007, 19, 4205–4212. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals.; physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef]
- Yin, S.; Ryan, R.; Harris, M.; Tan, Z. Subcritical hydrothermal liquefaction of cattle manure to bio-oil: Effects of conversion parameters on bio-oil yield and characterization of bio-oil. Bioresour. Technol. 2010, 101, 3657–3664. [Google Scholar] [CrossRef]
- Liu, L.; Neubauer, N.A. Gasification. In High Temperature Processes in Chemical Engineering; Lackner, M., Ed.; Process Engineering GmbH: Vienna, Austria, 2010. [Google Scholar]
- Carter, B.; Gilcrease, P.C.; Menkhausy, T.J. Removal and Recovery of Furfural, 5-Hydroxymethylfurfural, and Acetic Acid from Aqueous Solutions Using a Soluble Polyelectrolyte. Biotechnol. Bioenergy 2011, 108, 2046–2052. [Google Scholar] [CrossRef]
- Peterson, A.; Vogel, F.; Lahance, M. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Bergius, F. Chemical Reactions under High Pressure; 1991 Lecture Note; Nobel Foundation: Stockholm, Sweden, 1931; pp. 1–33. [Google Scholar]
- Llorach-Massana, P.; Lopez-Capel, E.; Peña, J.; Rieradevall, J.; Montero, J.; Puy, N. Technical feasibility and carbon footprint of biochar co-production with tomato plant residue. Waste Manag. 2017, 67, 121–130. [Google Scholar] [CrossRef]
- Savidov, N. Biochar: Growing a Sustainable Medium—Greenhouse Canada. 2019. Available online: www.greenhousecanada.com/technology-issues-growing-a-sustainable (accessed on 29 April 2022).
- World Health Organization (WHO). Guidelines for the Safe Use of Wastewater, Excreta and Greywater. Volume 2: Wastewater Use in Agriculture; World Health Organization (WHO): Geneva, Switzerland; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy; United Nations Environment Program (UNEP): Nairobi, Kenya, 2006. [Google Scholar]
- Basso, D.; Casello, D.; Baratiri, M.; Flori, L. Hydrothermal Carbonization of Waste Biomass: Progress Report and Prospect. In Proceedings of the 21st European Biomass Conference and Exhibition, Copenhagen, Denmark, 3–7 June 2013; Available online: https://www.researchgate.net/publication/257989805 (accessed on 29 April 2022).
- Peter, K.; Eckhard, D. An assessment of supercritical water oxidation (SCWO): Existing problems, possible solutions and new reactor concepts. Chem. Eng. J. 2001, 83, 207–214. [Google Scholar] [CrossRef]
- Wen, J.; Xiao, L.; Sun, Y.; Sun, S.; Xu, F.; Sun, R.; Zhang, X. Comparative study of alkali-soluble hemicelluloses isolated from bamboo (Bambusa rigida). Carbohydr. Res. 2011, 346, 111–120. [Google Scholar] [CrossRef]
- Harper, S.H.T.; Lynch, J.M. The Chemical Components and Decomposition of Wheat Straw Leaves, Internodes and Nodes. J. Sci. Food Agric. 1981, 32, 1057–1062. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Uddin, H.M.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Saddawi, A.; Jones, J.M.; Williams, A.; Le Coeur, C. Commodity fuels from biomass through pretreatment and torrefaction: Effects of mineral content on torrefied fuel characteristics and quality. Energy Fuels 2012, 26, 6466–6474. [Google Scholar] [CrossRef]
- Minaret, J.; Dutta, A. Comparison of liquid and vapor hydrothermal carbonization of corn husk for the use as a solid fuel. Bioresour. Technol. 2016, 200, 804–811. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. Strength, storage.; combustion characteristics of densified lignocellulosic biomass produced via torrefaction and hydrothermal carbonization. Appl. Energy 2014, 135, 182–191. [Google Scholar] [CrossRef]
- Uddin, A.H.; Khalid, R.S.; Alaama, M.; Abdualkader, A.M.; Kasmuri, A.; Abbas, S.A. Comparative study of three digestion methods for elemental analysis in traditional medicine products using atomic absorption spectrometry. J. Anal. Sci. Technol. 2016, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Ang, H.; Lee, K.L. Analysis of mercury in Malaysian herbal preparations: A peer-review. J. Med. Biomed. Sci. 2005, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kruse, A.; Dahmen, N. Water—A magic solvent for biomass conversion. J. Supercrit. Fluids 2015, 96, 36–45. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Graphical-statistical method for the study of structure and reaction processes of coal. Fuel 1950, 29, 269–284. [Google Scholar]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Joan, G.; Lynam, J.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Contescu, C.; Adhikari, S.; Gallego, N.; Evans, N.; Biss, B. Activated Carbons Derived from High-Temperature Pyrolysis of Lignocellulosic Biomass. J. Carbon Res. 2018, 4, 51. [Google Scholar] [CrossRef] [Green Version]
- Demirbas, A. Relationships between lignin contents and heating values of biomass. Energy Convers. Manag. 2001, 42, 183–188. [Google Scholar] [CrossRef]
- Menegazzo, F.; Elena Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) Production from Real Biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambo, H.; Dutta, A. Comparative evaluation of torrefaction and hydrothermal carbonization of lignocellulosic biomass for the production of solid biofuel. Energy Convers. Manag. 2015, 105, 746–755. [Google Scholar] [CrossRef]
- Bykov, I. Characterization of Natural and Technical Lignins Using FTIR Spectroscopy. Independent Thesis. 2008. Available online: http://www.diva-portal.org/smash/get/diva2:1016107/FULLTEXT01.pdf (accessed on 23 June 2021).
