About Hydrophobicity of Lignin: A Review of Selected Chemical Methods for Lignin Valorisation in Biopolymer Production
Abstract
1. Introduction
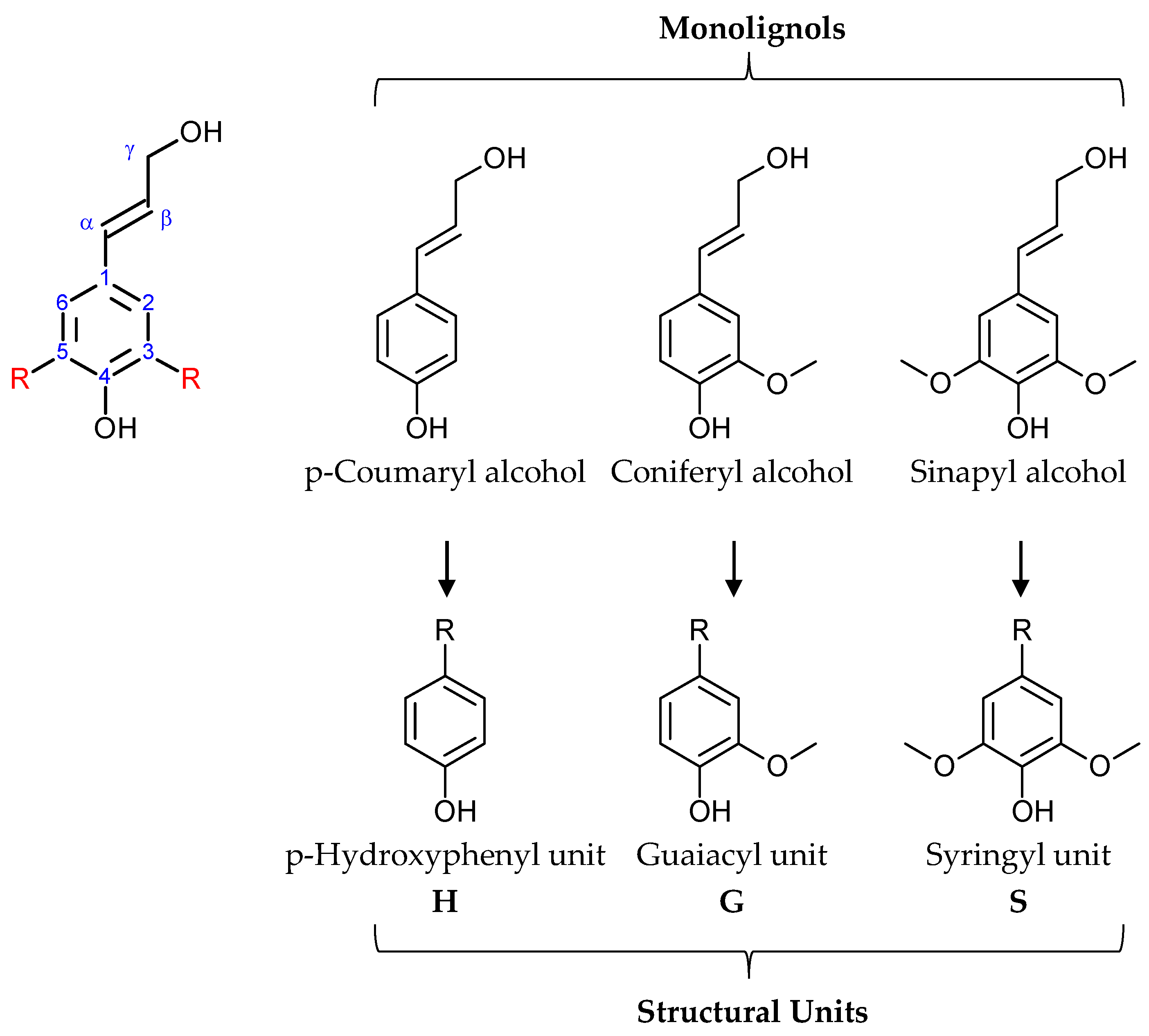
2. Lignin
2.1. Origin and Structure of Lignin
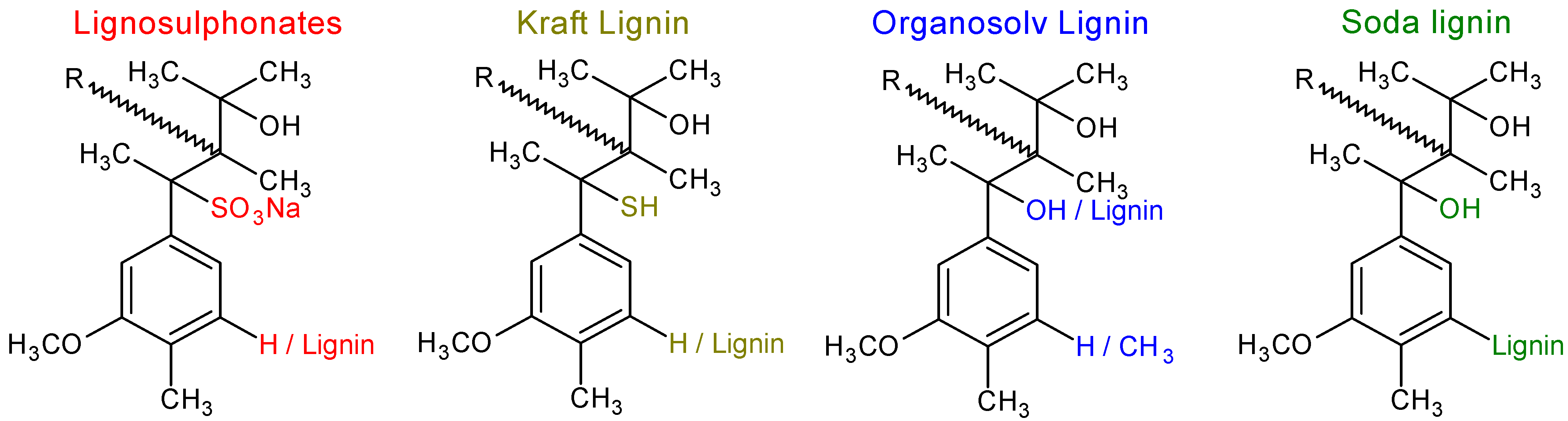
2.2. Isolation and Processing
2.2.1. Soda
2.2.2. Sulphite
2.2.3. Kraft (Sulphate)
2.2.4. Organosolv
2.2.5. Lignin-Centred Fractionation of Biomass
2.3. Effect of Delignification on Structure
3. Modification of Lignin
3.1. Creation of New Active Sites
3.1.1. Hydroxyalkylation
3.1.2. Amination
3.1.3. Nitration
3.1.4. Sulphometylation
3.1.5. Sulphonation
3.1.6. Other Reactions
3.2. Modification of Hydroxyl Groups
3.2.1. Alkylation
3.2.2. Esterification
3.2.3. Etherification
3.2.4. Phenolation
3.2.5. Urethanation
3.3. Grafting of Polymers
3.3.1. Grafting From
3.3.2. Grafting to
3.4. Functionalisation of Lignin
3.4.1. Purification and Fractionation
3.4.2. Depolymerisation
4. Hydrophobicity
4.1. Hydrogen Bond to Hydrophobicity
4.2. Hydrophobicity and Hydrophility of Lignin
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bar-On, Y.M.; Phillips, R.; Milo, R. The Biomass Distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
- Klett, A.S. Purification, Fractionation and Characterization of Lignin from Kraft Black Liquor for Use as a Renewable Biomaterial. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2017. [Google Scholar]
- Johannes, R.; Gosselink, A. Lignin as a Renewable Aromatic Resource for the Chemical Industry; Wageningen University and Research: Wageningen, The Netherlands, 2011; ISBN 9789461731005. [Google Scholar]
- Tobimatsu, Y.; Schuetz, M. Lignin Polymerization: How Do Plants Manage the Chemistry so Well? Curr. Opin. Biotechnol. 2019, 56, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Structure and Characteristics of Lignin. Lignin Chem. Appl. 2019, 25–50. [CrossRef]
- Huang, J.; Fu, S.; Gan, L. Lignin Chemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128139417. [Google Scholar]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin Depolymerisation Strategies: Towards Valuable Chemicals and Fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.; Balakshin, M. Industrial Lignins. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 315–336. ISBN 9780444595614. [Google Scholar]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From Lignin to Valuable Products–Strategies, Challenges, and Prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Gellerstedt, G.L.F.; Henriksson, E.G. Lignins: Major Sources, Structure and Properties. Monomers Polym. Compos. Renew. Resour. 2008, 201–224. [Google Scholar] [CrossRef]
- Kumar, A.; Anushree; Kumar, J.; Bhaskar, T. Utilization of Lignin: A Sustainable and Eco-Friendly Approach. J. Energy Inst. 2020, 93, 235–271. [Google Scholar] [CrossRef]
- Demirbas, A. Higher Heating Values of Lignin Types from Wood and Non-Wood Lignocellulosic Biomasses. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 592–598. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Alén, R.; Paleologou, M.; Kannangara, M.; Kihlman, J. Lignin Recovery from Spent Alkaline Pulping Liquors Using Acidification, Membrane Separation, and Related Processing Steps: A Review. BioResources 2019, 14, 2300–2351. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Poornima Devi, T.; Gunasekaran, M.; Kim, S.-H.H.; Kumar, G. A Review on Biopolymer Production via Lignin Valorization. Bioresour. Technol. 2019, 290, 121790. [Google Scholar] [CrossRef]
- Tomani, P. The Lignoboost Process. Cellul. Chem. Technol. 2010, 44, 53–58. [Google Scholar]
- Tanzid, M.; Andersson, M.A.; Sun, J.; Stake, J. Microwave Noise Characterization of Graphene Field Effect Transistors. Appl. Phys. Lett. 2014, 104, 013502. [Google Scholar] [CrossRef]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C.C. Global Lignin Supply Overview and Kraft Lignin Potential as an Alternative for Petroleum-Based Polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Chauhan, P.S. Lignin Nanoparticles: Eco-Friendly and Versatile Tool for New Era. Bioresour. Technol. Rep. 2020, 9, 100374. [Google Scholar] [CrossRef]
- Bertella, S.; Luterbacher, J.S. Lignin Functionalization for the Production of Novel Materials. Trends Chem. 2020, 2, 440–453. [Google Scholar] [CrossRef]
- Holtz, A.; Weidener, D.; Leitner, W.; Klose, H.; Grande, P.M.; Jupke, A. Process Development for Separation of Lignin from OrganoCat Lignocellulose Fractionation Using Antisolvent Precipitation. Sep. Purif. Technol. 2020, 236, 116295. [Google Scholar] [CrossRef]
- da Silva, S.H.F.; Gordobil, O.; Labidi, J. Organic Acids as a Greener Alternative for the Precipitation of Hardwood Kraft Lignins from the Industrial Black Liquor. Int. J. Biol. Macromol. 2020, 142, 583–591. [Google Scholar] [CrossRef]
- Schorr, D.; Diouf, P.N.; Stevanovic, T. Evaluation of Industrial Lignins for Biocomposites Production. Ind. Crops Prod. 2014, 52, 65–73. [Google Scholar] [CrossRef]
- Renders, T.; Van Den Bosch, S.; Koelewijn, S.F.; Schutyser, W.; Sels, B.F. Lignin-First Biomass Fractionation: The Advent of Active Stabilisation Strategies. Energy Environ. Sci. 2017, 10, 1551–1557. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Weng, J.K.; Chapple, C. The Origin and Evolution of Lignin Biosynthesis. New Phytol. 2010, 187, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Biotechnology of Lignocellulose: Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9789400768987. [Google Scholar]
- Novo-Uzal, E.; Pomar, F.; Gómez Ros, L.V.; Espiñeira, J.M.; Ros Barceló, A. Evolutionary History of Lignins. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 61, ISBN 9780124160231. [Google Scholar]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A Review on Lignin Structure, Pretreatments, Fermentation Reactions and Biorefinery Potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef]
- McCarthy, J.L.; Islam, A. Lignin Chemistry, Technology, and Utilization: A Brief History. ACS Symp. Ser. 1999, 742, 2–66. [Google Scholar] [CrossRef]
- Sundin, J. Precipitation of Kraft Lignin under Alkaline Conditions. Ph.D. Thesis, Royal Institute of Technology, Stockholm, Sweden, 2000. [Google Scholar]
- Manara, P.; Zabaniotou, A.; Vanderghem, C.; Richel, A. Lignin Extraction from Mediterranean Agro-Wastes: Impact of Pretreatment Conditions on Lignin Chemical Structure and Thermal Degradation Behavior. Catal. Today 2014, 223, 25–34. [Google Scholar] [CrossRef]
