Abstract
Nowadays, sustainable and renewable energy production is a global priority. Over the past decade, several Power-to-X (PtX) technologies have been proposed to store and convert the surplus of renewable energies into chemical bonds of chemicals produced by different processes. CO2 is a major contributor to climate change, yet it is also an undervalued source of carbon that could be recycled and represents an opportunity to generate renewable energy. In this context, PtX technologies would allow for CO2 valorization into renewable fuels while reducing greenhouse gas (GHG) emissions. With this work we want to provide an up-to-date overview of biomethanation as a PtX technology by considering the biological aspects and the main parameters affecting its application and scalability at an industrial level. Particular attention will be paid to the concept of CO2-streams valorization and to the integration of the process with renewable energies. Aspects related to new promising technologies such as in situ, ex situ, hybrid biomethanation and the concept of underground methanation will be discussed, also in connection with recent application cases. Furthermore, the technical and economic feasibility will be critically analyzed to highlight current options and limitations for implementing a sustainable process.
1. Introduction
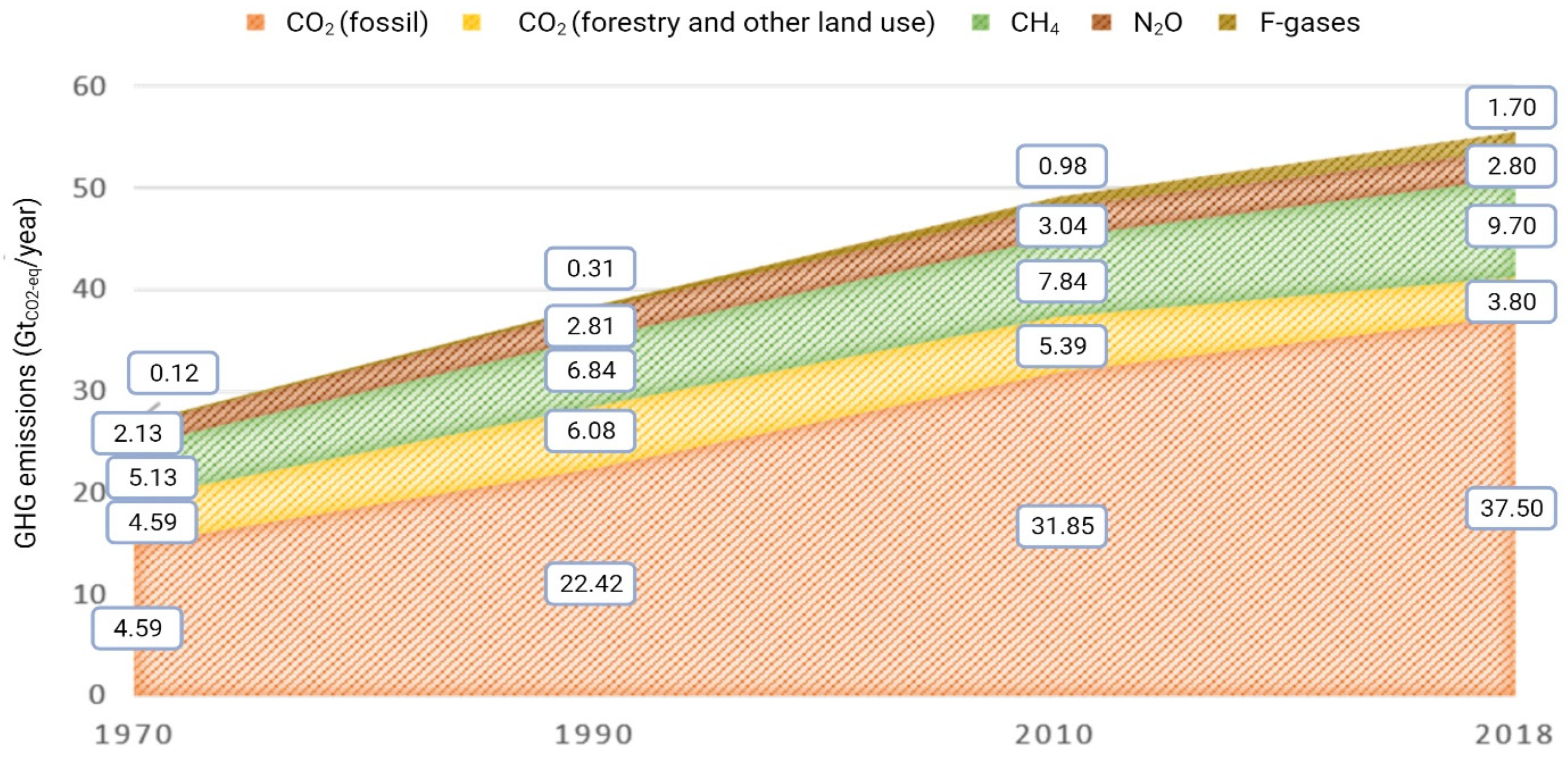
The effects of anthropic GHG production on climate change and global warming are nowadays well known world-wide. In 2019, GHG levels were reduced by 24% compared to those reported in 1990 (European Commission, 2018/2020). Nonetheless, global GHG emissions are still a pressing issue, with CO2 being the most abundant among GHG and accounting for 75% of total anthropogenic GHG emissions in 2018 (17% comes from CH4, 5% from N2O and 3% from fluorinated gases (F-gases) [1]) (Figure 1).
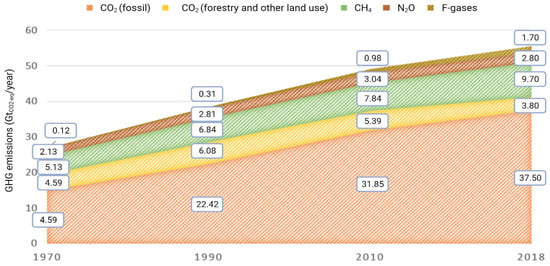
Figure 1.
Increase in global emissions of GHG in GtCO2-eq/yr between 1970 and 2018, adapted from [1].
A key role in reducing GHG emissions is played by renewable and sustainable energy sources. In 2004, the European Renewable Energies Council (EREC) launched a call, at the European level, for a binding target for the deployment of at least 20% of renewable energies by 2020, including all the main sectors of application such as electricity, transport, heating and cooling [2]. In September 2020, the European commission promoted a further reduction in CO2 and GHGs emissions to 55% and an increase in the renewable energies share from 20 to 32% by 2030. Moreover, long-term plans to achieve carbon neutrality by 2050 are under evaluation (European Commission, 2018/2020). However, renewable energies are fluctuating and intermittent by nature, with their deployment requiring high capacity and long-term storage to actually undertake this transition. Over the past decade, PtX technologies demonstrated the possibility to store the excess of renewable energies in chemical bonds of different chemicals produced during the process [3].
Despite being a major contributor to climate change, CO2 represents an opportunity to achieve renewable energy storage and, in a carbon-based global economy, represents a currently undervalued mass of carbon that could be recycled. While strategies for emission control, sequestration and fixation are required, the recovery and recycling of CO2 into renewable fuels can offer valuable options for GHG reduction and valorization [4,5]. Within the third generation biofuel technologies, interest is increasing in gas fermentation, consisting in the fermentation of gaseous substrates such as H2, CO and CO2, performed by anaerobic microorganisms, for the conversion of both industrial off gases and recalcitrant feedstocks, if coupled to their gasification into synthesis gas [5].
The biological conversion of CO2 and H2 into CH4, commonly referred to as biomethanation (Equation (1)), has gained a lot of attention and has been widely investigated in the last 10 years:
Indeed, this technology addresses both CO2 valorization, through its conversion into CH4, and the storage of the energy surplus generated by renewable sources (i.e., wind and solar power) into chemicals. Considering the H2 requirement for the reaction, the surplus of wind and solar power would be used to generate the needed H2 through water electrolysis. Although H2 could play a valuable role as clean fuel, its energy content is considerably lower (10.88 MJ/m3) than that of CH4 (36 MJ/m3) [6].
Different studies report how biomethanation processes can lead to the production of biomethane, the CH4 levels of which are similar to those of natural gas (NG), where CH4 often exceeds 95% of the gas mixture, making it fully compatible with the existing infrastructures. Thus, biomethane could be used as a replacement for NG and directly injected into the gas grid and storage systems, significantly reducing the initial investment [7,8,9]. Biomethanation’s main advantages include: (i) high robustness against impurities in the final product and in the feed gas; (ii) the possibility of intermittent operation; (iii) biomethanation takes place in the liquid phase, which helps to buffer load effects; and (iv) the ability of the biocatalyst to renew itself given appropriate conditions [10,11]. Moreover, the natural occurrence of methanogens in underground environments such as depleted hydrocarbon reservoirs and deep saline aquifers might offer the possibility to use such geological structures as bioreactor systems [12,13].
Different surveys reported that, as of 2019, there were 33 different biomethanation projects at different stages of realization. Such projects rely on H2, produced by means of water electrolysis powered by the surplus of electrical power generated by renewable energies, and CO2 mainly obtained from wastewater and sewage gasses (i.e., SYMBIO, Electrochaea GmbH), or from bottled gasses when considering lab-scale applications. Furthermore, the few available examples of biomethanation plants at commercial scale are reported to re-inject the produced biomethane directly into the gas grid or to store it locally [14,15]. The biomethanation process has been extensively investigated, and a substantial amount of studies frequently discuss the possible implementation and prospect of such technology, with several institutions recognizing its importance as a mean to store and deliver renewable energy. Nonetheless, examples of process up-scaling and its utilization at commercial scale are still lacking.
The purpose of this review is to provide a comprehensive and up-to-date overview of the biomethanation process and provide a deep description of the involved microorganisms, the physical and chemical parameters influencing the process and performances of the biocatalysts, current applications and future perspectives. The review also considers biomethanation’s viability through a technical and economic analysis.
2. Methanogenic Archaea: Microbiological and Biochemical Background
Microorganisms capable of conserving energy by CH4 formation are defined as methanogens. Methanogens are obligated methane producers, which means that they cannot use alternative electron acceptors for their respiration metabolism nor their fermentative process. They are the main actors of the biological reduction of CO2 by H2. Methanogenic microorganisms were first cultured in the 1933 [16,17], although the first evidence of biological methanation dates back to 1776, when Alessandro Volta hypothesized that flammable freshwater swamp gas was emanated by the decay of organic matter [18]. Currently, methanogens are divided into seven orders (i.e., Methanococcales, Methanobacteriales, Methanomicrobiales, Methanopyrales, Methanocellales, Methanomassiliicoccales, Methanosarcinales) recognized by mcrA and ribosomal gene phylogeny and belonging exclusively to the phylum Euryarcheota [19,20,21,22,23,24,25]. Members of the same genera display different morphological and physiological characteristics, allowing methanogens to adapt to different anaerobic habitats distinguished into three main types: anaerobic biotopes (i.e., anaerobic digesters), digestive tracts (i.e., gastro-intestinal tract of humans, ruminants and insects) and geothermal springs (i.e., freshwater sediments, hydrothermal vents and geothermal habitats) [26,27].
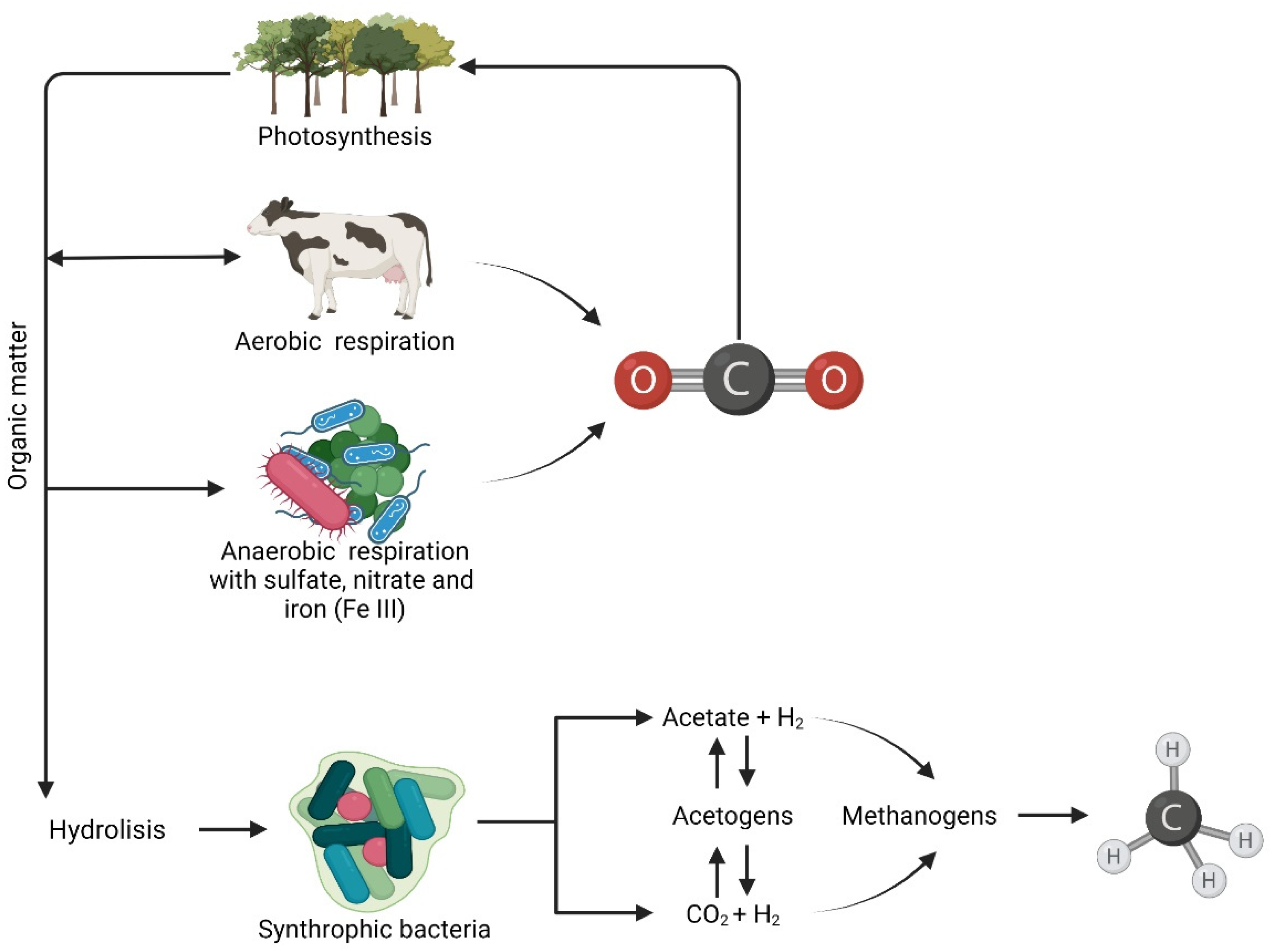
In methanogenic habitats, complex organic matter is degraded to CH4 by symbiotic relationships between different groups of anaerobic microbes. Substrates used for CH4 production include CO2, H2, acetic acid and methyl-group-containing molecules. Such compounds are naturally produced during the anaerobic degradation of organic matter, in which methanogens occupy the terminal niche of the electron transfer chain [28] (Figure 2). In addition, methanogenic habitats are poor in electron acceptors such as O2, NO3−, Fe3+ and SO42− due to their rapid consumption during biomass degradation. Moreover, bacteria such as de-nitrifiers and sulfate reducers are thermodynamically more efficient than the methanogens with which they compete for electron acceptors (i.e., NO3− and SO42−), thus inducing the inhibition of methanogens [29,30].
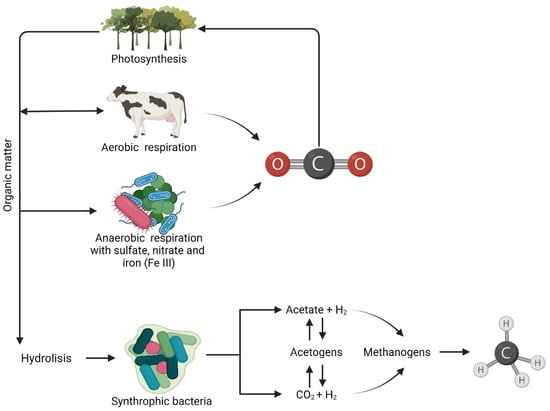
Figure 2.
Organic matter degradation cycle.
