Hydrogen Production from Enzymatic Hydrolysates of Alkali Pre-Treated Giant Reed (Arundo donax L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Experimental Design
2.3. Pre-Treatment, Fibre Recovery, Hydrolysis and Sugar Content Determination
2.4. Dark Fermentation
2.5. Analytical Methods
2.6. Statistical Analysis
3. Results
3.1. Biomass Fractionation Yields Following the Pre-Treatment
3.2. Composition of the Materials Utilised in Dark Fermentation
3.3. Dark Fermentation Kinetics and Parameters
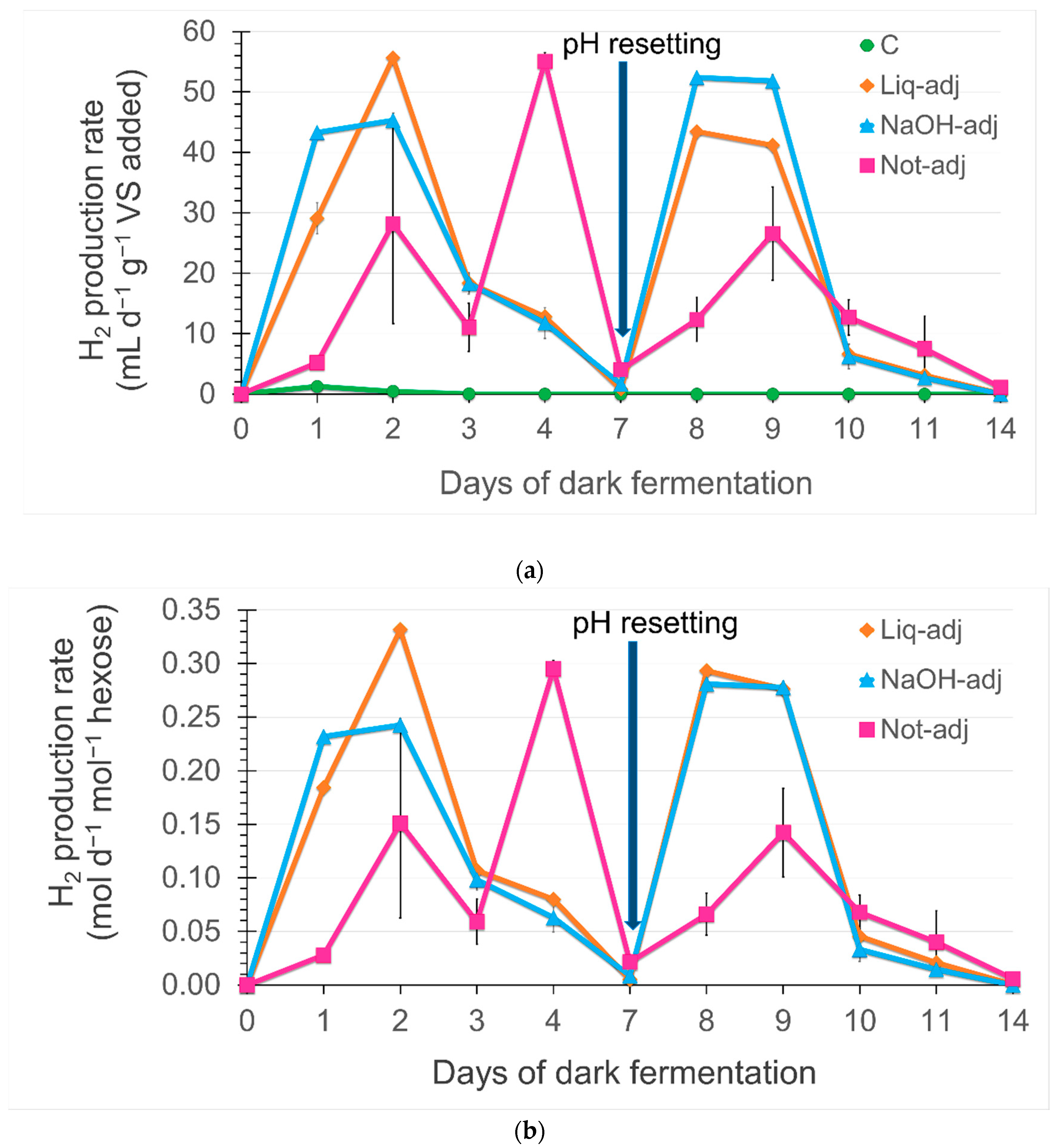
3.4. Time-Course of the Daily Rate of H2 Accumulation in Dark Fermentation
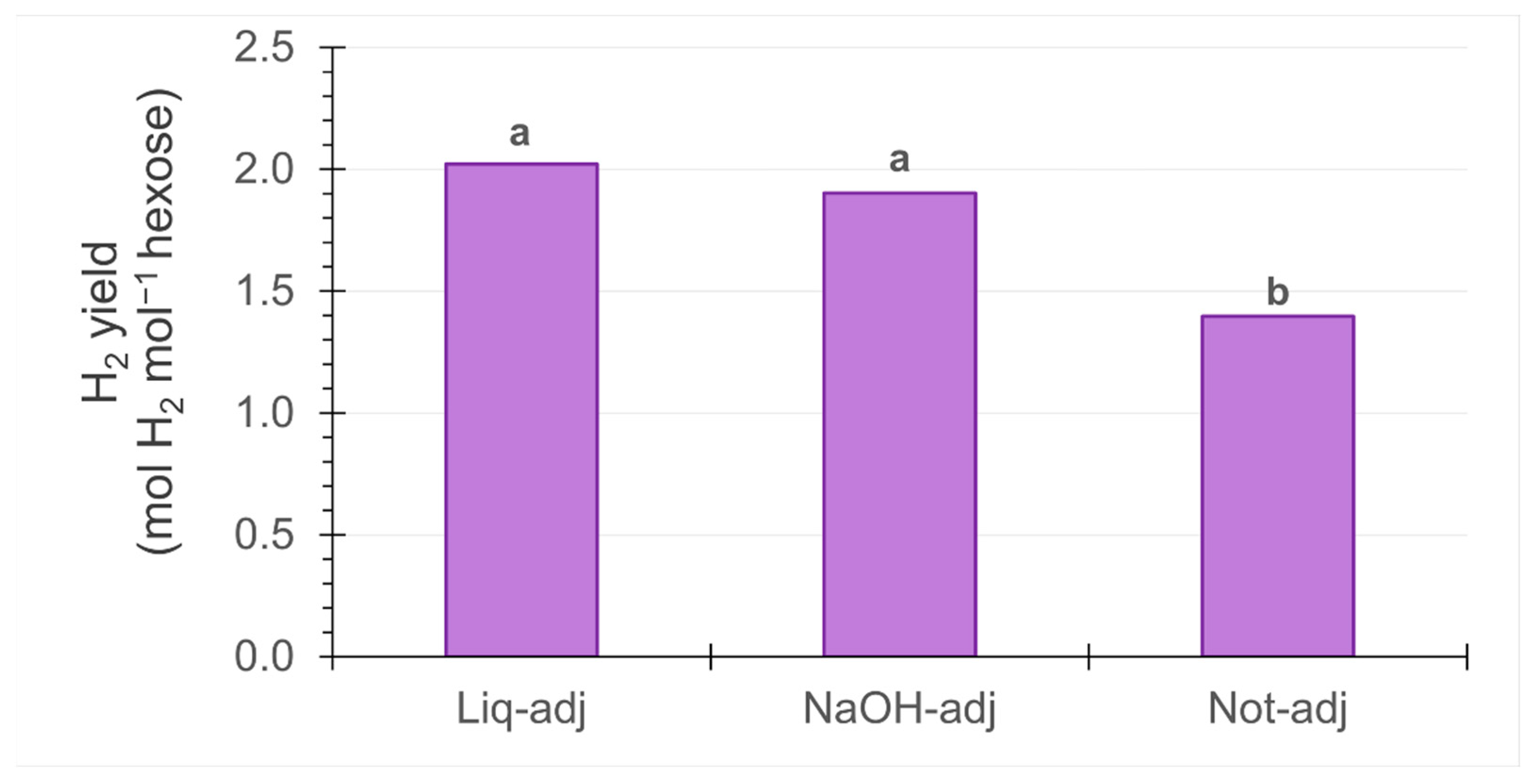
3.5. Hydrogen Yield Per Mole of Hexose in Dark Fermentation
4. Discussion
4.1. Biomass Fractionation Yields Following the Pre-Treatment
4.2. Composition of the Materials Utilized in Dark Fermentation
4.3. Dark Fermentation Kinetics and Parameters
4.4. Daily Rate of H2 Accumulation in Dark Fermentation
4.5. Hydrogen Yield Per Mole of Hexose in Dark Fermentation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McAllister, S.; Chen, J.-Y.; Fernandez-Pello, A.C. Fundamentals of Combustion Processes; Springer: New York, NY, USA, 2011; pp. 227–241. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen production technologies: Current state and future developments. Conf. Pap. Sci. 2013, 2013, 690627. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.E.; Kumar, G.; Yang, Y.H. Renewable biohydrogen production from lignocellulosic biomass using fermentation and integration of systems with other energy generation technologies. Sci. Total Environ. 2021, 765, 144429. [Google Scholar] [CrossRef] [PubMed]
- Delvar, M.A.; Wang, J. Numerical investigation of pH control on dark fermentation and hydrogen production in a microbioreactor. Fuel 2021, 292, 120355. [Google Scholar] [CrossRef]
- Balachandar, G.; Khanna, N.; Das, D. Biohydrogen production from organic wastes by dark fermentation. In Biohydrogen, 1st ed.; Pandey, A., Chang, J.-S., Hallenbeck, P., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 103–144. [Google Scholar] [CrossRef]
- Singh, R.; Tevatia, R.; White, D.; Demirel, Y.; Blum, P. Comparative kinetic modeling of growth and molecular hydrogen overproduction by engineered strains of Thermotoga maritima. Int. J. Hydrogen Energy 2019, 44, 7125–7136. [Google Scholar] [CrossRef] [Green Version]
