Synthesis of Biodiesel from Ricinus communis L. Seed Oil, a Promising Non-Edible Feedstock Using Calcium Oxide Nanoparticles as a Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Calcium Oxide Nanoparticle Preparation
2.2. XRD and SEM Analysis of CaO Nanoparticles
2.3. Oil Extraction from Feedstock
2.3.1. Chemical Extraction through Soxhlet
- W1 = empty flask weight
- W2 = fine powder sample weight
- W3 = flask + weight of extracted oil
- W4 = extracted oil weight
2.3.2. Mechanical Extraction of Oil
2.4. Oil Filtration
2.5. Free Fatty Acid (FFA) Content Determination
- A = potassium hydroxide (KOH) volume used in the sample titration.
- B = potassium hydroxide (KOH) volume used in blank titration.
- C = potassium hydroxide (KOH) concentration (g/L).
- V = volume of oil sample.
2.6. Biodiesel Synthesis
2.7. Analysis of Fuel Properties
2.8. Chemical Assessment of Biodiesel
2.8.1. Fourier Transform Infrared (FT-IR) Analysis of RCB
2.8.2. NMR Spectroscopy of the Ricinus communis Biodiesel (RCB)
- C = oil to biodiesel conversion percentage
- AMe = integration value of methoxy protons in biodiesel
- ACH2 = α-methylene protons’ integration value of methylene protons in biodiesel
2.8.3. FAMEs Determination through GC-MS
3. Results and Discussion
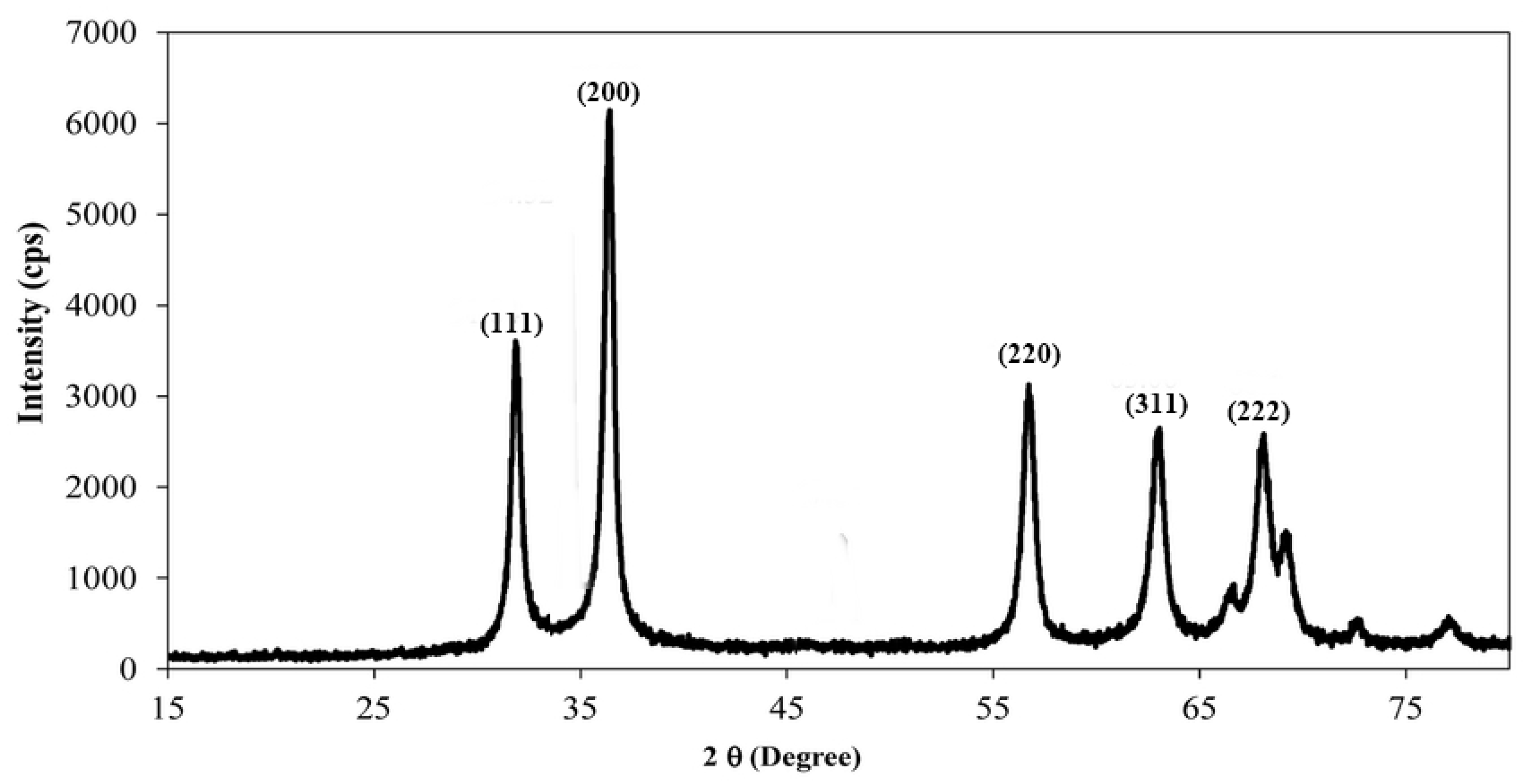
3.1. X-ray Diffraction of CaO Nanocatalysts
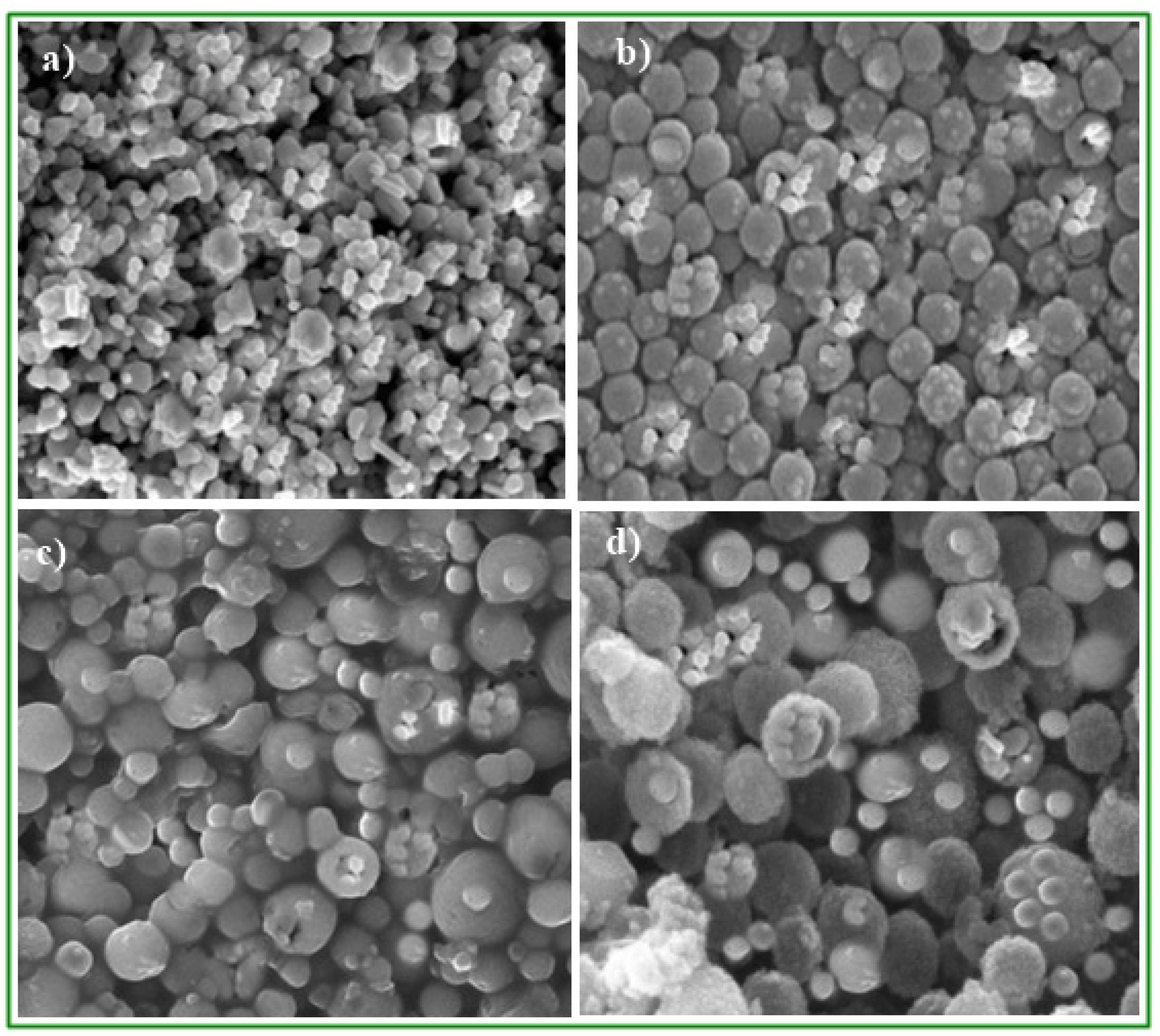
3.2. Scanning Electron Microscopic Study of CaO
3.3. Oil Extraction and Free Fatty Acids Content Determination
3.4. Biodiesel Synthesis through Transesterification Technique
3.4.1. Oil-to-Methanol Ratio
3.4.2. Catalyst Concentration
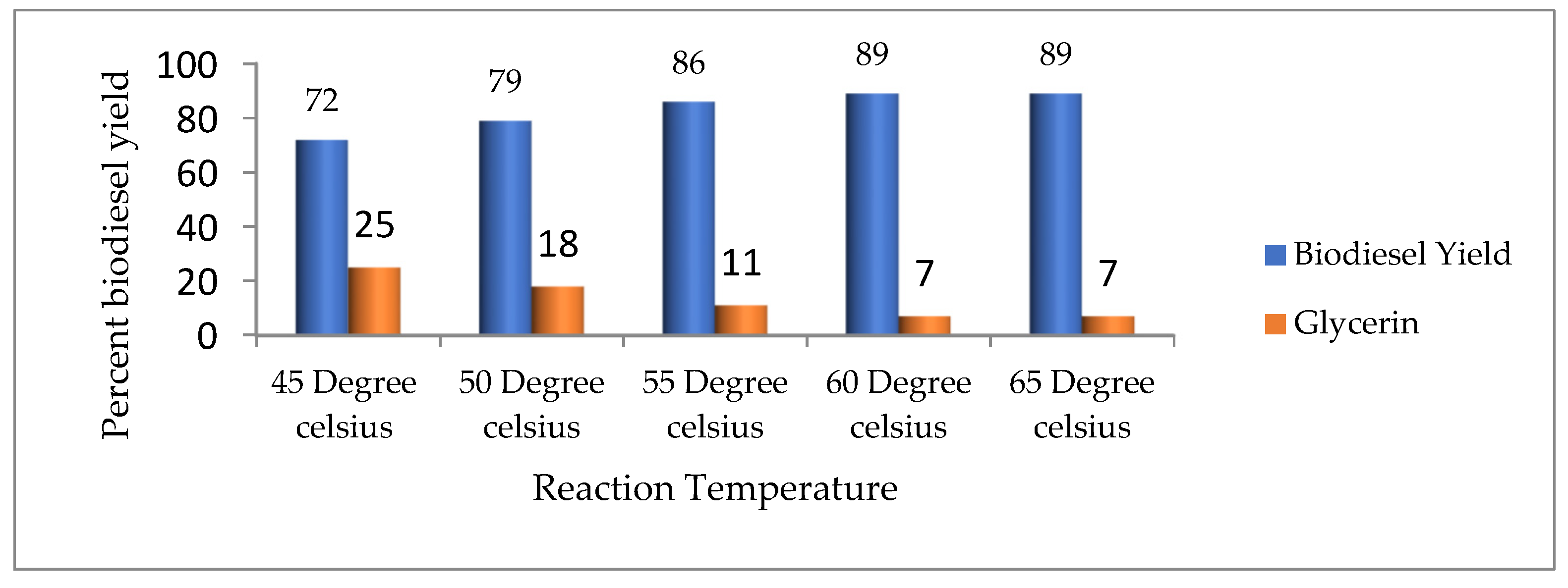
3.4.3. Reaction Temperature
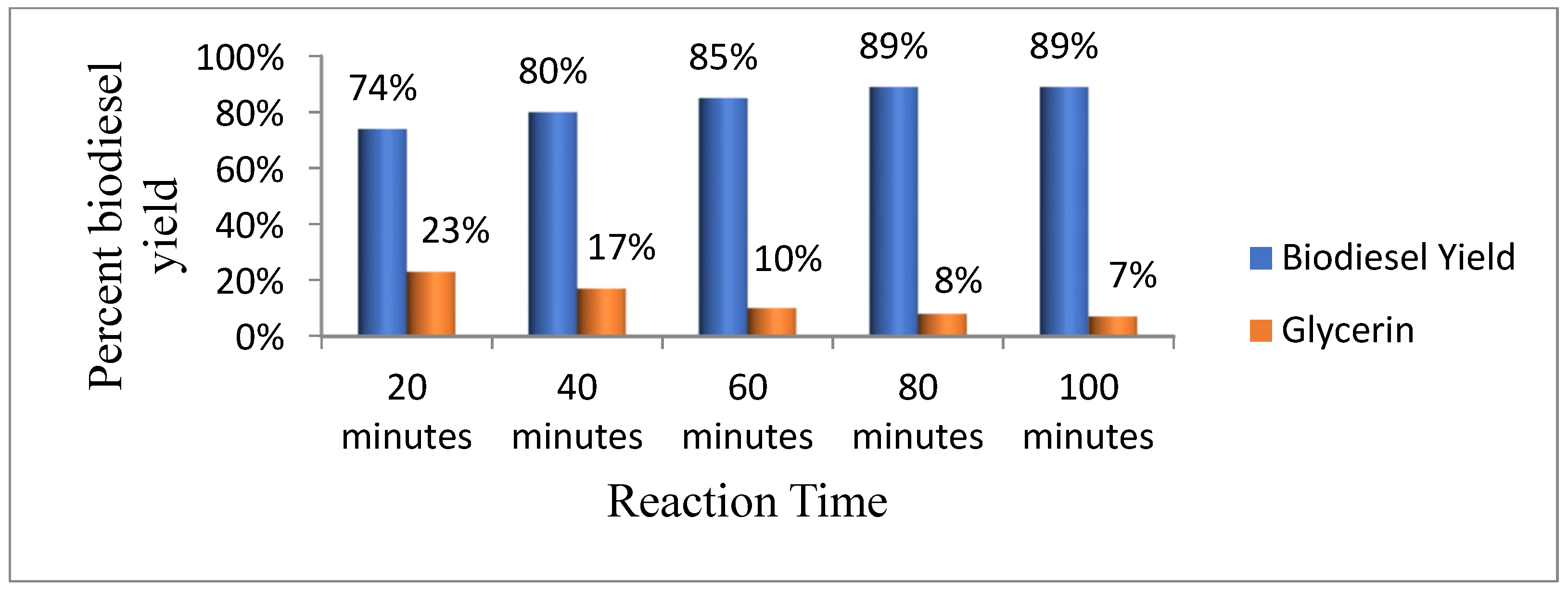
3.4.4. Reaction Time
3.5. Physical and Fuel Properties of Biodiesel
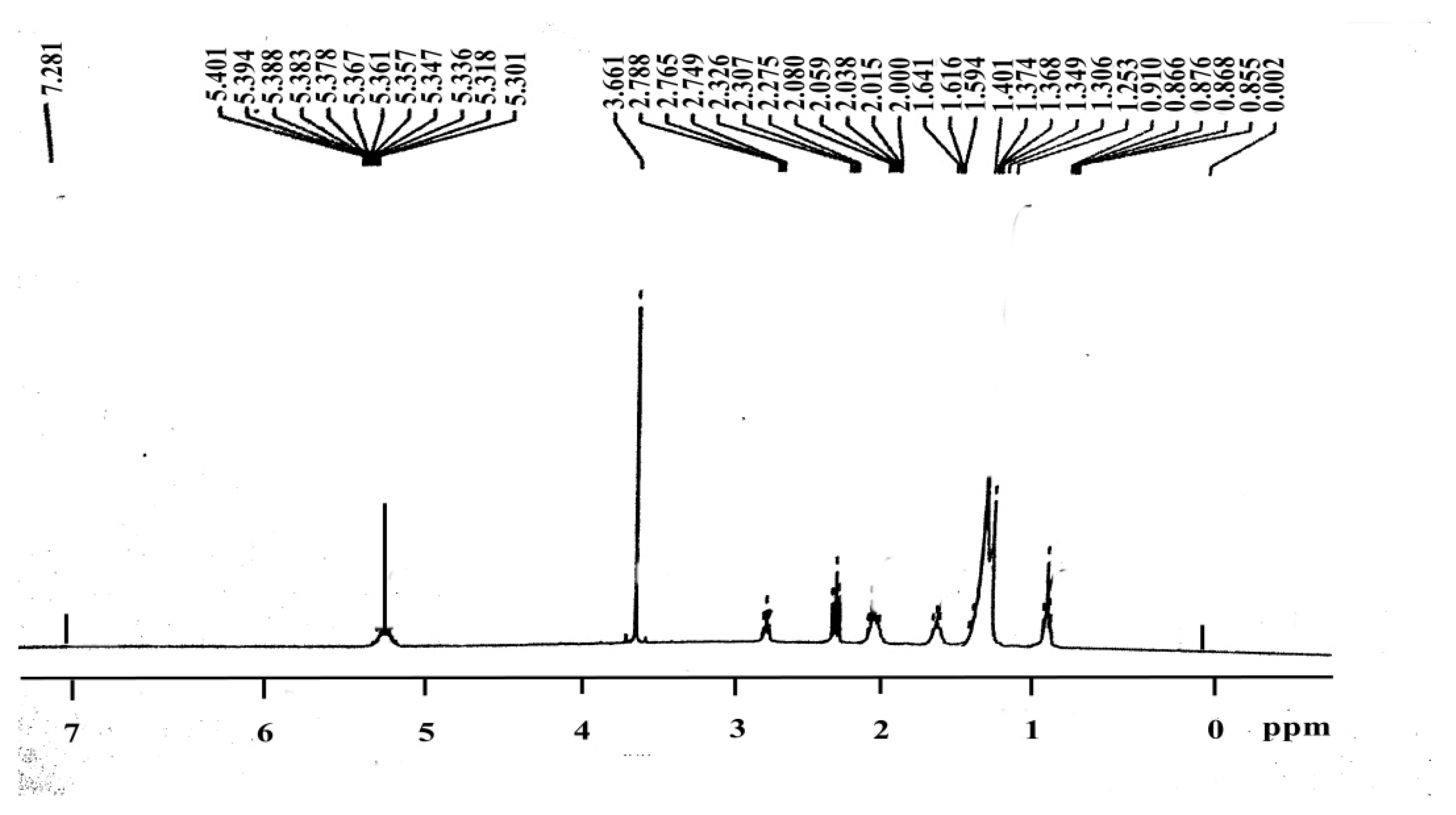
3.6. H NMR of Ricinus communis Biodiesel
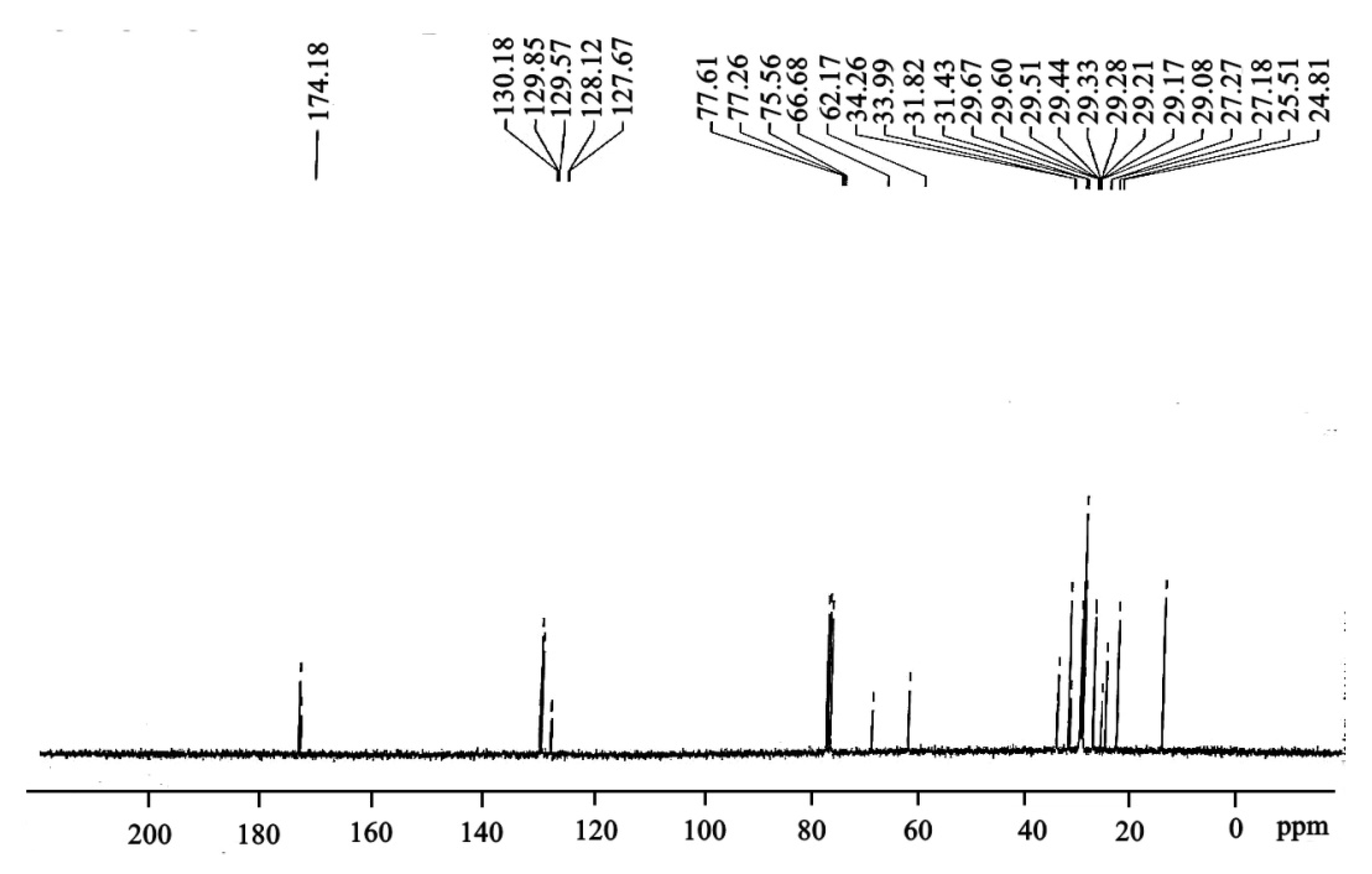
3.7. 13. C-NMR of Ricinus communis Biodiesel
