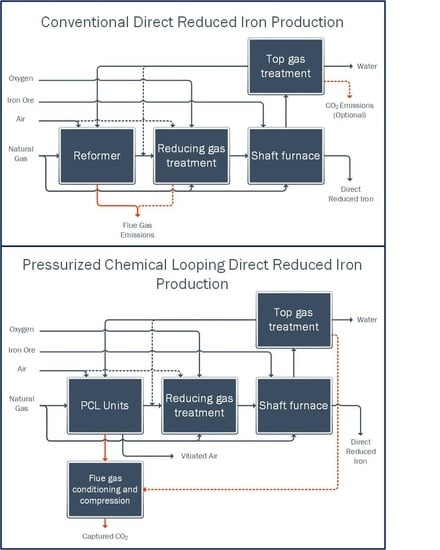
3. Results
3.1. Base Case Agreement to Literature Values
3.1.1. Base—M
A mass balance on the literature data provided in [
12] for the shaft furnace in the Contrecoeur facility showed that the reported flow of top gas exiting the shaft furnace was incorrect. To achieve a correct mass balance, the mass flow of top gas was increased by 7% relative to the literature data and the composition was slightly adjusted. This discrepancy is believed to be a result of errors in measurement of the high-temperature process gas stream. Note that this additional mass flow all ends up in the excess purge stream (stream 14) and thus has no impact on the idealized process performance. A comparison between the reducing gas composition generated by the model and the target literature value shows good agreement (
Table 4).
While no operating data for the balance of the plant is provided in [
12], the operating conditions of the reformer in Base—M can be compared to typical ranges that would be expected for a Midrex reformer, where dry reforming reactions dominate.
Table 5 shows that all model parameters fall within the expected range except for the steam to carbon ratio. In the model, this ratio is lower than the expected value, however, it falls within the same magnitude and results in dry reforming. It is not possible to increase the steam to carbon ratio in the model without exceeding the specified steam content for the reducing gas in
Table 4. Overall, the model provides a good representation of the Midrex reformer, and given that the same conditions are used in PCL-DRI—M, will not impact differential performance observations between Base—M and PCL-DRI—M.
3.1.2. Base—E
Verification of the mass balance around the shaft furnace did not show any major discrepancies for the data provided in Zulgiano et al. [
31], thus no adjustments to the model’s input top gas composition were required. The reducing gas composition calculated by the model for the Base—E case shows good agreement with the literature values, as shown in
Table 6.
Table 7 shows the reformer operating conditions for Base—E in comparison to a typical SMR. The reformed gas outlet temperature and the steam to carbon ratio in the feed are both within expected ranges. The steam to carbon ratio was set near the minimum of the range because, unlike for H
2 production, DRI requires a larger yield of CO to act as a reductant in the shaft furnace. SMRs are typically operated at high pressure to limit downstream compression requirements at the hydrogen production facility, however, the reactions are less thermodynamically favorable as the pressure increases [
33,
34]. Given the Energiron shaft furnace operates at 600–800 kPa [
12], there is no need to increase the reformer pressure beyond what is required as allowance for pressure drop across downstream processing units between the reformer and the shaft furnace.
3.2. Comparison of Process Performance
The PCL-DRI cases are equivalent or superior to the base cases in many aspects of DRI process performance as shown in
Table 8. First, comparing Base—M to PCL-DRI—M, the overall natural gas consumption of the two processes, which serves only to supplement the feed gas to the reformer tubes and provide direct contact heating of the reducing gas in the duct burner, is identical. This is because the chemistry and the conditions of the recycle gas loop are maintained constant between both cases. Likewise, the oxygen consumption is identical. The required fuel consumption (in the form of top gas) to the fuel reactor of the PCL unit compared to the reformer firebox is less in the PCL-DRI—M case due to the lower operating temperature of the PCL unit, thus requiring less sensible heating of the gases. Since heat losses in the recycled gas loop are not considered in these idealized cases, there is an excess top gas stream that is required neither as a fuel for heating, nor as a recycle feed to the reformer. For the purpose of these simulations, this excess gas is directed to a purge stream and it is excluded from emissions considerations or its impact on the thermal efficiency of the plant in this work. When factoring in practical operating considerations, this stream would not exist, because all of the top gas would be required to be combusted as fuel in the Midrex firebox to account for heat losses, with further supplementation of natural gas. To avoid introduction of additional uncertainty through the estimate of heat losses for the PCL-DRI and Base—E configurations, no heat losses in the recycle gas and reforming loop are considered for any of the cases. Thus, fuel consumption and CO
2 production rates represent the minimum possible for these configurations.
Comparing the fuel consumption for Base—E and PCL-DRI—E, again, there is a reduction in the fuel consumption for the PCL fuel reactor due to its lower operating temperature. In these configurations, all of the available top gas plus additional natural gas is required to fulfill the heating requirement of the PCL unit/firebox; thus, this translates directly to an 8% reduction in the battery limit natural gas requirement for the process.