- Corneli, E.; Dragoni, F.; Adessi, A.; De Philippis, R.; Bonari, E.; Ragaglini, G. Energy conversion of biomass crops and agroindustrial residues by combined biohydrogen/biomethane system and anaerobic digestion. Bioresour. Technol. 2016, 211, 509–518. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zhang, H.; He, C.; Zhang, Q. Potential use and the energy conversion efficiency analysis of fermentation effluents from photo and dark fermentative bio-hydrogen production. Bioresour. Technol. 2017, 245, 884–889. [Google Scholar] [CrossRef]
- Vasmara, C.; Pindo, M.; Micheletti, D.; Marchetti, R. Initial pH influences microbial communities composition in dark fermentation of scotta permeate. Int. J. Hydrogen Energy 2018, 43, 8707–8717. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Malhotra, B.D.; Gupta, V.K.; Ramteke, P.W.; Silva, R.N.; Shukla, P.; Dubey, K.K.; Mishra, P.K. Nanoengineered cellulosic biohydrogen production via dark fermentation: A novel approach. Biotechnol. Adv. 2019, 37, 107384. [Google Scholar] [CrossRef]
- Scordia, D.; Testa, G.; Cosentino, S.L. Perennial grasses as lignocellulosic feedstock for second-generation bioethanol production in Mediterranean environment. Ital. J. Agron. 2014, 9, 84–92. [Google Scholar] [CrossRef]
- Fike, J.H.; Parrish, D.J.; Fike, W.B. Sustainable cellulosic grass crop production. In Biofuel Crop Sustainability, 1st ed.; Singh, B.P., Ed.; John Wiley & Sons: Chichester, UK, 2013; pp. 109–164. [Google Scholar] [CrossRef]
- Ge, X.M.; Xu, F.Q.; Vasco-Correa, J.; Li, Y.B. Giant reed: A competitive energy crop in comparison with miscanthus. Renew. Sustain. Energy Rev. 2016, 54, 350–362. [Google Scholar] [CrossRef] [Green Version]
- Ceotto, E.; Castelli, F.; Moschella, A.; Diozzi, M.; Di Candilo, M. Cattle slurry fertilization to giant reed (Arundo donax L.): Biomass yield and nitrogen use efficiency. Bioenergy Res. 2015, 8, 1252–1262. [Google Scholar] [CrossRef]
- Ceotto, E.; Vasmara, C.; Marchetti, R.; Cianchetta, S.; Galletti, S. Biomass and methane yield of giant reed (Arundo donax L.) as affected by single and double annual harvest. Glob. Chang. Biol. Bioenergy 2021, 13, 393–407. [Google Scholar] [CrossRef]