- Prinsen, P.; Rencoret, J.; Gutiérrez, A.; Liitiä, T.; Tamminen, T.; Colodette, J.L.; Berbis, M.Á.; Jiménez-Barbero, J.; Martínez, Á.T.; Del Río, J.C. Modification of the Lignin Structure during Alkaline Delignification of Eucalyptus Wood by Kraft, Soda-AQ, and Soda-O2 Cooking. Ind. Eng. Chem. Res. 2013, 52, 15702–15712. [Google Scholar] [CrossRef]
- Kalliola, A.; Kuitunen, S.; Liitiä, T.; Rovio, S.; Ohra-Aho, T.; Vuorinen, T.; Tamminen, T. Lignin Oxidation Mechanisms under Oxygen Delignification Conditions. Part 1. Results from Direct Analyses. Holzforschung 2011, 65, 567–574. [Google Scholar] [CrossRef]
- Kienberger, M.; Maitz, S.; Pichler, T.; Demmelmayer, P. Systematic Review on Isolation Processes for Technical Lignin. Processes 2021, 9, 804. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Theliander, H. The LignoBoost Process: Solubility of Lignin. In Proceedings of the International Chemical Recovery Conference, Williamsburg, VA, USA, 29 March–1 April 2010; pp. 33–42. [Google Scholar]
- Prado, R.; Erdocia, X.; Serrano, L.; Labidi, J. Lignin Purification with Green Solvents. Cellul. Chem. Technol. 2012, 46, 221–225. [Google Scholar]
- El Mansouri, N.E.; Salvadó, J. Structural Characterization of Technical Lignins for the Production of Adhesives: Application to Lignosulfonate, Kraft, Soda-Anthraquinone, Organosolv and Ethanol Process Lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Carvajal, J.C.; Gómez, Á.; Cardona, C.A. Comparison of Lignin Extraction Processes: Economic and Environmental Assessment. Bioresour. Technol. 2016, 214, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Naron, D.R.; Collard, F.X.; Tyhoda, L.; Görgens, J.F. Characterisation of Lignins from Different Sources by Appropriate Analytical Methods: Introducing Thermogravimetric Analysis-Thermal Desorption-Gas Chromatography–Mass Spectroscopy. Ind. Crops Prod. 2017, 101, 61–74. [Google Scholar] [CrossRef]
- Roy, R.; Rahman, M.S.; Raynie, D.E. Recent Advances of Greener Pretreatment Technologies of Lignocellulose. Curr. Res. Green Sustain. Chem. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Moreno, A.D.; Alvira, P.; Ibarra, D.; Tomás-pejó, E. Production of Platform Chemicals from Sustainable Resources; Springer: Singapore, 2017; pp. 375–410. ISBN 978-981-10-4171-6. [Google Scholar]
- Gellerstedt, G.; Majtnerova, A.; Zhang, L. Towards a New Concept of Lignin Condensation in Kraft Pulping. Initial Results. Comptes Rendus. Biol. 2004, 327, 817–826. [Google Scholar] [CrossRef]
- Singh, S.K. Ionic Liquids and Lignin Interaction: An Overview. Bioresour. Technol. Rep. 2022, 17, 100958. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards Lignin-Based Functional Materials in a Sustainable World. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Bhattacharya, P.K.; Todi, R.K.; Tiwari, M.; Bhattacharjee, C.; Bhattacharjee, S.; Datta, S. Studies on Ultrafiltration of Spent Sulfite Liquor Using Various Membranes for the Recovery of Lignosulphonates. Desalination 2005, 174, 287–297. [Google Scholar] [CrossRef]
- Zhu, W.; Westman, G.; Theliander, H. Investigation and Characterization of Lignin Precipitation in the Lignoboost Process. J. Wood Chem. Technol. 2014, 34, 77–97. [Google Scholar] [CrossRef]
- Helander, M.; Theliander, H.; Lawoko, M.; Henriksson, G.; Zhang, L.; Lindström, M.E. Fractionation of Technical Lignin: Molecular Mass and PH Effects. BioResources 2013, 8, 2270–2282. [Google Scholar] [CrossRef]
- Minu, K.; Jiby, K.K.; Kishore, V.V.N. Isolation and Purification of Lignin and Silica from the Black Liquor Generated during the Production of Bioethanol from Rice Straw. Biomass Bioenergy 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, D. Chemical and Thermal Characteristics of Lignins Isolated from Siam Weed Stem by Acetic Acid and Formic Acid Delignification. Ind. Crops Prod. 2010, 32, 284–291. [Google Scholar] [CrossRef]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.M.; Sels, B.F.; Wang, F. Guidelines for Performing Lignin-First Biorefining. Energy Environ. Sci. 2021, 14, 262–292. [Google Scholar] [CrossRef]
- Wu, X.; Fan, X.; Xie, S.; Lin, J.; Cheng, J.; Zhang, Q.; Chen, L.; Wang, Y. Solar Energy-Driven Lignin-First Approach to Full Utilization of Lignocellulosic Biomass under Mild Conditions. Nat. Catal. 2018, 1, 772–780. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, Y.; Qiu, S.; Wang, M.; Ju, C.; Cao, H.; Fang, Y.; Tan, T. Lignin-First Biorefinery: A Reusable Catalyst for Lignin Depolymerization and Application of Lignin Oil to Jet Fuel Aromatics and Polyurethane Feedstock. Sustain. Energy Fuels 2018, 2, 637–647. [Google Scholar] [CrossRef]
- Cao, Z.; Dierks, M.; Clough, M.T.; Daltro de Castro, I.B.; Rinaldi, R. A Convergent Approach for a Deep Converting Lignin-First Biorefinery Rendering High-Energy-Density Drop-in Fuels. Joule 2018, 2, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Renders, T.; Van den Bossche, G.; Vangeel, T.; Van Aelst, K.; Sels, B. Reductive Catalytic Fractionation: State of the Art of the Lignin-First Biorefinery. Curr. Opin. Biotechnol. 2019, 56, 193–201. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, J.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Effective Release of Lignin Fragments from Lignocellulose by Lewis Acid Metal Triflates in the Lignin-First Approach. ChemSusChem 2016, 9, 3262–3267. [Google Scholar] [CrossRef]
- Bhalla, A.; Cai, C.M.; Xu, F.; Singh, S.K.; Bansal, N.; Phongpreecha, T.; Dutta, T.; Foster, C.E.; Kumar, R.; Simmons, B.A.; et al. Performance of Three Delignifying Pretreatments on Hardwoods: Hydrolysis Yields, Comprehensive Mass Balances, and Lignin Properties. Biotechnol. Biofuels 2019, 12, 213. [Google Scholar] [CrossRef]
- Ying, W.; Yang, J.; Zhang, J. In-Situ Modification of Lignin in Alkaline-Pretreated Sugarcane Bagasse by Sulfomethylation and Carboxymethylation to Improve the Enzymatic Hydrolysis Efficiency. Ind. Crops Prod. 2022, 182, 114863. [Google Scholar] [CrossRef]
- Park, G.W.; Gong, G.; Joo, J.C.; Song, J.; Lee, J.; Lee, J.P.; Kim, H.T.; Ryu, M.H.; Sirohi, R.; Zhuang, X.; et al. Recent Progress and Challenges in Biological Degradation and Biotechnological Valorization of Lignin as an Emerging Source of Bioenergy: A State-of-the-Art Review. Renew. Sustain. Energy Rev. 2022, 157, 112025. [Google Scholar] [CrossRef]
- Bujanovic, B.; Ralph, S.A.; Reiner, R.S.; Atalla, R.H. Erratum: Lignin Modification in the Initial Phase of Softwood Kraft Pulp Delignification with Polyoxometalates (POMs) (Holzforschung 61, (492–498)). Holzforschung 2007, 61, 731. [Google Scholar] [CrossRef]