The PCL-DRI cases have potential value-added by-products. Process water is produced at a rate of 2.6 times greater for the Midrex-type cases and 1.7 times greater for the Energiron-type cases. This water is recovered from the fuel reactor flue gas during heat recovery/cooling, compression, and drying. This additional water stream can be evaluated for use as boiler feed water, for cooling and scale removal in the hot rolling mill, or for other applications at the iron and steel facility [
37]. Additionally, there is production of a nitrogen stream from the air reactor, which may also be considered for cooling in the hot rolling mill to offset some of the water requirement.
CO2 capture does have additional costs, which become evident when examining the increased power import and cooling duty of the PCL-DRI cases relative to the base cases. The net power imports of the PCL-DRI processes are more than double the power requirements for their respective base cases, despite the offset of power requirement achieved through the use of an expansion turbine on the nitrogen stream produced from the air reactor. Power consumption of PCL-DRI—M is greater than PCL-DRI—E due to the greater compression requirement for the top gas used as fuel in the fuel reactor. The Midrex top gas is near atmospheric pressure when it exits the shaft furnace, whereas the Energiron top gas is already near the operating pressure of the PCL units.
The two major draws for power in the PCL-DRI process are (1) the air compressors supplying air to the air reactor and (2) the CO2 compression train that brings the captured CO2 to pipeline pressure. To understand the impact of these two main power consumers, the power consumption for PCL-DRI is presented both including and excluding CO2 conditioning and compression. A partial capture case (Base—E + comp) also shows the impact of adding a smaller CO2 compression unit to the CO2 stream that is separated from the CO2 absorber that is inherently a part of Energiron-type DRI processes. CO2 compression represents 23 and 34% of the total power import for PCL-DRI—M and PCL-DRI—E respectively, while it adds 46% for partial CO2 capture from Base—E. With the push toward electrification and toward reducing the carbon intensity of the power grid, the increased power import of the PCL-DRI processes does not have to be considered as a negative impact; it is merely a shift in the type of energy that the process consumes. A detailed techno-economic assessment (TEA) is required to assess how this shift may affect operating costs of the DRI facility, as well as how the cost of carbon capture compares to competing CO2 capture technologies. Further optimization can be performed to minimize power import, if desired.
Considering the cooling duty of the PCL-DRI processes relative to their respective base cases, the cooling duty excluding CO2 conditioning and compression is quite similar. The addition of conditioning in a DCC, drying, and intercooling between the CO2 compressor stages results in a 123% increase in the total process cooling duty for PCL-DRI—M and a 5% increase for PCL-DRI—E. The relatively small change for PCL-DRI—E results from the much higher cooling duty already required for Energiron-type processes, both in the condenser of the CO2 absorber and in the cooling of the syngas exiting the reformer for water knock-out, prior to re-heating the reducing gas for entry to the shaft furnace. Even after heat recovery from the flue gas, the stream is still relatively hot (528 °C for PCL-DRI—M and 130 °C for PCL-DRI—E) and contains a significant amount of waste heat that must be cooled before compression can occur.
Chemical looping has often been described as a carbon capture technology with little to no energy penalty [
38]. This is observed in the comparison of thermal efficiencies of PCL-DRI—M to Base—M, in which there is no loss in efficiency with integration of PCL. For PCL-DRI—E and Base—E + Comp, there is a small drop in efficiency that can be attributed to the increase in power and cooling duty for the CO
2 compression units, though this effect is much smaller than would be the case if amine capture were applied to all flue gas streams.
4. Discussion
The Base—M and Base—E cases were developed to have a reasonable point of comparison for PCL-DRI to current industrial standards; however, each of these models was built based on a single operating point and may not be representative of all Midrex or Energiron-III processes. If more industrial data were available at alternative operating points, or if a detailed shaft furnace model could be developed and integrated with the current models, future work could focus on broadening the understanding of PCL-DRI process performance under a range of common operating conditions. To evaluate the accuracy of the developed models, they were compared to emissions data in literature. The Midrex process typically produces 0.65 t CO
2/t DRI, while Energiron III produces 0.56 t CO
2/t DRI [
7]. In this work, the idealized Base—M configuration emits 0.39 t CO
2/t DRI, however, if one considers the combustion of the excess top gas as fuel in the reformer to make up for heat losses, as would be done in a non-idealized plant, this value rises to 0.52 t CO
2/t DRI. The Base—E configuration likewise undershoots the expected value, at 0.45 t CO
2/t DRI, but again, the difference would likely be made up if heat losses were considered.
