Differences in Macromolecular Structure Evolution during the Pyrolysis of Vitrinite and Inertinite Based on In Situ FTIR and XRD Measurements
Abstract
:1. Introduction
2. Samples Preparation and Methods
2.1. Samples Preparation
2.2. Experimental Process
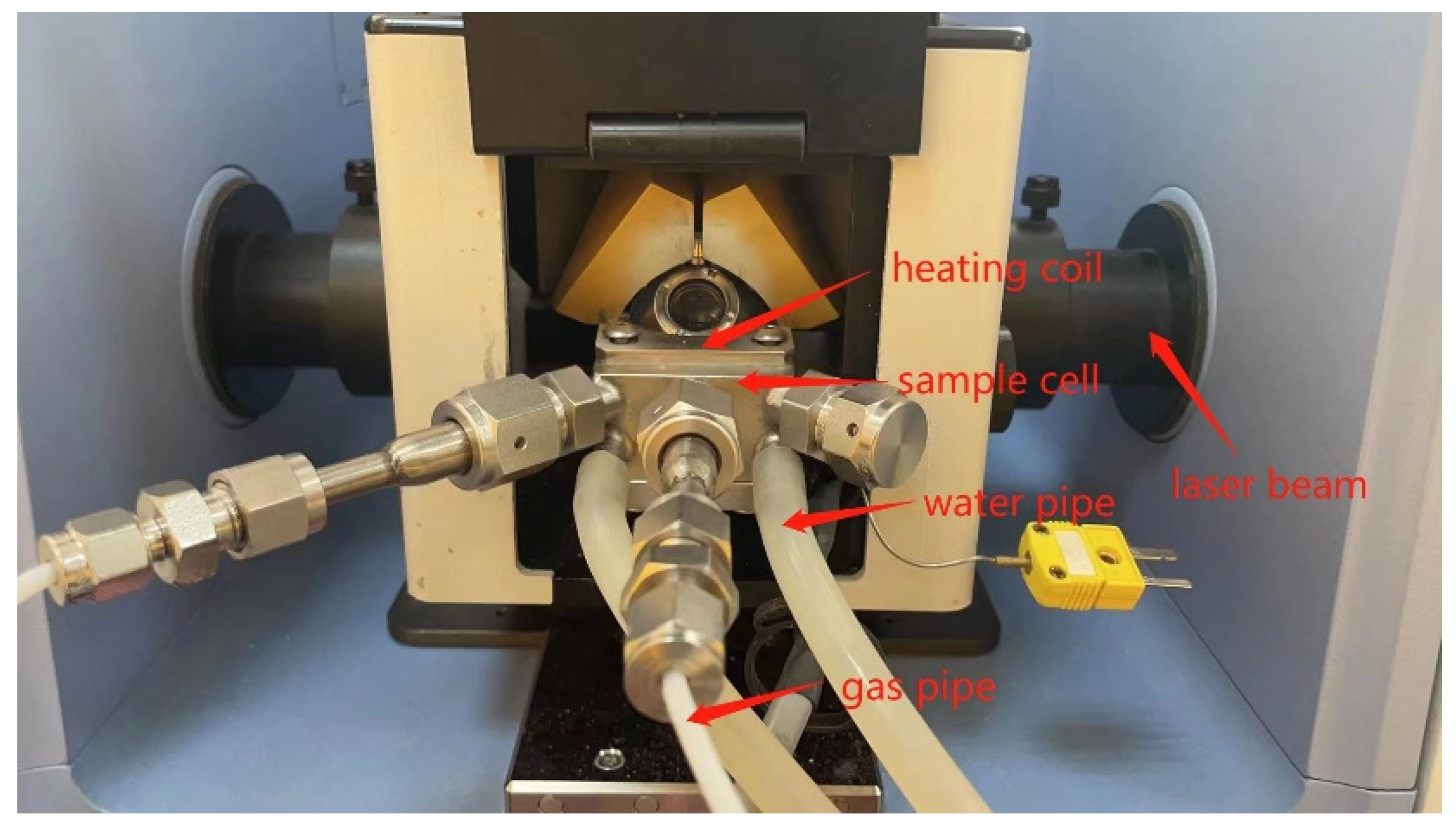
2.2.1. In Situ FTIR Spectroscopy
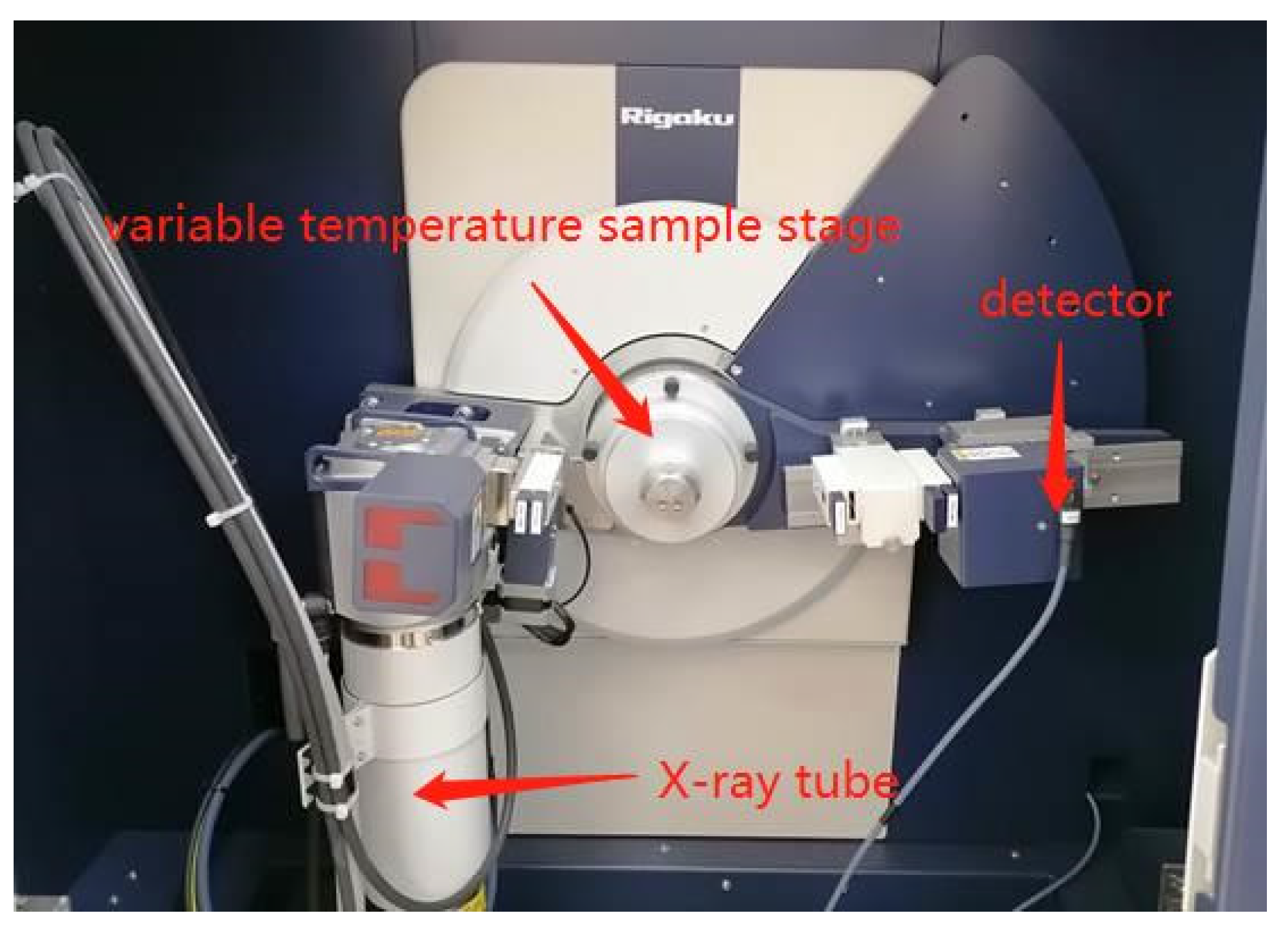
2.2.2. In Situ XRD
3. Results and Analysis
3.1. Analysis of the In Situ FTIR Results
3.1.1. In Situ FTIR Spectrum Characteristics