In anaerobic environments, insoluble organic polymers are initially hydrolyzed to simple sugars, lactate, volatile fatty acids and alcohols by hydrolytic organisms. Hydrolysis is followed by acidogenesis, consisting of the anaerobic conversion of complex organic matter via hydrogenation and dehydrogenation to volatile fatty acids (VFAs) (e.g., acetate, propionate, butyrate) and alcohols (e.g., methanol and ethanol) with H2, CO2 and NH4 being generated during the process. CH4 production is enhanced by the presence of syntrophic acetate oxidizers (SAOs) converting acetate into acetic acid, H2 and CO2 and syntrophic fatty acid oxidizers (sFAOs), promoting the oxidation of higher fatty acids (LCFAs) to acetate [31,32]. Methanogenesis has been traditionally linked to the Wood-Ljundgdahl (WL) pathway, which is one of the most ancient metabolisms for energy generation and carbon fixation in the Archaea. The WL pathway can act in two different ways: The first one consists of energy generation and carbon fixation. Most bacteria produce acetate as the end product (acetogens), whereas most archaea produce methane (CO2-reducing methanogens). The second consists of the reverse WL pathway, which produces reducing power from the oxidation of organic matter [33]. For acidogenesis to occur, the H2 partial pressures have to be below 102 Pa. Due to the presence of methanogens, which rapidly metabolize H2, the H2 partial pressure remains below 10 Pa. This exchange of electrons by means of hydrogen and formate among syntrophic organisms, which produce and consume H2, respectively, has been defined as interspecies hydrogen transfer [28,32] The H2, CO2, acetate and alcohols produced during acidogenesis and acetogenesis are substrates for hydrogenotrophic (HM), acetoclastic (AM) and methylotrophic (MeM) methanogens, whose metabolisms are resumed by Equations (1)–(3), respectively. The Gibbs free energy (ΔGo′) was calculated from the free energy of the formation of the most abundant ionic species at pH 7, as reported by [29,34]:
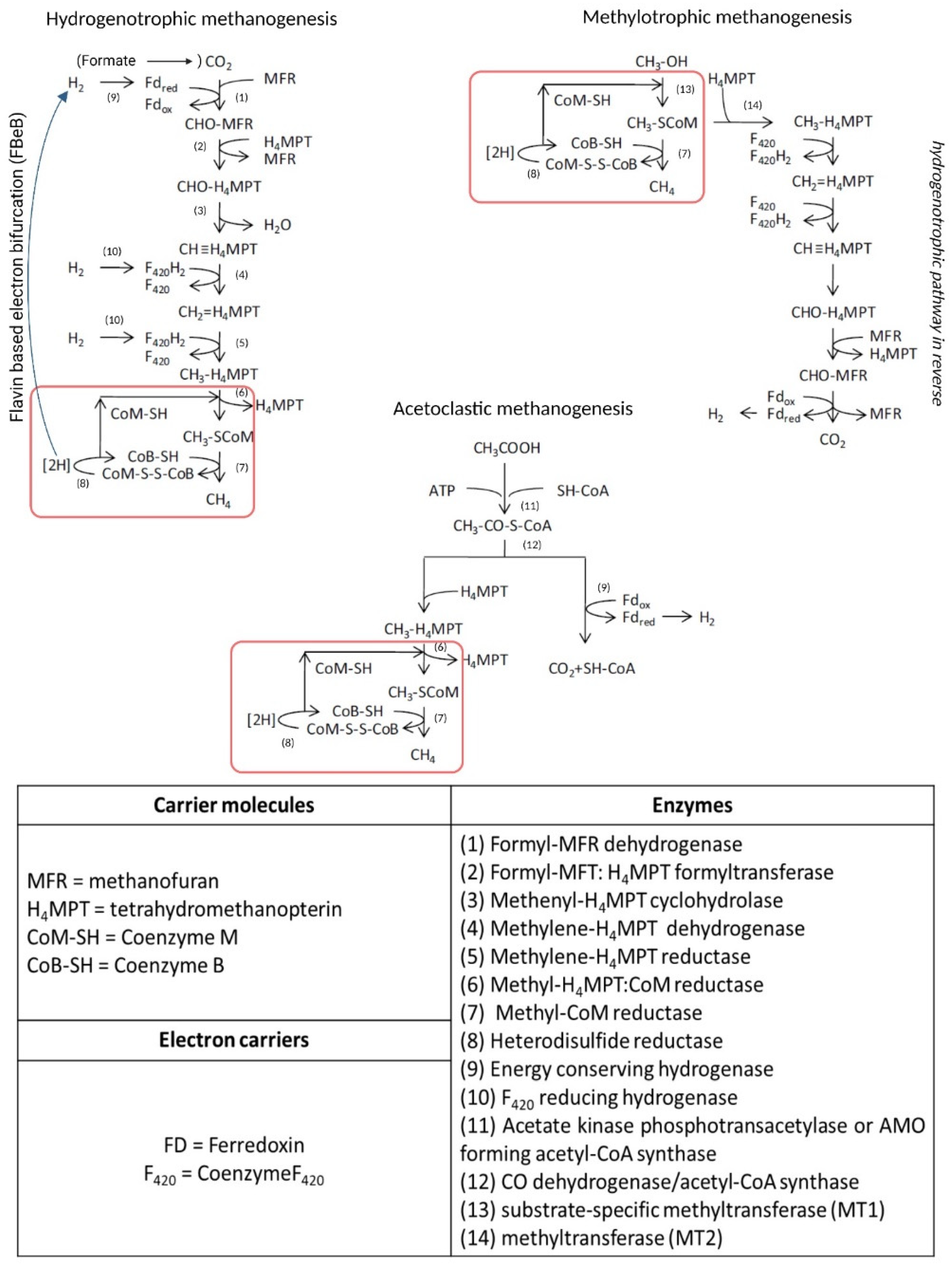
Although capable of growing on a limited variety of substrates (i.e., acetate, methylated compounds CO2 and H2), methanogens have a quite complex biochemistry. The biosynthetic pathway of biomethanation consists of seven different metabolic steps driven by specific enzymes and cofactors that lead to the reduction of CO2 to CH4, while the energy conservation system is different, especially for HM due to the lack of cytochromes [34,35]. The metabolic apparatus for methanogenesis is encoded by about 200 genes [35]. A schematization of the three metabolic pathway is shown in Figure 3, also reporting the list of enzymes and coenzymes involved in the methanogenic metabolisms.
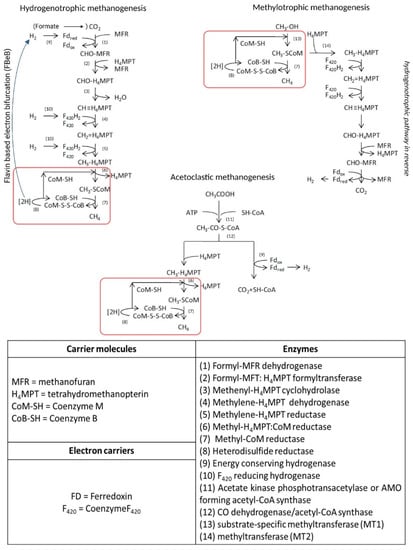
Figure 3.
Metabolic pathways, enzymes and co-enzymes involved in methanogenesis. Red squares indicate metabolic reactions common to all three methanogenic pathways. Adapted from [36].
Hydrogenotrophic Methanogens as Biocatalyst for Biomethanation: Metabolic Pathway and Physiological Aspects
HMs are the key organisms for the conversion of H2 and CO2 into CH4, and they are present in almost all methanogenic orders, except for the Methanomassiliicoccales. Most of the knowledge regarding the hydrogenotrophic metabolism comes from pure culture studies of two major thermophilic strains Methanotermobacter thermoautotrophicum and Methanothermobacter marburgensis [37].
In hydrogenotrophic methanogenesis, energy conservation depends on the flavin based electron bifurcation (FBeB), as shown in the pathway detailed in Figure 3. HMs are classified as cytochrome-free methanogens, in which an oxidative and a reductive energy system can be distinguished. The reductive process involves the H2-dependent exergonic reduction of CoM-S-S-CoB catalyzed by a complex consisting of a methyl-viologen-reducing hydrogenase and heterodisulfide reductase (Mvh-Hdr). This exergonic reaction drives the bifurcation of the electron flow, coupled to the endergonic reduction of the oxidized Fred [38,39] (Figure 3).
Concentration and availability of macro- and micronutrients have a crucial role in many synthetic pathways and for the correct function of key enzymes (Table 1).

Table 1.
Role of macro- and micronutrients in methanogenic archaea.
C, H, O, N, P and S are the main constituents of cells, and along with Mg, Na, Ca and K, concur to basic cell function and should always be available [40]. Metals are required in trace amounts (trace elements; TE); they cover the roles of electron donors or acceptors in the energetic metabolism and are fundamental as cofactors or parts of enzymes. According to the literature, several metals are required during methanogenesis, including iron (Fe), nickel (Ni), cobalt (Co), tungsten/molybdenum (W/Mo) and zinc (Zn). In addition, studies on the physiology of pure cultures of methanogens growing on H2/CO2 showed that the required optimal concentration of TE for these microorganisms is higher than those reported in natural environments [41]. Examples of Fe-containing proteins in methanogenic archaea include the formyl-methanofuran dehydrogenase (FMD/FWD), ferredoxins (FD), [Ni-Fe] hydrogenases (frhABG), [Fe] hydrogenase f and heterodisulfide reductase (HDR) complexed with the F420-non-reducing hydrogenase (MVH) a [Ni-Fe] hydrogenase. Ni is found in the active site of CO dehydrogenase/Acetyl-CoA synthase and in the tetrapyrrole ring system of methyl coenzyme M reductase (MCR). A Co ion is present in the subunit A of the methyl-H4MPT [42]. Metal ions are also important in methanogens’ transcription regulatory systems. As an example, M. thermoautotrophicus ΔH harbors a nickel-responsive transcriptional regulation system controlling the transcription of gene encoding for the synthesis of nickel-containing enzymes [43].
Along with the mineral nutrients, vitamins such as biotin, para-aminobenzoic acid, riboflavin and different B-group vitamins have been reported to be required or to stimulate the activity and growth of methanogenic archaea; all nutrients have been reviewed in detail by [44].
3. State of the Art and Current Advancements in Biomethanation of H2 and CO2
The biomethanation of H2 and CO2 has been thoroughly investigated as a biotechnological process, and several factors, including adopted set-up strategies (i.e., in situ, ex situ), choice of the biocatalyst (i.e., pure or mixed microbial culture) and several chemo-physical parameters, have been demonstrated to affect both the process productivity and performance. Such parameters of interest will be discussed in the following paragraphs.
3.1. Development of In Situ and Ex Situ Strategies for Biomethanation
In the last decade, biological carbon fixation emerged as a promising technology aiming at removing and transforming CO2 and reducing GHG emissions while operating in mild temperature and pressure conditions without using chemicals, thus offering a remarkable advantage compared to traditional physical-chemical technologies for CO2 capture [45,46,47]. Moreover, in addition to capturing CO2, this technology can also convert it into a valuable product, such as CH4. Biomethanation is an extremely versatile technology leading to the conversion of different gaseous substrates, such as rich gaseous streams of CO2, CO and H2 generated by different processes (e.g., anaerobic digestion (AD) of organic matter to biogas, thermochemical gasification of non-fermentable biomass to syngas, and natural or industrial processes). Biogas used as substrate for biomethanation can be obtained from numerous organic-matter-rich, non-food-related feedstocks, including the organic fraction of municipal solid waste, sewage sludge from wastewater treatment plants, manure from livestock, energy crops, organic industrial and commercial wastes and waste and sewage from agriculture [48]. Biogas is typically composed of mainly CH4 (50–70%) and CO2 (50–30%), while containing only residual amounts of other undesired compounds, such as N2, O2, H2S and NH3. The calorific value of CH4 stands at 36 MJ/m3-CH4; CO2 and residual compounds present in biogas lowers it to ~20 MJ/m3-biogas [49].
Similarly, syngas derived from the thermochemical gasification of lignocellulosic residues, non-fermentable by-products of bio-refineries and organic municipal wastes contains CO, CO2, CH4 and H2 in variable concentrations [49,50]. For these reasons, the biomethanation of biogas and syngas to higher CH4 content increases their calorific values, thus broadening their spectrum of potential applications and paving the way to the use of biomethane as an alternative to NG [7,49].
As discussed in the previous section, HM requires H2 as electron donor to reduce CO2 to CH4. The use of renewable energies, such as solar and wind, is expanding worldwide, and variable weather conditions may result in an uneven distribution of energy production. Thus, the surplus of electricity can be used to hydrolyze water for the production of green H2 [9]. Alternatively, H2 can be obtained from biomass gasification, biological H2 production or residual unconverted H2 from biomethanation process [51]
Nevertheless, because of its low volumetric energy content, H2 poses some challenges related to storage and distribution [52,53]. For these reasons, the utilization of H2 generated from surplus electricity as electron donor to reduce the CO2 derived from biogas, syngas or other industrial processes offers a valuable solution to produce a clean and cheap energy carrier such as biomethane while reducing atmospheric CO2 emissions.
3.1.1. In Situ Biomethanation
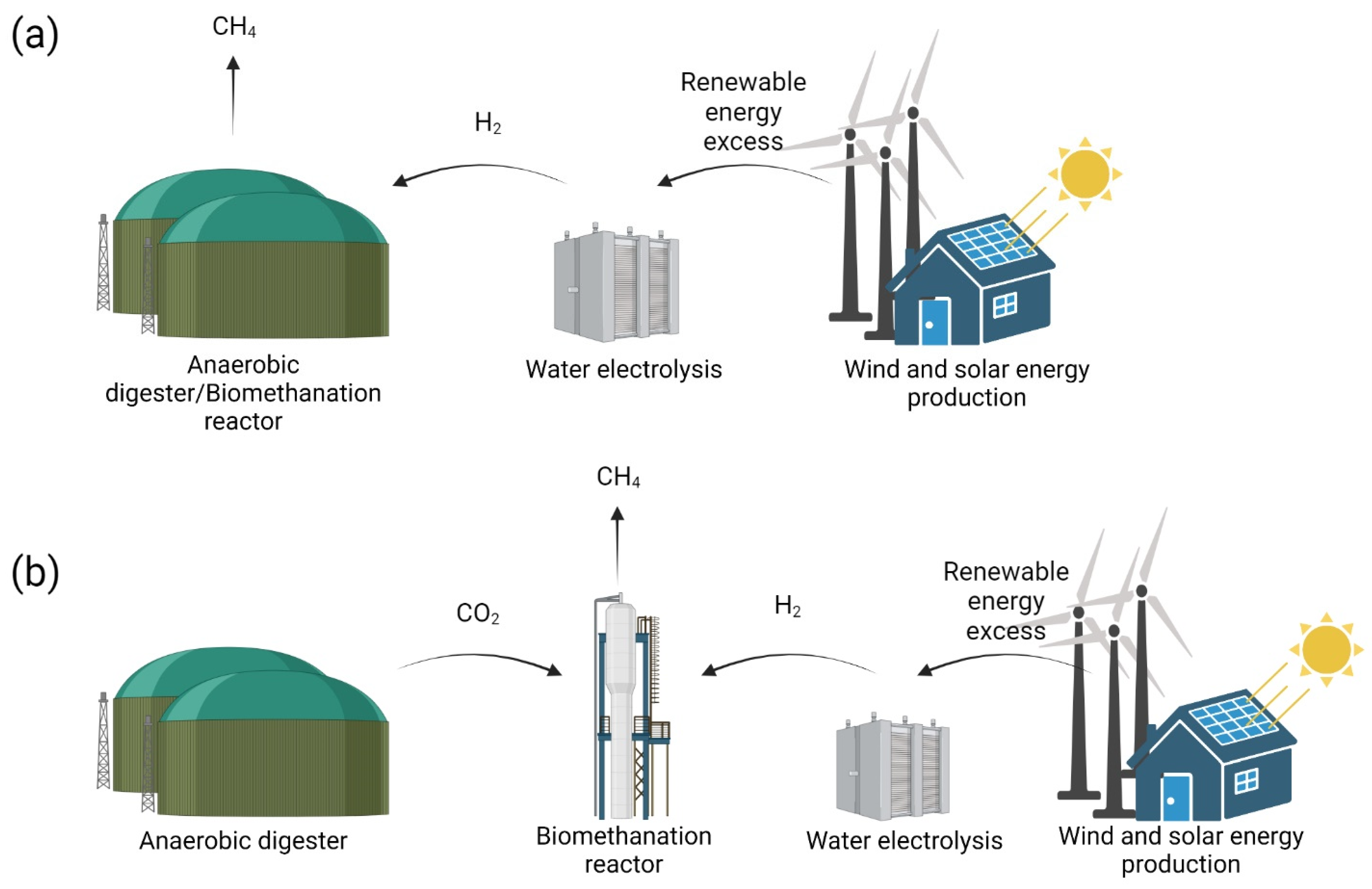
In situ biomethanation can be achieved by injecting H2 derived from external sources directly inside a biogas reactor. Along with the CO2 produced during AD in the biogas reactor, H2 is converted into CH4 by the activity of indigenous HM (Figure 4; in situ biogas upgrading) [54,55].
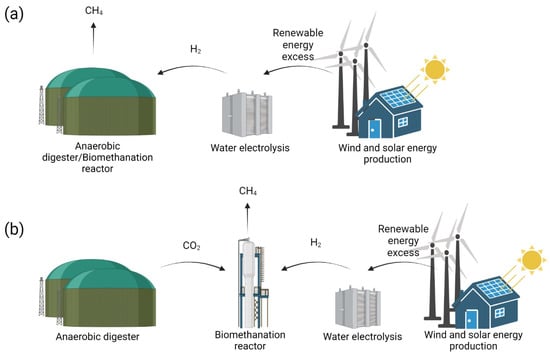
Figure 4.
Scheme representing in situ (a) and ex situ (b) biomethanation technologies.
During biomethanation, the injection of additional H2 results in a selective pressure, leading to radical changes in the relative abundance and richness of the different microbial taxa characterizing the microbial consortium responsible for the process. Previous studies utilized comparative bioinformatic tools to elucidate the effect of the H2 on complex communities, pointing out a decrease in species involved in the fermentation and hydrolysis together with AM and a concomitant increase in the relative abundance of HM and syntrophic bacteria (e.g., SAOB or homoacetogens) [56]. For this reason, biomethanation communities are typically characterized by a very low diversity, with few main genera involved in CH4 production, i.e., Methanoculleus, Methanothermobacter and Methanosarcina. Nevertheless, the coexistence of different closely related taxa able to replace the less-adapted ones has been demonstrated [57].
Previous studies [47,49,58] provided a comprehensive overview of successful in situ biogas upgrading works. In recent years, a remarkable effort has been dedicated to providing valuable solutions to major challenges related to in situ biomethanation technology.
Specifically, one of the main technical issues is the remarkable pH increase determined by CO2 removal. If the pH exceeds 8.5, it can inhibit methanogenesis [56,59,60]. CO2 dissolved in the liquid phase dissociates into H+ and HCO3−, playing a fundamental role in buffering the process. Therefore, CO2 removal results in reduced H+ levels with a consequent pH increase. More details on the optimal pH range and pH control during methanogenesis will be provided in the next paragraph.
Among the works aiming at containing the increase in pH upon addition of H2, Luo and Angelidaki tested the co-digestion of manure and cheese whey, which can counteract the increase in pH and maintain it within optimal levels for methanogenesis while achieving up to 85% CO2 removal [59].