- Dos Santos, P.S.B.; Da Silva, S.H.F.; Erdociaa, X.; Gatto, D.A.; Labidi, J. Characterization of Kraft Lignin Precipitated with Different Alcohols. Chem. Eng. Trans. 2015, 43, 469–474. [Google Scholar] [CrossRef]
- Bouxin, F.P.; David Jackson, S.; Jarvis, M.C. Isolation of High Quality Lignin as a By-Product from Ammonia Percolation Pretreatment of Poplar Wood. Bioresour. Technol. 2014, 162, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, A.; Gellerstedt, G.; Tamminen, T. Determination of Phenolic Hydroxyl Groups in Residual Lignin Using a Modified UV-Method. Nord. Pulp Pap. Res. J. 1999, 14, 163–170. [Google Scholar] [CrossRef]
- Jablonský, M.; Kočiš, J.; Ház, A.; Šima, J. Characterization and Comparison by UV Spectroscopy of Precipitated Lignins and Commercial Lignosulfonates. Cellul. Chem. Technol. 2015, 49, 267–274. [Google Scholar]
- Goldmann, W.M.; Ahola, J.; Mankinen, O.; Kantola, A.M.; Komulainen, S.; Telkki, V.V.; Tanskanen, J. Determination of Phenolic Hydroxyl Groups in Technical Lignins by Ionization Difference Ultraviolet Spectrophotometry (∆ε-IDUS Method). Period. Polytech. Chem. Eng. 2017, 61, 93–101. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, S.; Chen, Y. Basic Understanding of the Color Distinction of Lignin and the Proper Selection of Lignin in Color-Depended Utilizations. Int. J. Biol. Macromol. 2020, 147, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and Chemical Modifications of Lignin: Towards Lignin-Based Nanomaterials for Biomedical Applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Eraghi Kazzaz, A.; Fatehi, P. Technical Lignin and Its Potential Modification Routes: A Mini-Review. Ind. Crops Prod. 2020, 154, 112732. [Google Scholar] [CrossRef]
- Matsushita, Y. Conversion of Technical Lignins to Functional Materials with Retained Polymeric Properties. J. Wood Sci. 2015, 61, 230–250. [Google Scholar] [CrossRef]
- Ang, A.F.; Ashaari, Z.; Lee, S.H.; Md Tahir, P.; Halis, R. Lignin-Based Copolymer Adhesives for Composite Wood Panels—A Review. Int. J. Adhes. Adhes. 2019, 95, 102408. [Google Scholar] [CrossRef]
- Ha, J.-M.; Hwang, K.-R.; Kim, Y.-M.; Jae, J.; Kim, K.H.; Lee, H.W.; Kim, J.-Y.; Park, Y.-K. Recent Progress in the Thermal and Catalytic Conversion of Lignin. Renew. Sustain. Energy Rev. 2019, 111, 422–441. [Google Scholar] [CrossRef]
- Daniel, D.; Khachatryan, L.; Astete, C.; Asatryan, R.; Marculescu, C.; Boldor, D. Sulfur Contaminations Inhibit Depolymerization of Kraft Lignin. Bioresour. Technol. Rep. 2019, 8, 100341. [Google Scholar] [CrossRef]
- Nan, N.; Hu, W.; Wang, J. Lignin-Based Porous Biomaterials for Medical and Pharmaceutical Applications. Biomedicines 2022, 10, 747. [Google Scholar] [CrossRef]
- Shorey, R.; Gupta, A.; Mekonnen, T.H. Hydrophobic Modification of Lignin for Rubber Composites. Ind. Crops Prod. 2021, 174, 114189. [Google Scholar] [CrossRef]
- Attia, A.A.M.; Abas, K.M.; Ahmed Nada, A.A.; Shouman, M.A.H.; Šišková, A.O.; Mosnáček, J. Fabrication, Modification, and Characterization of Lignin-Based Electrospun Fibers Derived from Distinctive Biomass Sources. Polymers 2021, 13, 2277. [Google Scholar] [CrossRef]
- Lisperguer, J.; Nuñez, C.; Perez-Guerrero, P. Structure and Thermal Properties of Maleated Lignin-Recycled Polystyrene Composites. J. Chil. Chem. Soc. 2013, 58, 1937–1940. [Google Scholar] [CrossRef]
- Londoño Zuluaga, C.; Du, J.; Chang, H.-M.; Jameel, H.; Gonzalez, R.W. Lignin Modifications and Perspectives towards Applications of Phenolic Foams: A Review. BioResources 2018, 13, 9158–9179. [Google Scholar] [CrossRef]
- Eraghi Kazzaz, A.; Hosseinpour Feizi, Z.; Fatehi, P. Grafting Strategies for Hydroxy Groups of Lignin for Producing Materials. Green Chem. 2019, 21, 5714–5752. [Google Scholar] [CrossRef]
- Effendi, A.; Gerhauser, H.; Bridgwater, A.V. Production of Renewable Phenolic Resins by Thermochemical Conversion of Biomass: A Review. Renew. Sustain. Energy Rev. 2008, 12, 2092–2116. [Google Scholar] [CrossRef]
- Çetin, N.S.; Özmen, N. Use of Organosolv Lignin in Phenol-Formaldehyde Resins for Particleboard Production: I. Organosolv Lignin Modified Resins. Int. J. Adhes. Adhes. 2002, 22, 477–480. [Google Scholar] [CrossRef]
- Siddiqui, H. Production of Lignin-Based Phenolic Resin Using De-Polymerized Kraft Lignin and Process Optimization. Master’s Thesis, The University of Western Ontario, London, ON, USA, 2013. [Google Scholar]
- Çetin, N.S.; Özmen, N. Use of Organosolv Lignin in Phenol-Formaldehyde Resins for Particleboard Production: II. Particleboard Production and Properties. Int. J. Adhes. Adhes. 2002, 22, 481–486. [Google Scholar] [CrossRef]
- Kalami, S.; Arefmanesh, M.; Master, E.; Nejad, M. Replacing 100% of Phenol in Phenolic Adhesive Formulations with Lignin. J. Appl. Polym. Sci. 2017, 134, 45124. [Google Scholar] [CrossRef]
- da Silva, C.G.; Grelier, S.; Pichavant, F.; Frollini, E.; Castellan, A. Adding Value to Lignins Isolated from Sugarcane Bagasse and Miscanthus. Ind. Crops Prod. 2013, 42, 87–95. [Google Scholar] [CrossRef]
- Verdini, F.; Gaudino, E.C.; Canova, E.; Tabasso, S.; Behbahani, P.J.; Cravotto, G. Lignin as a Natural Carrier for the Efficient Delivery of Bioactive Compounds: From Waste to Health. Molecules 2022, 27, 3598. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, M.; Liu, G.; Wang, J.; Chen, Y.; Zhang, Z. Alkylated Lignin with Graft Copolymerization for Enhancing Toughness of PLA. J. Mater. Sci. 2022, 57, 8687–8700. [Google Scholar] [CrossRef]
- Hofmann, K.; Glasser, W.G. Engineering Plastics from Lignin. 21.1 Synthesis and Properties of Epoxidized Lignin- Poly (Propylene Oxide) Copolymers. J. Wood Chem. Technol. 1993, 13, 73–95. [Google Scholar] [CrossRef]
- Pan, H.; Sun, G.; Zhao, T. Synthesis and Characterization of Aminated Lignin. Int. J. Biol. Macromol. 2013, 59, 221–226. [Google Scholar] [CrossRef]
- Du, X.; Li, J.; Lindström, M.E. Modification of Industrial Softwood Kraft Lignin Using Mannich Reaction with and without Phenolation Pretreatment. Ind. Crops Prod. 2014, 52, 729–735. [Google Scholar] [CrossRef]
- Rong, Y.; Ji, N.; Yu, Z.; Diao, X.; Li, H.; Lei, Y.; Lu, X.; Fukuoka, A. Lignin Amination Valorization: Heterogeneous Catalytic Synthesis of Aniline and Benzylamine from Lignin-Derived Chemicals. Green Chem. 2021, 23, 6761–6788. [Google Scholar] [CrossRef]