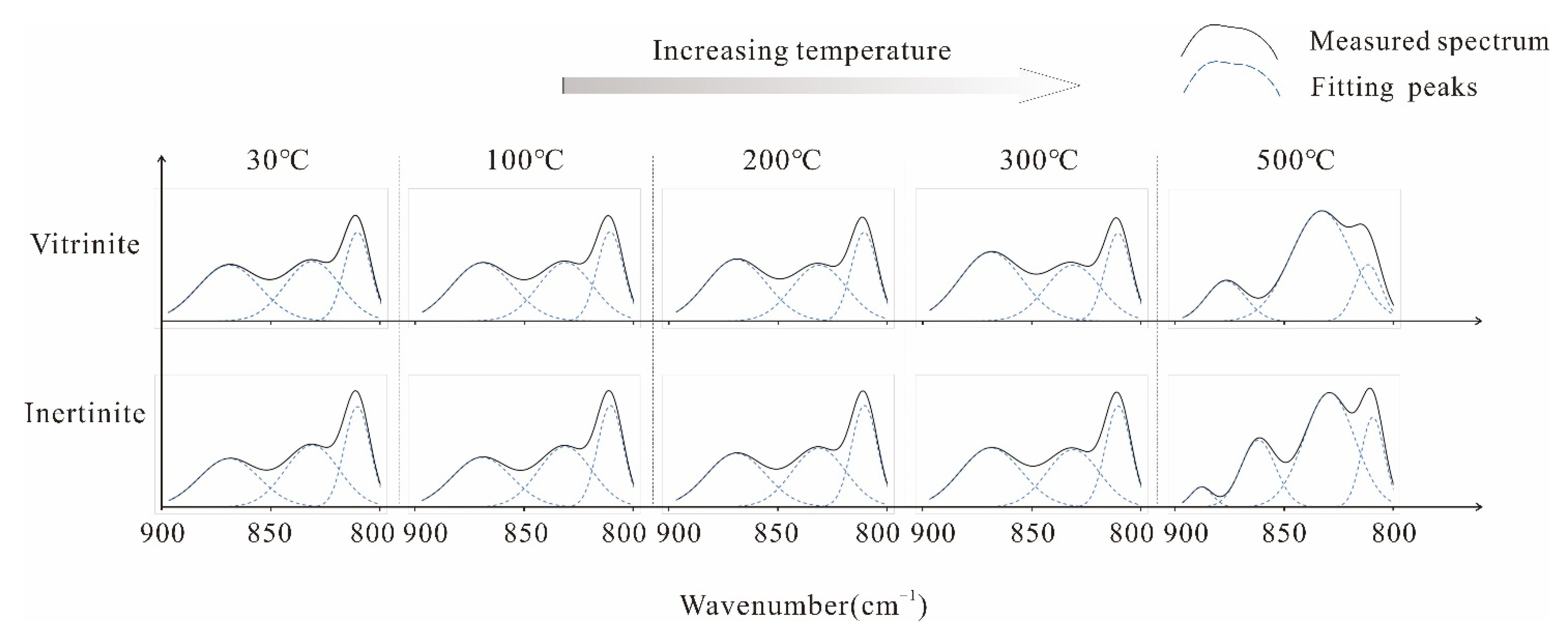
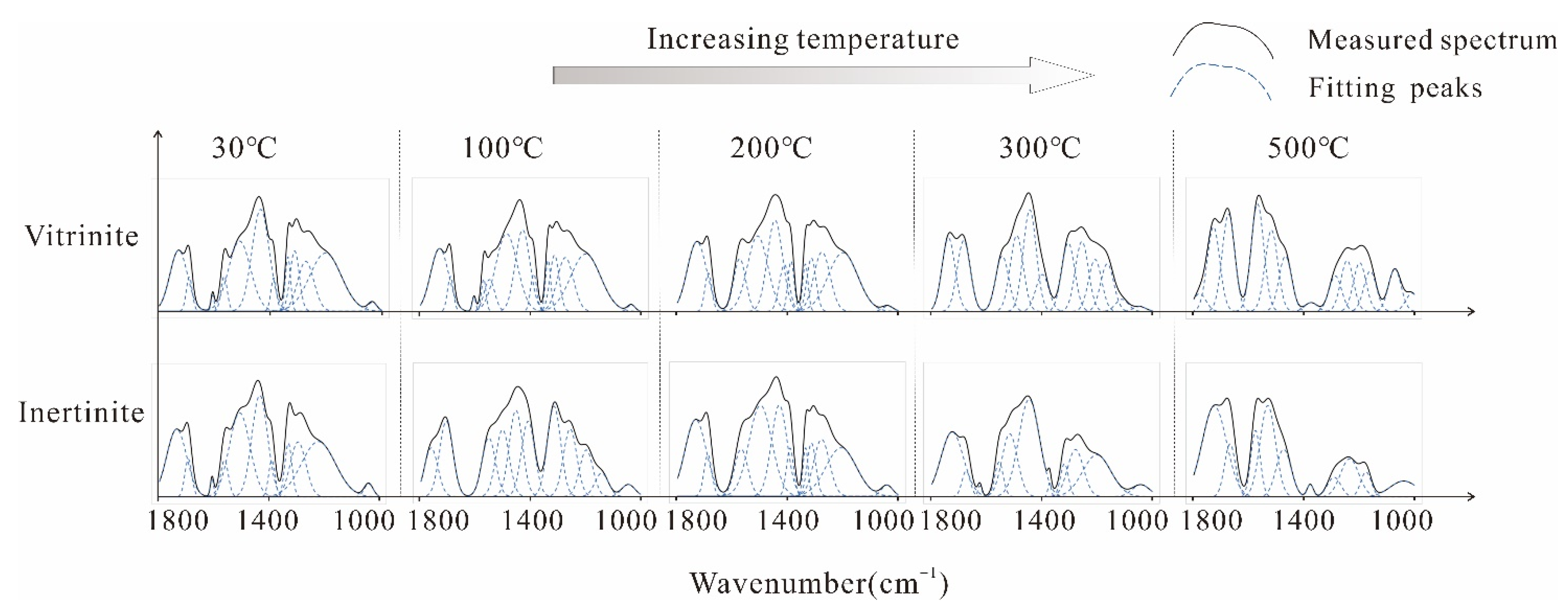
Aromatic Structure Absorption Bands
Oxygen-Containing Functional Groups Absorption Band
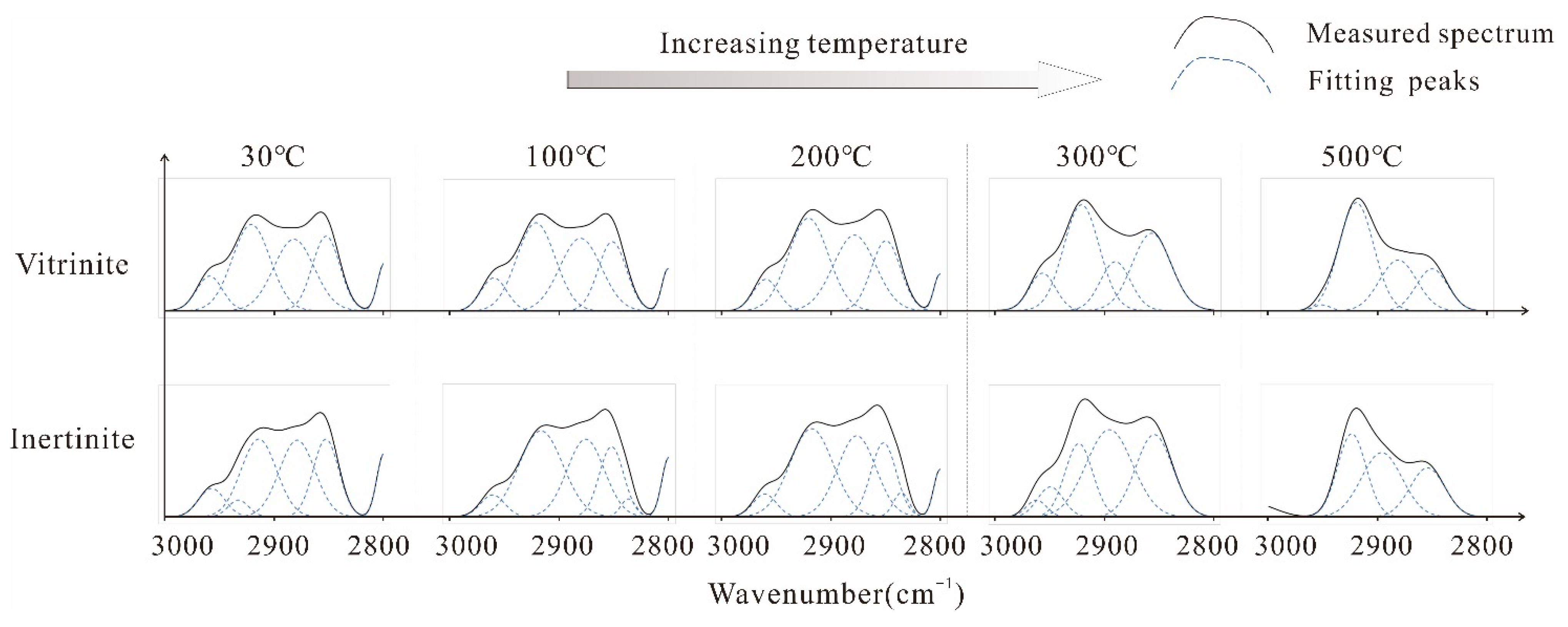
Aliphatic Structure Absorption Bands
Hydroxyl Absorption Band
3.1.2. In Situ FTIR Structural Parameter Characteristics
Aromatic Structure
Aliphatic Structure
3.2. Analysis of In Situ XRD Results
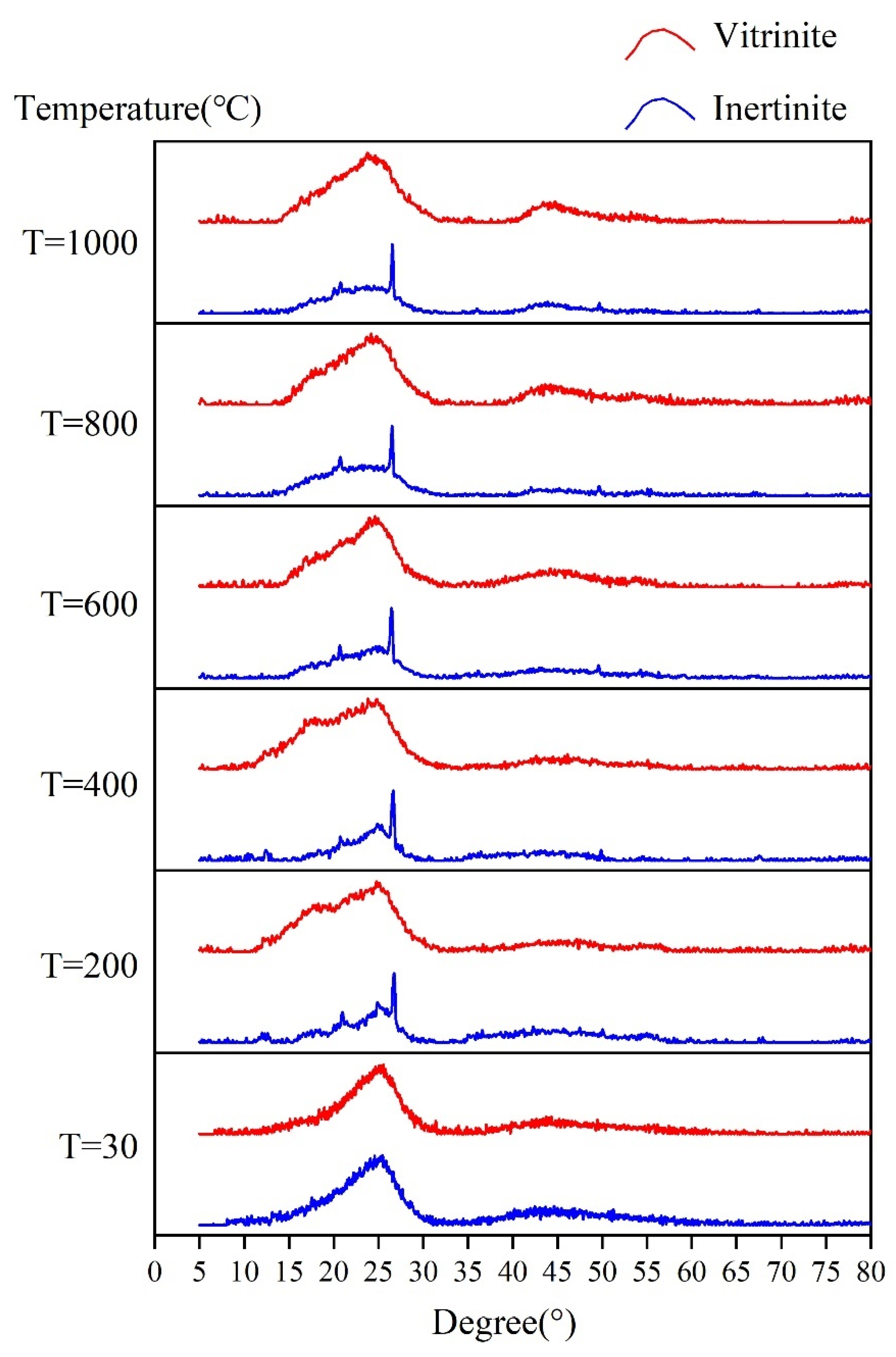
3.2.1. In Situ XRD Pattern Characteristics
3.2.2. In Situ XRD Structural Parameters
4. Discussion
- (1)
- During pyrolysis, the macromolecular structures of vitrinite and inertinite change with increasing temperature. The DOC of vitrinite and inertinite increases, and the plane extensibility and the Lc increase continuously, indicating that both the vitrinite and inertinite of low–middle rank coals have an evolution trend of increasing degree of aromatization in the early stages of pyrolysis. The increase in aromatization is reflected mainly in the continuous expansion of the aromatic structural units. The differences in the structural evolution of vitrinite and inertinite are reflected mainly by the continuous decrease in the d002 peak of vitrinite with increasing temperature, in which the reduced value is 0.013 nm, but there is no obvious change in inertinite. Second, the I, DOC, La, and Lc of inertinite are always higher than those of vitrinite, indicating that the degree of inertinite aromatization is always higher than that of vitrinite.