- Wang, W.; Chen, W.; Jang, M.-F. Characterization of Hydrochar Produced by Hydrothermal Carbonization of Organic Sludge. Future Cities Environ. 2021, 6, 13. [Google Scholar] [CrossRef]
- Santos, J.I.; Fillat, U.; Martin-Sampedro, R.; Ballesteros, I.; Manzanares, P.; Ballesteros, M.; Eugenio, M.E.; Ibarra, D. Lignin-enriched Fermentation Residues from Bioethanol Production of Fast-growing Poplar and Forage Sorghum. Bioresources 2015, 10, 5203–5214. [Google Scholar] [CrossRef] [Green Version]
- Miliotti, E.; Rosi, L.; Bettucci, L.; Lotti, G.; Maria Rizzo, A.; Chiaramonti, D. Characterization of Chemically and Physically Activated Carbons from Lignocellulosic Ethanol Lignin-Rich Stream vis Hydrothermal Carbonization and Slow Pyrolysis Pretreatment. Energies 2021, 13, 4101. [Google Scholar] [CrossRef]
- Colomba, A. Production of Activated Carbons from Pyrolytic Char For Environmental Applications. Ph.D. Thesis, University of Western Ontario, London, ON, Canada, 2015. [Google Scholar]
- Elad, Y.; David, D.; Harel, Y.; Borenshtein, M.; Kalifa, H.; Silber, A.; Graber, E. Induction of systemic resistance in plants by biochar, a soil-applied C sequestering agent. Phytopathology 2010, 100, 913–921. [Google Scholar] [CrossRef] [Green Version]
- Elad, Y.; Cytryn, E.; Harel, Y.; Lew, B. The biochar effect: Plant resistance to biotic stresses. Phytopathol. Mediterr. 2012, 50, 335–349. [Google Scholar] [CrossRef]
- Bai, M.; Wilske, B.; Buegger, F.; Esperschütz, J.; Kammann, C.; Eckhardt, C. Degradation kinetics of biochar from pyrolysis and hydrothermal carbonization in temperate soils. Plant Soil. 2013, 372, 375–387. [Google Scholar] [CrossRef]
- Malghani, S.; Gleixner, G.; Trumbore, S.E. Chars produced by slow pyrolysis and hydrothermal carbonization vary in carbon sequestration potential and greenhouse gases emissions. Soil Biol. Biochem. 2013, 62, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Qayyum, M.F.; Steffens, D.; Reisenauer, H.P.; Schubert, S. Kinetics of carbon mineralization of biochars compared with wheat straw in three soils. J. Environ. Qual. 2012, 41, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Kammann, C.; Ratering, S.; Eckhard, C.; Müller, C. Biochar and Hydrochar Effects on Greenhouse Gas (Carbon Dioxide, Nitrous Oxide, and Methane) Fluxes from Soils. J. Environ. Qual. 2012, 41, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Gajić, A.; Ramke, H.G.; Hendricks, A.; Koch, H.J. Microcosm study on the decomposability of hydrochars in a Cambisol. Biomass Bioenergy 2012, 47, 250–259. [Google Scholar] [CrossRef]
- Perez-Mercado, L.; Lalandera, C.; Joel, A.; Ottoson, J.; Dalahmeh, S.; Vinneråsa, B. Biochar filters as an on-farm treatment to reduce pathogens when irrigating with wastewater-polluted sources. J. Environ. Manag. 2019, 248, 109295. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Taylor & Francis Group: Abingdon, UK, 2015; 994p. [Google Scholar]