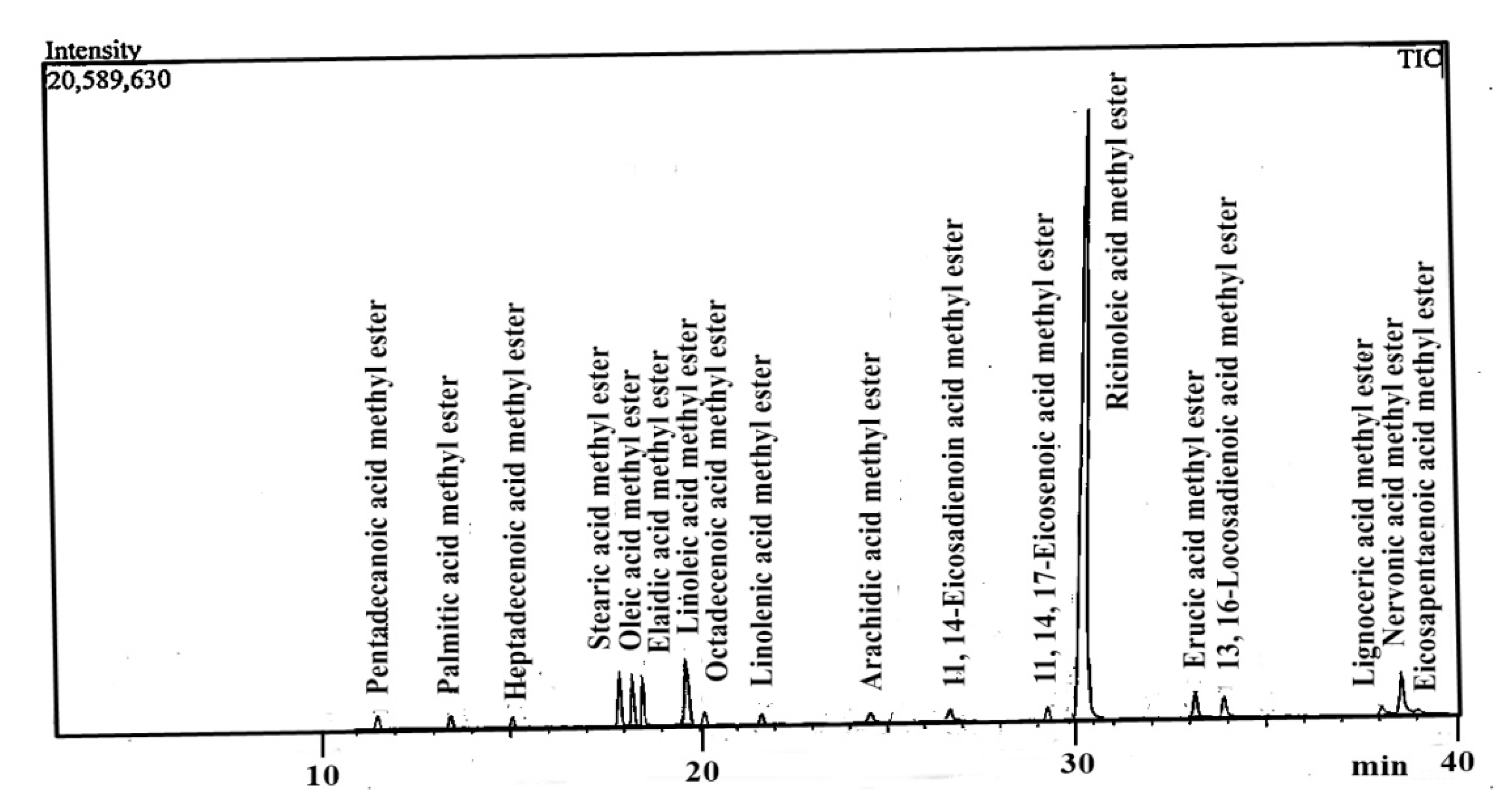
3.8. GC-MS Analysis of Ricinus communis Biodiesel
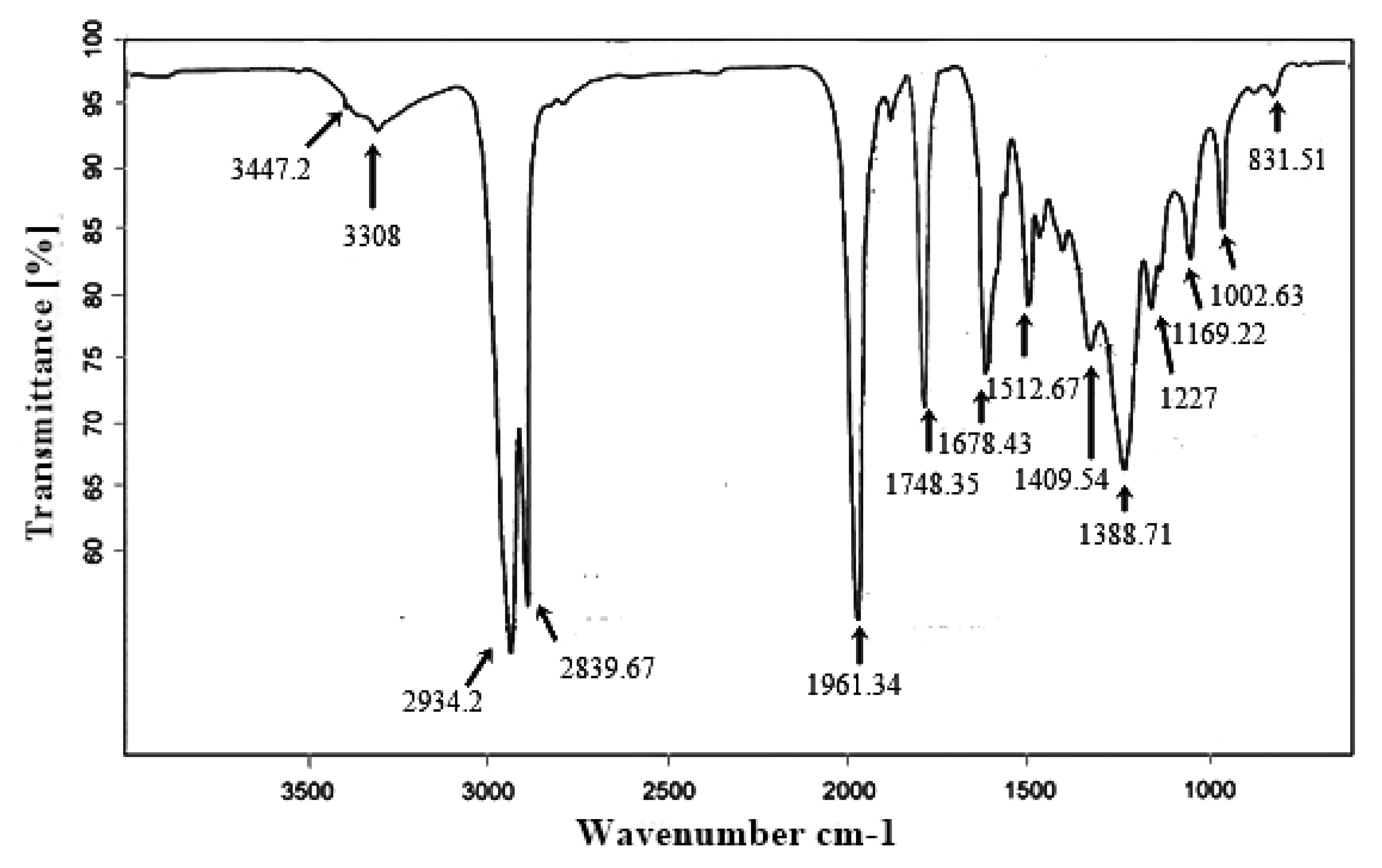
3.9. FT-IR Analysis of the Ricinus communis Biodiesel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASTM | American Society for Testing and Materials |
| CP | Cloud Point |
| FAME | Fatty Acid Methyl Esters |
| FFA | Free Fatty Acid |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| FT-IR spectroscopy | Fourier Transform Infrared Spectroscopy |
| H and C-NMR | Nuclear Magnetic Resonance |
| HHV | Higher Heating Value |
| PMCC | Flash Point (Pensky-Martens Closed Cup) |
| PP | Pour Point |
| SEM | Scanning Electron Microscopy |
| SMB | Silybum Marianum Biodiesel |
| XRD | X-ray Diffraction |
| ZnO | Zinc Oxide |
References
- Qadeer, M.U.; Ayoub, M.; Komiyama, M.; Daulatzai, M.U.K.; Mukhtar, A.; Saqib, S.; Bokhari, A. Review of biodiesel synthesis technologies, current trends, yield influencing factors and economical analysis of supercritical process. J. Clean. Prod. 2021, 309, 127388. [Google Scholar] [CrossRef]
- Bagher, A.M.; Vahid, A.; Mohsen, M.; Reza, B.M. Effect of Using Renewable Energy in Public Health. Am. J. En. Sci. 2016, 3, 1–9. [Google Scholar]
- Narasimhan, M.; Chandrasekaran, M.; Govindasamy, S.; Aravamudhan, A. Heterogeneous nanocatalysts for sustainable biodiesel production: A review. J. Environ. Chem. Eng. 2021, 9, 104876. [Google Scholar] [CrossRef]
- Sharma, V.; Duraisamy, G. Production and characterization of bio-mix fuel produced from a ternary and quaternary mixture of raw oil feedstock. J. Clean. Prod. 2019, 221, 271–285. [Google Scholar] [CrossRef]
- Sharma, V.; Hossain, A.K.; Duraisamy, G. Experimental Investigation of Neat Biodiesels’ Saturation Level on Combustion and Emission Characteristics in a CI Engine. Energies 2021, 14, 5203. [Google Scholar] [CrossRef]
- Ramirez-Ortiz, J.; Martinez, M.; Flores, H. Metakaolinite as a catalyst for biodiesel production from waste cooking oil. Front. Chem. Sci. Eng. 2012, 6, 403–409. [Google Scholar] [CrossRef]