Additionally, the increase in PH2 (>10 Pa) resulting from the injection of H2 in the system could also cause methanogenesis inhibition alongside the accumulation of volatile fatty acids (VFAs) [6,60,61,62]. As it will be detailed in the next paragraph, the accumulation of VFAs could lead to process failure due to the consequent decrease in pH; thus, a careful monitoring and control of VFA levels is required to maintain the process efficiency.
Ref. [63] specifically applied isotope analysis to investigate the effect of the excess of H2 on in-batch, in situ biomethanation performance at thermophilic conditions. The results showed how the excess of H2 led to its accumulation in reactor liquid phase as dissolved H2, resulting in the inhibition of VFAs’ degradation and the stimulation of the homoacetogenic pathway for the production of acetate from CO2 and H2. Nevertheless, VFAs’ degradation and methanogenesis resumed once the excess of H2 was removed from the system. Similarly, [64] tested mesophilic batch reactors exposed to pulse H2 injections at levels exceeding stoichiometric ratio for CO2 and H2. The authors report reversible acetate accumulation, mostly attributed to homoacetogenic activity, suggesting the possibility of exploiting acetate as a temporary H2 storage before methanogenesis restoration.
3.1.2. Ex Situ Biomethanation
Ex situ biomethanation consists of the injection of H2 and CO2 from external sources inside an anaerobic reactor containing enriched or pure hydrogenotrophic methanogenic cultures (Figure 4) [54,65,66,67]. Biomethanation can be decoupled from biogas production, resulting in a higher process flexibility and stability. In fact, CO2 derived from multiple sources, e.g., biogas production, biomass gasification and industrial process, can be applied to the process, ensuring a stable substrate supply independently from the biomass availability and geographical location of each. Moreover, biomethanation being carried out in a dedicated reactor, the system can benefit from higher stability, as no biomass degradation (i.e., hydrolysis and acetogenesis) is required, avoiding the technical limitations of in situ biomethanation. At the same time, the conversion efficiency of acclimatized hydrogenotrophic cultures allows for a reduction in operative costs, thanks to the possibility of providing the bioreactor with a high input of gas flow rates while keeping limited working volumes. Comprehensive overviews of tested ex situ biomethanation configurations are reported in the literature [47,49,68].
Interestingly, these studies investigated ex situ biomethanation based on biogas or CO2 as a carbon source. As mentioned above, this technology offers the possibility of sustaining the biological CO2 conversion of gas mixtures derived from different origins. Previous studies highlight the possibility of providing anaerobic reactors with syngas to biologically convert H2 and CO2 to CH4 [69]. When using mixed cultures this could be achieved by the pairing of carboxydotrophic-mediated H2 production followed by the methanogenic reduction of CO2 by HM or, alternatively, through homoacetogenesis from CO followed by acetoclastic methanogenesis or oxidation of CO to formic acid followed by its reduction to CH4 [70]. Biomethanation from syngas offers the opportunity of utilizing the fraction of organic waste remaining unused during biogas production (~50%) through gasification, followed by its reintroduction into the anaerobic reactor, with the heat produced from AD covering the power consumptions of gasification [71].
Despite the mentioned advantages associated to ex situ biomethanation technology compared to in situ biomethanation and the differences in system design and operation, both approaches are affected by a common parameter: the gas transfer to reactor liquid phase, where the biochemical reactions take place [66,70]. Because H2 is 500 times less soluble in water than CO2 [72], H2 availability for methanogens represents a remarkable limiting factor for methanogenesis [6,55,65]. Previous studies reported the existence of a correlation between H2 gas transfer coefficient (kLa), which is directly proportional to the gas–liquid mass transfer rate (rt), and in turn, the gas–liquid mass transfer depends on operational parameters such as reactor configuration, mixing speed, gas recirculation flow rate and applied gas diffusion device [49,65]. This has been demonstrated by several works aiming at optimizing H2 uptake and conversion to CH4 through the modulation of the aforementioned factors, reaching H2 conversion efficiencies close to 100% and upgrading the system CH4 content up to 98% [6,54,65,73].
A deeper insight into the rt correlation with the most important operational parameters will be provided in the next section.
3.1.3. Hybrid In Situ and Ex Situ Biomethanation
Recently, a new technology has been proposed integrating in situ and ex situ concepts in a single hybrid configuration, aiming at exploiting advantages of acclimatized hydrogenotrophic cultures for ex situ biomethanation and limiting the drawbacks of in situ technology, and some examples will be briefly illustrated below. Within this concept, upgraded biogas resulting from in situ H2 addition in a conventional biogas reactor is further polished to higher CH4 content by the action of acclimatized hydrogenotrophic cultures [74]. An example is the hybrid configuration presented by [75] and consisting of two separate chambers: the first dedicated to H2-assisted in situ biogas upgrading and upgrading the biogas up to 86% CH4 content, and the second receiving the biogas from the first chamber and further upgrading it to >91% CH4.
A similar concept was described by [76] proposing a hybrid system composed of a 10 L CSTR for in situ biomethanation and a 2 L chamber filled with packing material carrying out the ex situ process. The results showed that the operation of this hybrid reactor led to a 28% higher CH4 yield and a twofold higher H2 consumption rate compared to the in situ concept alone and a 76% higher CH4 yield compared to the non-H2-fed reactor, with an overall 62% H2 consumption rate.
An alternative design has been proposed by [74], in which an H2-fed reactor for in situ biogas upgrading was supplemented by additional external biogas and H2 to demonstrate the resilience of the system to increased input gas flow rate and simulate the biomethanation of gas derived from different sources. In situ biomethanation resulted in biogas upgraded at >93% CH4 content, which also remained stable upon external gas injection, with an overall >3-fold increase in CH4 yield.
3.2. Characteristics and Productivity of Pure Cultures vs. Methanogenic Consortia
As extensively discussed in the previous section, HMs are the key players during the biomethanation of H2 and CO2. Previous works presenting an overview of biomethanation studies carried out using hydrogenotrophic pure cultures and methanogenic microbial consortia demonstrated the feasibility of both approaches [58,77]. Both technologies have advantages and drawbacks related to the process operation, performance, operative costs and system sustainability. Specifically, the use of pure cultures in industrial applications may offer some advantages in terms of process predictability and ease of control. Conversely, enriched mixed cultures require a long adaptation time and a specific procedure. Moreover, unwanted side reactions taking place within a complex consortium could interfere with the process [77].
Pure cultures typically require more stringent conditions, in terms of nutrient content and control parameters when compared to the robustness characterizing mixed adapted cultures. In fact, during the operation of complex consortia systems, nutrients can be provided through their source substrate, without the need for sterility [78]. Moreover, in the context of industrial application, the uneven distribution of feedstock gas along the year, due to weather conditions and biomass availability, has to be considered. Recent studies [79,80] have shown the remarkable robustness and short recovery time of mixed cultures upon starvation/excess of input gas rate and oxygenation. In fact, in the presence of changing conditions, the best-adapted microorganisms will grow and become dominant. Conversly, less adapted species can survive through spore formation or utilizing residual biomass without the addition of any nutrient [78,79]. Mixed cultures are also capable of performing a variety of biochemical reactions entertaining inter-species communication, which explains the coexistence of different microbial groups in H2/CO2-fed methanogenic systems [47].
Compared to pure cultures, they also offer the possibility of polishing gas mixtures from residual components other than CO2 and H2, such as those contained in biogas or in flue gases emissions [54,77].
Additionally, mixed cultures offer advantages in terms of operative and startup costs, as specific nutrient media and stringent cultivation conditions are not required.
To improve CO2 and H2 conversion efficiency of complex methanogenic consortia, several strategies, such as bio-augmentation with pure cultures or enrichment of existing hydrogenotrophic consortia by specific nutrient or gas mixture supply, can be envisaged. However, both strategies increase operative costs, and the optimization of biochemical conditions has to be carefully considered in order to meet consortia nutrient requirements [6].
3.3. Critical Control Points: Physical/Chemical Parameters Affecting the Process
Despite the well-known advantages of the biological route for CO2 removal and conversion in terms of economic and environmental costs [77], an efficient biomethanation process demands a punctual setup and constant monitoring of the operational parameters [49,56]. In this section, we provide a list of the major factors that must be considered when designing and developing a biomethanation process and during system operation.
3.3.1. Temperature
In natural environments, methane formation occurs for a wide range of temperatures, going from ≤25 °C of psychrophilic methanogens to >60 °C of hyperthermophilic [29]. Nevertheless, most applications rely on mesophilic (25–45 °C) or thermophilic (45–60 °C) processes [81]. Previous studies compared reactor performances at different temperature conditions, reporting a different impact of the temperature on CH4 production and CO2 conversion efficiency.
For example, [6] demonstrated that, in batch assay, an enriched thermophilic culture resulted in >60% higher CO2 conversion compared to the mesophilic one. Conversely, tests conducted in a continuous stirred tank reactor (CSTR) showed higher CO2 conversion efficiency at 37 °C. Then, a remarkably higher CH4 production rate and yield were detected at 55 °C [56]. Similarly, [82] reported a comparable methane content in the output gas of mesophilic and thermophilic systems, despite the higher CH4 production rate detected at thermophilic conditions. Moreover, upon H2 application, several studies reported a higher diversity of methanogenic population at mesophilic conditions [79,82].
As detailed below, the higher conversion efficiency detected at mesophilic conditions can be reasonably explained by the well-known inverse correlation between gas solubility and temperature defined by Henry’s Law [83].
Finally, a study conducted at batch level, comparing process performances at thermophilic and hyperthermophilic conditions, showed that a temperature increase from 55 °C to 65 °C resulted in higher CH4 content and productivity [84].
Regarding mesophilic and thermophilic H2-adapted communities, previous studies underlined the existence of two clearly distinct populations, responding to H2 pressure in different ways, with mesophilic communities undergoing a more radical reduction in microbial diversity upon H2 exposure. Nevertheless, both adapted populations could rely on highly specialized consortia oriented towards methanogenic functions (Methanoculleus spp., Methanothermobacter spp., Methanosarcina spp.) [56,57]. Similar results were presented by [85] referring to a decrease in mesophilic population diversity and a completely different composition of the community between the two temperature conditions. According to β diversity analysis, thermophilic communities exhibiting higher CH4 production yields and conversion efficiencies were more sensitive to H2 addition. Moreover, phylogenetic analysis suggested that biomethanation occurred directly through hydrogenotrophic methanogenesis only at thermophilic conditions (Methanoculleus spp., Methanobacterium spp.), whereas homoacetogenesis and acetoclastic methanogenesis (Methanosaeta spp.) determined the major methanogenic pathway in mesophilic conditions, with SAOB only being detected in thermophilic reactors [85].
Despite the differences in process performance described here, several works reported successful biomethanation outcomes for a wide spectrum of temperatures and reactor sizes, using both pure and mixed cultures as inoculum [45,55,59,67,73,86,87,88]. Notably, among these studies, only two [67,86] were conducted at hyperthermophilic conditions, and none of them employed microbial consortia, with only the work of [84] testing hyperthermophilic conditions with mixed hydrogenotrophic culture. Specifically, they tested the activity of M. marburgensis and M. thermoautotrophicus at 65 and 60 °C in 10 and 3.5 L working volume, respectively, reaching up to 85% CH4 content, together with 950 mmol/L×h and ~50 L/L culture-day CH4 production rate.
3.3.2. pH
Biomethanation typically takes place in a pH range from 6.5 to 8.5 with an optimum at pH 6.5–7.5, and variations in pH were shown to directly affect archaeal growth and activity [29,89]. During the biomethanation process, the control and monitoring of the pH play a fundamental role, being the object of several studies. A specific methanogenic activity (SMA) test conducted on enriched mesophilic and thermophilic hydrogenotrophic cultures exposed to different pH conditions (from 6 to 10), pointed out that biomethanation was feasible up to pH 8.5, even though methanogenic activity was significantly reduced. Conversly, complete process inhibition was observed at pH levels >8.5 [56].
pH control strategies during biomethanation are mainly based on the injection of pH buffering solutions (NaOH and HCl) in order to stabilize the values in the theoretical optimal range for methanogenesis [61,75,82,90]. Ref. [73] report that, during continuous biomethanation of H2:CO2 at mesophilic conditions, fine control of the pH might be achieved by adjusting the CO2 flow rate in the input gas, using real time data of CO2 conversion efficiency and pH.
Among the compounds known to strongly affect the pH level of methanogenic reactors systems, VFA and ammonia (NH3) were identified, with VFA accumulation leading to reactor acidification, and NH3, generated from protein or urea degradation, resulting in higher pH [89]. Because the hydrolysis rate of organic matter increases with temperature, great attention must be paid to pH control and monitoring during methanogenesis at thermophilic or hyperthermophilic conditions.
3.3.3. VFA Concentration
During biomethanation, VFAs, produced from the hydrolysis of organic matter, can be utilized by AM or SAOB in syntrophic association with HM for CH4 production [89,91,92]. Inhibition of the activity of such classes of microorganisms, for instance, due to an increase in PH2 during in situ biomethanation, may lead to VFA accumulation and consequent reactor acidification. This could possibly result in process imbalance, reduced gas conversion efficiency and production rate or even system failure.
The literature offers a wide spectrum of works reporting temporary VFAs accumulation during both in situ and ex situ biomethanation, carried out at both mesophilic and thermophilic conditions [61,79,93,94]. Although the absence of organic feedstocks should keep the levels of VFAs relatively low in gas-fed biomethanation reactors, the literature reports the accumulation of VFAs, especially in thermophilic conditions. For example, during thermophilic ex situ biogas upgrading with methanogenic consortia, [54] reported a significant reduction in the activity of AM and the predominance of hydrogenotrophic taxa due to the decrease in the pH and the accumulation of acetate.
3.3.4. Ammonia Concentration
Ammonia concentration is another critical factor affecting the activity of the methanogenic archaea. Below a threshold concentration, NH3 ensures the buffering capacity of the reactor medium, increasing the stability of the process [95]. Nevertheless, its excess was reported as one of the main causes for process imbalance or reactor failure due to the inhibitory effect on the microbial population [96].
In anaerobic environments, ammonia is released from the hydrolysis of organic compounds, such as proteins and urea, causing an increase in the pH and counteracting the acidification induced by the acidogenesis. In aqueous solution, NH3 can be present as free un-ionized ammonia nitrogen (FAN) and ammonium nitrogen (NH4+). The dissociation balance between the two forms is strongly influenced by temperature and pH. It was reported how, at high temperature and pH, the dissociation balance tends to shift towards the FAN form [97], which is the most likely cause of process inhibition, due to FAN’s ability to permeate bacterial cell membranes [98].
AM and HM seem to respond in a different way to the stress induced by the excess of ammonia, resulting in a shift in the metabolic pathway and changes in the methanogenic population.
Ref. [92] reported that high ammonia concentrations are responsible for the inhibition of AM, resulting in competition for acetate, possibly enhancing the growth and the activity of SAOBs. SAOBs are known to form syntrophic relations with HM for the oxidation of acetate by the former and the consequent utilization of H2 and CO2 by the latter. Despite the slow SAOB growth rate, which can be a disadvantage in the competition for acetate with the AM, the high tolerance of HMs and SAOBs to ammonia favors these microbial groups at high ammonia levels [92]. Moreover, the SAO pathway is also energetically favorable at elevated temperature, which is a condition further forcing NH3 dissociation towards the FAN form [92].
While testing anaerobic digestion under different ammonia levels, [99] demonstrated that high ammonia concentrations (2.8–4.57 g NH4+/L) favor SAO and hydrogenotrophic methanogenesis (i.e., orders Methanomicrobiales and Methanobacteriales at thermophilic and mesophilic conditions, respectively). Conversely, acetoclastic methanogenesis (order Methanosaetaceae) was promoted at low ammonia levels (1.21 g NH4+/L). Similarly, it was subsequently confirmed that HM possesses higher tolerance to ammonia compared to acetoclastic methanogenic archaea [99].
These results may suggest the resilience of hydrogen-mediated methanation at high ammonia concentrations, with higher tolerance to ammonia compared to the AD process. Moreover, in gas-fed chemostats, the curtailment of organic feed should reduce the amount of NH3 present in the system, making its effect negligible. This statement is in agreement with ex situ biomethanation studies reporting a decrease in NH3 levels, following a short period of accumulation, attributable to the degradation of the residual biomass [79].
3.3.5. Salinity
During the last decade, the rising interest for AD from high-salt-content substrates, such as marine macroalgae, fish wastewater and brackish aquaculture sludge, has driven the development of several studies aiming at defining the range of salinity allowing for efficient biomethanation performances [100,101,102].
These studies pointed out the methanogenic inoculum adaptation to increasing salt contents as a crucial requirement for a successful AD process, with methanogens being considered as the most sensitive microbial group within the consortium [100,101,102,103]. In fact, salinity affects several biochemical processes occuring at the cellular level. For example, hyperionic and hyperosmotic stresses can cause dehydration and cell lysis. Moreover, intracellular and extracellular enzyme inhibition and cell membrane impairment may result in altered cell functioning [104,105].
Na+ has been suggested as the main methanogenesis inhibitor, compromising the process at levels as low as 6–13 g Na+/L when applied to non-acclimatized inocula [102,104,106]. More specifically, for methanogens, concentrations of 3.5–5.5 g Na+/L were reported to cause moderate inhbition, which became severe at >8 g Na+/L [107]. However, several Methanosarcina species are halotolerant, being detected at up to 18 g Na+/L and, similarly to Methanosaeta, dominating a high-salinity anaerobic digester over hydrogenotrophic methanogens [100,103,107]. In addition, Cl−, which is the most common counterpart of Na+, may be responsible for plant deterioration through the corrosion of steel components [108]. Conversely, low Na+ concentrations (≤0.35 g/L) are essential for methanogens, as this ion is involved in ATP syntesis and NADH oxidation [107,109].