- Van Dik, J.S.; Pletschke, B. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Larroche, C.; Dussap, C.G. Comprehensive assessment of 2G bioethanol production. Bioresour. Technol. 2020, 313, 123630. [Google Scholar] [CrossRef]
- Cianchetta, S.; Di Maggio, B.; Burzi, P.L.; Galletti, S. Evaluation of selected white-rot fungal isolates for improving the sugar yield from wheat straw. Appl. Biochem. Biotechnol. 2014, 173, 609–623. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Singh, J.; Suhag, M.; Dhaka, A. Augmented digestion of lignocellulose by steam explosion, acid and alkaline pretreatment methods: A review. Carbohydr. Polym. 2015, 117, 624–631. [Google Scholar] [CrossRef]
- Cianchetta, S.; Nota, M.; Polidori, N.; Galletti, S. Alkali pre-treatment and enzymatic hydrolysis of Arundo donax for single cell oil production. Environ. Eng. Manag. J. 2019, 18, 1693–1701. [Google Scholar]
- Cianchetta, S.; Polidori, N.; Vasmara, C.; Ceotto, E.; Marchetti, R.; Galletti, S. Single cell oil production from hydrolysates of alkali pre-treated giant reed: High biomass-to-lipid yields with selected yeasts. Ind. Crops Prod. 2022, 178, 114596. [Google Scholar] [CrossRef]
- McIntosh, S.; Vancov, T. Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw. Biomass Bioenergy 2011, 35, 3094–3103. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Salakkam, A.; Plangklang, P.; Sittijunda, S.; Kongkeitkajorn, M.B.; Lunprom, S.; Reungsang, A. Bio-hydrogen and methane production from lignocellulosic materials. In Biomass for Bioenergy-Recent Trends and Future Challenges, 1st ed.; Abomohra, A.E., Ed.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Vasmara, C.; Marchetti, R. Initial pH influences in-batch hydrogen production from scotta permeate. Int. J. Hydrogen Energy 2017, 42, 14400–14408. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Biohydrogen Production from Organic Wastes; Springer Nature: Singapore, 2017; pp. 69–121. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 2, 934–951. [Google Scholar] [CrossRef] [PubMed]
- Toscano, G.; Zuccaro, G.; Ausiello, A.; Micoli, L.; Turco, M.; Pirozzi, D. Production of hydrogen from giant reed by dark fermentation. Chem. Eng. 2014, 37, 331–336. [Google Scholar] [CrossRef]
- Ausiello, A.; Micoli, L.; Pirozzi, D.; Toscano, G.; Turco, M. Biohydrogen production by dark fermentation of Arundo donax for feeding fuel cells. Chem. Eng. Trans. 2015, 43, 385–390. [Google Scholar] [CrossRef]