- (2)
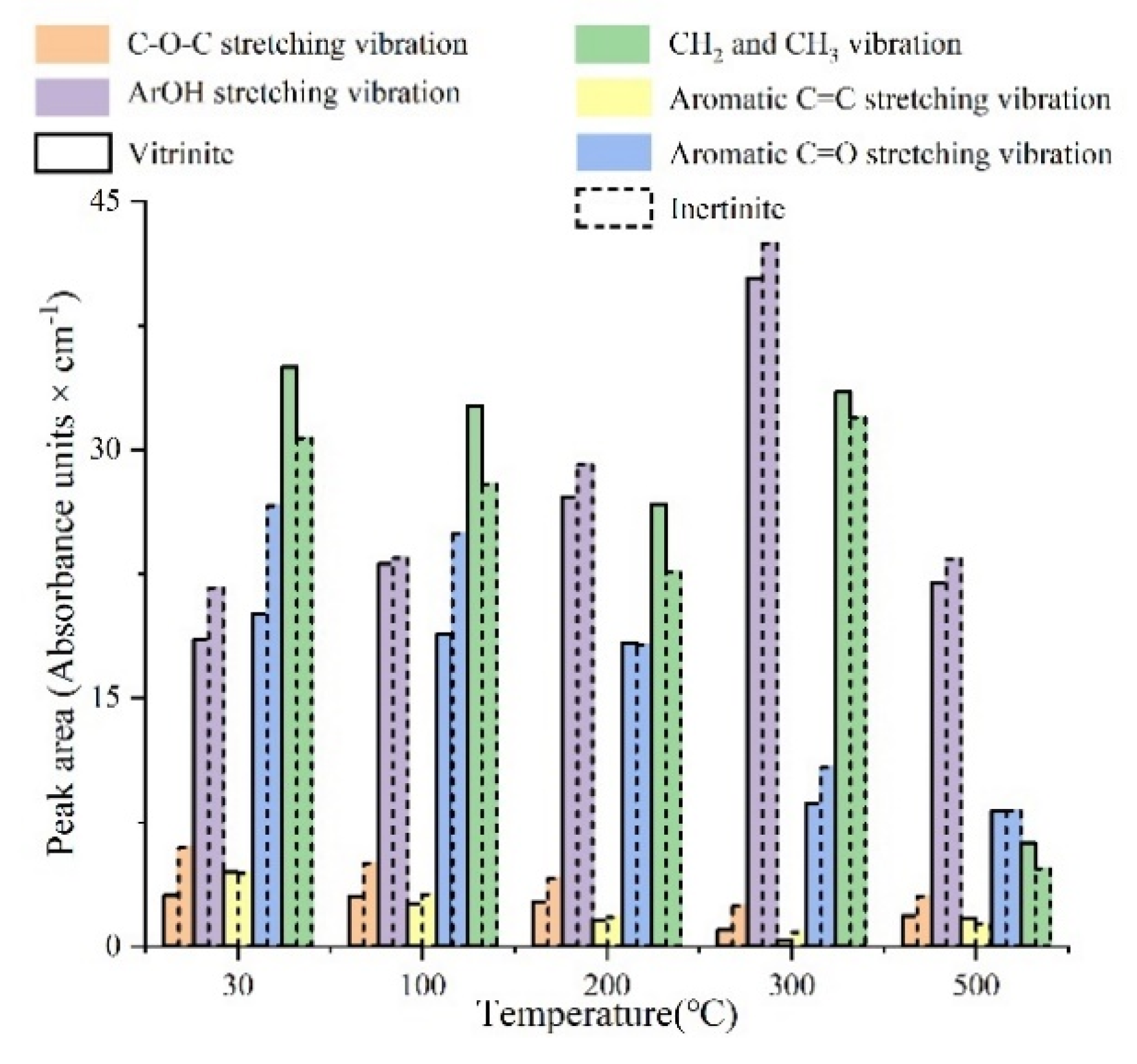
- The pyrolysis process of vitrinite and inertinite can be divided into three stages. Between 30 °C and 200 °C, the content of aromatic groups in vitrinite and inertinite shows no obvious change, while that of aliphatic groups increases slightly. The total amount of oxygen-containing functional groups decreases gradually. The content of C–O–C groups, aromatic C=O, and aromatic ring C=C has a small decrease in a relatively stable situation. The content of aliphatic CH2 and CH3 decreases significantly, and that of Ar–OH groups increases significantly (Figure 17). The content of O–H–π-H bonds increases greatly, and that of free O–H groups decreases greatly. There is no significant change in the I and DOC (Figure 13), which suggests that aliphatic and aromatic structures are enriched slightly at this stage. The enlargement of the aromatic structural system is not obvious in this stage, and the detachment of oxygen-containing functional groups and the enrichment of aliphatic hydrocarbons are the dominating chemical reactions [45].
5. Conclusions
- (1)
- During the pyrolysis process, both the inertinite and vitrinite show an increasing degree of aromatization. With increasing pyrolysis temperature, aromaticity (I), polycondensation degree of aromatic rings (DOC), average lateral sizes (La) of BSU, and stacking heights (Lc) of BSU in vitrinite and inertinite increase, but the aromatization level of inertinite has always been higher than that of vitrinite.
- (2)
- In the temperature range of 30–500 °C for the in situ FTIR spectroscopy, the macromolecular structure evolution of vitrinite and inertinite is divided into three stages: 30–200 °C, 200–300 °C, and 300–500 °C. At 30–200 °C, the detachment of oxygen-containing functional groups and the enrichment of aliphatic hydrocarbons occurs. The 200–300 °C stage is filled mainly by the synergistic effects of aliphatic and aromatic groups. The stage of 300–500 °C is dominated by the aromatization and condensation of macromolecules. The substituents of the aromatic system gradually detach, leading to an increase in I and DOC.
