Dynamic Electric Simulation Model of a Proton Exchange Membrane Electrolyzer System for Hydrogen Production
Abstract
:1. Introduction
- Brezak et al. [30] analyzed the PEM electrolytic stack, not considering its capacitive effect and the power supply variations;
- Yigit and Selamet [27] analyzed the PEM electrolyzer, but did not consider the capacitive effect of the PEM electrolytic stack and the real DC/DC converter, which feeds the PEM electrolyzer.
2. Numerical Simulation Model
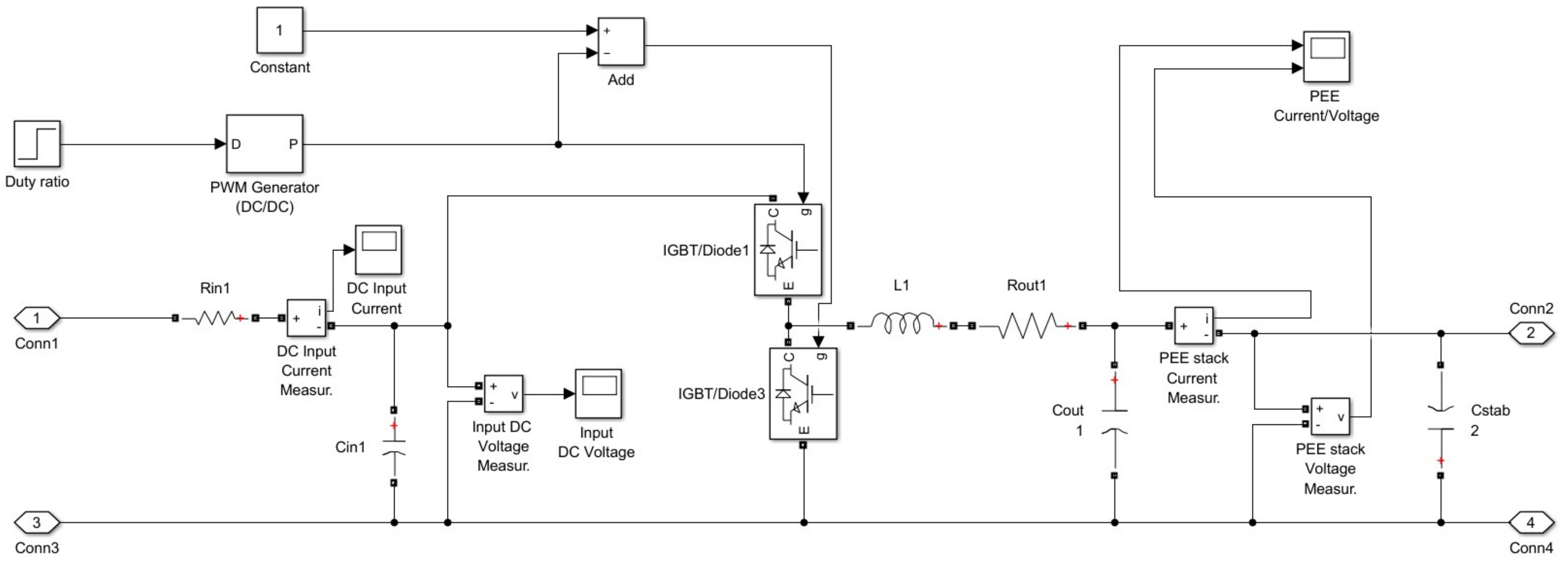
2.1. DC/DC Buck Converter
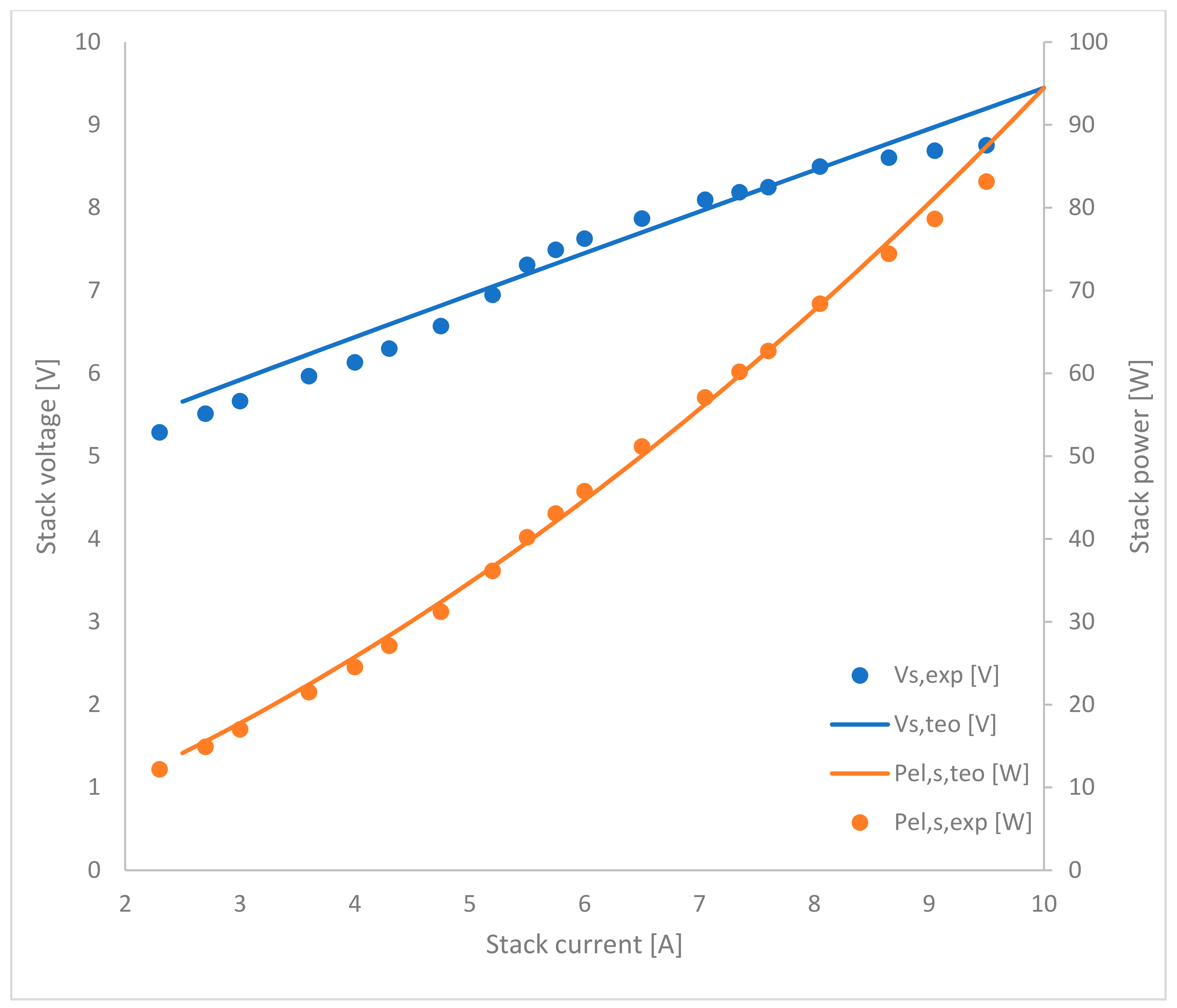
2.2. PEM Electrolytic Stack