Consistently, during the AD of food waste leachate supplemented with 0.5 and 2 g/L NaCl (corresponding to 0.2 and 0.8 g/L Na+, respectively), a 10% higher CH4 yield was observed with 2 g/L NaCl compared to 0.5 g/L [110]. This effect was attributed to the preliminary adaptation of the methanogenic inoculum to 1.2 g/L Na+. Nevertheless, further increasing NaCl levels to 5 and 10 g/L (corresponding to 2 and 4 g/L Na+, respectively) resulted in a ~40% lower CH4 yield [110].
Among naturally high salinity substrates suitable for CH4 production, Zhang and coworkers [101] reported that the AD of marine macroalgae using an adapted inoculum was achievable at salinity levels ≤35 g/L, whereas methanogenesis was seriously affected at salt concentrations >55 g/L. Notably, they observed that the best performances were achieved at the salinity of 15 g/L, suggesting an enhancement of methanogenesis at this salt level. Regarding the methanogenic archaeal community, acetoclastic Methanosaeta and Methanosarcina were detected at remarkable relative abundance at salinity ≤35 g/L, tolerating salt levels up to 55–65 g/L and being considered as moderated halophiles. Nevertheless, the dominance of hydrogenotrophic Mehanobacterium was observed at all salt levels tested, up to 85 g/L, with a maximum at 52.65 g/L [101].
Similarly, Letelier-Gordo and colleagues [102] have recently evaluated different co-digestion scenarios of fish wastewater and manure at salinities up to 35 g/L, successfully overcoming the CH4 production rates achievable with cow manure mono-digestion and pointing out a statistical correlation between process inhibition and the level of salinity.
3.3.6. Nutrient Content
In addition to carbon sources, different elements are involved and required for microbial metabolisms. In fact, the main cell constituents such as C, H, O, N, P and S, along with Mg, Na, Ca and K, concurring to basic cell functions should always be available [40]. Moreover, metallic elements, such as Fe, Ni, Co, Mo, W and Se, despite being available in lower amounts, play a fundamental role as cofactors or as part of an enzyme [111].
Nutrient composition and availability depends on substrate source, with different methanogenic feedstocks being characterized by peculiar mineral compositions. The authors of [112] reported that plants fed with mixtures of animal manure and different fractions of organic waste displayed higher concentrations of mineral nutrients when compared to plants largely fed with industrial by-products (i.e., glycerol).
Ref. [42] collected many studies investigating the physiology, media demand and productivity of different methanogenic strains. In the context of anaerobic digestion (AD), it is well established that variable concentrations of TE have significant effects on the production of CH4, where the archaea community was found to be more responsive than other bacterial community members [113]. However, only few studies cover the effect of heavy metals on pure cultures of methanogens.
Ref. [114] demonstrated that Methanococcus maripaludis growth was inhibited by specific concentration of Zn ione (2.5 and 3.5 mmol/L), while Cu concentrations of 1.9 µmol/L, 4.4 and 6.3 reduced growth and delayed biomass production. More interesting is the combined effect of Zn and Cu iones, where the addition of 1 mmol/L Zn can prevent the toxicity effect of Cu.
Another important aspect lacking insight, concerns the connection between TE and the physiological and biotechnological characteristics of methanogens. It was demonstrated that TE limitation could lead to low productivity during biomethanation in mixed cultures [115], but studies on pure cultures are rare. The growth and productivity of M. marburgensis was maximized by applying the exponential feeding of TE, different medium and sulphide dilution rates and different gas inflow rates. With the right combination of these parameters, the greatest ever specific growth rate (µmax) of 0.69 h−1 and methane evolution rate (MER) of 476 mmol/L × h were achieved [116]. Other studies demonstrated that the concentration of Fe, Cu, Ni and Zn, with the exception of Co, should be increased by 100 times than the conventional method to achieve high productivity of methane with acclimated-methanogens [117,118].
Along with the mineral nutrients, vitamins such as biotin, para-aminobenzoic acid, riboflavin and different B-group vitamins were reported to be required or to stimulate the activity and growth of methanogenic archaea [44]. In a recent study [119], it was highlighted that only some methanogens, such as Methanobacteriacae and M.maripaludis, are able to grow on minimal and optimized TE solution without cysteine or vitamins, while hyperthermophylic methanogens with high MERs require a combination of a rich TE composition with additional cysteine and/or vitamins [119].
3.3.7. Gas Solubility and Gas Transfer Coefficient (kLa)
The solubility of gases in aqueous environments is described by Henry’s Law stating that, at constant temperature, the amount of gas that dissolves in a liquid is proportional to the partial pressure of the gas in equilibrium with the liquid. Henry’s law can be expressed as Equation (4):
where C represents the gas solubility concentration at a certain temperature in a specific solvent, Hk is the Henry’s Law constant and P is the gas partial pressure at a given volume and temperature [83].
Different works underline the relationship between the Henry’s constant for a specific gas and the temperature of the system, when the partial pressure is considered in equilibrium, allowing for the calculation of the molar fraction of gas dissolved in liquid phase [83,120]. Data reported by these studies unequivocally show that the temperature increase in a specific system leads to the reduction in the solubility of the injected gases. This is mainly caused by the fact that gas solubilization is an exothermic process in which the gas dissolution releases heat to the system. A temperature rise leads to an increase in the kinetic energy of the gases’ molecules, and this may limit the formation of intermolecular bonds between the solute and the solvent [121].
As mentioned in the previous section, during biomethanation, the low solubility of H2 is one of the most relevant limiting factors for H2/CO2 conversion efficiency [47,68].
At the same time, the gas transfer coefficient (kLa) and the temperature are positively correlated [122]. Specifically, higher solvent viscosity (µa) was reported to retard diffusion of gases in Newtonian fluids [123,124]. In AD digestate, which is considered as a non-Newtonian fluid, µa changes with temperature, shear forces and solid content. While higher solids content was proven to increase µa [125], an increase in either temperature or shear stress was reported to decrease it [126].
Thus, the higher the temperature, the lower the µa and the higher the diffusivity of the gases.
Diffusivity and temperature can be correlated as per Equation (5):
where DL is the diffusivity of the solute at infinite dilution, µ is the viscosity of the solution and T is the absolute temperature. Diffusivity, temperature and viscosity are correlated and affect the gas transfer coefficient (kLa) [122], which determines the gas–liquid mass transfer rate (rt) as defined by Equation (6):
where 22.4 is the molar volume, kLa is the gas transfer coefficient, H2g is the H2 concentration in the gas phase and H2l is the H2 concentration in the liquid phase.
kLa comprises two other coefficients, where kL is defined as the film coefficient, depending on gas and liquid physicochemical features, and a is the interface area per unit volume of liquid [122]. Therefore, in order to take into account the dual role of temperature, the overall gas–liquid mass transfer rate should include both gas solubility and gas transfer coefficient, as defined by Equation (7) [47]:
Due to the lower solid content in the digestate or medium typically utilized during the ex situ biomethanation process compared to anaerobic sludge used for in situ methanogenesis, rt is expected to favor the ex situ process [77].
Despite the attempts to describe gas behavior and diffusion in a liquid [127,128], the obtained models appear too simplistic, especially when applied to a chemostat for biomethanation, in which a tri-phasic system (liquid–solid–gas) is constantly affected by the activity of the microorganisms in the developing community. In such a system, where interactions appear somewhat chaotic and hence difficult to predict, parameters can be considered individually in order to improve gas solubility.
3.3.8. Pressure
As described in the previous section, pressure is directly proportional to gas solubility in a liquid. The higher the volume of gas in a close system, the higher its solubility. The higher the number of gas molecules at the gas–liquid interface, the higher the interface contact; thus, the gas availability for microorganisms [122]. The resistance and improved performance and growth rate of hydrogenotrophic methanogens at extreme pressures (>100 atm) were reported [122]. A batch assay conducted on a lithotrophic strain pointed out a positive correlation between CO2 conversion efficiency and pressure, with higher conversion efficiencies being observed for a pressure increase from 1 (70 μMCH4) to 50 (3500 μMCH4) and 100 atm (7000 μMCH4) [129]. Similarly, by increasing the reactor pressure from 101 kPa to 122 kPa, CH4 production increases from 50 LCH4/Lculture/day to 65.6 LCH4/Lculture/day was reported during biomethanation using M. thermoautotrophicus pure culture [67].
3.3.9. Gas Hold-Up
The time a gas resides in a reactor can be defined as the gas hold-up. The longer the gas residence time, the longer the contact is between the gas and the liquid phase where metabolic reactions take place.
Gas hold-up can be modulated using gas or liquid recirculation [55,65] or through mixing speed [6], acting on the velocity of the bubbles in the reactor.
In fact, vigorous gas–liquid dispersion generated by the movement of the gas bubbles in the liquid medium results in turbulent flow, responsible for improving the gas contact with liquid phase in the reactor [130]. Moreover, gas recirculation can enhance gas–liquid mass transfer because it increases the overall gas injection rate to the liquid, thus also increasing the gas–liquid interface area [81].
4. Perspectives in Underground Biomethanation
A new line of research for PtG technology is represented by underground methanation, a slow and spontaneous phenomenon resulting from the conversion of the mixture of H2 and CO2 into CH4 in deep geological structures due to the presence of methanogenic archaea in native microbial populations.
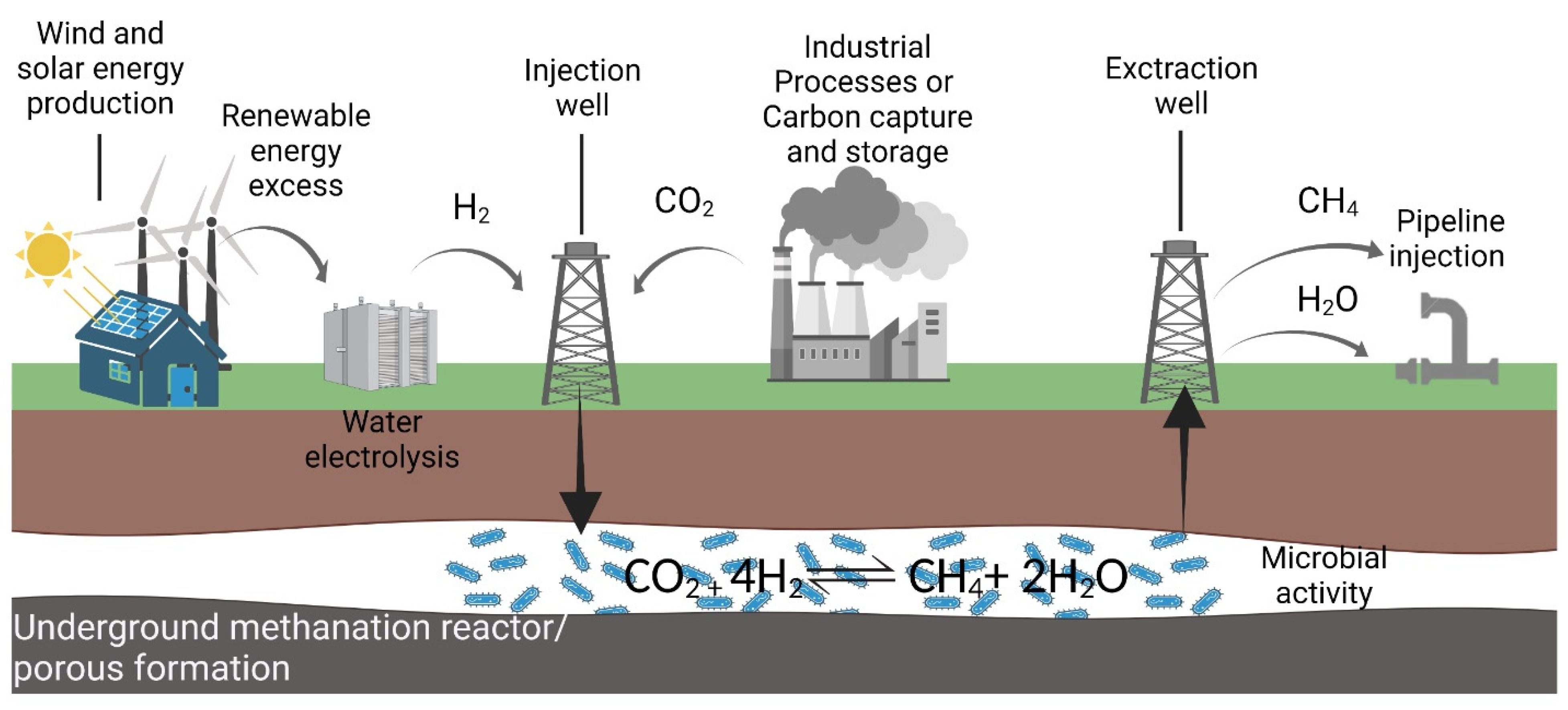
The concept of an underground methanation reactor (UMR) involves the enhancement of the natural process by H2 and CO2 injection into an underground gas storage site to act as a bioreactor for bioconversion, catalyzed by methanogenic archaea. This approach could be seen as a complement to Power-to-X technology in terms of the storage and conversion of the H2 generated by using the renewable energies surplus for electrolysis. The CO2 could be captured at a point-source or from the atmosphere. In this view, the injection, carried out during the energy peak, allows for partial conversion of the H2:CO2 mixture into CH4 and H2O with subsequent withdrawal of the CH4-enriched mixture through a producing well during the energy demand period. The gas mixture, mainly composed of CH4 and H2, will be further treated and delivered via pipelines to the end users (Figure 5). This transformation increases the energy potential of the stored gas, which is higher for CH4 than for H2, and has a promising potential for a new energy storage and enrichment technology [131,132].
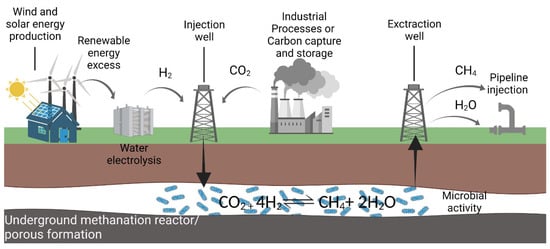
Figure 5.
Schematic representation of the underground methanation reactor (UMR) concept.
Microbial populations—specifically selected or evolved methanogenic strains—capable of competing for hydrogen as an energy source for the reduction of CO2 to CH4 could also be artificially introduced into a geological structure to implement the UMR concept.
The development of UMR technologies is dependent on the presence of two main elements: first, adequate surface facilities for the supply of renewable H2 and CO2; second, a suitable and proximate geological site to be used as bioreactor. With regard to above-ground infrastructures, the presence of a well-established system such as an electrolyzer for hydrogen generation is essential, preferably close to the renewable energy sources to be used for its supply (i.e., wind or photovoltaic plant) so as to reduce the costs of the energy transport chain. At the same time, there must be an accessible source of CO2 to be injected into the underground site. This source could be a direct air capture plant to collect CO2 from the atmosphere by means, for example, of modular CO2 collectors, a biogas plant, or an industrial plant with CO2 gaseous waste. In the latter cases, the CO2 will have to be purified by membrane-based or chemical capture systems prior to injection into the geological site. The site chosen for the UMR process should also be equipped with all the infrastructures for gas injection and withdrawal and, preferably, with existing pipelines to allow the distribution of the produced methane directly to consumers. For this reason, an existing storage of natural gas could be a preferred choice for a UMR.
The main microbiological, environmental and geological features of an underground site suitable to be exploited as a UMR will be illustrated hereafter, alongside with the main research projects currently focused on underground biomethanation technology.
4.1. Geological, Environmental and Microbiological Features
The geological formations (i.e., depleted hydrocarbon reservoirs and deep saline aquifers) used for gas storage could be converted from storage sites to bioreactors. The choice of the type of reservoir depends on two main issues: the sealing capacity of the cap rock (i.e., the impermeable layer overlaying the reservoir or the aquifer) with respect to the stored gas mixture and the reservoir potential for microbial activity.
With the geological structure acting as a natural trap, its impermeable cap rock must be able to prevent the vertical migration of the gas. Depleted reservoirs proved they can contain hydrocarbons for geological eras but need to be tested for H2 and CO2 confinement; conversely, thorough investigations must be carried out to ensure aquifers can safely store any gas mixture. Cap rocks characterized by shales are well suited for this purpose [13]. The interactions with the reservoir rocks and potential subsequent changes of the storage properties (e.g., porosity, permeability, mechanical properties) must also be carefully evaluated, alongside with the potential issues related to the reactivity of mineral elements and the corrosion/damage of the technical equipment [133]. To avoid or limit reactivity between the injected gas and the rock components, siliciclastic rocks, such as sandstones, are the most favorable rock types. Porous rock formations with a porosity above 20% are preferred [13]. The ideal thickness and permeability of the reservoir should be larger than 20 m (but 50 m or more are preferable) and 200 mD, respectively. Limited thickness and low permeability values (below 50 mD) are to be avoided because they do not grant sufficient well injectivity. In depleted reservoirs, water saturation should be above 10% to allow microorganisms to access nutrients and biomass accumulation.
Depleted hydrocarbon reservoirs are considered optimal candidates for UMR as they are well-known systems, they can be managed at operating pressures below their original one and typically have favorable conditions for microbial growth along with a proven storage capacity and are, in principle, the cheapest pre-existing technology to be converted into hydrogen storage and conversion sites. Currently, the only three projects that have demonstrated biomethanation technology in the field (detailed in the next section) have leveraged depleted natural gas reservoirs. Deep saline aquifers might have a great potential as storage sites mainly because of their wide distribution underground and potentially very large storage capacity, but their exploitation requires huge costs to define the extension and geometry of the geological structure to characterize the rock properties and to assess the cap rock sealing capacity. Furthermore, fluid injection into aquifers implies exceeding the original pressure of the formation to displace the water, thus posing additional safety issues to be addressed. Eventually, salt caverns, proved to be successful for pure hydrogen storage due to their unique sealing characteristics but have rather limited potential for methanogenesis due to their high salinity [12,13,132].