- Toledo Arana, J.; Torres, J.J.; Acevedo, D.F.; Illanes, C.O.; Ochoa, N.A.; Pagliero, C.L. One-step synthesis of CaO-ZnO efficient catalyst for biodiesel production. Int. J. Chem. Eng. 2019, 2019, 1806017. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Gogate, P.R.; Pandit, A.B. Ultrasound-assisted synthesis of biodiesel from palm fatty acid distillate. Ind. Eng. Chem. Res. 2009, 48, 7923–7927. [Google Scholar] [CrossRef]
- Gole, V.L.; Gogate, P.R. Intensification of synthesis of biodiesel from nonedible oils using sonochemical reactors. Ind. Eng. Chem. Res. 2012, 51, 11866–11874. [Google Scholar] [CrossRef]
- Sharma, V.; Hossain, A.K.; Duraisamy, G.; Vijay, M. Transesterification of Pyrolysed Castor Seed Oil in the Presence of CaCu(OCH3)2 Catalyst. Energies 2021, 14, 6064. [Google Scholar] [CrossRef]
- Tahvildari, K.; Anaraki, Y.N.; Fazaeli, R.; Mirpanji, S.; Delrish, E. The study of CaO and MgO heterogenic nano-catalyst coupling on transesterification reaction efficacy in the production of biodiesel from recycled cooking oil. J. Environ. Health Sci. Eng. 2015, 13, 73. [Google Scholar] [CrossRef] [Green Version]
- Bharti, P.; Bhaskar, S.; Dey, R.K. Process optimization of biodiesel production catalyzed by CaO nanocatalyst using response surface methodology. J. Nanostructure Chem. 2019, 9, 269–280. [Google Scholar] [CrossRef]
- Ullah, K.; Jan, H.A.; Ahmad, M.; Ullah, A. Synthesis and structural characterization of biofuel from Cocklebur sp., using zinc oxide nano-particle: A novel energy crop for energy industry. Front. Bioeng. Biotech. 2020, 8, 756. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, Q.; Knothe, G.; Lu, M. Direct transesterification of spent coffee grounds for biodiesel production. Fuel 2017, 199, 157–161. [Google Scholar] [CrossRef]
- Seffati, K.; Honarvar, B.; Esmaeili, H.; Esfandiari, N. Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 2019, 235, 1238–1244. [Google Scholar] [CrossRef]