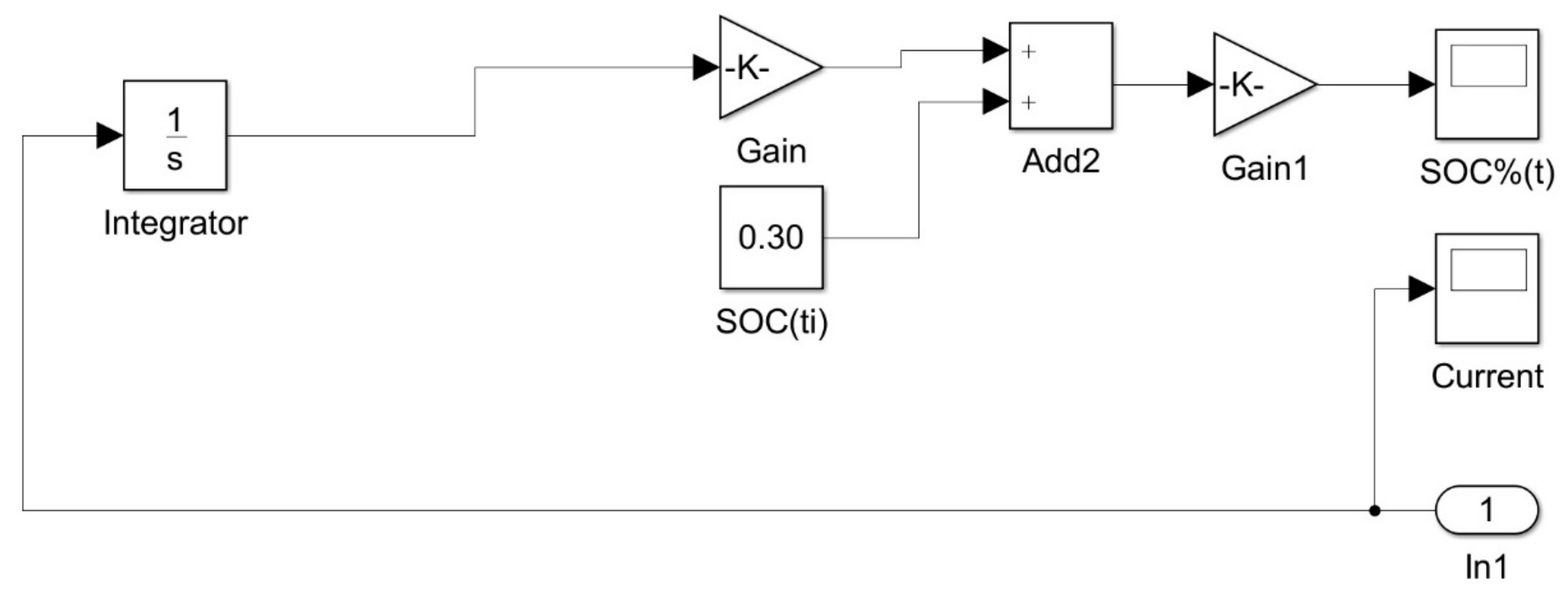
2.3. Hydrogen Storage System
2.4. PEM Electrolyzer System
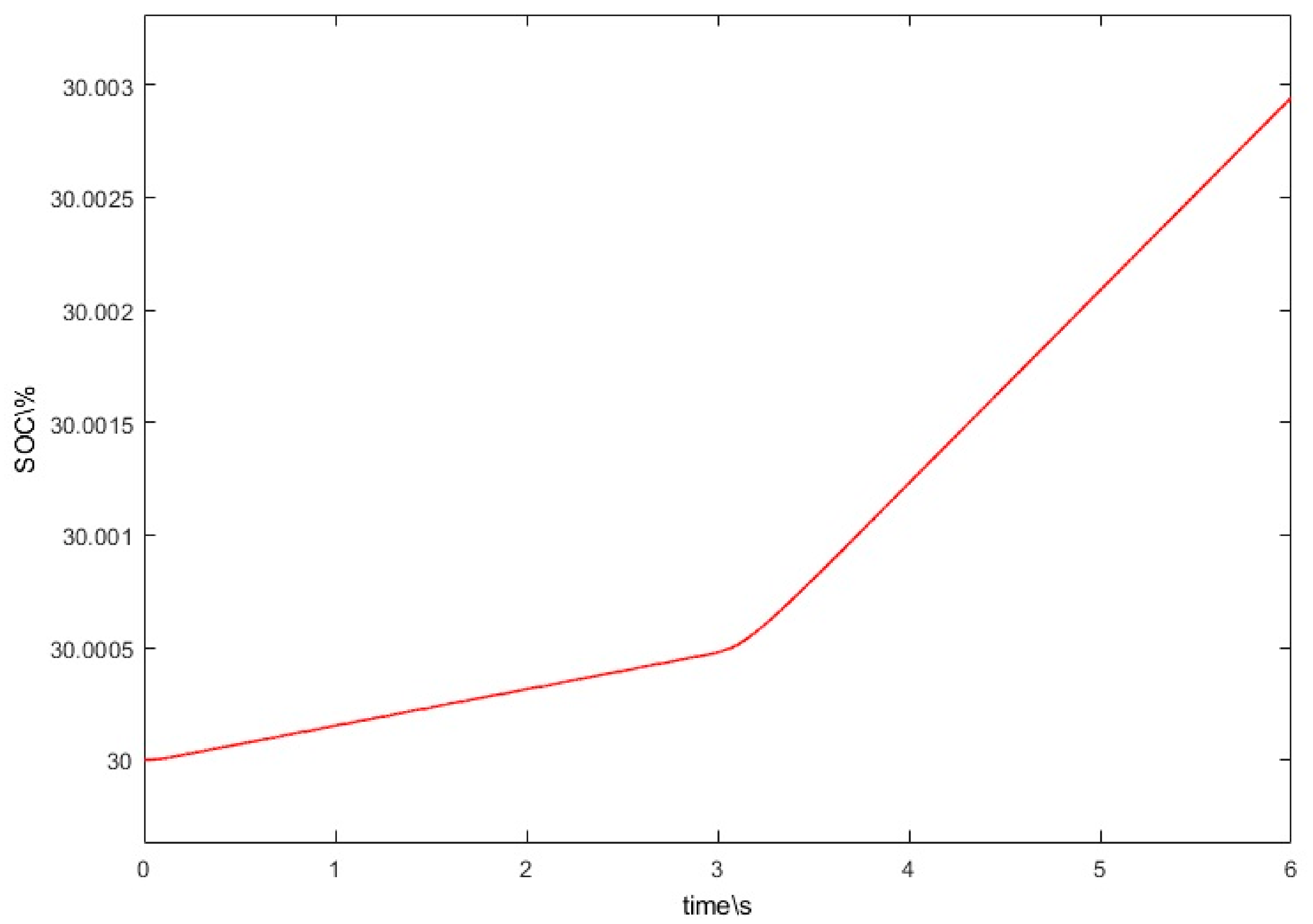
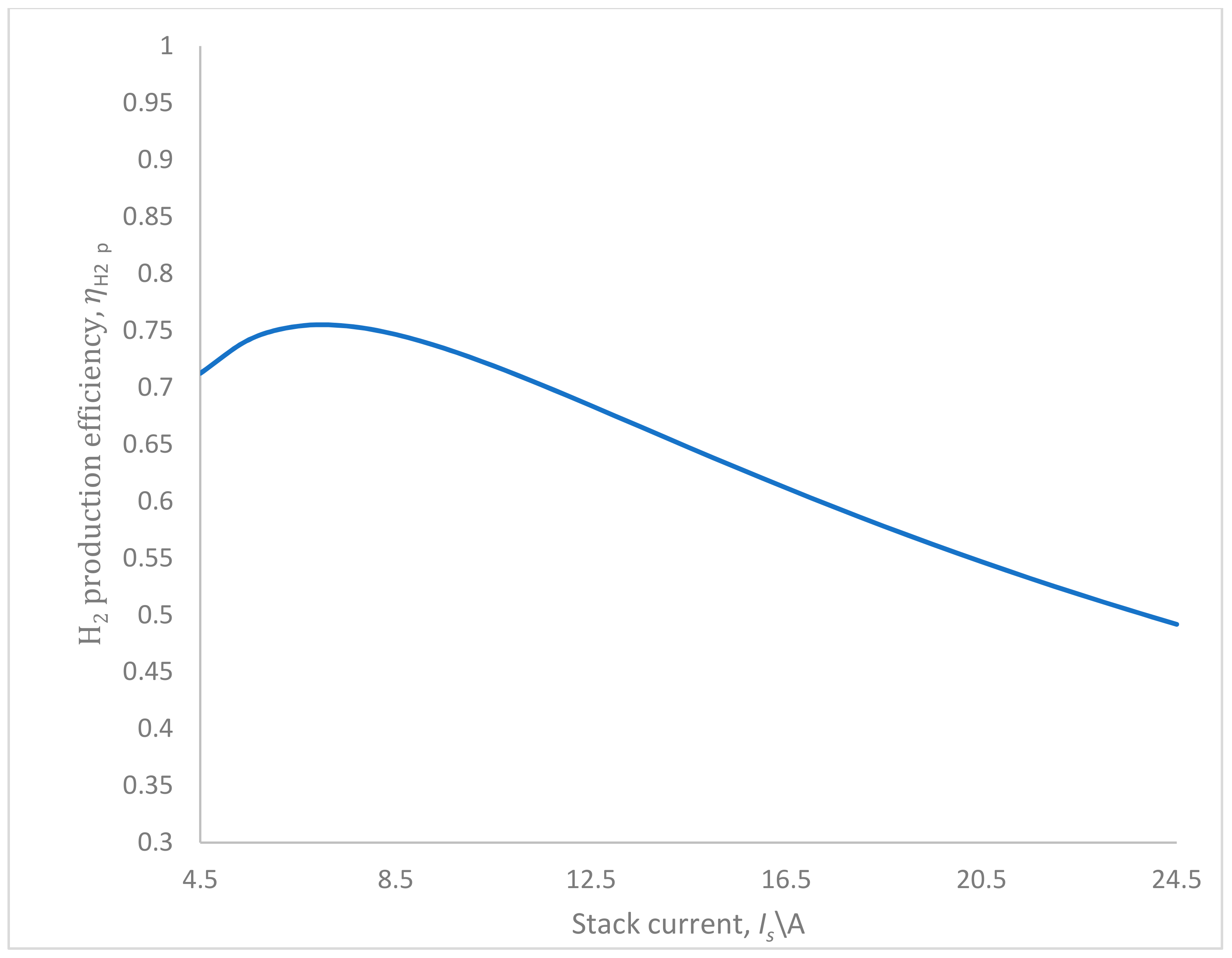
3. Numerical Simulations and Results Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| SYMBOLS | Units | |
| L | inductance | H |
| V | voltage | V |
| D | duty ratio | - |
| f | frequency | Hz |
| ΔI | current variation | A |
| C | capacitance | Fa |
| ΔV | voltage variation or over-voltage | V |
| n | phase number | - |
| t | duration or time | s |
| I | current | A |
| OCV | open circuit voltage | V |