- Ntakirutimana, S.; Xu, T.; Liu, H.; Cui, J.-Q.; Zong, Q.-J.; Liu, Z.-H.; Li, B.-Z.; Yuan, Y.-J. Amine-Based Pretreatments for Lignocellulose Fractionation and Lignin Valorization: A Review. Green Chem. 2022, 24, 5460–5478. [Google Scholar] [CrossRef]
- Ott, M.W.; Dietz, C.; Trosien, S.; Mehlhase, S.; Bitsch, M.J.; Nau, M.; Meckel, T.; Geissler, A.; Siegert, G.; Huong, J.; et al. Co-Curing of Epoxy Resins with Aminated Lignins: Insights into the Role of Lignin Homo Crosslinking during Lignin Amination on the Elastic Properties. Holzforschung 2021, 75, 390–398. [Google Scholar] [CrossRef]
- Chen, J.; An, L.; Bae, J.H.; Heo, J.W.; Han, S.Y.; Kim, Y.S. Green and Facile Synthesis of Aminated Lignin-Silver Complex and Its Antibacterial Activity. Ind. Crops Prod. 2021, 173, 114102. [Google Scholar] [CrossRef]
- Chen, J.; An, L.; Heo, J.W.; Bae, J.H.; Jeong, H.; Kim, Y.S. Utilization of Aminated Lignin as an Adsorbent to Remove Cationic and Anionic Dyes from Aqueous Solutions. J. Wood Chem. Technol. 2022, 42, 114–124. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, M.; Zhao, C.; Li, B.; Peng, H.; Zhang, Y.; Shao, Q.; Hassan, M. Application of Lignin in Preparation of Slow-Release Fertilizer: Current Status and Future Perspectives. Ind. Crops Prod. 2022, 176, 114267. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, L.; Huang, M.; Mersal, G.A.M.; Ibrahim, M.M.; El-Bahy, Z.M.; Shi, X.; Jiang, Q. Aminated Lignin by Ultrasonic Method with Enhanced Arsenic (V) Adsorption from Polluted Water. Adv. Compos. Hybrid Mater. 2022, 5, 1044–1053. [Google Scholar] [CrossRef]
- Dong, R.; Yang, Z.; Fu, Y.; Chen, Z.; Hu, Y.; Zhou, Y.; Qin, H. Aminated Lignin Chelated Metal Derived Bifunctional Electrocatalyst with High Catalytic Performance. Appl. Surf. Sci. 2022, 580, 152205. [Google Scholar] [CrossRef]
- Graglia, M.; Pampel, J.; Hantke, T.; Fellinger, T.P.; Esposito, D. Nitro Lignin-Derived Nitrogen-Doped Carbon as an Efficient and Sustainable Electrocatalyst for Oxygen Reduction. ACS Nano 2016, 10, 4364–4371. [Google Scholar] [CrossRef]
- Suota, M.J.; Kochepka, D.M.; Ganter Moura, M.G.; Pirich, C.L.; Matos, M.; Magalhães, W.L.E.; Ramos, L.P. Lignin Functionalization Strategies and the Potential Applications of Its Derivatives—A Review. BioResources 2021, 16, 6471–6511. [Google Scholar] [CrossRef]
- Ahmad, Z.; Paleologou, M.; Xu, C.C. Oxidative Depolymerization of Lignin Using Nitric Acid under Ambient Conditions. Ind. Crops Prod. 2021, 170, 113757. [Google Scholar] [CrossRef]
- Chen, W.-J.; Zhao, C.-X.; Li, B.-Q.; Yuan, T.-Q.; Zhang, Q. Lignin-Derived Materials and Their Applications in Rechargeable Batteries. Green Chem. 2022, 24, 565–584. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L. Effects of NCO/OH Molar Ratio on Structure and Properties of Graft-Interpenetrating Polymer Networks from Polyurethane and Nitrolignin. Polymer 2002, 43, 2287–2294. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J. Effects of Hard-Segment Compositions on Properties of Polyurethane-Nitrolignin Films. J. Appl. Polym. Sci. 2001, 81, 3251–3259. [Google Scholar] [CrossRef]
- Ma, X.; Chen, J.; Zhu, J.; Yan, N. Lignin-Based Polyurethane: Recent Advances and Future Perspectives. Macromol. Rapid Commun. 2021, 42, e2000492. [Google Scholar] [CrossRef]
- Madzhidova, V.E.; Dalimova, G.N.; Abduazimov, K.A. Sulfomethylation of Lignins. Chem. Nat. Compd. 1998, 34, 179–181. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Khorasani, Z.; Hamzeh, Y.; Momenbeik, F.; Zadeh, Z.E.; Sun, F.; Madadi, M.; Zhang, X.M. Valorization of Bagasse Alkali Lignin to Water-Soluble Derivatives through Chemical Modification. 2022. Available online: https://link.springer.com/article/10.1007/s13399-022-02935-x (accessed on 10 July 2022).
- Paek, N.H.; Ri, S.H.; Jong, H.C.; Guo, Z.Y. Study on Production of Cation Asphalt Emulsifier for Micro-Surfacing by Using Sulfomethylated Lignin. 2022. Available online: https://www.sciencedirect.com/science/article/pii/S2046043022000053?via%3Dihub (accessed on 10 July 2022).
- Büyükdere, B.K.; Ünlü, C.H.; Atıcı, O.G. Synthesis of Surface Active Agents from Natural Waste Phenolics. Tenside Surfactants Deterg. 2022, 59, 192–203. [Google Scholar] [CrossRef]
- Kanwar, V.S.; Sharma, S.K.; Prakasam, C. 3rd International Conference on Innovative Technologies for Clean and Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2020; Volume 32, ISBN 9783030939359. [Google Scholar]
- Hong, N. Pickering Emulsions Stabilized by an Alkyl Chain-Bridged Lignin-Based Polymer without Additives and Organic Solvents. J. Agric. Food Chem. 2022, 70, 1196–1202. [Google Scholar] [CrossRef]
- Bertella, S.; Bernardes Figueirêdo, M.; De Angelis, G.; Mourez, M.; Bourmaud, C.; Amstad, E.; Luterbacher, J.S. Extraction and Surfactant Properties of Glyoxylic Acid-Functionalized Lignin. ChemSusChem 2022, 15, e202200270. [Google Scholar] [CrossRef]
- Li, M.; Yuan, Y.; Zhu, Y.; Jiang, B.; Wu, W.; Wu, S.; Jin, Y. Comparison of Sulfomethylated Lignin from Poplar and Masson Pine on Cellulase Adsorption and the Enzymatic Hydrolysis of Wheat Straw. Bioresour. Technol. 2022, 343, 126142. [Google Scholar] [CrossRef]
- Ouyang, X.; Ke, L.; Qiu, X.; Guo, Y.; Pang, Y. Sulfonation of Alkali Lignin and Its Potential Use in Dispersant for Cement. J. Dispers. Sci. Technol. 2009, 30, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, M.; Tang, Y.; Yang, J.; Shen, F.; Qi, X.; Yu, Y. Synthesis of Sulfonated Lignin-Derived Ordered Mesoporous Carbon for Catalytic Production of Furfural from Xylose. Int. J. Biol. Macromol. 2021, 187, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gao, W.; Kong, F.; Fatehi, P. One-Pot Preparation of Zwitterion-Type Lignin Polymers. Int. J. Biol. Macromol. 2019, 140, 429–440. [Google Scholar] [CrossRef]
- Puumala, L.S.; Fatehi, P. Dispersion Performance of Hydroxypropyl Sulfonated Lignin in Aluminum Oxide Suspension. Sep. Purif. Technol. 2021, 276, 119247. [Google Scholar] [CrossRef]
- Xiao, X.; Jiang, J.; Wang, Y.; Wang, B.; Yuan, T.Q.; Shi, Q.; Liao, X.; Shi, B.; Sun, R.C. Microwave-Assisted Sulfonation of Lignin for the Fabrication of a High-Performance Dye Dispersant. ACS Sustain. Chem. Eng. 2021, 9, 9053–9061. [Google Scholar] [CrossRef]
- Varão, L.H.R.; Silva, T.A.L.; Zamora, H.D.Z.; de Morais, L.C.; Pasquini, D. Synthesis of Methyl Biodiesel by Esterification Using Magnetic Nanoparticles Coated with Sulfonated Lignin. 2022. Available online: https://link.springer.com/article/10.1007/s13399-021-02214-1 (accessed on 10 July 2022).