- Knothe, G. Analyzing biodiesel: Standards and other methods. J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Antolın, G.; Tinaut, F.V.; Briceno, Y.; Castano, V.; Perez, C.; Ramırez, A.I. Optimisation of biodiesel production by sunflower oil transesterification. Bioresour. Technol. 2002, 83, 111–114. [Google Scholar] [CrossRef]
- Holser, R.A.; O’Kuru, R.H. Transesterified milkweed (Asclepias) seed oil as a biodiesel fuel. Fuel 2006, 85, 2106–2110. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Alagar, M. Nano sized copper particles by electrolytic synthesis and characterizations. Int. J. Phy. Sci. 2011, 6, 3662–3671. [Google Scholar] [CrossRef]
- Fan, M.; Liu, Y.; Zhang, P.; Jiang, P. Blocky shapes Ca-Mg mixed oxides as a water-resistant catalyst for effective synthesis of biodiesel by transesterification. Fuel Process. Technol. 2016, 149, 163–168. [Google Scholar] [CrossRef]
- Awual, M.R.; Yaita, T.; Shiwaku, H. Design a novel optical adsorbent for simultaneous ultra-trace cerium (III) detection, sorption and recovery. Chem. Eng. J. 2013, 228, 327–335. [Google Scholar] [CrossRef]
- Flemmer, A.C.; Franchini, M.C.; Lindström, L.I. Description of safflower (Carthamus tinctorius) phenological growth stages according to the extended BBCH scale. Ann. App. Biol. 2015, 166, 331–339. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Akhtar, M.N.; Nisar, N.; Rashid, N. Optimized production and advanced assessment of biodiesel: A review. Int. J. Energy Res. 2018, 42, 2070–2083. [Google Scholar] [CrossRef]
- Worapun, I.; Pianthong, K.; Thaiyasuit, P. Two-step biodiesel production from crude Jatropha curcas L. oil using ultrasonic irradiation assisted. J. Oleo Sci. 2012, 61, 165–172. [Google Scholar] [CrossRef]
- Kumar, K. Standardization of non-edible Pongamia pinnata oil methyl ester conversion using hydroxyl content and GC–MS analysis. J. Taiwan Inst. Chem. Eng. 2013, 45, 1485–1489. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q. Biodiesel production from heterotrophic microalgal oil. Bioresour. Technol. 2006, 97, 841–846. [Google Scholar] [CrossRef]