| R | resistance | Ω |
| F | molar flow | mol s−1 |
| Fa | Faraday constant | C mol−1 |
| p | pressure | bar |
| N | number | - |
| SOC | State Of Charge for hydrogen storage system | - |
| P | power | W |
| MW | molecular weight | kg mol−1 |
| η | efficiency | - |
| HHV | high heating value | J kg−1 |
| Vol | Volume | L |
| SUBSCRIPTS | ||
| 1,2 | indices | |
| in, out | at the inlet and at the outlet | |
| sw | switch | |
| L1 | referred to inductance L1 | |
| min, max | minimum and maximum values | |
| step | load step | |
| act | activation polarization | |
| conc | concentration polarization | |
| s | stack | |
| id | ideal | |
| mem | membrane electrolyte | |
| an | anode | |
| cat | cathode | |
| cyl | cylinders | |
| mol | moles of hydrogen | |
| H2 | hydrogen | |
| p | produced or production | |
| el | electric | |
| sys | PEM electrolyzer system | |
| aux | auxiliary | |
| c | electrolytic cell | |
References
- European Commission: Climate Action, Key Targets for 2030. Available online: https://ec.europa.eu/clima/policies/strategies/2030_en (accessed on 19 July 2022).
- European Commission. «Accelerating Clean Energy Innovation» EUR-Lex, Brussels. 2016. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52016DC0763 (accessed on 19 July 2022).
- European Commission. «Delivering a New Deal for Energy Consumers» EUR-Lex, Brussels. 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52015DC0339 (accessed on 19 July 2022).
- Directorate-General for Energy (European Commission); Joint Research Centre (European Commission). «Transforming the European Energy System through Innovation—Integrated Strategic Energy Technology (SET) Plan: Progress in 2016» Luxembourg: Publications Office of the European Union. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/set-plan_progress_2016.pdf (accessed on 19 July 2022).