- Yuan, Z.; Shang, X.; Fang, J.; Li, H. A Simple Method for Preparation of Lignin/TiO2 Nanocomposites by Sulfonation Degree Regulation and Their Application in Polyurethane Films. Int. J. Biol. Macromol. 2022, 198, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Liu, M.; Lin, L.; Pu, X.; Ge, C.; Zhang, T.; Liu, G. Sulfonated Lignin Modified with Silane Coupling Agent as Biodegradable Shale Inhibitor in Water-Based Drilling Fluid. J. Pet. Sci. Eng. 2022, 208, 109618. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, X.; Wu, H.; Wang, X.; Shi, B.; Fan, C.; Kong, Y.; Jiang, Z. Sulfonated Lignin Intercalated Graphene Oxide Membranes for Efficient Proton Conduction. J. Membr. Sci. 2022, 644, 120126. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Shen, J.; Wu, B.; He, Y. Azo Coupling Reaction Induced Macromolecular Self-Assembly in Aqueous Solution. ACS Macro Lett. 2018, 7, 437–441. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Liang, R.; Wu, B.; He, Y. Synthesis and Characterization of Water-Soluble PEGylated Lignin-Based Polymers by Macromolecular Azo Coupling Reaction. Chin. Chem. Lett. 2018, 29, 143–146. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, Y.; Qian, Y.; Zhang, W.; Qiu, X. Preparation of Photoresponsive Azo Polymers Based on Lignin, a Renewable Biomass Resource. ACS Sustain. Chem. Eng. 2015, 3, 1111–1116. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Gupta, P.; Patidar, R.; Srivastava, V.C. Microbial Peroxide Producing Cell Mediated Lignin Valorization. Int. J. Biol. Macromol. 2022, 202, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xie, S.; Pu, Y.; Zhang, R.; Huang, F.; Ragauskas, A.J.; Yuan, J.S. Synergistic Enzymatic and Microbial Lignin Conversion. Green Chem. 2016, 18, 1306–1312. [Google Scholar] [CrossRef]
- Bajpai, P. Pretreatment of Lignocellulosic Biomass for Biofuel Production; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Li, C.; Chen, C.; Wu, X.; Tsang, C.-W.; Mou, J.; Yan, J.; Liu, Y.; Lin, C.S.K. Recent Advancement in Lignin Biorefinery: With Special Focus on Enzymatic Degradation and Valorization. Bioresour. Technol. 2019, 291, 121898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, X.; Lin, J.; Zhao, G.; Chang, H.M.; Jameel, H. Reactivity Improvement by Phenolation of Wheat Straw Lignin Isolated from a Biorefinery Process. New J. Chem. 2019, 43, 2238–2246. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and Analysis of the Molecular Weight of Lignin for Biorefining Studies. Biofuels Bioprod. Biorefining 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Li, F.; Zhao, H.; Shao, R.; Zhang, X.; Yu, H. Enhanced Fenton Reaction for Xenobiotic Compounds and Lignin Degradation Fueled by Quinone Redox Cycling by Lytic Polysaccharide Monooxygenases. J. Agric. Food Chem. 2021, 69, 7104–7114. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Ge, M.; Liu, B.; Chen, S.; Zhang, D.; Gao, L. Converting Lignin into Long-Chain Fatty Acids with the Electro-Fenton Reaction. GCB Bioenergy 2021, 13, 1290–1302. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Z.; Liu, H.; Wu, T.; Han, B. Dehydroxyalkylative Halogenation of C(Aryl)-C Bonds of Aryl Alcohols. Chem. Commun. 2020, 56, 7120–7123. [Google Scholar] [CrossRef]
- Milstead, R.P.; Remucal, C.K. Molecular-Level Insights into the Formation of Traditional and Novel Halogenated Disinfection Byproducts. ACS EST Water 2021, 1, 1966–1974. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Synthesis, Thermal Properties, Rheological and Mechanical Behaviors of Lignins-Grafted-Poly(ε-Caprolactone). Polymer 2013, 54, 3882–3890. [Google Scholar] [CrossRef]
- Zhou, S.-J.J.; Wang, H.-M.M.; Xiong, S.-J.J.; Sun, J.-M.M.; Wang, Y.-Y.Y.; Yu, S.; Sun, Z.; Wen, J.-L.L.; Yuan, T.-Q.Q. Technical Lignin Valorization in Biodegradable Polyester-Based Plastics (BPPs). ACS Sustain. Chem. Eng. 2021, 9, 12017–12042. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X.; Shi, Y. A Review on Lignin Antioxidants: Their Sources, Isolations, Antioxidant Activities and Various Applications. Int. J. Biol. Macromol. 2022, 210, 716–741. [Google Scholar] [CrossRef] [PubMed]
- Rubens, M.; Van Wesemael, M.; Feghali, E.; Lufungula, L.L.; Blockhuys, F.; Vanbroekhoven, K.; Eevers, W.; Vendamme, R. Exploring the Reactivity of Aliphatic and Phenolic Hydroxyl Groups in Lignin Hydrogenolysis Oil towards Urethane Bond Formation. Ind. Crops Prod. 2022, 180, 114703. [Google Scholar] [CrossRef]
- Faris, A.H.; Rahim, A.A.; Ibrahim, M.N.M.; Alkurdi, A.M.; Shah, I. Combination of Lignin Polyol-Tannin Adhesives and Polyethylenimine for the Preparation of Green Water-Resistant Adhesives. J. Appl. Polym. Sci. 2016, 133, 43437. [Google Scholar] [CrossRef]
- Cao, M.; Hu, Y.; Cheng, W.; Huan, S.; Bai, T.; Niu, Z.; Zhao, Y.; Yue, G.; Zhao, Y.; Han, G. Lignin-Based Multi-Scale Cellular Aerogels Assembled from Co-Electrospun Nanofibers for Oil/Water Separation and Energy Storage. Chem. Eng. J. 2022, 436, 135233. [Google Scholar] [CrossRef]
- Sakai, H.; Kuroda, K.; Muroyama, S.; Tsukegi, T.; Kakuchi, R.; Takada, K.; Hata, A.; Kojima, R.; Ogoshi, T.; Omichi, M.; et al. Alkylated Alkali Lignin for Compatibilizing Agents of Carbon Fiber-Reinforced Plastics with Polypropylene. Polym. J. 2018, 50, 281–284. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Matsakas, L.; Rova, U.; Christakopoulos, P. Melt Stable Functionalized Organosolv and Kraft Lignin Thermoplastic. Processes 2020, 8, 1108. [Google Scholar] [CrossRef]
- Wang, S.; Bai, J.; Innocent, M.T.; Wang, Q.; Xiang, H.; Tang, J.; Zhu, M. Lignin-Based Carbon Fibers: Formation, Modification and Potential Applications. Green Energy Environ. 2021, 7, 578–605. [Google Scholar] [CrossRef]
- Karthäuser, J.; Biziks, V.; Mai, C.; Militz, H. Lignin and Lignin-Derived Compounds for Wood Applications—A Review. Molecules 2021, 26, 2533. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, Z.; Ji, X.; Ma, H.; Yang, G.; He, M.; Dai, L.; Xu, T.; Si, C. Alkylation Modification for Lignin Color Reduction and Molecular Weight Adjustment. Int. J. Biol. Macromol. 2022, 201, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, S.; Wang, W.; Deng, T.; Wang, H. Empowering Alkali Lignin with High Performance in Pickering Emulsion by Selective Phenolation for the Protection and Controlled-Release of Agrochemical. J. Clean. Prod. 2022, 339, 130769. [Google Scholar] [CrossRef]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How Far Is Lignin from Being a Biomedical Material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K. Preparation and Characterization of Demethylated Lignin-Polyethylenimine Adhesives. J. Adhes. 2006, 82, 593–605. [Google Scholar] [CrossRef]
- Zhen, X.; Li, H.; Xu, Z.; Wang, Q.; Xu, J.; Zhu, S.; Wang, Z.; Yuan, Z. Demethylation, Phenolation, and Depolymerization of Lignin for the Synthesis of Lignin-Based Epoxy Resin via a One-Pot Strategy. Ind. Crops Prod. 2021, 173, 114135. [Google Scholar] [CrossRef]
- Gao, C.; Li, M.M.; Zhu, C.; Hu, Y.; Shen, T.; Li, M.M.; Ji, X.; Lyu, G.; Zhuang, W. One-Pot Depolymerization, Demethylation and Phenolation of Lignin Catalyzed by HBr under Microwave Irradiation for Phenolic Foam Preparation. Compos. Part B Eng. 2021, 205, 108530. [Google Scholar] [CrossRef]
- Sivasankarapillai, G.; McDonald, A.G.; Li, H. Lignin Valorization by Forming Toughened Lignin-Co-Polymers: Development of Hyperbranched Prepolymers for Cross-Linking. Biomass Bioenergy 2012, 47, 99–108. [Google Scholar] [CrossRef]
- Sivasankarapillai, G.; McDonald, A.G. Synthesis and Properties of Lignin-Highly Branched Poly (Ester-Amine) Polymeric Systems. Biomass Bioenergy 2011, 35, 919–931. [Google Scholar] [CrossRef]
- Park, C.W.; Han, S.Y.; Bandi, R.; Dadigala, R.; Lee, E.A.; Kim, J.K.; Cindradewi, A.W.; Kwon, G.J.; Lee, S.H. Esterification of Lignin Isolated by Deep Eutectic Solvent Using Fatty Acid Chloride, and Its Composite Film with Poly(Lactic Acid). Polymers 2021, 13, 2149. [Google Scholar] [CrossRef]
- Chemical Modification of Lignin. Lignin Chem. Appl. 2019, 51–78. [CrossRef]