- Ausiello, A.; Micoli, L.; Turco, M.; Toscano, G.; Florio, C.; Pirozzi, D. Biohydrogen production by dark fermentation of Arundo donax using a new methodology for selection of H2-producing bacteria. Int. J. Hydrogen Energy 2017, 42, 30599–30612. [Google Scholar] [CrossRef]
- Jiang, D.; Ge, X.; Zhang, T.; Chen, Z.; Zhang, Z.; He, C.; Zhang, Q.; Li, Y. Effect of alkaline pretreatment on photo-fermentative hydrogen production from giant reed: Comparison of NaOH and Ca(OH)2. Bioresour. Technol. 2020, 304, 123001. [Google Scholar] [CrossRef]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Ceotto, E.; Galletti, S. Potassium Hydroxide Pre-Treatment Enhances Methane Yield from Giant Reed (Arundo donax L.). Energies 2021, 14, 630. [Google Scholar] [CrossRef]
- Hinkelmann, K.; Kempthorne, O. Design and Analysis of Experiments: Introduction to Experimental Design, 2nd ed.; Wiley-Interscience: New York, NY, USA, 2007; Volume 1, p. 631. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Cianchetta, S.; Galletti, S.; Burzi, P.L.; Cerato, C. A novel microplate-based screening strategy to assess the cellulolytic potential of Trichoderma strains. Biotechnol. Bioeng. 2010, 107, 461–468. [Google Scholar] [CrossRef]
- Kádár, Z.; de Vrije, T.; van Noorden, G.E.; Budde, M.A.; Szengyel, Z.; Réczey, K.; Claassen, P.A. Yields from glucose, xylose, and paper sludge hydrolysate during hydrogen production by the extreme thermophile Caldicellulosiruptor saccharolyticus. Appl. Biochem. Biotechnol. 2004, 113–116, 497–508. [Google Scholar] [CrossRef]
- Vasmara, C.; Cianchetta, S.; Marchetti, R.; Galletti, S. Biogas production from wheat straw pre-treated with ligninolytic fungi and co-digestion with pig slurry. Environ. Eng. Manag. J. 2015, 14, 1751–1760. Available online: http://www.eemj.icpm.tuiasi.ro/pdfs/vol14/no7/Full/28_1073_Vasmara_14.pdf (accessed on 1 April 2022). [CrossRef]
- Marchetti, R.; Vasmara, C. Co-digestion of deproteinized dairy waste with pig slurry: Effect of recipe and initial pH on biogas and volatile fatty acid production. BioEnergy Res. 2020, 13, 643–658. [Google Scholar] [CrossRef]