- Fragiacomo, P.; De Lorenzo, G.; Corigliano, O. Performance analysis of an Intermediate Temperature SOE Test bench under a CO2-H2O feeding stream. Energies 2018, 11, 2276. [Google Scholar] [CrossRef]
- De Lorenzo, G.; Corigliano, O.; Fragiacomo, P. Analysing thermal regime and transient by using numerical modelling for solid oxide electrolyser aided by solar radiation. Int. J. Therm. Sci. 2022, 177, 107545. [Google Scholar] [CrossRef]
- Fragiacomo, P.; De Lorenzo, G.; Corigliano, O. Design of an SOFC/SOE station: Experimental test campaigns. Energy Procedia 2018, 148, 543–550. [Google Scholar] [CrossRef]
- Fragiacomo, P.; Astorino, E.; Chippari, G.; De Lorenzo, G.; Czarnetzki, W.T.; Schneider, W. Anion Exchange Membrane Fuel Cell modeling. Int. J. Sustain. Energy 2018, 37, 340–353. [Google Scholar] [CrossRef]
- Fragiacomo, P.; Astorino, E.; Chippari, G.; De Lorenzo, G.; Czarnetzki, W.T.; Schneider, W. Dynamic Modeling of a Hybrid Electric System based on an Anion Exchange Membrane Fuel Cell. Cogent Eng. 2017, 4, 1357891. [Google Scholar] [CrossRef]
- De Lorenzo, G.; Milewski, J.; Fragiacomo, P. Theoretical and experimental investigation of syngas-fueled Molten Carbonate Fuel Cell for Assessment of its performance. Int. J. Hydrogen Energy 2017, 42, 28816–28828. [Google Scholar] [CrossRef]
- De Lorenzo, G.; Fragiacomo, P. Technical Analysis of an eco-friendly hybrid plant with a micro gas turbine and a MCFC system. Fuel Cells 2010, 10, 194–208. [Google Scholar] [CrossRef]
- Calderón, A.J.; Vivas, F.J.; Segura, F.; Andújar, J.M. Integration of a Multi-Stack Fuel Cell System in Microgrids: A Solution Based on Model Predictive Control. Energies 2020, 13, 4924. [Google Scholar] [CrossRef]
- Silaa, M.Y.; Derbeli, M.; Barambones, O.; Cheknane, A. Design and Implementation of High Order Sliding Mode Control for PEMFC Power System. Energies 2020, 13, 4317. [Google Scholar] [CrossRef]
- De Lorenzo, G.; Andaloro, L.; Sergi, F.; Napoli, G.; Ferraro, M.; Antonucci, V. Numerical simulation model for the preliminary design of hybrid electric city bus propulsion system with polymer electrolyte fuel cell. Int. J. Hydrogen Energy 2014, 39, 12934–12947. [Google Scholar] [CrossRef]
- De Luca, D.; Fragiacomo, P.; De Lorenzo, G.; Czarnetzki, W.T.; Schneider, W. Strategies for Dimensioning Two-Wheeled Fuel Cell Hybrid Electric Vehicles Using Numerical Analysis Software. Fuel Cells 2016, 16, 628–639. [Google Scholar] [CrossRef]
- Fragiacomo, P.; Piraino, F. Fuel cell hybrid powertrains for use in Southern Italian railways. Int. J. Hydrogen Energy 2019, 44, 27930–27946. [Google Scholar] [CrossRef]
- Nazir, M.S.; Ahmad, I.; Khan, M.J.; Ayaz, Y.; Armghan, H. Adaptive Control of Fuel Cell and Supercapacitor Based Hybrid Electric Vehicles. Energies 2020, 13, 5587. [Google Scholar] [CrossRef]
- Sampietro, J.L.; Puig, V.; Costa-Castelló, R. Optimal Sizing of Storage Elements for a Vehicle Based on Fuel Cells, Supercapacitors and Batteries. Energies 2019, 12, 925. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442. [Google Scholar] [CrossRef]
- Mandal, M.; Valls, A.; Gangnus, N.; Secanell, M. Analysis of Inkjet Printed Catalyst Coated Membranes for Polymer Electrolyte Electrolyzers. J. Electrochem. Soc. 2018, 165, F543–F552. [Google Scholar] [CrossRef]
- Li, R.; Cai, Y.; Reimer, U.; Wippermann, K.; Shao, Z.; Lehnert, W. CrN/Cr-Coated Steel Plates for High-Temperature Polymer Electrolyte Fuel Cells: Performance and Durability. J. Electrochem. Soc. 2020, 167, 144507. [Google Scholar] [CrossRef]
- Liang, M.; Fu, C.; Xiao, B.; Luo, L.; Wang, Z. A fractal study for the effective electrolyte diffusion through charged porous media. Int. J. Heat Mass Transf. 2019, 137, 365–371. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, W.; Zhang, X.; Long, G.; Fan, F.; Chen, H.; Deng, L. A novel fractal solution for permeability and Kozeny-Carman constant of fibrous porous media made up of solid particles and porous fibers. Powder Technol. 2019, 349, 92–98. [Google Scholar] [CrossRef]