- Rajan, K.; Mann, J.K.; English, E.; Harper, D.P.; Carrier, D.J.; Rials, T.G.; Labbé, N.; Chmely, S.C. Sustainable Hydrogels Based on Lignin-Methacrylate Copolymers with Enhanced Water Retention and Tunable Material Properties. Biomacromolecules 2018, 19, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ruan, X.; Cheng, X.; Lü, Q. Liquefaction of Lignin by Polyethyleneglycol and Glycerol. Bioresour. Technol. 2011, 102, 3581–3583. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhou, M.; Wang, S.; Lou, H.; Yang, D.; Qiu, X. Synthesis, Structure, and Dispersion Property of a Novel Lignin-Based Polyoxyethylene Ether from Kraft Lignin and Poly(Ethylene Glycol). ACS Sustain. Chem. Eng. 2014, 2, 1902–1909. [Google Scholar] [CrossRef]
- Orebom, A.; Di Francesco, D.; Shakari, P.; Samec, J.S.M.; Pierrou, C. Thermal and Mechanical Properties of Esterified Lignin in Various Polymer Blends. Molecules 2021, 26, 3219. [Google Scholar] [CrossRef]
- Adjaoud, A.; Dieden, R.; Verge, P. Sustainable Esterification of a Soda Lignin with Phloretic Acid. Polymers 2021, 13, 637. [Google Scholar] [CrossRef]
- Saffian, H.A.; Yamaguchi, M.; Ariffin, H.; Abdan, K.; Kassim, N.K.; Lee, S.H.; Lee, C.H.; Shafi, A.R.; Alias, A.H. Thermal, Physical and Mechanical Properties of Poly(Butylene Succinate)/Kenaf Core Fibers Composites Reinforced with Esterified Lignin. Polymers 2021, 13, 2359. [Google Scholar] [CrossRef]
- Shorey, R.; Mekonnen, T.H. Sustainable Paper Coating with Enhanced Barrier Properties Based on Esterified Lignin and PBAT Blend. Int. J. Biol. Macromol. 2022, 209, 472–484. [Google Scholar] [CrossRef]
- Jedrzejczyk, M.A.; Van den Bosch, S.; Van Aelst, J.; Van Aelst, K.; Kouris, P.D.; Moalin, M.; Haenen, G.R.M.M.; Boot, M.D.; Hensen, E.J.M.; Lagrain, B.; et al. Lignin-Based Additives for Improved Thermo-Oxidative Stability of Biolubricants. ACS Sustain. Chem. Eng. 2021, 9, 12548–12559. [Google Scholar] [CrossRef]
- Liu, L.Y.; Chen, S.; Ji, L.; Jang, S.K.; Renneckar, S. One-Pot Route to Convert Technical Lignin into Versatile Lignin Esters for Tailored Bioplastics and Sustainable Materials. Green Chem. 2021, 23, 4567–4579. [Google Scholar] [CrossRef]
- Anugwom, I.; Lahtela, V.; Hedenström, M.; Kiljunen, S.; Kärki, T.; Kallioinen-Mänttäri, M. Esterified Lignin from Construction and Demolition Waste (CDW) as a Versatile Additive for Polylactic-Acid (PLA) Composites—The Effect of Artificial Weathering on Its Performance. Glob. Chall. 2022, 6, 2100137. [Google Scholar] [CrossRef]
- Dong, C.; Meng, X.; Leu, S.Y.; Xu, L.; Wu, Z.; Cravotto, G.; Fang, Z. Enhancing α-Etherification of Lignin in Eucalyptus Diol Pretreatment to Improve Lignin Monomer Production. Ind. Crops Prod. 2022, 185, 115130. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, J.; Du, X.; Hu, Z.; Chang, H.M.; Jameel, H. Phenolation to Improve Lignin Reactivity toward Thermosets Application. ACS Sustain. Chem. Eng. 2018, 6, 5504–5512. [Google Scholar] [CrossRef]
- Zhang, H.N.; Ren, H.; Zhai, H.M. Analysis of Phenolation Potential of Spruce Kraft Lignin and Construction of Its Molecular Structure Model. Ind. Crops Prod. 2021, 167, 113506. [Google Scholar] [CrossRef]
- Liang, D.; Zhu, X.; Dai, P.; Lu, X.; Guo, H.; Que, H.; Wang, D.; He, T.; Xu, C.; Robin, H.M.; et al. Preparation of a Novel Lignin-Based Flame Retardant for Epoxy Resin. Mater. Chem. Phys. 2021, 259, 124101. [Google Scholar] [CrossRef]
- Bertella, S.; Luterbacher, J.S. Simultaneous Extraction and Controlled Chemical Functionalization of Hardwood Lignin for Improved Phenolation. Green Chem. 2021, 23, 3459–3467. [Google Scholar] [CrossRef]
- Maree, C.; Görgens, J.F.; Tyhoda, L. Lignin Phenol Formaldehyde Resins Synthesised Using South African Spent Pulping Liquor. Waste Biomass Valorization 2022, 13, 3489–3507. [Google Scholar] [CrossRef]
- Cao, H.; Liu, R.; Li, B.; Wu, Y.; Wang, K.; Yang, Y.; Li, A.; Zhuang, Y.; Cai, D.; Qin, P. Biobased Rigid Polyurethane Foam Using Gradient Acid Precipitated Lignin from the Black Liquor: Revealing the Relationship between Lignin Structural Features and Polyurethane Performances. Ind. Crops Prod. 2022, 177, 114480. [Google Scholar] [CrossRef]
- Arefmanesh, M.; Nikafshar, S.; Master, E.R.; Nejad, M. From Acetone Fractionation to Lignin-Based Phenolic and Polyurethane Resins. Ind. Crops Prod. 2022, 178, 114604. [Google Scholar] [CrossRef]
- Henry, C.; Gondaliya, A.; Thies, M.; Nejad, M. Studying the Suitability of Nineteen Lignins as Partial Polyol Replacement in Rigid Polyurethane/Polyisocyanurate Foam. Molecules 2022, 27, 2535. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Valencia, C.; Ibarra, D.; Ballesteros, I.; Franco, J.M. Lignin-Enriched Residues from Bioethanol Production: Chemical Characterization, Isocyanate Functionalization and Oil Structuring Properties. Int. J. Biol. Macromol. 2022, 195, 412–423. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, S.; Gibril, M.E.; Kong, F. The Copolymer of Polyvinyl Acetate Containing Lignin-Vinyl Acetate Monomer: Synthesis and Characterization. Eur. Polym. J. 2020, 123, 109411. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin Biopolymers in the Age of Controlled Polymerization. Polymers 2019, 11, 1176. [Google Scholar] [CrossRef]
- Panesar, S.S.; Jacob, S.; Misra, M.; Mohanty, A.K. Functionalization of Lignin: Fundamental Studies on Aqueous Graft Copolymerization with Vinyl Acetate. Ind. Crops Prod. 2013, 46, 191–196. [Google Scholar] [CrossRef]
- Jung, H.Y.; Lee, J.S.; Han, H.T.; Jung, J.; Eom, K.; Lee, J.T. Lignin-Based Materials for Sustainable Rechargeable Batteries. Polymers 2022, 14, 673. [Google Scholar] [CrossRef] [PubMed]
- Goliszek, M.; Podkościelna, B.; Sevastyanova, O.; Fila, K.; Chabros, A.; Pączkowski, P. Investigation of Accelerated Aging of Lignin-Containing Polymer Materials. Int. J. Biol. Macromol. 2019, 123, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Belgacem, M.N. Optimization Study of Lignin Oxypropylation in View of the Preparation of Polyurethane Rigid Foams. Ind. Eng. Chem. Res. 2009, 48, 2583–2589. [Google Scholar] [CrossRef]
- Nadji, H.; Bruzzèse, C.; Belgacem, M.N.; Benaboura, A.; Gandini, A. Oxypropylation of Lignins and Preparation of Rigid Polyurethane Foams from the Ensuing Polyols. Macromol. Mater. Eng. 2005, 290, 1009–1016. [Google Scholar] [CrossRef]
- Kai, D.; Jiang, S.; Low, Z.W.; Loh, X.J. Engineering Highly Stretchable Lignin-Based Electrospun Nanofibers for Potential Biomedical Applications. J. Mater. Chem. B 2015, 3, 6194–6204. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, W.; Yang, Z.; Mai, Z.; Huang, Z.; Bie, Y.; Wu, S.; Dong, X.; Fu, X.; Ko, F.; et al. Electrospun Nanofibrous Membrane with Antibacterial and Antiviral Properties Decorated with Myoporum Bontioides Extract and Silver-Doped Carbon Nitride Nanoparticles for Medical Masks Application. SSRN Electron. J. 2022, 298, 121565. [Google Scholar] [CrossRef]
- Fitzgerald, C.L.; Thies, M.C. Continuous Recovery of High-Purity Kraft Lignin from Black Liquor via Simultaneous, Liquid-Phase Acidification and Purification. Ind. Crops Prod. 2022, 184, 115084. [Google Scholar] [CrossRef]
- Roy, P.S.; Garnier, G.; Allais, F.; Saito, K. Effective Lignin Utilization Strategy: Major Depolymerization Technologies, Purification Process and Production of Valuable Material. Chem. Lett. 2021, 50, 1123–1130. [Google Scholar] [CrossRef]
- Hasanov, I.; Shanmugam, S.; Kikas, T. Extraction and Isolation of Lignin from Ash Tree (Fraxinus Exselsior) with Protic Ionic Liquids (PILs). Chemosphere 2022, 290, 133297. [Google Scholar] [CrossRef] [PubMed]
- Resende, F.L.P.; Fraley, S.A.; Berger, M.J.; Savage, P.E. Noncatalytic Gasification of Lignin in Supercritical Water. Energy Fuels 2008, 22, 1328–1334. [Google Scholar] [CrossRef]
- Salimi, M.; Nejati, B.; Karimi, A.; Tavasoli, A. Hydrothermal Gasification Performance of Iranian Rice Straw in Supercritical Water Media for Hydrogen-Rich Gas Production. BioResources 2016, 11, 6362–6377. [Google Scholar] [CrossRef][Green Version]