- Owen, W.F.; Stuckey, D.C.; Healy, J.B., Jr.; Young, L.Y.; McCarty, P.L. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- Vasmara, C.; Marchetti, R. Biogas production from biodegradable bioplastics. Environ. Eng. Manag. J. 2016, 15, 2041–2048. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral-detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Vasmara, C.; Marchetti, R. Spent coffee grounds from coffee vending machines as feedstock for biogas production. Environ. Eng. Manag. J. 2018, 17, 2813–2821. Available online: http://www.eemj.icpm.tuiasi.ro/pdfs/vol17/full/no10/12_108_Vasmara_18.pdf (accessed on 1 April 2022).
- Vasmara, C.; Marchetti, R.; Carminati, D. Wastewater from the production of lactic acid bacteria as feedstock in anaerobic digestion. Energy 2021, 229, 120740. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: https://palaeo-electronica.org/2001_1/past/past.pdf (accessed on 1 April 2022).
- Cianchetta, S.; Bregoli, L.; Galletti, S. Microplate-based evaluation of the sugar yield from giant reed, giant Miscanthus and switchgrass after mild chemical pre-treatments and hydrolysis with tailored Trichoderma enzymatic blends. Appl. Biochem. Biotechnol. 2017, 183, 876–892. [Google Scholar] [CrossRef]
- Jiang, D.; Ge, X.; Zhang, Q.; Li, Y. Comparison of liquid hot water and alkaline pretreatments of giant reed for improved enzymatic digestibility and biogas energy production. Bioresour. Technol. 2016, 216, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemões, J.S.; e Silva, C.F.L.; Avila, S.P.F.; Montero, C.R.S.; e Silva, S.D.D.A.; Samios, D.; Peralba, M.D.C.R. Chemical pretreatment of Arundo donax L. for second-generation ethanol production. Electron. J. Biotechnol. 2018, 31, 67–74. [Google Scholar] [CrossRef]
- Panagiotopoulos, I.A.; Bakker, R.R.; de Vrije, T.; van Niel, E.W.J.; Koukios, E.G.; Claassen, P.A.M. Exploring critical factors for fermentative hydrogen production from various types of lignocellulosic biomass. J. Jpn. Inst. Energy 2011, 90, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Panagiotopoulos, I.A. Dark fermentative hydrogen production from ligno-cellulosic biomass. In Production of Hydrogen from Renewable Resources. Biofuels and Biorefineries, 1st ed.; Fang, Z., Smith, R., Jr., Qi, X., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 5, pp. 3–40. [Google Scholar] [CrossRef]