With respect to the microbiological aspects, the physical/chemical characteristics limiting the growth of methanogenic archaea in reservoirs are the same that determine the feasibility of the UMR technology. These characteristics are schematically summarized in Table 2, together with the geological features previously described and discussed below. It is necessary to consider that a certain variability exists depending on the different environmental conditions and the various adaptation strategies that archaea, known to be functionally and metabolically flexible microorganisms, are able to adopt in order to survive even in hostile conditions. Among these strategies, one of the most important is the ability to establish syntrophic relationships with different species of bacteria so as to benefit from each other and proliferate even in conditions of nutrient deficiency.

Table 2.
Environmental factors and geological characteristics for a UMR site as reported by [12,13,132].
Methanogens are usually strictly anaerobic, capable of performing the methanogenesis reaction at redox potential, measured in volts (V), between −0.4 and 0.2 V. With regard to the main physical parameters characterizing the ideal geological site, the temperature should be below 60–70 °C, considering that the optimal temperature range for mesophilic methanogens is between 27 °C and 47 °C and that for thermophilic/hyperthermophilic strains is between 50 °C and 80 °C. The pressure should not exceed 150 bar, whilst pH values close to neutrality are preferable to favor the methanogenic metabolism. In underground environments characterized by temperatures compatible with mesophilic microorganisms, several species of the order Methanomicrobiales are commonly found, alongside the genera Methanosarcina, Methanomassiliicoccus and Methanomethylovorans. A characteristic genus that dominates in deep formations characterised by high temperatures is the thermophilic hydrogenotrophic Methanothermobacter [12].
Salinity has been largely reported as one of the main limiting factors for methanogenesis, particularly for hydrogenotrophic and acetoclastic species, which mostly prefer low NaCl concentrations, below 90 g/L [12]. Indeed, most halophilic or highly salt-tolerant species are methylotrophic types, belonging to the Methanosarcinaceae family. In particular, key strains of halophilic methanogens isolated from saline sediments or soda lakes belong to the genera Methanohalobium, Methanohalophilus, Methanosalum, Methanolobus, Methanomethylovorans and Methanosarcina [134]. Recently, a new genus belonging to the halophilic Methanosarcinaceae family was isolated from the sediments of a hypersaline industrial saltern; it was sequenced and named Methanosalis [135]. However there are some exceptions, and hydrogenotrophic mesophilic strains, such as Methanocalculus halotolerans [136] and Methanocalculus natronophilus [137], belonging to the small Methanocalculaceae family, were characterized in highly saline environments. Methanosarcinaceae and Methanocalculaceae are the only two families of methanogens in which halophilic species were characterized.
4.2. Underground Biomethanation Research Projects
To the best of the authors’ knowledge, only three research projects focused on field testing for underground biomethanation are currently underway. They are reported in the following.
4.2.1. The Hychico-BRGM Pilot Project
In 2010, the Argentinian Hychico, focused on power and H2 generation from renewable resources, began geological studies to start an Underground Hydrogen Storage project in a depleted gas reservoir located near its hydrogen facilities. The Hychico pilot-project in Patagonia [138] was aimed at performing in situ tests of H2:NG subsurface injection in a depleted gas field, directly connected with a H2 pipeline reaching the electrolysis production facilities. Three storage cycles of H2:NG were performed in the field: (i) first, to confirm properties and seals of the system, NG was injected until the original reservoir pressure was reached and then was withdrawn; (ii) a mixture H2:NG with 10% of H2 was injected to study the behavior of the reservoir at intermediate pressures; and (iii) NG was injected again to increase the pressure and assess the tightness to hydrogen of the reservoir cap rock at the original pressure. The storage cycles were associated with the analysis of the changes in reservoir properties and gas composition. Following H2 injection, a partial conversion of the stored gas to methane was detected [138].
Further steps are currently aimed at evaluating biomethanation potential of the underground storage site. This new Hychico-BRGM Pilot Project is being conducted in collaboration with the French Geological Survey (BRGM) and envisages microbial characterization, laboratory and field tests and modeling analysis. The target is to find the key factors to control and optimize the process of biological conversion of methane in the reservoir. Characterization of the microbial population and an in-depth analysis of all the major physico-chemical parameters potentially inhibiting the biomethanation process (e.g., nutrient availability, redox conditions, temperature, pH, etc.) are considered and analyzed in both formation fluid samples and core sections, collected from a well. Preliminary results suggest that the chosen site has a good potential for biomethanation [132]
4.2.2. The Underground Sun Conversion–Flexible Storage Project
Started in December 2020, the ERA-Net “Underground Sun Conversion–Flexible Storage” (USC-FlexStore; https://www.underground-sun-conversion.at/en/, accessed on 8 April 2022) aims at investigating and developing a seasonal, high-volume storage and transformation of large quantities of renewable energy to be made available year round.
The aim of the project is to implement RAG Austria AG’s patented “Underground Sun Conversion” (USC) technology, focused on the underground methanation of CO2 and green H2, to the next level and designing services based on it. Field tests are planned at RAG’s research facility in Pilsbach (Upper Austria) [139,140]. Excess renewable energy will be safely stored in a gaseous form in underground facilities at depths of over 1000 m and will be utilized via existing infrastructure in all energy demanding sectors. In addition to RAG Austria, the project consortium also includes an energy supplier company and various research partners. Investigations are centered on the technological, commercial, energy-sector and legal requirements for a cross-sector approach, aiming at buffering the current need for substantial imports and use of fossil energy during winter, when demand is stronger.
The combination of the power to methane process with geological storage of flexible shares of feed and product gases provides flexibility and storage capacity for the future energy system. Thus, the goal is to enhance the inter-seasonal capacity of the “USC-FlexStore” storage system with a view to developing a commercial service. The development of related services together with need-owners complete the project, which is expected to end in 2023.
4.2.3. The Bio-UGS–Biological Conversion of Carbon Dioxide and Hydrogen to Methane Potential Analysis of Underground Bio-Methanation Project
The “Bio-UGS–Biological conversion of carbon dioxide and hydrogen to methane in porous underground gas storage facilities” (https://co2-utilization.net/en/projects/chemical-and-biotechnological-reduction-of-co2/bio-ugs/, accessed on 8 April 2022) project is funded by the German Federal Ministry of Education and Research as part of the funding measure “CO2 as a sustainable source of carbon–Pathways to industrial application (CO2 -WIN)”. The measure supports projects that utilize carbon dioxide as raw material for the German economy.
The Bio-UGS project, started in February 2020, aims at exploiting existing infrastructure for large-scale conversion processes and at investigating the targeted conversion of CO2 and green H2 to CH4 in underground storage sites in geological formations by using naturally existing microorganisms.
The independent microbiological laboratory MicroPro GmbH is responsible to investigate the microbiological issues related to the project, which include the characterization of the reservoir’s indigenous microbial population, the risk assessment of microbial processes and the possibility of stimulating an economic methanation. Currently, the main achievements of the project are related to: (i) lithological and mineralogical characterization of UGS sites in Germany; (ii) identification and pre-selection of favorable conditions for underground biomethanation; (iii) successful collection of representative materials and analysis of rock samples, formation waters and various microbial cultures; (iv) successful bio-methanation in laboratory microcosmos observed for some cultures; (v) conceptualization of reservoir models resembling different lithological and reservoir mechanical conditions; (vi) bio-methanation modeling in DuMuX [141], workflow assembly in STARS and integrated methanogenesis and bio-reactive transport, bacterial growth and decay dependent on nutrient supply [142]. The final goal of the project, which is expected to end in 2023, is to catalogue and evaluate potential storage sites in porous and permeable formations in Germany in the perspectives of UMR.
The concept of underground biomethanation is a promising technological solution that could make the PtG system more competitive and sustainable. Although early experimental field trials are encouraging, it is also clear that this technology still needs technical detailed studies and especially a thorough site-specific feasibility study before being implemented, as its success is strictly dependent on the geological, environmental and microbiological requirements described above.
Mineralogical characterization and microbiological investigation, based on metagenomic analysis, represent fundamental tools through which pre-existing mathematical and biogeochemical models [131,143] to assess the feasibility and large-scale impact of the CO2:H2 storage and bioconversion process can be implemented.
The main limitations to the implementation of this technology are, on the one hand, intrinsic to the biological process of biomethanation and, on the other hand, related to the large-scale availability of renewable energy. The first type of limitations can be summarized as follows: low solubility of H2 in water (limiting H2 availability for microbial metabolism), microbial competition for H2 (especially if sulfate-reducing bacteria are present, resulting in decreased production of H2S and pH, which in turn can cause the inhibition of methanogenesis) and, eventually, the formation of biofilm during the growth and accumulation of bacterial biomass (potentially leading to the obstruction of the rock pores) [131,144,145,146]. As mentioned previously, microbial competition can occur for reduced substrate availability, e.g., HM and acetogens compete for both H2 and CO2 availability, as well as the competition between hydrogenotrophic methanogenesis and sulphate reduction has been largely investigated. It is known that HM is less favorable from a thermodynamic point of view, and thus, in the presence of sulphate, the methanogens can be outcompeted by SRBs. However, many studies have documented coexistence of these microbial groups in complex sedimentary systems [144,147]
Indeed, methanogens possess strong adaptive capability to different physical-chemical conditions, even very limiting ones, and the targeted bioaugmentation strategies could be implemented to rebalance or strengthen the native methanogenic community. To date, the limitations related to the availability of large quantities of H2 produced by renewable energies to be injected into reservoirs is essentially an economic limitation, which must be overcome through the implementation of renewable technologies in the near future.
It is also worth noting that, in the view of the feasibility of UMR technology, it is necessary not to underestimate the environmental safety aspects that may arise and that are to be carefully considered on a case-by-case basis during the implementation of the technology. Safety issues are not only associated with possible methane leakage but also with the potential leakage of hydrogen. In fact, as previously mentioned, the geological formations used as UMRs are already naturally suited to contain methane, but the risks associated with the injection of gas mixtures containing H2, which is more reactive, must be carefully evaluated [148].
Finally, microbiological aspects of the technology need to be assessed during long-term storage procedures. Particularly, with microorganisms reported to associate in biofilm structures in natural environment, the possibility of potential clogging and occlusion of porous rock material caused by microbial proliferation should be considered along with efficient strategies to control and influence the activity of the indigenous microbial population [12].
5. Technical and Economical Assessments for the Biomethanation of CO2 and H2 and Currently Available Industrial-Scale Processes
To date, as previously described, the biomethanation of CO2 and H2 represents a valuable solution for both the reduction in CO2 emissions and the valorization of CO2 through its conversion into bio-methane, which, due to its CH4 content (≤95%), could be used as a direct substitute of natural gas. Nonetheless, the International Energy Agency (IEA) reported that in 2018 the combined production of biomethane and biogas was calculated around 35 million tonnes of oil equivalent (Mtoe) while the overall estimated potential of biomethane only was calculated around 730 Mtoe. Moreover, according to IEA estimations biomethane utilization could avoid the generation of 1000 million tonnes of GHG, including CO2, by 2040. Despite the considerable progress made in the field of biomethanation, as of 2018, the biomethane share of the global natural gas demand was accounted as 0.1% [149], and its potential and the economic feasibility of the process still appear unclear. Moreover, the information provided by the available literature mainly refers to the profitability of chemo-physical-based methodologies for upgrading biogas (i.e., membrane separation; amine scrubbing), regarded as the main feedstock for biomethane production, whilst it does not yet consider the possibility to use the biological route [150,151,152,153]. In order to find clarity about the economic feasibility of the process, data collection related to available in situ and ex situ biomethanation processes and techno-economic analyses from previous works were performed.
5.1. Data Regarding In Situ and Ex Situ Biomethanation Technologies at Demo-Scale
As previously reported, biomethanation could be distinguished between in situ and ex situ technologies. Regarding the in situ technologies, aiming at producing biomethane by injecting H2 in pre-existing anaerobic digesters, the main expense would be represented by the installation of a water electrolysis system in direct proximity to the anaerobic digestion plant. The authors of [15] reported projections of capital expenditures (CAPEX) until 2050 for different types of water electrolyzers, including alkaline electrolyzer (AE), proton exchange membranes (PEM) and high-temperature electrolyzers (HtE), indicating that a gradual reduction in the cost of electrolyzers is expected. Prices for both AE and PEM systems are expected to fall below 500 EUR/kWel in 2050 from 2017 values reported at 1300 and 1900 EUR/kWel, respectively, with the greatest price reduction being expected for HtE systems, whose price should fall from 3570 EUR/kWel to an average of 535 EUR/kWel [15]. In situ biomethanation would seemingly only be affected by electrolyzer costs under an economical point of view due to the well-established presence of reactors for anaerobic digestion (AD), while the injection of H2 in pre-existing anaerobic digesters could bring some challenges from the technical point of view.
First and foremost, H2 injection would bring a considerable reduction in CO2 in the reactor due to its conversion into CH4. However, a decrease in CO2 levels would affect the concentration of HCO3−, which acts as a buffering agent for the process, leading to pH increases. Moreover, the loss of the buffering action promoted by HCO3− could bring an increase in VFA concentrations, which might inhibit HM activity [63]. To counteract such possibility, acidic wastes (i.e., food waste, whey wastewater) could be added to level pH values within the optimal range for biomethanation [154,155].
Second, H2 gas–liquid mass transfer might represent another issue related to in situ biomethanation. In fact, the level of H2 and its concentration as cations in the digester should respect the optimal stoichiometric ratio with CO2 for CH4 formation, which is reported to be 4:1 (H2:CO2) [93]. Nonetheless, due to H2’s reduced solubility in aqueous environments, actions should be considered in order to increase its mass transfer.
On the basis of the literature, the H2 mass transfer issue is often solved through optimization of the following aspects: reactor design (i.e., Up-flow Anaerobic Sludge Blanket—UASB—reactors), gas recirculation and an increase in the mixing speed of the liquid phase [6,55,58,75].
Concerning reactor design, UASB represents the most diffused configuration worldwide [146]. The system relies on the generation of a blanket due to the formation of a granular sludge with water moving bottom-up and the reactor dimension being dependent on the amount of waste to be treated and the area that they are serving. Nonetheless, at an industrial level, UASB height is reported between 5.5 and 6.5 m with a height-to-area ratio of 0.2–0.5 and an up-flow velocity of 0.5–0.8 m/h [156,157,158]. The UASB system allows for teh treatment of different wastewaters with organic loading rates (OLR) between 1.3 and 72.1 gCOD/Linfluent * day and methane yield ranging from 0.16 to 2.3 Lbiogas/Lreactor/day. The literature reports that depending on the feedstock nature, considerable differences in CH4 production could be achieved when using lipids (0.99 LCH4/g), proteins (0.63 LCH4/g) or carbohydrates (0.42 LCH4/g) [159,160]. UASB’s design ensures high contact between microorganisms and injected H2, allowing for the high conversion and quality of the CH4 produced (CH4 = 91%) when compared to the performance of standard CSTR (CH4 ≤ 85%) [55,75]. Despite the numerous lab-scale studies, only a few examples of in situ biomethanation at full scale have been reported to date. Microbenergy GmbH reported that during in situ operations, H2 injection (20 m3/h) biogas produced by a 100 m3 reactor was enriched in CH4 with its concentration rising from 53 to 60%, leading to a biomethane production of 198 m3/h.
In another study involving a standard CSTR (1110 m3; T = 55 °C), the application of gas recirculation from the headspace shows conversion values of H2 up to 41–50% due to the increase in residence time of H2 in the liquid phase of the system, allowing for a more thorough contact between gases and microbes; the absence of recirculation resulted in H2 conversion values of 2–4% only. Nonetheless, it was calculated that the employment of two pumps for liquid recirculation with a power consumption of 38 kW increased three times the H2 energy transfer up to 81 mol H2 kW/h [58,161]. Despite the technical advantage of using a recirculation system, the total investment costs of the process increased. Moreover, both the above-mentioned full-scale systems required a further CH4 upgrading before injection in the gas grid because the quality of the biogas was still below the NG constraints (CH4 > 97%) [49].
Ex situ biomethanation is just in the early stages of its application. Although having been widely investigated at a lab scale, only few examples of industrial-scale applications are available and reported herein.
MicrobEnergy GmbH, and more specifically, the Viessman group (Germany), carried out tests in a 5 m3 reactor operating at temperatures and pressures between 50 and 80 °C and 5 and 15 bar, respectively, under continuous stirring at 400 rpm. H2 was provided by two 150 kW PEM electrolyzers with an input of 1 LH2O/H2 m3 and capable of delivering a maximum of 60 H2 m3/h. Injection of 15 m3 of H2 alongside 30 m3 of biogas (50% CO2) produced 15 m3 gas with 98% CH4 and less than 1.5% H2. The long-term plans of the company are to reach the conversion of streams of 450 H2 m3/h, with CO2 being provided by the treatment of residual gases from different treatment plants [47]; www.microbenergy.de/aktuelles (accessed on 4 March 2022).
Electrochaea GmbH developed a trademark process based on the activity of M. thermoautotrophicus. Pre-commercial tests were realized with a 10 m3 CSTR operating at 65 °C and at pressures between 1 and 10 bars treating raw biogas as a source of CO2. During the Store&GO project (2015–2019), a 1 MW ex situ fully automated biomethanation demonstration plant was built in Solothurn (Switzerland). The installed 3.5 m3 reactors operate at 62 °C and 9 bars and obtains the needed CO2 (15 Nm3/h at 50% load) from the biogas of a nearby wastewater treatment, while H2 (60 Nm3/h at 50% load) was provided by a 350 kW PEM electrolyzer. The plant itself allowed for the production of biomethane (98% CH4), with a final yield of 14.3 m3CH4/m3reactor/h and a heat generation of 320 kW/h, with the produced gas being directly injected into the gas grid. As long-term plans, the company aims to develop a 10,000 MW plant in Hungary, although the realization timeline has not yet been specified [14,15,47]; store&go.info.