- (3)
- In the temperature range of 30–1000 °C by in situ XRD, the difference in macromolecular structure evolution between vitrinite and inertinite is manifested mainly by the arrangement of aromatic layers, which tends to be more and more ordered in vitrinite regular during the pyrolysis process, while there is no significant change in inertinite. However, the aromatic layers of inertinite are always more compact than that of vitrinite. In addition, the aliphatic side chains of inertinite are more stable than that of vitrinite during pyrolysis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, D.; Wei, Y.; Wang, A.; Wang, L.; Liu, Z.; Qin, R.; Shu, Z.; Chen, G. The evolution difference of macromolecular structures and its dynamic mechanism of coal macerals: Research status and prospect. Coal Geol. Explor. 2021, 49, 12–20, (In Chinese with English Abstract). [Google Scholar]
- Louw, E.B.; Mitchell, G.D.; Wang, J.; Winans, R.; Mathews, J.P. Constitution of drop-tube-generated coal chars from vitrinite-and inertinite-rich South African coals. Energy Fuels 2016, 30, 112–120. [Google Scholar] [CrossRef]
- Gao, M.; Li, X.; Ren, C.; Wang, Z.; Pan, Y.; Guo, L. Construction of a multicomponent molecular model of Fugu coal for ReaxFF-MD pyrolysis simulation. Energy Fuels 2019, 33, 2848–2858. [Google Scholar] [CrossRef]
- Gao, M.; Li, X.; Guo, L. Pyrolysis simulations of Fugu coal by large-scale ReaxFF molecular dynamics. Fuel Process. Technol. 2018, 178, 197–205. [Google Scholar] [CrossRef]
- Wang, A.; Wei, Y.; Cao, D.; Ding, L.; Zhao, M. Effect of different functional groups on CH4 adsorption heat and surface free energy of vitrinite during coalification. Appl. Surf. Sci. 2022, 597, 153748. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Pan, J.; Wang, Z.; Niu, Q.; Du, M. Research on molecular structure characteristics of vitrinite and inertinite from bituminous coal with FTIR, micro-Raman, and XRD spectroscopy. Energy Fuels 2021, 35, 1322–1335. [Google Scholar] [CrossRef]
- Kidena, K.; Katsuyama, M.; Murata, S.; Nomura, M.; Chikada, T. Study on plasticity of maceral concentrates in terms of their structural features. Energy Fuels 2002, 16, 1231–1238. [Google Scholar] [CrossRef]
- Van Niekerk, D.; Mathews, J. Molecular representations of Permian-aged vitrinite-rich and inertinite-rich South African coals. Fuel 2010, 89, 73–82. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Taulbee, D.N.; Andrésen, J.M.; Hower, J.C.; Snape, C.E. Quantitative 13C NMR study of structural variations within the vitrinite and inertinite maceral groups for a semifusinite-rich bituminous coal. Fuel 1998, 77, 805–813. [Google Scholar] [CrossRef]
- Van Niekerk, D.; Pugmire, R.; Solum, M.S.; Painter, P.C.; Mathews, J.P. Structural characterization of vitrinite-rich and inertinite-rich Permian-aged South African bituminous coals. Int. J. Coal Geol. 2008, 76, 290–300. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Cao, D.; Wei, Y.; Ding, L.; Zhao, M. Comparison of nanopore structure evolution in vitrinite and inertinite of tectonically deformed coals: A case study in the Wutongzhuang coal mine of Hebei province, North China. Front. Earth Sci. 2022, 10, 93. [Google Scholar] [CrossRef]
- Wang, A.; Cao, D.; Wei, Y.; Liu, Z. Macromolecular structure controlling micro mechanical properties of vitrinite and inertinite in tectonically deformed coals—A case study in Fengfeng coal mine of Taihangshan fault zone (North China). Energies 2020, 13, 6618. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, S.; Longhurst, P.; Yang, W.; Zheng, S. Molecular structure characterization of bituminous coal in Northern China via XRD, Raman and FTIR spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 119724. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Lei, Z.; Li, Z.; Wang, Z.; Ren, S.; Kang, S.; Wang, X.; Shui, H. Molecular structure characterization of low-medium rank coals via XRD, solid state 13C NMR and FTIR spectroscopy. Fuel 2020, 268, 117038. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, W.; Cheng, Y.; Liu, Z.; Zhang, Q.; Zhao, K. Molecular structure characterization of middle-high rank coal via XRD, Raman and FTIR spectroscopy: Implications for coalification. Fuel 2019, 239, 559–572. [Google Scholar] [CrossRef]
- Zhao, X.; Zong, Z.; Cao, J.; Ma, Y.; Han, L.; Liu, G.; Zhao, W.; Wei, X. Difference in chemical composition of carbon disulfide-extractable fraction between vitrinite and inertinite from Shenfu-Dongsheng and Pingshuo coals. Fuel 2008, 87, 565–575. [Google Scholar] [CrossRef]