- Komolwanich, T.; Tatijarern, P.; Prasertwasu, S.; Khumsupan, D.; Chaisuwan, T.; Luengnaruemitchai, A.; Wongkasemjit, S. Comparative potentiality of Kans grass (Saccharum spontaneum) and Giant reed (Arundo donax) as lignocellulosic feedstocks for the release of monomeric sugars by microwave/chemical pretreatment. Cellulose 2014, 21, 1327–1340. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L.; Lee, J.W.; Jeffries, T.W. Dilute oxalic acid pretreatment for biorefining giant reed (Arundo donax L.). Biomass Bioenergy 2011, 35, 3018–3024. [Google Scholar] [CrossRef]
- Torrado, I.; Bandeira, F.; Shatalov, A.A.; Carvalheiro, F.; Duarte, L.C. The impact of particle size on the dilute acid hydrolysis of giant reed biomass. Electron. J. Energy Environ. 2014, 2, 9–17. [Google Scholar]
- Davidek, T.; Gouezec, E.; Devaud, S.; Blank, I. Origin and yields of acetic acid in pentose-based Maillard reaction systems. Ann. N. Y. Acad. Sci. 2008, 1126, 241–243. [Google Scholar] [CrossRef] [Green Version]
- Shatalov, A.A.; Morais, A.R.C.; Duarte, L.C.; Carvalheiro, F. Selective single-stage xylan-to-xylose hydrolysis and its effect on enzymatic digestibility of energy crops giant reed and cardoon for bioethanol production. Ind. Crops Prod. 2017, 95, 104–112. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L.; Lee, J.W.; Jeffries, T.W. Bioconversion of giant reed (Arundo donax L.) hemicellulose hydrolysate to ethanol by Scheffersomyces stipitis CBS6054. Biomass Bioenergy 2012, 39, 296–305. [Google Scholar] [CrossRef]
- Mars, A.E.; Veuskens, T.; Budde, M.A.W.; van Doeveren, P.F.N.M.; Lips, S.J.; Bakker, R.R.; de Vrije, T.; Claassen, P.A.M. Biohydrogen production from untreated and hydrolyzed potato steam peels by the extreme thermophiles Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Int. J. Hydrogen Energy 2010, 35, 7730–7737. [Google Scholar] [CrossRef]
- Cao, G.; Ren, N.; Wang, A.; Lee, D.-J.; Guo, W.; Liu, B.; Feng, Y.; Zhao, Q. Acid hydrolysis of corn stover for biohydrogen production using Thermoanaerobacterium thermosaccharolyticum W16. Int. J. Hydrogen Energy 2009, 34, 7182–7188. [Google Scholar] [CrossRef]
- Singh, N.; Sarma, S. Biological routes of hydrogen production: A critical assessment. In Handbook of Biofuels, 1st ed.; Sahay, S., Ed.; Academic Press: London, UK, 2022; Volume 1, pp. 419–434. [Google Scholar] [CrossRef]