- Cai, Z.; Long, J.; Li, Y.; Ye, L.; Yin, B.; France, L.J.; Dong, J.; Zheng, L.; He, H.; Liu, S.; et al. Selective Production of Diethyl Maleate via Oxidative Cleavage of Lignin Aromatic Unit. Chem 2019, 5, 2365–2377. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; Teunissen, W.; van Dam, J.E.G.; de Jong, E.; Gellerstedt, G.; Scott, E.L.; Sanders, J.P.M. Lignin Depolymerisation in Supercritical Carbon Dioxide/Acetone/Water Fluid for the Production of Aromatic Chemicals. Bioresour. Technol. 2012, 106, 173–177. [Google Scholar] [CrossRef]
- Schoenherr, S.; Ebrahimi, M.; Czermak, P. Lignin Degradation Processes and the Purification of Valuable Products. In Lignin—Trends and Applications; IntechOpen: London, UK, 2018; 308p. [Google Scholar] [CrossRef]
- Mimini, V.; Sykacek, E.; Hashim, S.N.A.; Holzweber, J.; Hettegger, H.; Fackler, K.; Potthast, A.; Mundigler, N.; Rosenau, T. Compatibility of Kraft Lignin, Organosolv Lignin and Lignosulfonate with PLA in 3D Printing. J. Wood Chem. Technol. 2019, 39, 14–30. [Google Scholar] [CrossRef]
- Ponnuchamy, V.; Sandak, J.; Sandak, A. Revealing of Supercritical Water Gasification Process of Lignin by Reactive Force Field Molecular Dynamics Simulations. Processes 2021, 9, 714. [Google Scholar] [CrossRef]
- Gieseking, B.; Jäck, B.; Preis, E.; Jung, S.; Forster, M.; Scherf, U.; Deibel, C.; Dyakonov, V. Excitation Dynamics in Low Band Gap Donor-Acceptor Copolymers and Blends. Polym. Polym. Compos. 2012, 16, 101–113. [Google Scholar] [CrossRef]
- Law, K.Y. Water-Surface Interactions and Definitions for Hydrophilicity, Hydrophobicity and Superhydrophobicity. Pure Appl. Chem. 2015, 87, 759–765. [Google Scholar] [CrossRef]
- Law, K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Stokely, K.; Mazza, M.G.; Stanley, H.E.; Franzese, G. Effect of Hydrogen Bond Cooperativity on the Behavior of Water. Proc. Natl. Acad. Sci. USA 2010, 107, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Gilormini, P.; Verdu, J. On the Role of Hydrogen Bonding on Water Absorption in Polymers. Polymer 2018, 142, 164–169. [Google Scholar] [CrossRef]
- He, Y.; Zhu, B.; Inoue, Y. Hydrogen Bonds in Polymer Blends. Prog. Polym. Sci. 2004, 29, 1021–1051. [Google Scholar] [CrossRef]
- Ago, M.; Okajima, K.; Jakes, J.E.; Park, S.; Rojas, O.J. Lignin-Based Electrospun Nanofibers Reinforced with Cellulose Nanocrystals. Biomacromolecules 2012, 13, 918–926. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J. Hydrophilic Polymers. Polymers 2019, 11, 693. [Google Scholar] [CrossRef]
- Kung, C.H.; Sow, P.K.; Zahiri, B.; Mérida, W. Assessment and Interpretation of Surface Wettability Based on Sessile Droplet Contact Angle Measurement: Challenges and Opportunities. Adv. Mater. Interfaces 2019, 6, 1–27. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Sui, T.; Li, A.; Chen, D. Investigation on Hydrophobicity of Lotus Leaf: Experiment and Theory. Plant Sci. 2009, 176, 687–695. [Google Scholar] [CrossRef]
- Geils, J.; Patzelt, G.; Kesel, A. The Larger the Contact Angle, the Lower the Adhesion? 2019. Available online: https://www.researchgate.net/profile/Judith_Geils/publication/330556275_The_larger_the_contact_angle_the_lower_the_adhesion/links/5c4831e1299bf12be3dcac4f/The-larger-the-contact-angle-the-lower-the-adhesion.pdf (accessed on 19 July 2022).
- Zhou, X.F.; Lu, X.J. Structural Characterization of Kraft Lignin for Its Green Utilization. Wood Res. 2014, 59, 583–592. [Google Scholar]
- Li, B.; You, S.; Qi, W.; Wang, Y.; Su, R.; He, Z. Structure-Tunable Assembly of Lignin Sub-Micro Spheres by Modifying the Amphiphilic Interfaces of Lignin via n-Alkane. Eur. Polym. J. 2020, 126, 109539. [Google Scholar] [CrossRef]
- Antonino, L.D.; Gouveia, J.R.; de Sousa Júnior, R.R.; Garcia, G.E.S.; Gobbo, L.C.; Tavares, L.B.; dos Santos, D.J. Reactivity of Aliphatic and Phenolic Hydroxyl Groups in Kraft Lignin towards 4,4′ MDI. Molecules 2021, 26, 2131. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Pan, X. Correlation between Lignin Physicochemical Properties and Inhibition to Enzymatic Hydrolysis of Cellulose. Biotechnol. Bioeng. 2016, 113, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Methylation of Softwood Kraft Lignin with Dimethyl Carbonate. Green Chem. 2015, 17, 1077–1087. [Google Scholar] [CrossRef]
- Diop, A.; Mijiyawa, F.; Koffi, D.; Kokta, B.V.; Montplaisir, D. Study of Lignin Dispersion in Low-Density Polyethylene. J. Thermoplast. Compos. Mater. 2015, 28, 1662–1674. [Google Scholar] [CrossRef]
- Gao, Y.; Qu, W.; Liu, Y.; Hu, H.; Cochran, E.; Bai, X. Agricultural Residue-derived Lignin as the Filler of Polylactic Acid Composites and the Effect of Lignin Purity on the Composite Performance. J. Appl. Polym. Sci. 2019, 136, 47915. [Google Scholar] [CrossRef]
- Gordobil, O.; Delucis, R.; Egüés, I.; Labidi, J. Kraft Lignin as Filler in PLA to Improve Ductility and Thermal Properties. Ind. Crops Prod. 2015, 72, 46–53. [Google Scholar] [CrossRef]
- Hajirahimkhan, S.; Xu, C.C.; Ragogna, P.J. Ultraviolet Curable Coatings of Modified Lignin. ACS Sustain. Chem. Eng. 2018, 6, 14685–14694. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Zhu, Z.; Fu, S. High-Value Utilization of Hydroxymethylated Lignin in Polyurethane Adhesives. Int. J. Biol. Macromol. 2020, 152, 775–785. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W.; Knozowska, K. Hydrophobic Ceramic Membranes for Water Desalination. Appl. Sci. 2017, 7, 402. [Google Scholar] [CrossRef]
- Kudanga, T.; Prasetyo, E.N.; Sipilä, J.; Guebitz, G.M.; Nyanhongo, G.S. Reactivity of Long Chain Alkylamines to Lignin Moieties: Implications on Hydrophobicity of Lignocellulose Materials. J. Biotechnol. 2010, 149, 81–87. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Wenger, J.; Haas, V.; Stern, T. Why Can We Make Anything from Lignin Except Money? Towards a Broader Economic Perspective in Lignin Research. Curr. For. Rep. 2020, 6, 294–308. [Google Scholar] [CrossRef]
- Hemmilä, V.; Trischler, J.; Sandberg, D. Lignin—An Adhesive Raw Materials of the Future or a Waste of Research Energy? In Northern European Network for Wood Science and Engineering, Proceedings of the WSE–9th Meeting, Hannover, Germany, 11–12 September 2013; Leibniz Universität: Hannover, Germany, 2013; pp. 98–103. [Google Scholar]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of Lignin in Polymer and Composite Systems for Advanced Engineering Applications—A Review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Vives, M.B.; Thuvander, J.; Arkell, A.; Lipnizki, F. Low-Molecular-Weight Lignin Recovery with Nanofiltration in the Kraft Pulping Process. Membranes 2022, 12, 310. [Google Scholar] [CrossRef]
- Wong, S.S.; Shu, R.; Zhang, J.; Liu, H.; Yan, N. Downstream Processing of Lignin Derived Feedstock into End Products. Chem. Soc. Rev. 2020, 49, 5510–5560. [Google Scholar] [CrossRef]
- Korányi, T.I.; Fridrich, B.; Pineda, A.; Barta, K. Development of ‘Lignin-First’ Approaches for the Valorization of Lignocellulosic Biomass. Molecules 2020, 25, 2815. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Slabon, A.; Sipponen, M.H. Lignin–Inorganic Interfaces: Chemistry and Applications from Adsorbents to Catalysts and Energy Storage Materials. ChemSusChem 2020, 13, 4344–4355. [Google Scholar] [CrossRef]
- José Borges Gomes, F.; de Souza, R.E.; Brito, E.O.; Costa Lelis, R.C. A Review on Lignin Sources and Uses. J. Appl. Biotechnol. Bioeng. 2020, 7, 100–105. [Google Scholar] [CrossRef]
- Chaves, A.V.; Waghorn, G.C.; Tavendale, M.H. A Simplified Method for Lignin Measurement in a Range of Forage Species. In Proceedings of the New Zealand Grassland Association; New Zealand Grassland Association: Dunedin, New Zealand, 2002; pp. 129–133. [Google Scholar] [CrossRef]
- Poke, F.S.; Wright, J.K.; Raymond, C.A. Predicting Extractives and Lignin Contents in Eucalyptus Globulus Using Near Infrared Reflectance Analysis. J. Wood Chem. Technol. 2005, 24, 55–67. [Google Scholar] [CrossRef]
- Wu, X.; Li, G.; Liu, X.; He, F. Rapid Non-destructive Analysis of Lignin Using NIR Spectroscopy and Chemo-metrics. Food Energy Secur. 2021, 10, e289. [Google Scholar] [CrossRef]
- Moretti, C.; Corona, B.; Hoefnagels, R.; Vural-Gürsel, I.; Gosselink, R.; Junginger, M. Review of Life Cycle Assessments of Lignin and Derived Products: Lessons Learned. Sci. Total Environ. 2021, 770, 144656. [Google Scholar] [CrossRef] [PubMed]