- Wang, H.; Shi, X.; Tian, J.; Pan, J.; Yao, L.; Feng, X. Macromolecular Structure Changes of Tectonically Deformed Coal: Evidence from Coal Pyrolysis, 13C NMR, and XRD Experiments. Energy Fuels 2021, 35, 8711–8722. [Google Scholar] [CrossRef]
- Yu, X.; Yu, C.; Jiang, Q.; Ran, Q.; Liu, J.; Yang, B. In-situ X-ray Diffraction Analysis on the Role of Hardening Accelerator in Early Hydration of Cement. Mater. Rep. 2020, 34, 2058–2062, (In Chinese with English Abstract). [Google Scholar]
- Tay, F.H.; Kazarian, S. Study of petroleum heat-exchanger deposits with ATR-FTIR spectroscopic imaging. Energy Fuels 2009, 23, 4059–4067. [Google Scholar] [CrossRef]
- Wang, A.; Wei, Y.; Yuan, Y.; Li, C.; Li, Y.; Cao, D. Coalbed methane reservoirs’ pore-structure characterization of different macrolithotypes in the southern Junggar Basin of Northwest China. Mar. Pet. Geol. 2017, 86, 675–688. [Google Scholar] [CrossRef]
- De Souza, A.V.A.; da Silva, J.F.C. Biodiesel synthesis evaluated by using real-time ATR-FTIR. Org. Process. Res. Dev. 2013, 17, 127–132. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Morozov, E.V.; Subramani, V.; Martyanov, O.N.; Kazarian, S.G. Chemical visualization of asphaltenes aggregation processes studied in situ with ATR-FTIR spectroscopic imaging and NMR imaging. J. Phys. Chem. C 2015, 119, 2646–2660. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Wei, Y.; Yang, C.; Nie, J.; Cao, D. Gas migration for terrestrial gas hydrates in the Juhugeng mining area of Muli basin, Qilian Mountains, Northwest China. Energy Explor. Exploit. 2020, 38, 989–1013. [Google Scholar] [CrossRef]
- Wang, A.; Cao, D.; Wei, Y.; Nie, J.; Qin, R. Comparison of nanopore evolution in vitrinite and inertinite in coalbed methane reservoirs during coalification. J. Nat. Gas Sci. Eng. 2020, 78, 103289. [Google Scholar] [CrossRef]
- Wu, D.; Liu, G.; Sun, R.; Fan, X. Investigation of structural characteristics of thermally metamorphosed coal by FTIR spectroscopy and X-ray diffraction. Energy Fuels 2013, 27, 5823–5830. [Google Scholar]
- Guo, Y.; Bustin, R. Micro-FTIR spectroscopy of liptinite macerals in coal. Int. J. Coal Geol. 1998, 36, 259–275. [Google Scholar] [CrossRef]
- Mastalerz, M.; Bustin, R. Application of reflectance micro-Fourier transform infrared spectrometry in studying coal macerals: Comparison with other Fourier transform infrared techniques. Fuel 1995, 74, 536–542. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, H.; Dong, Y.; Yang, C. Nanoscale Microscopic Features and Evolution Sequence of Coal-Based Graphite. J. Nanosci. Nanotechnol. 2017, 17, 6276–6283. [Google Scholar] [CrossRef]
- Bragg, W.H.; Bragg, W.L. X-rays and Crystal Structure; George Bell and Sons, Limited: London, UK, 1915. [Google Scholar]
- Bragg, W.H. IX Bakerian Lecture—X-rays and crystal structure. In Philosophical Transactions of the Royal Society of London. Series A, Containing Papers of a Mathematical or Physical Character; Royal Society: London, UK, 1915; Volume 215, pp. 253–274. [Google Scholar]
- Debye, P.; Scherrer, P. Interferenzen an regellos orientierten Teilchen im Röntgenlicht. I. Nachr. Ges. Wiss. Göttingen Math. Phys. Kl. 1916, 1916, 1–15. [Google Scholar]
- Lu, L.; Sahajwalla, V.; Kong, C.; Harris, D. Quantitative X-ray diffraction analysis and its application to various coals. Carbon 2001, 39, 1821–1833. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, H.; Wei, H.; Wang, W.; Wang, H.; Li, K.; Lu, X.; Tan, B. Investigation into the thermal behavior and FTIR micro-characteristics of re-oxidation coal. Combust Flame 2020, 216, 354–368. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, Y.; Ning, S.; Jia, X.; Qin, R.; Cao, D. The differences of element geochemical characteristics of the main coal seams in the Ningdong coalfield, Ordos Basin. J. Geochem. Explor. 2019, 202, 77–91. [Google Scholar]
- Chen, Y.; Mastalerz, M.; Schimmelmann, A. Characterization of chemical functional groups in macerals across different coal ranks via micro-FTIR spectroscopy. Int. J. Coal Geol. 2012, 104, 22–33. [Google Scholar] [CrossRef]
- Qin, R.; Wang, A.; Cao, D.; Wei, Y.; Ding, L.; Li, J. Effect of peat mire evolution on pore structure characteristics in thick coal seam: Examples from Xishanyao Formation (Middle Jurassic), Yili Basin, China. Energy Explor. Exploit. 2020, 38, 1484–1514. [Google Scholar] [CrossRef]
- Ibarra, J.; Munoz, E.; Moliner, R. FTIR study of the evolution of coal structure during the coalification process. Org. Geochem. 1996, 24, 725–735. [Google Scholar] [CrossRef]