- De Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zou, W.; Zhang, K.; Ye, G.; Yang, J. Advances and applications of Clostridium co-culture systems in biotechnology. Front. Microbiol. 2020, 11, 560223. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Miyahara, T.; Noike, T. Effect of pH on microbial hydrogen fermentation. J. Chem. Technol. Biotechnol. 2002, 77, 694–698. [Google Scholar] [CrossRef]
- Nissilä, M.E.; Lay, C.H.; Puhakka, J.A. Dark fermentative hydrogen production from lignocellulosic hydrolyzates—A review. Biomass Bioenergy 2014, 67, 145–159. [Google Scholar] [CrossRef]
- Cieciura-Włoch, W.; Borowski, S.; Domański, J. Dark fermentative hydrogen production from hydrolyzed sugar beet pulp improved by iron addition. Bioresour Technol. 2020, 314, 123713. [Google Scholar] [CrossRef]
- Khanal, S.K.; Chen, W.H.; Li, L.; Sung, S. Biological hydrogen production: Effects of pH and intermediate products. Int. J. Hydrogen Energy 2004, 29, 1123–1131. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Influence of initial uncontrolled pH on acidogenic fermentation of brewery spent grains to biohydrogen and volatile fatty acids production: Optimization and scale-up. Bioresour. Technol. 2021, 319, 124233. [Google Scholar] [CrossRef]
- Grzelak, J.; Œlêzak, R.; Krzystek, L.; Ledakowicz, S. Effect of pH on the production of volatile fatty acids in dark fermentation process of organic waste. Ecol. Chem. Eng. 2018, 25, 295. [Google Scholar] [CrossRef] [Green Version]
- de Vrije, T.; Bakker, R.R.; Budde, M.A.W.; Lai, M.H.; Mars, A.E.; Claassen, P.A.M. Efficient hydrogen production from the lignocellulosic energy crop Miscanthus by the extreme thermophilic bacteria Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Biotechnol. Biofuels 2009, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, N.; Dipasquale, L.; d’Ippolito, G.; Panico, A.; Lens, P.N.; Esposito, G.; Fontana, A. Hydrogen production by the thermophilic bacterium Thermotoga neapolitana. Int. J. Mol. Sci. 2015, 16, 12578–12600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
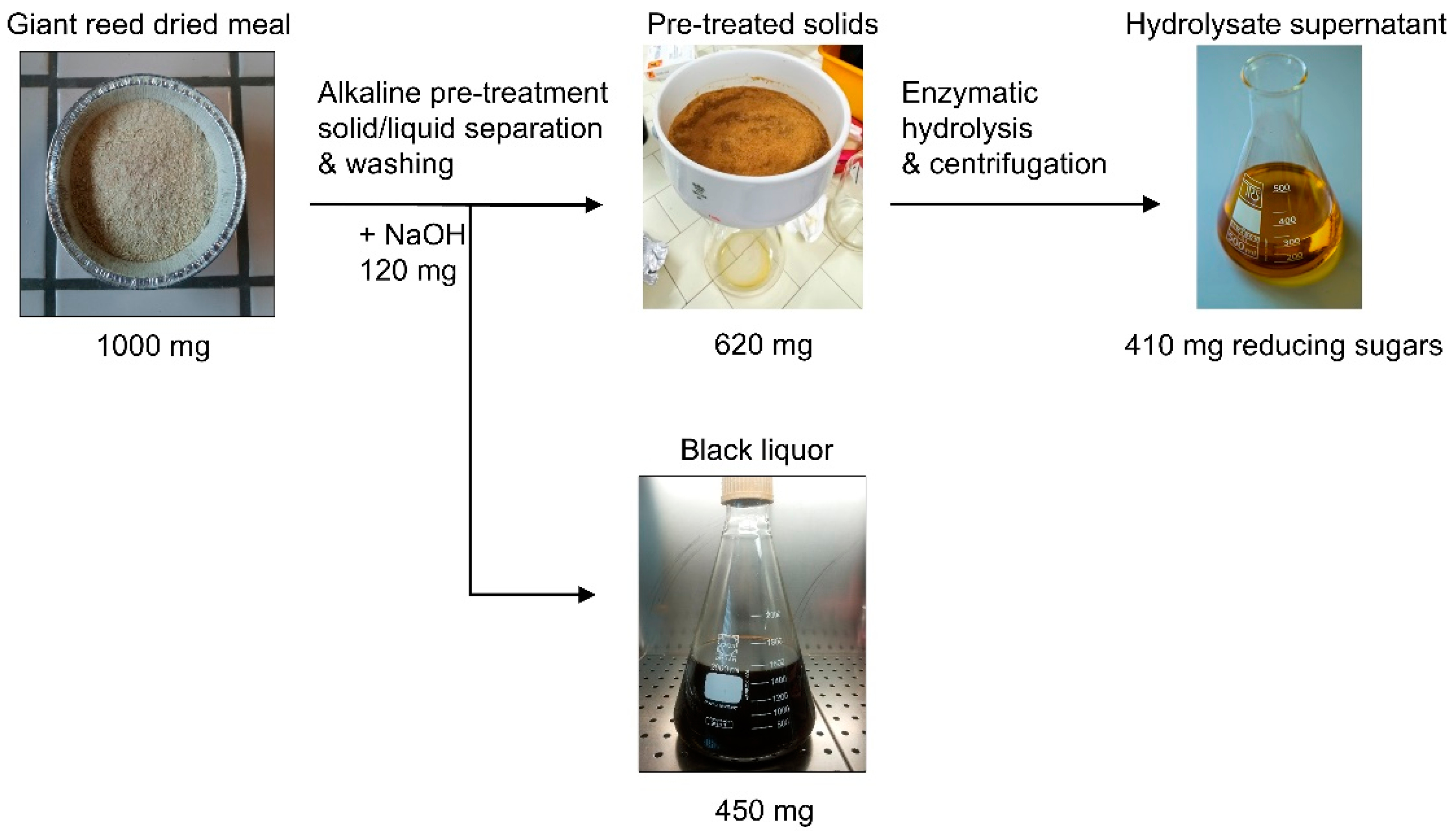

| Trait | Giant Reed Meal | Hydrolysate Supernatant | Black Liquor | Inoculum |
|---|---|---|---|---|
| Total solids (TS), % | 98.21 (0.4) | 6.03 (0.02) | 6.43 (0.02) | 6.33 (0.30) |