- Bojan, S.G.; Durairaj, S.K. Producing Biodiesel from High Free Fatty Acid Jatropha Curcas Oil by a Two Step Method—An Indian Case Study. J. Sustain. Energy Environ. 2012, 3, 63–66. [Google Scholar]
- Phan, A.N.; Phan, T.M. Biodiesel production from waste cooking oils. Fuel 2008, 87, 3490–3496. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef]
- Mathiyazhagan, M.; Ganapathi, A. Factors affecting biodiesel production. Res. Plant Biol. 2011, 1, 1–5. [Google Scholar] [CrossRef]
- Ávila Vázquez, V.; Díaz Estrada, R.A.; Aguilera Flores, M.M.; Escamilla Alvarado, C.; Correa Aguado, H.C. Transesterification of non-edible castor oil (Ricinus communis L.) from Mexico for biodiesel production: A physicochemical characterization. Biofuels 2020, 11, 753–762. [Google Scholar] [CrossRef]
- Wang, R.; Hanna, M.A.; Zhou, W.W.; Bhadury, P.S.; Chen, Q.; Song, B.A.; Yang, S. Production and selected fuel properties of biodiesel from promising non-edible oils: Euphorbia lathyris L., Sapium sebiferum L. and Jatropha curcas L. Bioresour. Technol. 2011, 102, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Kaisan, M.U.; Anafi, F.O.; Nuszkowski, J.; Kulla, D.M.; Umaru, S. Calorific value, flash point and cetane number of biodiesel from cotton, jatropha and neem binary and multi-blends with diesel. Biofuels 2017, 11, 321–327. [Google Scholar] [CrossRef]
- Refaat, A.A.; Attia, N.K.; Sibak, H.A.; El Sheltawy, S.T.; El Diwani, G.I. Production optimization and quality assessment of biodiesel from waste vegetable oil. Int. J. Environ. Sci. Technol. 2008, 5, 75–82. [Google Scholar] [CrossRef]
- Dias, J.M.; Alvim-Ferraz, M.; Almeida, M.F. Comparison of the performance of different homogeneous alkali catalysts during transesterification of waste and virgin oils and evaluation of biodiesel quality. Fuel 2008, 87, 3572–3578. [Google Scholar] [CrossRef]