- Manoj, B.; Kunjomana, A.; Chandrasekharan, K. Chemical leaching of low rank coal and its characterization using SEM/EDAX and FTIR. J. Miner. Mater. Charact. Eng. 2009, 8, 821–832. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, T.; Deng, J.; Shu, C.-M.; Zeng, Q.; Guo, T.; Zhang, Y. Microcharacteristic analysis of CH4 emissions under different conditions during coal spontaneous combustion with high-temperature oxidation and in situ FTIR. Energy 2020, 209, 118494. [Google Scholar] [CrossRef]
- Saikia, B.K.; Boruah, R.K.; Gogoi, P. FT-IR and XRD analysis of coal from Makum coalfield of Assam. J. Earth Syst. Sci. 2007, 116, 575–579. [Google Scholar] [CrossRef]
- Chen, C.; Gao, J.; Yan, Y. Observation of the type of hydrogen bonds in coal by FTIR. Energy Fuels 1998, 12, 446–449. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, H.; Hu, G.; Zhang, W. Fine characterization of the macromolecular structure of huainan coal using XRD, FTIR, 13C-CP/MAS NMR, SEM, and AFM techniques. Molecules 2020, 25, 2661. [Google Scholar] [CrossRef]
- Qu, X.; Wang, J. X-ray analysis of coal. Coal Geol. Explor. 1980, 2, 33–40, (In Chinese with English Abstract). [Google Scholar]
- Zhang, D.; Xian, X. X-ray analysis of coal structure. J. Xi’an Inst. Min. Technol. 1990, 3, 42–49, (In Chinese with English Abstract). [Google Scholar]
- Tissot, B.P.; du Petrole, E.N.S.; Welte, D.H. Petroleum Formation and Occurrence. A New Approach to Oil and Gas Exploration. [Book in German]; Springer: Berlin/Heidelberg, Germany, 1978. [Google Scholar]
- Wang, S.-H.; Griffiths, P.R. Resolution enhancement of diffuse reflectance ir spectra of coals by Fourier self-deconvolution: 1. CH stretching and bending modes. Fuel 1985, 64, 229–236. [Google Scholar] [CrossRef]
- Morga, R. Chemical structure of semifusinite and fusinite of steam and coking coal from the Upper Silesian Coal Basin (Poland) and its changes during heating as inferred from micro-FTIR analysis. Int. J. Coal Geol. 2010, 84, 1–15. [Google Scholar] [CrossRef]










| No. | Density Interval (g/cm3) | Ro,max (%) | Macerals (%) | Notes | ||
|---|---|---|---|---|---|---|
| Vitrinite | Inertinite | Liptinite | ||||
| 1 | 1.3–1.35 | 0.698 | 90 | 8 | 2 | Vitrinite |
| 2 | 1.35–1.4 | 1.526 | 5 | 95 | 0 | Inertinite |
| Wavenumber Range | Absorption Peak Assignment |
|---|---|
| 3600–3700 | free OH bonds |
| 3500 | OH–π–H bonds |
| 3350–3400 | self-associated OH bonds |
| 3300 | OH–ether O hydrogen bonds |
| 3180–3240 | cyclic OH groups |
| 3200–3000 | the C–H stretching vibration of the aromatic nucleus |
| 2940–3000 | aliphatic CH3 asymmetric stretching vibration |
| 2940–2900 | aliphatic CH2 asymmetric stretching vibration |
| 2863 | aliphatic CH3 symmetric stretching vibration |
| 2848 | aliphatic CH2 symmetric stretching vibration |
| 1700 | the stretching vibration of aromatic C=O |
| 1650−1520 | the stretching vibration of the aromatic ring C=C |
| 1440−1360 | aliphatic CH3, and CH2 deformation vibration |
| 1340–1200 | Ar–OH |
| 1100 | aryl ether |
| 1039 | alkyl ether |
| 868 | aromatic nucleus (CH), one adjacent H deformation |
| 810 | aromatic nucleus (CH), three adjacent H deformation |
| Maceral Category | Temperature (°C) | 2θ002 (°) | d002 (nm) | FWHM | Lc (nm) | 2θ100 (°) | La (nm) |
|---|---|---|---|---|---|---|---|
| Inertinite | 30 | 25.579 | 0.34796 | 6.841 | 1.191 | 43.104 | 1.093 |
| 200 | 26.052 | 0.34175 | 6.596 | 1.236 | 42.998 | 1.074 | |
| 400 | 25.594 | 0.34776 | 5.804 | 1.404 | 43.401 | 1.595 | |
| 600 | 25.354 | 0.35099 | 5.342 | 1.524 | 44.299 | 1.738 | |
| 800 | 25.573 | 0.34804 | 4.717 | 1.727 | 42.848 | 1.912 | |
| 1000 | 25.491 | 0.34915 | 3.552 | 2.293 | 42.955 | 2.720 | |
| Vitrinite | 30 | 24.320 | 0.36567 | 8.014 | 1.014 | 42.959 | 1.094 |
| 200 | 24.805 | 0.35864 | 6.204 | 1.311 | 44.388 | 1.301 | |
| 400 | 24.919 | 0.35703 | 5.233 | 1.555 | 44.251 | 1.350 | |
| 600 | 24.983 | 0.35613 | 5.619 | 1.448 | 43.511 | 1.503 | |
| 800 | 25.104 | 0.35444 | 5.138 | 1.584 | 42.764 | 1.742 | |
| 1000 | 25.229 | 0.35271 | 4.338 | 1.877 | 44.968 | 2.757 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Wang, A.; Cao, D.; Wei, Y.; Ding, L. Differences in Macromolecular Structure Evolution during the Pyrolysis of Vitrinite and Inertinite Based on In Situ FTIR and XRD Measurements. Energies 2022, 15, 5334. https://doi.org/10.3390/en15155334
Zhao M, Wang A, Cao D, Wei Y, Ding L. Differences in Macromolecular Structure Evolution during the Pyrolysis of Vitrinite and Inertinite Based on In Situ FTIR and XRD Measurements. Energies. 2022; 15(15):5334. https://doi.org/10.3390/en15155334
Chicago/Turabian StyleZhao, Meng, Anmin Wang, Daiyong Cao, Yingchun Wei, and Liqi Ding. 2022. "Differences in Macromolecular Structure Evolution during the Pyrolysis of Vitrinite and Inertinite Based on In Situ FTIR and XRD Measurements" Energies 15, no. 15: 5334. https://doi.org/10.3390/en15155334