| Volatile solids (VS), % | 92.90 (0.2) | 5.04 (0.02) | 3.22 (0.03) | 4.02 (0.02) |
| Volatile solids, % TS | 95 | 84 | 50 | 64 |
| Ash, % | 5.31 (0.23) | 0.99 (0.01) | 3.21 (0.02) | 2.31 (0.29) |
| pH | 5.80 (0.02) | 5.32 (0.01) | 11.12 (0.01) | 7.60 (0.01) |
| Cellulose, % TS | 39.11 (0.3) | n.d. | 2.38 (0.11) | 3.62 (0.85) |
| Hemicellulose, % TS | 23.14 (0.3) | n.d. | n.d. | 0.66 (0.07) |
| Lignin, % TS | 11.67 (0.1) | n.d. | 4.16 (0.08) | 3.78 (0.89) |
| Acetic acid, g L−1 | n.d. | n.d. | 10.48 (0.3) | 0.43 (0.02) |
| Total polyphenols, % TS | 0.05 (0.02) | n.d. | 21.77 (0.2) | n.d. |
| Reducing sugars, g L−1 | n.d. | 50.35 (0.2) | trace | n.d. |
| Dark Fermentation Parameter 2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment 1 | Initial pH | Total VS Added | Reducing Sugars | Cumulated H2 | Max Rate1 | Max Rate2 | Day 7 pH | H2 MAX1 conc. | H2 MAX2 conc. | Final pH |
| g/Reactor | g/Reactor | mL H2 STP g−1 VS | mL H2 STP d−1 g−1 VS | mL H2 STP d−1 g−1 VS | % | % | ||||
| Liq-Adj | 8.7 | 1.9 | 1.5 | 199 b 3 | 56 a | 43 b | 4.6 a | 58 a | 48 a | 4.5 a |
| NaOH-Adj | 8.7 | 1.5 | 1.5 | 237 a | 45 b | 52 a | 4.4 a | 55 a | 50 a | 4.6 a |
| Not-Adj | 5.3 | 1.5 | 1.5 | 174 c | 55 a | 27 c | 4.5 a | 53 a | 49 a | 4.7 a |
| C | 8.7 | 1.5 | trace | 2 d | 1 c | 0 d | 7.2 b | 10 b | 0 b | 7.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasmara, C.; Cianchetta, S.; Marchetti, R.; Ceotto, E.; Galletti, S. Hydrogen Production from Enzymatic Hydrolysates of Alkali Pre-Treated Giant Reed (Arundo donax L.). Energies 2022, 15, 4876. https://doi.org/10.3390/en15134876
Vasmara C, Cianchetta S, Marchetti R, Ceotto E, Galletti S. Hydrogen Production from Enzymatic Hydrolysates of Alkali Pre-Treated Giant Reed (Arundo donax L.). Energies. 2022; 15(13):4876. https://doi.org/10.3390/en15134876
Chicago/Turabian StyleVasmara, Ciro, Stefano Cianchetta, Rosa Marchetti, Enrico Ceotto, and Stefania Galletti. 2022. "Hydrogen Production from Enzymatic Hydrolysates of Alkali Pre-Treated Giant Reed (Arundo donax L.)" Energies 15, no. 13: 4876. https://doi.org/10.3390/en15134876
APA StyleVasmara, C., Cianchetta, S., Marchetti, R., Ceotto, E., & Galletti, S. (2022). Hydrogen Production from Enzymatic Hydrolysates of Alkali Pre-Treated Giant Reed (Arundo donax L.). Energies, 15(13), 4876. https://doi.org/10.3390/en15134876