- Sevilla, M.; Maciá-Agulló, J.A.; Fuertes, A.B. Hydrothermal carbonization of biomass as a route for the sequestration of CO2: Chemical and structural properties of the carbonized products. Biomass Bioenergy 2011, 35, 3152–3159. [Google Scholar] [CrossRef] [Green Version]





| Biomass | Extractives % | Hemicellulose % | Lignin % | Cellulose % | Ash % | HHV MJ/kg |
|---|---|---|---|---|---|---|
| Tomato plant | 14.1 ± 2.4 | 37.2 ± 2.1 | 11.9 ± 1.8 | 32.1 ± 2.6 | 4.7 ± 0.3 | 12.7 |
| Miscanthus [41] | 6.9 | 30.2 | 14.2 | 44.4 | 4.4 | 17.4 |
| Switch grass [41] | 13.6 | 33.7 | 8.4 | 35.3 | 9.1 | 15.3 |
| Corn stover [41] | 26.3 | 26.3 | 9.5 | 29.7 | 8.2 | 15.6 |
| (mg/kg) | % Removal | ||||||
|---|---|---|---|---|---|---|---|
| HC at 260 °C | HC at 220 °C | HC at 250 °C NP | Biomass | HC at 260 °C | HC at 220 °C | HC at 250 °C NP | |
| Na | 396.5 | 395.1 | 585.7 | 500 | 20.7 | 21.0 | |
| K | 1586.0 | 5926.5 | 37,094.98 | 76,900 | 97.9 | 92.3 | 51.8 |
| Mg | 991.3 | 987.8 | 5857.1 | 4300 | 77.0 | 77.0 | |
| Ca | 5749.4 | 1461.9 | 8180.4 | 37,700 | 84.8 | 96.1 | 78.3 |
| S% | 0.0055 | 0.0069 | 0.031 | ||||
| Material | Moisture % | VM % | FC % | Ash % | C % | H % | N % | S % | O % | H/C | O/C | HHV (MJ/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPB | 90.0 | 7.1 | 1.2 | 1.7 | 36.8 | 4.0 | 2.0 | 0.8 | 54.0 | 1.3 | 1.1 | 12.6 ± 1.2 |
| Grass * | 10.0–50.0 | 75.0–83.0 | 8.0–15.0 | 2.0–7.0 | 46.0–51.0 | 6.0–7.0 | 0.4–1.0 | <0.02 | 41.0–46.0 | 16.0–18.7 | ||
| A. Res. * | 5.0–40.0 | 70–85 | 8.0–20.0 | 4.6–21.0 | 45.0–51.0 | 6.2–7.2 | 0.2–1.3 | 0.3–0.5 | 41–47 | 15.0–18.0 | ||
| AA220 °C | 89.7 | 9.3 | 1.0 | 50.9 | 5.6 | 0.9 | 0.1 | 41.5 | 1.3 | 0.6 | 18.6 | |
| 220 °C (i) | 93.9 | 5.2 | 0.9 | 47.8 | 5.1 | 1.4 | 0.2 | 44.6 | 1.3 | 0.7 | 18.0 | |
| 220 °C (ii) | 87.8 | 11.3 | 0.9 | 49.0 | 5.2 | 0.8 | 0.04 | 44.1 | 1.3 | 0.7 | 23.1 | |
| AA260 °C | 86.8 | 12.5 | 0.7 | 50.6 | 5.6 | 0.8 | 0.06 | 42.2 | 1.3 | 0.6 | 25.9 | |
| np250 °C | 88.7 | 9.1 | 2.2 | 44.6 | 3.7 | 3.1 | 2.0 | 44.4 | 1.0 | 0.7 | 17.4 | |
| np230 °C | 90.0 | 8.4 | 1.6 | 42.2 | 4.0 | 3.3 | 0.7 | 48.2 | 1.1 | 0.9 | 19.3 | |
| 180 °C | 85.3 | 13.5 | 1.1 | 43.4 | 5.4 | 1.2 | 0.1 | 48.8 | 1.5 | 0.8 | 17.9 |
| Chemical Name (Pyrolite Library) | Formula | MW | Ret. Time (min) | Com. % | |
|---|---|---|---|---|---|
| from | to | ||||
| Gas | CO2 | 44 | 1.7 | 1.765 | 11.41 |
| Methyl glyoxal | C3H4O2 | 43 | 1.856 | 2.071 | 3.02 |
| Hydroxy acetaldehyde | C2H4O2 | 31 | 2.064 | 2.401 | 11.48 |
| Hydroxy acetone | C3H6O2 | 43 | 2.782 | 3.140 | 4.85 |
| Hydroxyethyl acetate | C4H8O3 | 43 | 4.012 | 4.358 | 1.3 |
| Succin-aldehyde | C4H6O2 | 29 | 4.113 | 4.513 | 3.46 |