- Weiß, A.; Siebel, A.; Bernt, M.; Shen, T.H.; Tileli, V.; Gasteiger, H.A. Impact of Intermittent Operation on Lifetime and Performance of a PEM Water Electrolyzer. J. Electrochem. Soc. 2019, 166, F487–F497. [Google Scholar] [CrossRef] [Green Version]
- Awasthi, A.; Scott, K.; Basu, S. Dynamic modeling and simulation of a proton exchange membrane electrolyzer for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 14779–14786. [Google Scholar] [CrossRef]
- Guilbert, D.; Vitale, G. Dynamic Emulation of a PEM Electrolyzer by Time Constant Based Exponential Model. Energies 2019, 12, 750. [Google Scholar] [CrossRef]
- Yigit, T.; Selamet, O.F. Mathematical modeling and dynamic Simulink simulation of high-pressure PEM electrolyzer system. Int. J. Hydrogen Energy 2016, 41, 13901–13914. [Google Scholar] [CrossRef]
- Görgün, H. Dynamic modelling of a proton exchange membrane (PEM) electrolyzer. Int. J. Hydrogen Energy 2006, 31, 29–38. [Google Scholar] [CrossRef]
- Hernandez-Gomez, A.; Ramirez, V.; Guilbert, D.; Saldivar, B. Development of an adaptive static-dynamic electrical model based on input electrical energy for PEM water electrolysis. Int. J. Hydrogen Energy 2020, 45, 18817–18830. [Google Scholar] [CrossRef]
- Brezak, D.; Kovač, A.; Firak, M. MATLAB/Simulink simulation of low-pressure PEM electrolyzer stack. Int. J. Hydrogen Energy 2022, in press. [Google Scholar] [CrossRef]
- Mohan, N.; Undeland, T.M.; Robbins, W.P. Power Electronics: Converters, Applications and Design, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Ejury, J.; Buck Converter Design. Infineon Technologies North America (IFNA) Corporation. Design Note DN 2013-01 V1.0 January 2013. Available online: https://www.infineon.com (accessed on 17 July 2022).
- Larminie, J.; Dicks, A. Fuel Cell Systems Explained, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2002. [Google Scholar]
- Hernandez-Gomez, A.; Ramirez, V.; Guilbert, D. Investigation of PEM Electrolyzer modeling: Electrical domain, efficiency and specific energy consumption. Int. J. Hydrogen Energy 2020, 45, 14625–14639. [Google Scholar] [CrossRef]
- Li, X. Principles of Fuel Cells, 2nd ed.; Taylor & Francis: New York, NY, USA, 2006. [Google Scholar]



| Parameter | Unit | Value |
|---|---|---|
| Rated electrical power | W | 400 |
| Stack operating voltage range | V | 7.5–8 |
| Stack current range | A | 0–50 |
| Output H2 pressure | bar | 0.1–10.5 |
| Cell number | - | 3 |
| Active area section | cm2 | 50 |
| Parameter | Unit | Value |
|---|---|---|
| Hz | 1000 | |
| H | 0.107 | |
| Ω | 0.02 | |
| Ω | 0.002 | |
| Fa | 0.023 | |
| Fa | 4.62 × 10−5 |
| Parameter | Unit | Value |
|---|---|---|
| A | 4.5 | |
| A | 24.5 | |
| Ω | 0.8299 | |
| Ω | −0.6774 | |
| Ω | 0.0119 | |
| Ω | −0.0075 | |
| Ω | 0.1607 | |
| Ω | −0.0001 | |
| Ω | 0.0465 | |
| Ω | −0.0303 | |
| - | 3 | |
| Fa | 0.05 | |
| Fa | 0.05 |
| Parameter | Unit | Value |
|---|---|---|
| - | 3 | |
| mol | 34 | |
| bar | 16 | |
| L | 50 | |
| - | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lorenzo, G.; Agostino, R.G.; Fragiacomo, P. Dynamic Electric Simulation Model of a Proton Exchange Membrane Electrolyzer System for Hydrogen Production. Energies 2022, 15, 6437. https://doi.org/10.3390/en15176437
De Lorenzo G, Agostino RG, Fragiacomo P. Dynamic Electric Simulation Model of a Proton Exchange Membrane Electrolyzer System for Hydrogen Production. Energies. 2022; 15(17):6437. https://doi.org/10.3390/en15176437
Chicago/Turabian StyleDe Lorenzo, Giuseppe, Raffaele Giuseppe Agostino, and Petronilla Fragiacomo. 2022. "Dynamic Electric Simulation Model of a Proton Exchange Membrane Electrolyzer System for Hydrogen Production" Energies 15, no. 17: 6437. https://doi.org/10.3390/en15176437