- Knothe, G. Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ. Sci. 2009, 2, 759–766. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.H.; Kalam, M.A.; Rasul, M.G.; Atabani, A.E.; Hazrat, M.A.; Mahmudul, H.M. Effect of biodiesel-diesel blending on physico-chemical properties of biodiesel produced from Moringa oleifera. Procedia Eng. 2015, 105, 665–669. [Google Scholar] [CrossRef]
- Killner, M.H.M.; Linck, Y.G.; Danieli, E.; Rohwedder, J.J.R.; Blümich, B. Compact NMR spectroscopy for real-time monitoring of a biodiesel production. Fuel 2015, 139, 240–247. [Google Scholar] [CrossRef]
- Dutra, E.D.; de Lima, T.A.; de Oliveira Souza, J.L.; Silva, J.G.V.; da Silva Aquino, K.A.; da Silva Aquino, F.; Menezes, R.S.C. Characterization of fat and biodiesel from mango seeds using 1HNMR spectroscopy. Biomass Convers. Biorefinery 2018, 8, 135–141. [Google Scholar] [CrossRef]
- Laskar, I.B.; Rokhum, L.; Gupta, R.; Chatterjee, S. Zinc oxide supported silver nanoparticles as a heterogeneous catalyst for production of biodiesel from palm oil. Environ. Prog. Sustain. Energy 2020, 39, e13369. [Google Scholar] [CrossRef]
- Kalanakoppal Venkatesh, Y.; Mahadevaiah, R.; Haraluru Shankaraiah, L.; Ramappa, S.; Basanagouda, A.S. Preparation of a CaO nanocatalyst and its application for biodiesel production using butea monosperma oil: An optimization study. J. Am. Oil Chem. Soc. 2018, 95, 635–649. [Google Scholar] [CrossRef]
- Berman, P.; Nizri, S.; Wiesman, Z. Castor oil biodiesel and its blends as alternative fuel. Biomass Bioenergy 2011, 35, 2861–2866. [Google Scholar] [CrossRef]
- Ola, P.D.; Karim, R.A.; Suherdin, M.F. The optimum condition for synthesis of biodiesel from castor (Ricinus communis) oil through transesterification reaction. J. Appl. Chem. Sci. 2013, 2, 267–272. [Google Scholar]