5.2. Studies on Techno-Economic Analysis for Biomethanation of CO2 and H2
Within the last 10 years, biomethane gained considerable interest as a renewable source of energy. Nonetheless, biological biogas upgrading technologies are still in the early development stage, with commercial applications only being represented by Electrochaea GmbH. To better define ex situ biomethanation process feasibility and promote its diffusion, different modeling and techno-economic studies have been presented in recent years. Most of these studies consider different scenarios and usually screen the economics of biological processes against biogas upgrading via membrane separation or amine-scrubbing [150,153,162]; others consider the ex situ biomethanation of syngas provided by biomass gasification [163]. Techno-economic data collected from different studies and regarding scenarios in which biogas/syngas are directly injected in the biomethanation systems are reported in Table 3.

Table 3.
Characteristics and parameters of biomethanation techno-economic analysis, parameters that were not specified from cited studies are reported as n.s. (not specified).
Although the reported studies included several parameters and factors, two of them can actually help to understand the techno-economic status and viability of the biomethanation process. At the economic level: (i) the production cost indicates the overall cost needed to generate a unit of the desired product, whilst (ii) the minimum selling price (MSP) indicates the price at which a specific product should be sold to achieve full payback of the initial investment.
Ref. [162] evaluated the possibility to couple an existing biogas plant to an ex situ biomethanation trickle bed system along with PEM water electrolyzer for H2 production with a CAPEX of 1.5 × 106 EUR and 1.0 × 103 EUR/kW, respectively, and compared it with a standard membrane upgrading system. Electricity consumption of the biomethanation system (0.16 kW/Nm3) was reported to be lower than the membrane one (0.26 kW/Nm3) with CAPEX related to the ex situ biomethanation being only 0.25 × 106 EUR higher than those reported for membrane upgrading. Moreover, the adoption of a biomethanation system almost doubled the amount of produced biomethane. Nonetheless, energy consumption of the electrolyzer for H2 production (5 kW/Nm3H2) increased the production costs of CH4 during ex situ biomethanation (0.73–0.8 EUR/Nm3 injected gas) when compared to membrane upgrading (0.65 EUR/Nm3 injected gas). The authors of [153] analyzed the application of ex situ biomethanation for upgrading of biogas generated by biogas plants treating grass silage and slurry and compared it with an amine scrubbing biogas upgrade system. Although data related to the installation and function of the electrolyzer were not reported, the costs of the electricity needed to generate H2 were accounted for. Utilization of an ex situ biomethanation plant of 5 MW with CAPEX of ≈2.5 × 106 EUR allowed for doubling the amount of biomethane produced, nonetheless even in this case production cost of generated biomethane (1.3 EUR/Nm3 injected gas) was reported higher than the one displayed by amine scrubbing methodologies (0.57 EUR/Nm3 injected gas). Along with this, the MSP for biomethane produced through ex situ biomethanation was reported at 1.43 EUR/Nm3 while the utilization of amine scrubbers lowered the MSP to 0.57 EUR/Nm3. Similarly to the two above-mentioned studies, [150] proposed the techno-economical assessment of an ex situ biomethanation plant (CAPEX 5.68 × 106 EUR) upgrading biogas directly injected from an anaerobic digestion plant treating 100,000 tsubstrate/year. Although costs of production related to upgrading via water scrubbing used in the considered biogas plant were still lower (0.225 EUR/Nm3) when compared to those achieved in ex situ biomethanation, by using high-grade biogas (CH4 65%, CO2 35%), it was possible to reach a biomethane production cost of 0.47 EUR/Nm3 due to reduction in the needed amount of H2 for the biological reaction and hence of the costs related to its production. Further reduction in biomethane production cost from 0.47 to 0.31 EUR/Nm3 was achieved when considering a biogas plant treating up to 250,000 tsubstrate/year. Despite production costs for biomethane generated by ex situ biomethanation being lower than the one previously described [153], its MSP was reported at 0.66 EUR/Nm3, which was still higher than the one for standard NG.
Another techno-economic study carried out by [163] compared syngas biomethanation and syngas purification for H2 generation. When coupling the gasification of dry biomass with either biomethanation or H2 production processes, it was found that syngas biomethanation provides much higher yield (0.39 CH4 Nm3) than H2 generation (0.07 H2 Nm3) per kg of gasified dry biomass. Moreover, due to a lower MSP (2.68 EUR/Nm3) biomethane production from syngas would allow for a faster economical return than H2 production (15.35 €/kg). Eventually, it has to be reported that CO2 concentration in the syngas fed to the biomethanation system needs to be reduced in order to match optimal stoichiometric conditions for the CH4 generation and the production capacity of the electrolyzer, with the excess of CO2 being lossed during the process [163].
Some general conclusions and considerations could be made regarding techno-economical aspects of biomethanation of CO2 and H2. Despite the environmental benefits of biomethanation (i.e., GHG and CO2 emission reduction) and higher CH4 yields than those achieved by chemo-physical technologies (i.e., amine and water scrubbing), the production cost of biomethane is usually reported to be higher (0.47–0.8 EUR/Nm3) than the one listed for the standard chemo-physical methodologies (0.225–0.65 EUR/Nm3) [150,153,162]. The main reason behind the higher production costs is usually the high energy consumption related to H2 generation. Water electrolyzer (i.e., PEM) commonly requires between 4.1–7.5 kWh/Nm3H2 [164], an energy demand that is way more consistent than the one requested for the biomethanation reactor (0.16–0.42 kWh/Nm3CH4). Moreover, it has to be considered that energy used for such operations has to be intended as derived from renewable sources and depending on renewable electricity price, curtailment and fluctuations in the cost of H2 needed for biomethanation may vary consistently, affecting biomethane’s final MSP and making it less competitive on the market when compared with the MPS of NG and biomethane produced through chemo-physical methods [150,153,162,164].
Although other ways to produce renewable H2 have been proposed and investigated, H2 production by means of water electrolysis is still the only one available at a commercial and industrial scale despite the high production costs (7.68–8.63 EUR/kgH2) [165]. Among the new technologies that would allow a strong reduction in renewable H2 production costs, biological dark fermentation (2.15 EUR/kgH2) and pyrolysis (1.38 EUR/kgH2) appear to be the most promising, but nonetheless, further improvement in both technologies and in their readiness at a commercial scale are still required [165,166,167].
Another aspect that deserves consideration is represented by the gaseous streams to be used as source of CO2 for the biomethanation process. Within this work, we presented techno-economic analysis in which the considered CO2 sources were represented by either biogas or syngas, and the final MSP of biomethane produced from biogas (0.66–1.43 EUR/Nm3) was consistently lower than the one produced from syngas (2.68 EUR/Nm3). The main reason behind such a difference could be attributed to the energy consumption of the gasifier section and higher CAPEX related to the realization of the syngas plant (Table 3). Moreover, in the case study here described, part of the CO2 is discharged in order to match H2 production from the electrolyzer, and a considerable amount of substrate for biomethane is lost, thus decreasing the final product yield when compared to the utilization of CO2 from biogas. Considering this, syngas biomethanation requires further improvement for what concerns both CO2 use and valorization and market competitiveness.
A concluding remark should be made regarding the importance that biologically produced CH4 could have in the near future. Despite data previously reported clearly indicating that biomethane is not competitive with NG so far due to its higher MSP, recent geo-political events led to a considerable increase in NG prices, especially in Europe, promoting increasing efforts to find new potential suppliers and sources of NG [167]. Although, nowadays, an efficient scale-up for biomethane production through biological methodologies is not possible due to the time required for project deployment (1.2–2 years), such a promising renewable and low-carbon sector is crucial to ensure both GHG emission reduction targeted in the mid- and long-term by the EU between 2030 and 2050 and to provide energy price buffering capacity.
6. Perspectives and Conclusions
In this review, we wanted to provide a comprehensive picture regarding the current knowledge, status and future perspectives for biomethanation of H2 and CO2, PtX technology for both GHG emission valorization/reduction and the storage of renewable energy excess.
The literature describes biomethanation as a flexible process, capable of operating in a wide range of T, neutral pH and standard P. The majority of the available papers indicates that the process is enhanced in thermophilic conditions (T < 60 °C) and by reactor pressurization due to their positive effects on reaction rate and gas solubilization, allowing for high product yields. Although pH control is frequently performed through the use of buffering agents (i.e., HCl, NaOH), biomethanation processes allow for direct pH control through modulation of the CO2 inlet flow, providing a valuable integration of system control and potentially reducing operational costs. HMs represent the main biocatalysts for the biomethanation process, with the available literature reporting their utilization both as pure cultures or enriched methanogenic consortia. The few available examples of biomethanation at demo/commercial scale relies strongly on the use of pure cultures (i.e., Electrochaea GmbH), probably due to their higher predictability when cultivated. Nonetheless, high robustness, less stringent conditions, capacity of intermittent work and fast recovery after process unbalance described for stable enriched methanogenic consortia would allow for process optimization with further studies required. In particular, substantial efforts should be focused on the conservation and stabilization of the enriched methanogenic community, its symbionts, performances and monitoring.
Information gathered in the present work surely identifies biomethanation as a technology in its early stages that, although deeply investigated at lab scale and displaying promising potential, shows only a few successful examples to date of demo/commercial-scale plants. The techno-economic analyses of different biomethanation scenarios frequently report that, although the feasibility of the process was confirmed, biomethane production costs are still considerably affected by the cost of H2 generation. In order for the biomethanation process to be fully renewable, the H2 needed for the reaction has to be produced from renewable sources. At present, water electrolysis is the only technology available at commercial scale, although its low efficiency and high power consumption lead to an increase in the H2 production price. Projections estimate that further technological development will improve the efficiency of water electrolyzers by 2030, and even more by 2050. Considering the consistent connection between water electrolysis and biomethanation, further integration of the two systems and their implementation should be investigated. Moreover, several technologies which have been proved promising for the production of cheaper H2 as feedstock for biomethanation (i.e., dark fermentation, pyrolysis) could be further implemented to provide alternative sources of green H2, allowing for a reduction in the production costs and an increase in biomethane’s competitiveness on the market.
Nowadays, the biomethanation potential is still under-expressed, and further efforts are required for its application at an industrial scale. With limitation posed by the production cost of H2 and the long time required for its optimization, future research should prioritize a reduction in costs related to the biomethanation process. Considering process characteristics, mesophilic and low temperature processes requiring a reduced amount of thermal energy should be implemented to reach high rate productivity. Moreover, the utilization of mixed methanogenic consortia requiring less stringent conditions and capable to grow in waste liquid effluents of different industrial processes (i.e., sewage sludge, whey and wastewaters) could further reduce process costs, increasing the market competitiveness of biomethanation. Finally, considering the exergonic nature of the biomethanation process energy released in form of heat could be recovered and re-deployed to sustain the process energetic requirements or H2 generation.
The recent increase in NG market prices and the need to reach energetic independence allows one to see this process as a viable mid-term and long-term solution for both GHG emission reduction and renewable energy storage. Nonetheless, a considerable amount of research is still required in order to further optimize the process, its efficient scale-up and integration in the energy grid.
Author Contributions
Conceptualization, R.B.; Software, R.B.; Validation, R.B., I.B., A.V., A.A.A., B.M. and N.S.V.; Resources, R.B., B.M., I.B. and A.V.; Writing—original draft preparation, R.B., I.B., A.V., A.A.A., B.M. and N.S.V.; Writing—review and editing, R.B. and B.M.; Visualization, R.B. and A.V.; Supervision, B.M., C.F.P. and F.V.; Project Administration, B.M., C.F.P. and F.V.; Funding Acquisition, B.M., C.F.P. and F.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research has not received any external funding. Internal fundings from the Italian institute of technology were used.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
All figures presented in this work have been created or modified with BioRender.com (accessed on 3 March 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Olivier, J.G.J.; Peters, J.A.H.W. Trends in Global CO2 and Total Greenhouse Gas Emissions: 2019 Report. PBL Neth. Environ. Assess. Agency 2020, 2020, 1–70. [Google Scholar]
- EREC. Mapping Renewable Energy Pathways towards 2020; European Renewable Energy Council: Brussels, Belgium, 2011; p. 28. [Google Scholar]
- de Vasconcelos, B.R.; Lavoie, J.M. Recent Advances in Power-to-X Technology for the Production of Fuels and Chemicals. Front. Chem. 2019, 7, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, R. CO2 Recovery and Reuse in the Energy Sector, Energy Resource Development and Others: Economic and Technical Evaluation of Large-Scale CO2 Recycling. Energy Environ. 2003, 14, 383–396. [Google Scholar] [CrossRef]
- Grimalt-Alemany, A.; Skiadas, I.V.; Gavala, H.N. Syngas Biomethanation: State-of-the-Art Review and Perspectives. Biofuels Bioprod. Biorefining 2018, 12, 139–158. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Integrated Biogas Upgrading and Hydrogen Utilization in an Anaerobic Reactor Containing Enriched Hydrogenotrophic Methanogenic Culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on Methanation—From Fundamentals to Current Projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Collet, P.; Flottes, E.; Favre, A.; Raynal, L.; Pierre, H.; Capela, S.; Peregrina, C. Techno-Economic and Life Cycle Assessment of Methane Production via Biogas Upgrading and Power to Gas Technology. Appl. Energy 2017, 192, 282–295. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, K.; Kumagai, N.; Izumiya, K.; Takano, H.; Shinomiya, H.; Sasaki, Y.; Yoshida, T.; Kato, Z. The Use of Renewable Energy in the Form of Methane via Electrolytic Hydrogen Generation Using Carbon Dioxide as the Feedstock. Appl. Surf. Sci. 2016, 388, 608–615. [Google Scholar] [CrossRef]
- Brooks, K.P.; Hu, J.; Zhu, H.; Kee, R.J. Methanation of Carbon Dioxide by Hydrogen Reduction Using the Sabatier Process in Microchannel Reactors. Chem. Eng. Sci. 2007, 62, 1161–1170. [Google Scholar] [CrossRef]
- Savvas, S.; Donnelly, J.; Patterson, T.P.; Dinsdale, R.; Esteves, S.R. Closed Nutrient Recycling via Microbial Catabolism in an Eco-Engineered Self Regenerating Mixed Anaerobic Microbiome for Hydrogenotrophic Methanogenesis. Bioresour. Technol. 2017, 227, 93–101. [Google Scholar] [CrossRef]
- Molíková, A.; Vítězová, M.; Vítěz, T.; Buriánková, I.; Huber, H.; Dengler, L.; Hanišáková, N.; Onderka, V.; Urbanová, I. Underground Gas Storage as a Promising Natural Methane Bioreactor and Reservoir? J. Energy Storage 2022, 47, 103631. [Google Scholar] [CrossRef]
- Strobel, G.; Hagemann, B.; Huppertz, T.M.; Ganzer, L. Underground Bio-Methanation: Concept and Potential. Renew. Sustain. Energy Rev. 2020, 123, 109747. [Google Scholar] [CrossRef]
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas Projects Review: Lab, Pilot and Demo Plants for Storing Renewable Energy and CO2. Renew. Sustain. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Thema, M.; Bauer, F.; Sterner, M. Power-to-Gas: Electrolysis and Methanation Status Review. Renew. Sustain. Energy Rev. 2019, 112, 775–787. [Google Scholar] [CrossRef]
- Back, K.J.; Lascelles, J.; Still, J.L. Hydrogenase. Aust. J. Sci. 1946, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Oremland, R.S. Biogeochemistry of Methanogenic Bacteria. In Biology of Anaerobic Microorganisms; John and Wiley and Sons: Hoboken, NJ, USA, 1988; pp. 641–705. [Google Scholar]
- Wolfe, R.S. An Historical Overview of Methanogenesis. In Methanogenesis; Springer: Boston, MA, USA, 1993; pp. 1–32. [Google Scholar] [CrossRef]
- Balch, W.E.; Fox, G.E.; Magrum, L.J.; Woese, C.R.; Wolfe, R.S. Methanogens: Reevaluation of a Unique Biological Group. Microbiol. Rev. 1979, 43, 260–296. [Google Scholar] [CrossRef]
- Stadtman, T.C.; Barker, H.A. Studies on the Methane Fermentation X: A New Formate-Decomposing Bacterium, Methanococcus vannielii. J. Bacteriol. 1951, 62, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Kurr, M.; Huber, R.; König, H.; Jannasch, H.W.; Fricke, H.; Trincone, A.; Kristjansson, J.K.; Stetter, K.O. Methanopyrus kandleri, gen. and sp. nov. Represents a Novel Group of Hyperthermophilic Methanogens, Growing at 110 °C. Arch. Microbiol. 1991, 156, 239–247. [Google Scholar] [CrossRef]
- Sakai, S.; Imachi, H.; Hanada, S.; Ohashi, A.; Harada, H.; Kamagata, Y. Methanocella paludicola gen. nov., sp. nov., a Methane-Producing Archaeon, the First Isolate of the Lineage “Rice Cluster I”, and Proposal of the New Archaeal Order Methanocellales ord. nov. Int. J. Syst. Evol. Microbiol. 2008, 58, 929–936. [Google Scholar] [CrossRef]
- Dridi, B.; Fardeau, M.L.; Ollivier, B.; Raoult, D.; Drancourt, M. Methanomassiliicoccus luminyensis gen. nov., sp. nov., a Methanogenic Archaeon Isolated from Human Faeces. Int. J. Syst. Evol. Microbiol. 2012, 62, 1902–1907. [Google Scholar] [CrossRef] [Green Version]
- Iino, T.; Tamaki, H.; Tamazawa, S.; Ueno, Y.; Ohkuma, M.; Suzuki, K.I.; Igarashi, Y.; Haruta, S. Candidatus Methanogranum Caenicola: A Novel Methanogen from the Anaerobic Digested Sludge, and Proposal of Methanomassiliicoccaceae fam. nov. and Methanomassiliicoccales ord. nov., for a Methanogenic Lineage of the Class Thermoplasmata. Microbes Environ. 2013, 28, 244–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, J.G.; Garrity, G.M.; Whitman, W.B. Euryarchaeota, Taxonomy of Methanogenic Archaea. 1994. Available online: https://doi.org/10.1007/978-0-387-21609-6_17 (accessed on 1 March 2022).