- Jablonsky, M.; Haz, A.; Majova, V. Assessing the Opportunities for Applying Deep Eutectic Solvents for Fractionation of Beech Wood and Wheat Straw. Cellulose 2019, 26, 7675–7684. [Google Scholar] [CrossRef]
- Ghareh Bagh, F.S.; Ray, S.; Seth, R. Optimizing Lignin Extraction from Kraft Black Liquor Using Protic Ionic Liquids. Biomass Bioenergy 2021, 154, 106249. [Google Scholar] [CrossRef]
- Szalaty, T.J.; Klapiszewski, Ł.; Jesionowski, T. Recent Developments in Modification of Lignin Using Ionic Liquids for the Fabrication of Advanced Materials–A Review. J. Mol. Liq. 2020, 301, 112417. [Google Scholar] [CrossRef]
- Sahoo, S.; Seydibeyoĝlu, M.Ö.; Mohanty, A.K.; Misra, M. Characterization of Industrial Lignins for Their Utilization in Future Value Added Applications. Biomass Bioenergy 2011, 35, 4230–4237. [Google Scholar] [CrossRef]
- Wang, M.; Dessie, W.; Li, H. Chemically Modified Lignin: Correlation between Structure and Biodegradability. J. Renew. Mater. 2021, 9, 2119–2128. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Collet, C.; Gaugler, M.; Lloyd-Jones, G. Integrating Softwood Biorefinery Lignin into Polyhydroxybutyrate Composites and Application in 3D Printing. Mater. Today Commun. 2019, 19, 286–296. [Google Scholar] [CrossRef]
- Tanase-Opedal, M.; Espinosa, E.; Rodríguez, A.; Chinga-Carrasco, G. Lignin: A Biopolymer from Forestry Biomass for Biocomposites and 3D Printing. Materials 2019, 12, 3006. [Google Scholar] [CrossRef]
- Kumar, M.; Hietala, M.; Oksman, K. Lignin-Based Electrospun Carbon Nanofibers. Front. Mater. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, J.; Dong, R.; Fu, Y.; Zhou, J.; Qin, H. High-Performance Electrospun Carbon Fiber Derived from Lignin and Metal Composite. Ionics 2022, 28, 1119–1127. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, H.; Cao, Q.; Zheng, H.; Xu, D.; Guo, H.; Wang, S.; Li, Y.; Zhou, J. Electrospun Lignin-Based Carbon Nanofibers as Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 12831–12841. [Google Scholar] [CrossRef]
- Roman, J.; Neri, W.; Derré, A.; Poulin, P. Electrospun Lignin-Based Twisted Carbon Nanofibers for Potential Microelectrodes Applications. Carbon 2019, 145, 556–564. [Google Scholar] [CrossRef]
- Mikeš, P.; Baker, D.A.; Uhlin, A.; Lukáš, D.; Kuželová-Košťáková, E.; Vidrich, A.; Valtera, J.; Kopřivová, B.; Asatiani, N.; Salmén, L.; et al. The Mass Production of Lignin Fibres by Means of Needleless Electrospinning. J. Polym. Environ. 2021, 29, 2164–2173. [Google Scholar] [CrossRef]
- Svinterikos, E.; Zuburtikudis, I.; Al-Marzouqi, M. Electrospun Lignin-Derived Carbon Micro- and Nanofibers: A Review on Precursors, Properties, and Applications. ACS Sustain. Chem. Eng. 2020, 8, 13868–13893. [Google Scholar] [CrossRef]
- Rivière, G.N.; Korpi, A.; Sipponen, M.H.; Zou, T.; Kostiainen, M.A.; Österberg, M. Agglomeration of Viruses by Cationic Lignin Particles for Facilitated Water Purification. ACS Sustain. Chem. Eng. 2020, 8, 4167–4177. [Google Scholar] [CrossRef]
- Hoyo, J.; Ivanova, K.; Torrent-Burgues, J.; Tzanov, T. Interaction of Silver-Lignin Nanoparticles with Mammalian Mimetic Membranes. Front. Bioeng. Biotechnol. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Dalton, N.; Lynch, R.P.; Collins, M.N.; Culebras, M. Thermoelectric Properties of Electrospun Carbon Nanofibres Derived from Lignin. Int. J. Biol. Macromol. 2019, 121, 472–479. [Google Scholar] [CrossRef]
- Huerta, R.R.; Silva, E.K.; Ekaette, I.; El-Bialy, T.; Saldaña, M.D.A. High-Intensity Ultrasound-Assisted Formation of Cellulose Nanofiber Scaffold with Low and High Lignin Content and Their Cytocompatibility with Gingival Fibroblast Cells. Ultrason. Sonochem. 2020, 64, 104759. [Google Scholar] [CrossRef]
- Rahman, M.S.; Roy, R.; Jadhav, B.; Hossain, M.N.; Halim, M.A.; Raynie, D.E. Formulation, Structure, and Applications of Therapeutic and Amino Acid-Based Deep Eutectic Solvents: An Overview. J. Mol. Liq. 2021, 321, 114745. [Google Scholar] [CrossRef]
- Pereira, A.D.E.S.; Luiz de Oliveira, J.; Maira Savassa, S.; Barbara Rogério, C.; Araujo de Medeiros, G.; Fraceto, L.F. Lignin Nanoparticles: New Insights for a Sustainable Agriculture. J. Clean. Prod. 2022, 345, 131145. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Qi, G.; Guo, J.; Xu, F.; Li, C.; Puglia, D.; Kenny, J.; Ma, P. Enhancing the Radical Scavenging Activity and UV Resistance of Lignin Nanoparticles via Surface Mannich Amination toward a Biobased Antioxidant. Biomacromolecules 2021, 22, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Tran, T.M.T.; Choi, J.W.; Won, K. Lignin for White Natural Sunscreens. Int. J. Biol. Macromol. 2019, 122, 549–554. [Google Scholar] [CrossRef] [PubMed]



| Delignification | Summary | Advantages | Disadvantages | References |
| Sulphite process | Lignosulphonate Hydrolytic process NaOH and Na2SO3 Temperature 120–180 °C High molecular weight product | Water-soluble product Soluble in polar organic solvents Used on mostly wood biomass | High sulphur content High ash content Low purity Nonselective separation High carbohydrates content | [35,36] |
| Kraft process | Kraft lignin Hydrolytic process NaOH and Na2S Temperature 150–180 °C Medium molecular weight product | Ideal for lignin removal Low ash content Alkali and organic solvent soluble lignin used on mostly wood biomass | Moderate sulphur content Long processing time High carbohydrates content without selective separation/purification | [11,14,37] |
| Organosolv | Organosolv lignin Hydrothermal process Organic solvent Temperature 90–210 °C Low molecular weight product | Sulphur free Low molecular weight Low changes to structure Hydrophobic lignin | High operational cost High solvent cost Aditional recovery of Hydrophobic lignin Pilot production scale | [35,38] |
| Soda process | Soda lignin Hydrolytic alkali process NaOH Temperature 90–150 °C Low molecular weight product | Sulphur free Low catalyst inhibitors content Low ash content Used on mostly non-wood biomass | High carbohydrate content High delignification agent consumption High heterogeneity of product | [39,40,41] |
| Enzymatic * | Enzymatic lignin Biological process Low temperature Fungi or bacteria Medium molecular weight product | Sulphur free Low energy cost | Long term processing Carbohydrate consumption by bioreagents | [29,42,43] |
| Ionic liquids * | Ionic liquid lignin Hydrothermal process Temperature 100–170 °C Low to medium molecular weight | Sulphur free Minimal changes to structure | High operational cost (high reagent cost) Additional stages for separation | [42,44,45] |
| Lignosulphonates | Kraft Lignin | Organosolv Lignin | Soda Lignin | |
|---|---|---|---|---|
| Solubility | Water | Alkali, Organic | Organic | Alkali |
| Mw (g/mol) | 1000–150,000 | 1000–20,000 | 500–5000 | 1000–3000 |
| Mn (g/mol) | 1500–50,000 | 1000–3000 | 800–3000 | 500–5000 |
| Polydispersity (-) | 6–8 | 2.5–3.5 | 1.5–2.5 | 2.5–3.5 |
| Tg (°C) | 130 | 140–150 | 90–110 | 140 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisý, A.; Ház, A.; Nadányi, R.; Jablonský, M.; Šurina, I. About Hydrophobicity of Lignin: A Review of Selected Chemical Methods for Lignin Valorisation in Biopolymer Production. Energies 2022, 15, 6213. https://doi.org/10.3390/en15176213
Lisý A, Ház A, Nadányi R, Jablonský M, Šurina I. About Hydrophobicity of Lignin: A Review of Selected Chemical Methods for Lignin Valorisation in Biopolymer Production. Energies. 2022; 15(17):6213. https://doi.org/10.3390/en15176213
Chicago/Turabian StyleLisý, Anton, Aleš Ház, Richard Nadányi, Michal Jablonský, and Igor Šurina. 2022. "About Hydrophobicity of Lignin: A Review of Selected Chemical Methods for Lignin Valorisation in Biopolymer Production" Energies 15, no. 17: 6213. https://doi.org/10.3390/en15176213
APA StyleLisý, A., Ház, A., Nadányi, R., Jablonský, M., & Šurina, I. (2022). About Hydrophobicity of Lignin: A Review of Selected Chemical Methods for Lignin Valorisation in Biopolymer Production. Energies, 15(17), 6213. https://doi.org/10.3390/en15176213