- Kundu, A.; Mukherjee, A.; Halder, G.; Datta, D. A kinetic study on acid catalyzed esterification of free fatty acids in Ricinus Communis oil for the production of biodiesel. Int. J. Res. Eng. Technol. 2016, 5, 31–44. [Google Scholar]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Castor oil biodiesel production and optimization. Egypt. J. Pet. 2018, 27, 979–984. [Google Scholar] [CrossRef]
- Palconite, C.L.; Edrolin, A.C.; Lustre, S.N.B.; Manto, A.A.; Caballero, J.R.L.; Tizo, M.S.; Arazo, R.O. Optimization and characterization of bio-oil produced from Ricinus communis seeds via ultrasonic-assisted solvent extraction through response surface methodology. Sustain. Environ. Res. 2018, 28, 444–453. [Google Scholar] [CrossRef]
- Gebrehiwot, H.; Zelelew, D. Ricinus communis Seed Oils as a Source of Biodiesel; A Renewable Form of Future Energy. J. Turk. Chem. Soc. Sect. Chem. 2022, 9, 339–354. [Google Scholar] [CrossRef]
- Soon, L.B.; AZ, M.R.; Hasan, S. Continuous biodiesel production using ultrasound clamp on tubular reactor. Int. J. Automot. Mech. Eng. 2013, 8, 1396–1405. [Google Scholar] [CrossRef]


| Fuel Property | ASTM Methods Used | ASTM D6751 Limit | RCB-B100% | HSD |
|---|---|---|---|---|
| Flash Point °C (PMCC) | D-93 | >130 | 84 | 60–80 |
| Density at 15 °C kg/L | D-4052 | 860–900 | 869 | 832.6 |
| Kinematic Viscosity at 40 °C cSt | D-445 | 1.9.2006 | 3.98 | 2.4 |
| Pour Point °C | D-97-12 | −15 to 16 | −9 | −7 |
| Cloud Point °C | D-2500-11 | −3 to 12 | −4 | −2 |
| Sulphur % wt | D-5453 | 0.005 | 0.0018 | 0.09 |
| Calorific Value kJ/kg | D-5865 | 35,000 | 34,815 | 45,200 |
| Cetane No. | D-613 | 40 | 51 | 48–52 |
| Identified FAME | Formula of FAME | Retention Time | Concentration % |
|---|---|---|---|
| Pentadecanoic acid methyl ester | C15:0 | 11.267 | 01.08 |
| Palmitic acid methyl ester | C16:0 | 13.424 | 01.39 |
| Heptadecenoic acid methyl ester | C17:1 | 15.943 | 0.27 |
| Stearic acid methyl ester | C18:0 | 17.897 | 04.08 |
| Oleic acid methyl ester | C18:1c | 18.375 | 03.49 |
| Elaidic acid methyl ester | C18:1n9t | 18.506 | 02.20 |