| 2-Furfural | C5H4O2 | 39 | 5.079 | 5.148 | 0.97 |
| 3-Hexene-2,5-diol | C6H12O2 | 43 | 5.408 | 1.0 | |
| 5-Methyl furan2(3H)-one | C5H6O2 | 98 | 5.806 | 6.211 | 2.37 |
| Levoglucosan | C6H10O5 | 60 | 10.450 | 10.889 | 10.42 |
| 1-Tritriacontene (mixed with 1.32 and n-Tritriacontene | C33H66, C33H64, | 57 | 18.735 | 19.131 | 3.75 |
| 1-Hentetra-acontene(mixed with 1.40 hentetra-acontene) | C41H82, C41H80, | 57 | 18.931 | 19.273 | 2.63 |
| 1-Hexatri-acontene (mixed with 1.35 and n-hexa-tri-acontene | C36H70, C36H74 | 57 | 22.231 | 23.05 | 6.24 |
| Feedstock | HC C Content (%) | Soil Type | HC Rate (g/kg Soil) | Emissions | Degradation Rate (%) | References | ||
|---|---|---|---|---|---|---|---|---|
| CO2 | N2O | CH4 | ||||||
| TPB | 47.8–50.5 | Potting soil | 5.0–10.0 | n.d. | n.d. | n.d. | n.d. | Present work |
| Miscanthus | 48.9 | Inceptisol | 3.4–9.7 | n.d. | n.d. | n.d. | 27–27 | [61] |
| Corn silage | 51.6 | Arabian soil | 5.2 | + | − | ++ | >50 | [62] |
| Bark | 51.0 | Oxisol | 11.3 | + | n.d. | n.d. | 10 | [63] |
| Glucose/yeast | 64.6/67.4 | Arabian soil | 7.5 | ++ | n.d. | n.d. | 3–9 | [64] |
| Beet root chips | 49.7 | Arabian soil | 39.8 | ++ | + | + | n.d. | [65] |
| Sugar beet pulp | 49.2–52.5 | Arabian soil | 4.8–5.2 | + | n.d. | n.d. | 12–32 | [66] |
| Parameters | GNF1 (mg/L) | HC Treated (mg/L) | % Reduction | GNF3 (mg/L) | HC Treated mg/L | % Reduction |
|---|---|---|---|---|---|---|
| NO3 & NO2-N | 226 | 202 | 10.62 | 319.5 | 238 | 25.51 |
| TKN | 231.5 | 211 | 8.86 | 165 | 128 | 22.42 |
| TN (mg/L) | 457.5 | 371 | 18.91 | 494.5 | 376 | 23.96 |
| Phosphate total PO4−3 | 132 | 116 | 12.12 | 315 | 211 | 33.02 |
| Phosphate reactive (orthophosphate) | 67 | 60 | 10.45 | 175 | 151 | 13.71 |
| Na | 61 | 52 | 14.75 | 70 | 68 | 2.86 |
| K | 670 | 580 | 13.43 | 614 | 432 | 29.64 |
| Mg | 81 | 76 | 6.17 | 182 | 129 | 29.12 |
| Ca | 291 | 211 | 27.49 | 329 | 296 | 10.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamal-Uddin, A.-T.; Salaudeen, S.A.; Dutta, A.; Zytner, R.G. Hydrothermal Conversion of Waste Biomass from Greenhouses into Hydrochar for Energy, Soil Amendment, and Wastewater Treatment Applications. Energies 2022, 15, 3663. https://doi.org/10.3390/en15103663
Jamal-Uddin A-T, Salaudeen SA, Dutta A, Zytner RG. Hydrothermal Conversion of Waste Biomass from Greenhouses into Hydrochar for Energy, Soil Amendment, and Wastewater Treatment Applications. Energies. 2022; 15(10):3663. https://doi.org/10.3390/en15103663
Chicago/Turabian StyleJamal-Uddin, Abu-Taher, Shakirudeen A. Salaudeen, Animesh Dutta, and Richard G. Zytner. 2022. "Hydrothermal Conversion of Waste Biomass from Greenhouses into Hydrochar for Energy, Soil Amendment, and Wastewater Treatment Applications" Energies 15, no. 10: 3663. https://doi.org/10.3390/en15103663