The performance of the PCL-DRI processes are competitive with traditional DRI processes, with the added benefit of full CO2 capture. There was also no optimization of the top gas recycle loop for PCL-DRI; it is possible that superior performance could be achieved if the overall process pressure and reformer feed conditions could be modified to better suit PCL. For example, the shaft furnace in PCL-DRI—E could be operated such that the top gas is at a pressure sufficient to supply the fuel reactor directly, without requiring an additional fuel gas compressor. For the PCL-DRI—M configuration, options could be explored to reduce the natural gas feed to the reformer through the use of the additional top gas that would be available due to the reduced fuel requirement in the fuel reactor. Optimization activities such as these would require a detailed kinetic model of the shaft furnace in order to predict performance with changing reducing gas compositions and pressures.
Despite its many advantages, the challenges and drawbacks associated with PCL-DRI must also be considered. As has been stated in
Section 3.2, the duty of the main air compressor(s) significantly increase the power consumption of the process. Due to their size, previous work has shown that the rotating equipment represents the largest portion of the capital costs of the plant, equal to or more than the cost of all other equipment combined [
25]. Scale-up may also be a challenge, given the very high duty and solids circulation rates that would be required to meet the needs of a typical iron and steel facility. In this work, heat recovery from the air reactor approaches 200 MW, depending on the case, and the required solids circulation rate is up to 1370 t/h (oxidized). It is anticipated that two or more PCL units would be required to meet this demand, allowing scale-up to take a modular approach. Choice of reactor type will likely be a key driver in the economics and feasibility of such a process scale. Future work will examine reactor sizing and economics for a novel design with no external solids transfer between the air and fuel reactors.
PCL-DRI is not the only way to eliminate CO
2 emissions from a DRI facility. Post combustion capture units, such as amine absorbers, can be employed, or alternative feedstocks such as low-carbon hydrogen or bio-methane can be used [
39,
40,
41,
42]. PCL has already been shown to be more efficient than conventional carbon capture technologies for other applications [
17,
24,
43]. Midrex and Energiron, as well as several other green steelmaking projects, have configurations under development that eliminate the reformer from the DRI process and allow direct use of H
2 as the reducing gas in the shaft furnace (purchased over-the-fence or produced on site); however, this alters the heat balance in the shaft furnace and requires additional heat input to make up the difference [
6,
39]. There is the additional caveat of the alteration of the composition of the DRI product when only hydrogen is used as the reductant. To aid in the efficiency of the downstream EAF, it is preferred to have 1.5–3 wt % carbon in the DRI. This carbon is then combusted with oxygen in the EAF to help melt the iron; thus, those CO
2 emissions would have to be managed downstream [
6]. In traditional DRI processes, the carbon in the DRI is incorporated in the shaft furnace via carbon deposition from the thermal decomposition of methane and the inverse Boudouard reaction [
8]. Switching to DRI-H
2 means that carbon cannot be deposited onto the DRI, and will increase the energy consumption of the EAF. PCL-DRI does not have this drawback, and will allow the EAF to operate without modification or loss of efficiency. Furthermore, PLC-DRI does not depend on the existence of a local low-carbon hydrogen producer or hydrogen distribution network, allowing net-zero DRI to be produced before the infrastructure for a hydrogen economy becomes available, as well as in remote regions where low-carbon hydrogen might be inaccessible.
This work considers the use of ilmenite ore as the oxygen carrier for PCL. This could be an attractive option for steelmakers, as there would be the potential for iron and titanium recovery from the spent oxygen carrier, thus valorizing an otherwise waste-product at its point of use. Iron ore can also be used directly as an oxygen carrier [
23], which would simplify the supply chain for the iron and steel facility, since oxygen carrier makeup could be supplied from the iron ore already brought in to feed the shaft furnace. Many other oxygen carriers bear consideration, both natural and synthetic, and typically contain some combination of Cu, Mn, Fe, Co, and Ni species [
24,
44,
45]. The choice of oxygen carrier will impact the heat balance of the PCL units as well as the flue gas purity, based on the reactivity of the oxygen carrier with the fuel. In the current work with ilmenite ore, the fuel reactor is net adiabatic (incoming sensible heat and exothermic reactions with CO and H
2 counterbalance the endothermic reactions with CH
4); however, with some oxygen carriers, heat is released in both the air and fuel reactors. This would alter the location of some of the heat recovery units in the PCL units, though at this stage of the analysis, it would not have a significant impact on the overall process performance. Ilmenite ore has a relatively low reactivity with CH
4 compared to CO and H
2 [
22,
46], and for this reason, the flue gas is expected to contain a fraction of unconverted methane (in this work, we assume 98.5% fuel conversion). Other oxygen carriers may have differing reactivities with the fuel, differing selectivities for products (resulting in differing yields of CO
2) or have oxygen uncoupling properties, all of which may impact flue gas treatment requirements to achieve purity specifications for transportation and storage.