- Sowers, K.R. Methanogenesis. Encyclopedia of Microbiology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 265–286. [Google Scholar] [CrossRef]
- Garcia, J.; Patel, B.K.C.; Ollivier, B. Taxonomic, Phylogenetic, and Ecological Diversity of Methanogenic Archaea. Anaerobe 2000, 6, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Nazaries, L.; Murrell, J.C.; Millard, P.; Baggs, L.; Singh, B.K. Methane, Microbes and Models: Fundamental Understanding of the Soil Methane Cycle for Future Predictions. Environ. Microbiol. 2013, 15, 2395–2417. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Whitman, W.B. Metabolic, Phylogenetic, and Ecological Diversity of Methanogenic Archaea. Ann. N. Y. Acad. Sci. 2008, 189, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Z.; Shao, N.; Akinyemi, T.; Whitman, W.B. Methanogenesis. Curr. Biol. 2018, 28, R727–R732. [Google Scholar] [CrossRef] [Green Version]
- Ferry, J.G. Fundamentals of Methanogenic Pathways that Are Key to the Biomethanation of Complex Biomass. Curr. Opin. Biotechnol. 2011, 22, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Basak, B.; Hwang, J.H.; Salama, E.S.; Chatterjee, P.K.; Jeon, B.H. Microbial Symbiosis: A Network towards Biomethanation. Trends Microbiol. 2020, 28, 968–984. [Google Scholar] [CrossRef]
- Borrel, G.; Adam, P.S.; Gribaldo, S. Methanogenesis and the Wood–Ljungdahl Pathway: An Ancient, Versatile, and Fragile Association. Genome Biol. Evol. 2016, 8, 1706–1711. [Google Scholar] [CrossRef] [Green Version]
- Zinder, S.H. Physiological Ecology of Methanogens. In Methanogenesis; Springer: Boston, MA, USA, 1993; pp. 128–206. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic Archaea: Ecologically Relevant Differences in Energy Conservation. Nat. Rev. Microbiol. 2008, 6, 579–591. [Google Scholar] [CrossRef]
- Enzmann, F.; Mayer, F.; Rother, M.; Holtmann, D. Methanogens: Biochemical Background and Biotechnological Applications. AMB Express 2018, 8, 1. [Google Scholar] [CrossRef]
- Kletzin, A. General Characteristics and Important Model Organisms. In Archaea; ASM Press: Washington, DC, USA, 2014; pp. 14–92. [Google Scholar] [CrossRef]
- Buckel, W.; Thauer, R.K. Energy Conservation via Electron Bifurcating Ferredoxin Reduction and Proton/Na+ Translocating Ferredoxin Oxidation. Biochim. Biophys. Acta (BBA)-Bioenerg. 2013, 1827, 94–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chellapandi, P.; Prathiviraj, R. Methanothermobacter Thermautotrophicus Strain ΔH as a Potential Microorganism for Bioconversion of CO2 to Methane. J. CO2 Util. 2020, 40, 101210. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the Anaerobic Biodegradability of Macropollutants. Re/Views Environ. Sci. Bio/Technol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Scherer, P.; Lippert, H.; Wolff, G. Composition of the Major Elements and Trace Elements of 10 Methanogenic Bacteria Determined by Inductively Coupled Plasma Emission Spectrometry. Biol. Trace Elem. Res. 1983, 5, 149–163. [Google Scholar] [CrossRef]
- Ünal, B.; Perry, V.R.; Sheth, M.; Gomez-Alvarez, V.; Chin, K.J.; Nüsslein, K. Trace Elements Affect Methanogenic Activity and Diversity in Enrichments from Subsurface Coal Bed Produced Water. Front. Microbiol. 2012, 3, 175. [Google Scholar] [CrossRef] [Green Version]
- Kaster, A.; Goenrich, M.; Seedorf, H.; Liesegang, H.; Wollherr, A.; Gottschalk, G.; Thauer, R.K. More than 200 Genes Required for Methane Formation from H2 and CO2 and Energy Conservation Are Present in Methanothermobacter marburgensis and Methanothermobacter thermautotrophicus. Archaea 2011, 2011, 973848. [Google Scholar] [CrossRef] [Green Version]
- Jarrell, K.F.; Kalmokoff, M.L. Nutritional Requirements of the Methanogenic Archaebacteria. Can. J. Microbiol. 1988, 34, 557–576. [Google Scholar] [CrossRef]
- Alitalo, A.; Niskanen, M.; Aura, E. Biocatalytic Methanation of Hydrogen and Carbon Dioxide in a Fixed Bed Bioreactor. Bioresour. Technol. 2015, 196, 600–605. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, J.H.; Chang, W.S.; Pak, D. Biological Conversion of CO2 to CH4 Using Hydrogenotrophic Methanogen in a Fixed Bed Reactor. J. Chem. Technol. Biotechnol. 2012, 87, 844–847. [Google Scholar] [CrossRef]
- Rafrafi, Y.; Laguillaumie, L.; Dumas, C. Biological Methanation of H2 and CO2 with Mixed Cultures: Current Advances, Hurdles and Challenges. Waste Biomass Valorization 2021, 12, 5259–5282. [Google Scholar] [CrossRef]
- Iglesias, R.; Muñoz, R.; Polanco, M.; Díaz, I.; Susmozas, A.; Moreno, A.D.; Guirado, M.; Carreras, N.; Ballesteros, M. Biogas from Anaerobic Digestion as an Energy Vector: Current Upgrading Development. Energies 2021, 14, 2742. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas Upgrading and Utilization: Current Status and Perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef] [Green Version]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable Hydrogen Production. Int. J. Energy Res. 2008, 32, 379–407. [Google Scholar] [CrossRef] [Green Version]
- Jürgensen, L.; Ehimen, E.A.; Born, J.; Holm-Nielsen, J.B. Utilization of Surplus Electricity from Wind Power for Dynamic Biogas Upgrading: Northern Germany Case Study. Biomass Bioenergy 2014, 66, 126–132. [Google Scholar] [CrossRef]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A Review on the State-of-the-Art of Physical/Chemical and Biological Technologies for Biogas Upgrading. Rev. Environ. Sci. Biotechnol. 2015, 14, 727–759. [Google Scholar] [CrossRef] [Green Version]
- Kougias, P.G.; Treu, L.; Benavente, D.P.; Boe, K.; Campanaro, S.; Angelidaki, I. Ex-Situ Biogas Upgrading and Enhancement in Different Reactor Systems. Bioresour. Technol. 2017, 225, 429–437. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Angelidaki, I. In-Situ Biogas Upgrading in Thermophilic Granular UASB Reactor: Key Factors Affecting the Hydrogen Mass Transfer Rate. Bioresour. Technol. 2016, 221, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas Upgrading via Hydrogenotrophic Methanogenesis in Two-Stage Continuous Stirred Tank Reactors at Mesophilic and Thermophilic Conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef]
- Treu, L.; Campanaro, S.; Kougias, P.G.; Sartori, C.; Bassani, I.; Angelidaki, I. Hydrogen-Fueled Microbial Pathways in Biogas Upgrading Systems Revealed by Genome-Centric Metagenomics. Front. Microbiol. 2018, 9, 1079. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.B.; Jensen, B.; Ottosen, L.D.M.; Kofoed, M.V.W. Integrating H2 Injection and Reactor Mixing for Low-Cost H2 Gas-Liquid Mass Transfer in Full-Scale in Situ Biomethanation. Biochem. Eng. J. 2021, 166, 107869. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Co-Digestion of Manure and Whey for in Situ Biogas Upgrading by the Addition of H2: Process Performance and Microbial Insights. Appl. Microbiol. Biotechnol. 2013, 97, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Alessi, A.M.; Zhang, Y.; Chong JP, J.; Heaven, S.; Banks, C.J. Simultaneous Biomethanisation of Endogenous and Imported CO2 in Organically Loaded Anaerobic Digesters. Appl. Energy 2019, 247, 670–681. [Google Scholar] [CrossRef]
- Wang, W.; Xie, L.; Luo, G.; Zhou, Q.; Angelidaki, I. Performance and Microbial Community Analysis of the Anaerobic Reactor with Coke Oven Gas Biomethanation and in Situ Biogas Upgrading. Bioresour. Technol. 2013, 146, 234–239. [Google Scholar] [CrossRef] [Green Version]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.; Siegrist, H.; Vavilin, V.A. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Mulat, D.G.; Mosbæk, F.; Ward, A.J.; Polag, D.; Greule, M.; Keppler, F.; Nielsen, J.L.; Feilberg, A. Exogenous Addition of H2 for an in Situ Biogas Upgrading through Biological Reduction of Carbon Dioxide into Methane. Waste Manag. 2017, 68, 146–156. [Google Scholar] [CrossRef]
- Agneessens, L.M.; Ottosen, L.D.M.; Voigt, N.V.; Nielsen, J.L.; de Jonge, N.; Fischer, C.H.; Kofoed, M.V.W. In-Situ Biogas Upgrading with Pulse H2 Additions: The Relevance of Methanogen Adaption and Inorganic Carbon Level. Bioresour. Technol. 2017, 233, 256–263. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Treu, L.; Porté, H.; Campanaro, S.; Angelidaki, I. Optimization of Hydrogen Dispersion in Thermophilic Up-Flow Reactors for Ex Situ Biogas Upgrading. Bioresour. Technol. 2017, 234, 310–319. [Google Scholar] [CrossRef]
- Díaz, I.; Pérez, C.; Alfaro, N.; Fdz-Polanco, F. A Feasibility Study on the Bioconversion of CO2 and H2 to Biomethane by Gas Sparging through Polymeric Membranes. Bioresour. Technol. 2015, 185, 246–253. [Google Scholar] [CrossRef]
- Martin, M.R.; Fornero, J.J.; Stark, R.; Mets, L.; Angenent, L.T. A Single-Culture Bioprocess of Methanothermobacter Thermautotrophicus to Upgrade Digester Biogas by CO2-to-CH4 Conversion with H2. Archaea 2013, 2013, 157529. [Google Scholar] [CrossRef] [Green Version]
- Jensen, M.B.; Ottosen, L.D.M.; Kofoed, M.V.W. H2 Gas-Liquid Mass Transfer: A Key Element in Biological Power-to-Gas Methanation. Renew. Sustain. Energy Rev. 2021, 147, 111209. [Google Scholar] [CrossRef]
- Guiot, S.R.; Cimpoia, R.; Carayon, G. Potential of Wastewater-Treating Anaerobic Granules for Biomethanation of Synthesis Gas. Environ. Sci. Technol. 2011, 45, 2006–2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henstra, A.M.; Sipma, J.; Rinzema, A.; Stams, A.J. Microbiology of Synthesis Gas Fermentation for Biofuel Production. Curr. Opin. Biotechnol. 2007, 18, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New Opportunities for Agricultural Digestate Valorization: Current Situation and Perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Ahern, E.P.; Deane, P.; Persson, T.; Ó Gallachóir, B.; Murphy, J.D. A Perspective on the Potential Role of Renewable Gas in a Smart Energy Island System. Renew. Energy 2015, 78, 648–656. [Google Scholar] [CrossRef]
- Savvas, S.; Donnelly, J.; Patterson, T.; Chong, Z.S.; Esteves, S.R. Biological Methanation of CO2 in a Novel Biofilm Plug-Flow Reactor: A High Rate and Low Parasitic Energy Process. Appl. Energy 2017, 202, 238–247. [Google Scholar] [CrossRef]
- Corbellini, V.; Kougias, P.G.; Treu, L.; Bassani, I.; Malpei, F.; Angelidaki, I. Hybrid Biogas Upgrading in a Two-Stage Thermophilic Reactor. Energy Convers. Manag. 2018, 168, 1–10. [Google Scholar] [CrossRef]
- Wahid, R.; Horn, S.J. Impact of Operational Conditions on Methane Yield and Microbial Community Composition during Biological Methanation in in Situ and Hybrid Reactor Systems. Biotechnol. Biofuels 2021, 14, 170. [Google Scholar] [CrossRef]
- Rittmann, S.K.M.R. A Critical Assessment of Microbiological Biogas to Biomethane Upgrading Systems. Adv. Biochem. Eng. Biotechnol. 2015, 151, 117–135. [Google Scholar] [CrossRef]
- Rachbauer, L.; Beyer, R.; Bochmann, G.; Fuchs, W. Characteristics of Adapted Hydrogenotrophic Community during Biomethanation. Sci. Total Environ. 2017, 595, 912–919. [Google Scholar] [CrossRef]
- Savvas, S.; Donnelly, J.; Patterson, T.; Chong, Z.S.; Esteves, S.R. Methanogenic Capacity and Robustness of Hydrogenotrophic Cultures Based on Closed Nutrient Recycling via Microbial Catabolism: Impact of Temperature and Microbial Attachment. Bioresour. Technol. 2018, 257, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, M.; Jordan, I.; Heinrich, S.; Behrens, J.; Ziesche, A.; Busch, G. Long Term and Demand-Oriented Biocatalytic Synthesis of Highly Concentrated Methane in a Trickle Bed Reactor. Appl. Energy 2019, 240, 818–826. [Google Scholar] [CrossRef]
- Jensen, M.B.; Strübing, D.; de Jonge, N.; Nielsen, J.L.; Ottosen, L.D.M.; Koch, K.; Kofoed, M.V.W. Stick or Leave—Pushing Methanogens to Biofilm Formation for Ex Situ Biomethanation. Bioresour. Technol. 2019, 291, 121784. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.M.; Sung, S.; Kang, S.; Kim, M.S.; Kim, D.H. Enrichment of Hydrogenotrophic Methanogens by Means of Gas Recycle and Its Application in Biogas Upgrading. Energy 2017, 135, 294–302. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s Law Constants (Version 4.0) for Water as Solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Guneratnam, A.J.; Ahern, E.; FitzGerald, J.A.; Jackson, S.A.; Xia, A.; Dobson, A.D.W.; Murphy, J.D. Study of the Performance of a Thermophilic Biological Methanation System. Bioresour. Technol. 2017, 225, 308–315. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Chen, Y.; Cao, Q.; Liu, X.; Li, D. Differences of Methanogenesis between Mesophilic and Thermophilic in Situ Biogas-Upgrading Systems by Hydrogen Addition. J. Ind. Microbiol. Biotechnol. 2019, 46, 1569–1581. [Google Scholar] [CrossRef]
- Seifert, A.H.; Rittmann, S.; Herwig, C. Analysis of Process Related Factors to Increase Volumetric Productivity and Quality of Biomethane with Methanothermobacter Marburgensis. Appl. Energy 2014, 132, 155–162. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K.; Chung, J. Reduction in Carbon Dioxide and Production of Methane by Biological Reaction in the Electronics Industry. Int. J. Hydrogen Energy 2013, 38, 3488–3496. [Google Scholar] [CrossRef]
- Burkhardt, M.; Koschack, T.; Busch, G. Biocatalytic Methanation of Hydrogen and Carbon Dioxide in an Anaerobic Three-Phase System. Bioresour. Technol. 2015, 178, 330–333. [Google Scholar] [CrossRef]
- Strübing, D.; Huber, B.; Lebuhn, M.; Drewes, J.E.; Koch, K. High Performance Biological Methanation in a Thermophilic Anaerobic Trickle Bed Reactor. Bioresour. Technol. 2017, 245, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Sun, L.; Müller, B.; Westerholm, M.; Schnürer, A. Syntrophic Acetate Oxidation in Industrial CSTR Biogas Digesters. J. Biotechnol. 2014, 171, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Westerholm, M.; Moestedt, J.; Schnürer, A. Biogas Production through Syntrophic Acetate Oxidation and Deliberate Operating Strategies for Improved Digester Performance. Appl. Energy 2016, 179, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Rachbauer, L.; Voitl, G.; Bochmann, G.; Fuchs, W. Biological Biogas Upgrading Capacity of a Hydrogenotrophic Community in a Trickle-Bed Reactor. Appl. Energy 2016, 180, 483–490. [Google Scholar] [CrossRef]
- Agneessens, L.M.; Ottosen, L.D.M.; Andersen, M.; Berg Olesen, C.; Feilberg, A.; Kofoed, M.V.W. Parameters Affecting Acetate Concentrations during In-Situ Biological Hydrogen Methanation. Bioresour. Technol. 2018, 258, 33–40. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A Critical Review on Inhibition of Anaerobic Digestion Process by Excess Ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Hejnfelt, A.; Angelidaki, I. Anaerobic Digestion of Slaughterhouse By-Products. Biomass Bioenergy 2009, 33, 1046–1054. [Google Scholar] [CrossRef]
- Fricke, K.; Santen, H.; Wallmann, R.; Hüttner, A.; Dichtl, N. Operating Problems in Anaerobic Digestion Plants Resulting from Nitrogen in MSW. Waste Manag. 2007, 27, 30–43. [Google Scholar] [CrossRef]
- Müller, T.; Walter, B.; Wirtz, A.; Burkovski, A. Ammonium Toxicity in Bacteria. Curr. Microbiol. 2006, 52, 400–406. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Fotidis, I.A.; Kissas, K.; Angelidaki, I. Effect of Different Ammonia Sources on Aceticlastic and Hydrogenotrophic Methanogens. Bioresour. Technol. 2018, 250, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Tao, Y.; Hu, J.; Liu, G.; Spanjers, H.; van Lier, J.B. Biomethanation and Microbial Community Changes in a Digester Treating Sludge from a Brackish Aquaculture Recirculation System. Bioresour. Technol. 2016, 214, 338–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Alam, M.A.; Kong, X.; Wang, Z.; Li, L.; Sun, Y.; Yuan, Z. Effect of Salinity on the Microbial Community and Performance on Anaerobic Digestion of Marine Macroalgae. J. Chem. Technol. Biotechnol. 2017, 92, 2392–2399. [Google Scholar] [CrossRef]