| Linoleic acid methyl ester | C18:2c | 19.659 | 05.21 |
| Octadecenoic acid methyl ester | C18:2t | 20.817 | 1.25 |
| Linolenic acid methyl ester | C18:3n3 | 21.743 | 0.91 |
| Arachidic acid methyl ester | C20:0 | 24.661 | 0.81 |
| 11, 14-Eicosadienoic acid methyl ester | C20:2 | 26.948 | 0.39 |
| 11, 14, 17-Eicosenoic acid methyl ester | C20:1n9 | 29.259 | 0.90 |
| Ricinoleic acid methyl ester | C18:1-OH | 30.189 | 67.35 |
| Erucic acid methyl ester | C22:1n9 | 33.91 | 02.41 |
| 13, 16-Docosadienoic acid methyl ester | C22:2c | 35.03 | 01.19 |
| Lignoceric acid methyl ester | C24:0 | 38.08 | 0.82 |
| Nervonic acid methyl ester | C24:1 | 38.611 | 4.97 |
| Eicosapentaenoic acid methyl ester | C20:5n3 | 39.28 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad Jan, H.; Šurina, I.; Zaman, A.; Al-Fatesh, A.S.; Rahim, F.; Al-Otaibi, R.L. Synthesis of Biodiesel from Ricinus communis L. Seed Oil, a Promising Non-Edible Feedstock Using Calcium Oxide Nanoparticles as a Catalyst. Energies 2022, 15, 6425. https://doi.org/10.3390/en15176425
Ahmad Jan H, Šurina I, Zaman A, Al-Fatesh AS, Rahim F, Al-Otaibi RL. Synthesis of Biodiesel from Ricinus communis L. Seed Oil, a Promising Non-Edible Feedstock Using Calcium Oxide Nanoparticles as a Catalyst. Energies. 2022; 15(17):6425. https://doi.org/10.3390/en15176425
Chicago/Turabian StyleAhmad Jan, Hammad, Igor Šurina, Akhtar Zaman, Ahmed S. Al-Fatesh, Fazli Rahim, and Raja L. Al-Otaibi. 2022. "Synthesis of Biodiesel from Ricinus communis L. Seed Oil, a Promising Non-Edible Feedstock Using Calcium Oxide Nanoparticles as a Catalyst" Energies 15, no. 17: 6425. https://doi.org/10.3390/en15176425
APA StyleAhmad Jan, H., Šurina, I., Zaman, A., Al-Fatesh, A. S., Rahim, F., & Al-Otaibi, R. L. (2022). Synthesis of Biodiesel from Ricinus communis L. Seed Oil, a Promising Non-Edible Feedstock Using Calcium Oxide Nanoparticles as a Catalyst. Energies, 15(17), 6425. https://doi.org/10.3390/en15176425