- Letelier-Gordo, C.O.; Mancini, E.; Pedersen, P.B.; Angelidaki, I.; Fotidis, I.A. Saline Fish Wastewater in Biogas Plants - Biomethanation Toxicity and Safe Use. J. Environ. Manag. 2020, 275, 111233. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hou, X.; Su, H. Exploration of the Relationship between Biogas Production and Microbial Community under High Salinity Conditions. Sci. Rep. 2017, 7, 1149. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Spanjers, H.; van Lier, J.B. Potentials and Limitations of Biomethane and Phosphorus Recovery from Sludges of Brackish/Marine Aquaculture Recirculation Systems: A Review. J. Environ. Manag. 2013, 131, 44–54. [Google Scholar] [CrossRef]
- Vyrides, I.; Stuckey, D.C. Adaptation of Anaerobic Biomass to Saline Conditions: Role of Compatible Solutes and Extracellular Polysaccharides. Enzym. Microb. Technol. 2009, 44, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Huzir, N.M.; Mahmood, N.A.N.; Muhammad, S.A.F.A.S.; Umor, N.A.; Ismail, S. Effect of Specific Methanogenic Activity (SMA) of Anaerobic Sludge under High Salinity. J. Adv. Res. Appl. Sci. Eng. Technol. 2019, 16, 35–40. [Google Scholar]
- De Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The Rediscovered Methanogen for Heavy Duty Biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Arends, J.B.A.; Van de Wiele, T.; Boon, N. Bioreactor Technology in Marine Microbiology: From Design to Future Application. Biotechnol. Adv. 2011, 29, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Feijoo, G.; Soto, M.; Méndez, R.; Lema, J.M. Sodium Inhibition in the Anaerobic Digestion Process: Antagonism and Adaptation Phenomena. Enzym. Microb. Technol. 1995, 17, 180–188. [Google Scholar] [CrossRef]
- Lee, D.H.; Behera, S.K.; Kim, J.W.; Park, H.S. Methane Production Potential of Leachate Generated from Korean Food Waste Recycling Facilities: A Lab-Scale Study. Waste Manag. 2009, 29, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Romero-Güiza, M.S.; Vila, J.; Mata-Alvarez, J.; Chimenos, J.M.; Astals, S. The Role of Additives on Anaerobic Digestion: A Review. Renew. Sustain. Energy Rev. 2016, 58, 1486–1499. [Google Scholar] [CrossRef]
- Schattauer, A.; Abdoun, E.; Weiland, P.; Plöchl, M.; Heiermann, M. Abundance of Trace Elements in Demonstration Biogas Plants. Biosyst. Eng. 2011, 108, 57–65. [Google Scholar] [CrossRef]
- Feng, X.M.; Karlsson, A.; Svensson, B.H.; Bertilsson, S. Industrial Waste Linking Process to Microbial Communities. FEMS Microbiol. Ecol. 2010, 74, 226–240. [Google Scholar] [CrossRef] [Green Version]
- Abdel Azim, A.; Rittmann, S.K.M.R.; Fino, D.; Bochmann, G. The Physiological Effect of Heavy Metals and Volatile Fatty Acids on Methanococcus maripaludis S2. Biotechnol. Biofuels 2018, 11, 301. [Google Scholar] [CrossRef]
- Glass, J.B.; Orphan, V.J. Trace Metal Requirements for Microbial Enzymes Involved in the Production and Consumption of Methane and Nitrous Oxide. Front. Microbiol. 2012, 3, 61. [Google Scholar] [CrossRef] [Green Version]
- Abdel Azim, A.; Pruckner, C.; Kolar, P.; Taubner, R.S.; Fino, D.; Saracco, G.; Sousa, F.L.; Rittmann, S.K.M.R. The Physiology of Trace Elements in Biological Methane Production. Bioresour. Technol. 2017, 241, 775–786. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Maekawa, T. Continuous Fermentation Using Acclimated-Methanogens Feed Substrate of Mixed Carbon Dioxide on and Hydrogen. J. Soc. Agric. Struct. 1993, 24, 207–214. [Google Scholar]
- Zhang, Y.; Zhang, Z.; Suzuki, K.; Maekawa, T. Uptake and Mass Balance of Trace Metals for Methane Producing Bact. Biomass-Bioenergy 2003, 25, 427–433. [Google Scholar] [CrossRef]
- Mauerhofer, L.M.; Zwirtmayr, S.; Pappenreiter, P.; Bernacchi, S.; Seifert, A.H.; Reischl, B.; Schmider, T.; Taubner, R.S.; Paulik, C.; Rittmann, S.K.M.R. Hyperthermophilic Methanogenic Archaea Act as High-Pressure CH4 Cell Factories. Commun. Biol. 2021, 4, 289. [Google Scholar] [CrossRef]
- Battino, R. The Ostwald Coefficient of Gas Solubility. Fluid Phase Equilib. 1984, 15, 231–240. [Google Scholar] [CrossRef]
- Lu, J.X.; Murray, J. Biochemistry, Dissolution and Solubility; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Rusmanis, D.; O’Shea, R.; Wall, D.M.; Murphy, J.D. Biological Hydrogen Methanation Systems—An Overview of Design and Efficiency. Bioengineered 2019, 10, 604–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özbek, B.; Gayik, S. The Studies on the Oxygen Mass Transfer Coefficient in a Bioreactor. Process Biochem. 2001, 36, 729–741. [Google Scholar] [CrossRef]
- Bajón Fernández, Y.; Cartmell, E.; Soares, A.; McAdam, E.; Vale, P.; Darche-Dugaret, C.; Jefferson, B. Gas to Liquid Mass Transfer in Rheologically Complex Fluids. Chem. Eng. J. 2015, 273, 656–667. [Google Scholar] [CrossRef]
- Goel, R.; Komatsu, K.; Yasui, H.; Harada, H. Process Performance and Change in Sludge Characteristics during Anaerobic Digestion of Sewage Sludge with Ozonation. Water Sci. Technol. 2004, 49, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hammadi, L.; Ponton, A.; Belhadri, M. Effects of Heat Treatment and Hydrogen Peroxide (H2O2) on the Physicochemical and Rheological Behavior of an Activated Sludge from a Water Purification Plant. Procedia Eng. 2012, 33, 293–302. [Google Scholar] [CrossRef] [Green Version]
- Alves, S.S.; Maia, C.I.; Vasconcelos, J.M.T. Gas-Liquid Mass Transfer Coefficient in Stirred Tanks Interpreted through Bubble Contamination Kinetics. Chem. Eng. Process. Process Intensif. 2004, 43, 823–830. [Google Scholar] [CrossRef]
- Montante, G.; Horn, D.; Paglianti, A. Gas-Liquid Flow and Bubble Size Distribution in Stirred Tanks. Chem. Eng. Sci. 2008, 63, 2107–2118. [Google Scholar] [CrossRef]
- Leu, J.Y.; Lin, Y.H.; Chang, F.L. Conversion of CO2 into CH4 by Methane-Producing Bacterium FJ10 under a Pressurized Condition. Chem. Eng. Res. Des. 2011, 89, 1879–1890. [Google Scholar] [CrossRef]
- Nielsen, J.; Villadsen, J. Bioreaction Engineering Principles, 2nd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Panfilov, M.; Reitenbach, V.; Ganzer, L. Self-Organization and Shock Waves in Underground Methanation Reactors and Hydrogen Storages. Environ. Earth Sci. 2016, 75, 313. [Google Scholar] [CrossRef]
- Amez, I.; Gonzalez, S.; Sanchez-Martin, L.; Ortega, M.F.; Llamas, B. Underground Methanation, a Natural Way to Transform Carbon Dioxide into Methane. In Climate Change Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 81–106. [Google Scholar] [CrossRef]
- Tarkowski, R. Underground Hydrogen Storage: Characteristics and Prospects. Renew. Sustain. Energy Rev. 2019, 105, 86–94. [Google Scholar] [CrossRef]
- Oren, A. Taxonomy of Halophilic Archaea: Current Status and Future Challenges. Extremophiles 2014, 18, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Bueno de Mesquita, C.P.; Zhou, J.; Theroux, S.M.; Tringe, S.G. Methanogenesis and Salt Tolerance Genes of a Novel Halophilic Methanosarcinaceae Metagenome-Assembled Genome from a Former Solar Saltern. Genes 2021, 12, 1609. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, B.; Fardeau, M.L.; Cayol, J.L.; Magot, M.; Patel, B.K.C.; Prensier, G.; Garcia, J.L. Methanocalculus halotolerans gen. nov., sp. nov., Isolated from an Oil- Producing Well. Int. J. Syst. Bacteriol. 1998, 48, 821–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhilina, T.N.; Zavarzina, D.G.; Kevbrin, V.V.; Kolganova, T.V. Methanocalculus natronophilus sp. nov., a New Alkaliphilic Hydrogenotrophic Methanogenic Archaeon from a Soda Lake, and Proposal of the New Family Methanocalculaceae. Microbiology 2013, 82, 698–706. [Google Scholar] [CrossRef]
- Pérez, A.; Pérez, E.; Dupraz, S.; Bolcich, J. Patagonia Wind-Hydrogen Project: Underground Storage and Methanation. In Proceedings of the WHEC 2016—21st World Hydrogen Energy Conference 2016, Zaragoza, Spain, 13–16 June 2016. [Google Scholar]
- RAG. Underground Sun Conversion—Project Description—Project. 2018. Available online: https://www.underground-sun-conversion.at/das-projekt/projektbeschreibung.html (accessed on 8 April 2022).
- RAG. Underground SUN Storage—Chemical Storage of Renewable Energy in Porous Subsurface Reservoirs with Exemplary Test Bed. January 2017. Available online: https://energieforschung.at/projekt/chemical-storage-of-renewable-energy-in-porous-subsurface-reservoirs-with-exemplary-testbed/ (accessed on 8 April 2022).
- Flemisch, B.; Darcis, M.; Erbertseder, K.; Faigle, B.; Lauser, A.; Mosthaf, K.; Müthing, S.; Nuske, P.; Tatomir, A.; Wolff, M.; et al. DuMux: DUNE for Multi-{phase, Component, Scale, Physics,...} Flow and Transport in Porous Media. Adv. Water Resour. 2011, 34, 1102–1112. [Google Scholar] [CrossRef]
- Nikolaev, D.S.; Moeininia, N.; Ott, H.; Bueltemeier, H. Investigation of Underground Bio-Methanation Using Bio-Reactive Transport Modeling. In Proceedings of the Society of Petroleum Engineers—SPE Russian Petroleum Technology Conference 2021, RPTC 2021, Virtual, 12–15 October 2021. [Google Scholar] [CrossRef]
- Eddaoui, N. Patterns in Bioreactive Transport in Underground Storage of Hydrogen: Impact of Natural and Induced eterogeneity, HAL Id: Tel-03564124 Soutenance et Mis à Disposition del’ Ensemble de La Contact: Ddoc-Theses-Contact@univ-Lorrai. 2022. Available online: https://hal.univ-lorraine.fr/tel-03564124/document (accessed on 4 March 2022).
- Gniese, C.; Bombach, P.; Rakoczy, J.; Hoth, N.; Schlömann, M.; Richnow, H.H.; Krüger, M. Relevance of Deep-Subsurface Microbiology for Underground Gas Storage and Geothermal Energy Production. Adv. Biochem. Eng. Biotechnol. 2014, 142, 95–121. [Google Scholar] [CrossRef]
- Toleukhanov, A.; Panfilov, M.; Kaltayev, A. Storage of Hydrogenous Gas Mixture in Geological Formations: Self-Organisation in Presence of Chemotaxis. Int. J. Hydrog. Energy 2015, 40, 15952–15962. [Google Scholar] [CrossRef]
- Eddaoui, N.; Panfilov, M.; Ganzer, L.; Hagemann, B. Impact of Pore Clogging by Bacteria on Underground Hydrogen Storage. Transp. Porous Media 2021, 139, 89–108. [Google Scholar] [CrossRef]
- Michas, A.; Harir, M.; Lucio, M.; Vestergaard, G.; Himmelberg, A.; Schmitt-Kopplin, P.; Lueders, T.; Hatzinikolaou, D.G.; Schöler, A.; Rabus, R.; et al. Sulfate Alters the Competition Among Microbiome Members of Sediments Chronically Exposed to Asphalt. Front. Microbiol. 2020, 11, 2420. [Google Scholar] [CrossRef]
- Van Der Valk, K.; Van Unen, M.; Brunner, L.; Gas, D. Inventory of Risks Associated with Underground Storage of Compressed Air (CAES) and Hydrogen (UHS), and Qualitative Comparison of Risks of UHS vs. Underground Storage of Natural Gas (UGS). 2020. Available online: https://repository.tno.nl/islandora/object/uuid%3A4a8bfbac-9b18-4499-945c-807c6ca7872e (accessed on 27 May 2022).
- IEA. Outlook for Biogas and Biomethane. Prospects for Organic Growth; World Energy Outlook Special Report; IEA: Paris, France, 2020; p. 93. [Google Scholar]
- Lawson, N.; Alvarado-Morales, M.; Tsapekos, P.; Angelidaki, I. Techno-Economic Assessment of Biological Biogas Upgrading Based on Danish Biogas Plants. Energies 2021, 14, 8252. [Google Scholar] [CrossRef]
- Junaidi, M.U.M.; Leo, C.P.; Ahmad, A.L. Biogas Upgrading System: Techno-Economic Analysis of Membrane Separation Process Implementation. Int. J. Appl. Eng. Res. 2016, 11, 973–4562. [Google Scholar]
- Cavaignac, R.S.; Ferreira, N.L.; Guardani, R. Techno-Economic and Environmental Process Evaluation of Biogas Upgrading via Amine Scrubbing. Renew. Energy 2021, 171, 868–880. [Google Scholar] [CrossRef]
- Vo, T.T.Q.; Wall, D.M.; Ring, D.; Rajendran, K.; Murphy, J.D. Techno-Economic Analysis of Biogas Upgrading via Amine Scrubber, Carbon Capture and Ex-Situ Methanation. Appl. Energy 2018, 212, 1191–1202. [Google Scholar] [CrossRef]
- Fontana, A.; Campanaro, S.; Treu, L.; Kougias, P.G.; Cappa, F.; Morelli, L.; Angelidaki, I. Performance and Genome-Centric Metagenomics of Thermophilic Single and Two-Stage Anaerobic Digesters Treating Cheese Wastes. Water Res. 2018, 134, 181–191. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Dong, R. Recent Progress towards In-Situ Biogas Upgrading Technologies. Sci. Total Environ. 2021, 800, 149667. [Google Scholar] [CrossRef]
- Mainardis, M.; Buttazzoni, M.; Goi, D. Up-Flow Anaerobic Sludge Blanket (Uasb) Technology for Energy Recovery: A Review on State-of-the-Art and Recent Technological Advances. Bioengineering 2020, 7, 43. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, T.H. Applicability and Trends of Anaerobic Granular Sludge Treatment Processes. Biomass Bioenergy 2014, 60, 189–202. [Google Scholar] [CrossRef]
- Lettinga, G. Anaerobic Digestion and Wastewater Treatment Systems. Antonie Van Leeuwenhoek 1995, 67, 3–28. [Google Scholar] [CrossRef]
- Nakasaki, K.; Nguyen, K.K.; Ballesteros, F.C.; Maekawa, T.; Koyama, M. Characterizing the Microbial Community Involved in Anaerobic Digestion of Lipid-Rich Wastewater to Produce Methane Gas. Anaerobe 2020, 61, 102082. [Google Scholar] [CrossRef]
- Alves, M.M.; Pereira, M.A.; Sousa, D.Z.; Cavaleiro, A.J.; Picavet, M.; Smidt, H.; Stams, A.J.M. Waste Lipids to Energy: How to Optimize Methane Production from Long-Chain Fatty Acids (LCFA). Microb. Biotechnol. 2009, 2, 538–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, M.B.; Kofoed, M.V.W.; Fischer, K.; Voigt, N.V.; Agneessens, L.M.; Batstone, D.J.; Ottosen, L.D.M. Venturi-Type Injection System as a Potential H2 Mass Transfer Technology for Full-Scale in Situ Biomethanation. Appl. Energy 2018, 222, 840–846. [Google Scholar] [CrossRef]
- Bekkering, J.; Zwart, K.; Martinus, G.; Langerak, J.; Tideman, J.; van der Meij, T.; Alberts, K.; van Steenis, M.; Nap, J.P. Farm-Scale Bio-Power-to-Methane: Comparative Analyses of Economic and Environmental Feasibility. Int. J. Energy Res. 2020, 44, 2264–2277. [Google Scholar] [CrossRef]
- Menin, L.; Vakalis, S.; Benedetti, V.; Patuzzi, F.; Baratieri, M. Techno-Economic Assessment of an Integrated Biomass Gasification, Electrolysis, and Syngas Biomethanation Process. Biomass Convers. Biorefin. 2021, 11, 445–459. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A Technological and Economic Review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef] [Green Version]
- Vidas, L.; Castro, R. Recent Developments on Hydrogen Production Technologies: State-of-the-Art Review with a Focus on Green-Electrolysis. Appl. Sci. 2021, 11, 11363. [Google Scholar] [CrossRef]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Biomass-Based Hydrogen Production: A Review and Analysis. Int. J. Hydrogen Energy 2009, 34, 8799–8817. [Google Scholar] [CrossRef]
- Abdul Mujeebu, M. Hydrogen and Syngas Production by Superadiabatic Combustion—A Review. Appl. Energy 2016, 173, 210–224. [Google Scholar] [CrossRef]
- IEA. A 10-Point Plan to Reduce the European Union’ s Reliance on Russian Natural Gas; IEA: Paris, France, 2022. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).