Experimental Study on the Effect of SDS and Micron Copper Particles Mixture on Carbon Dioxide Hydrates Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus
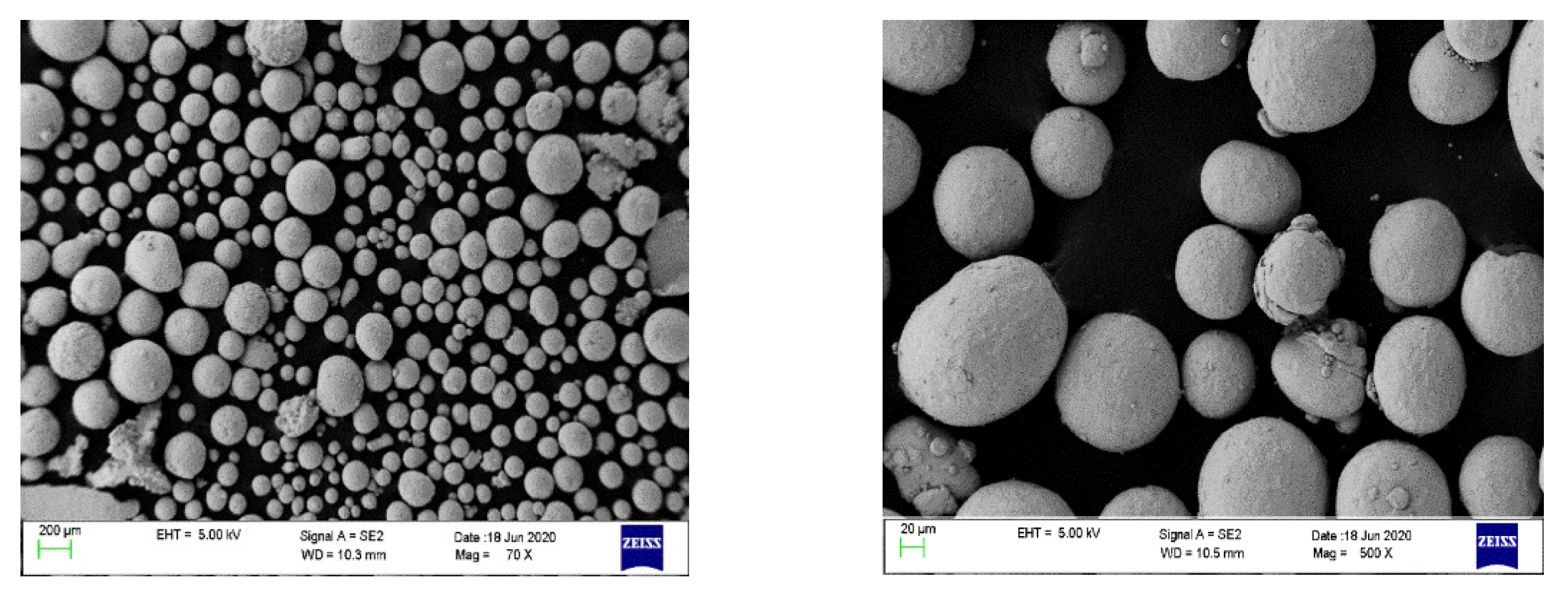
2.2. Materials
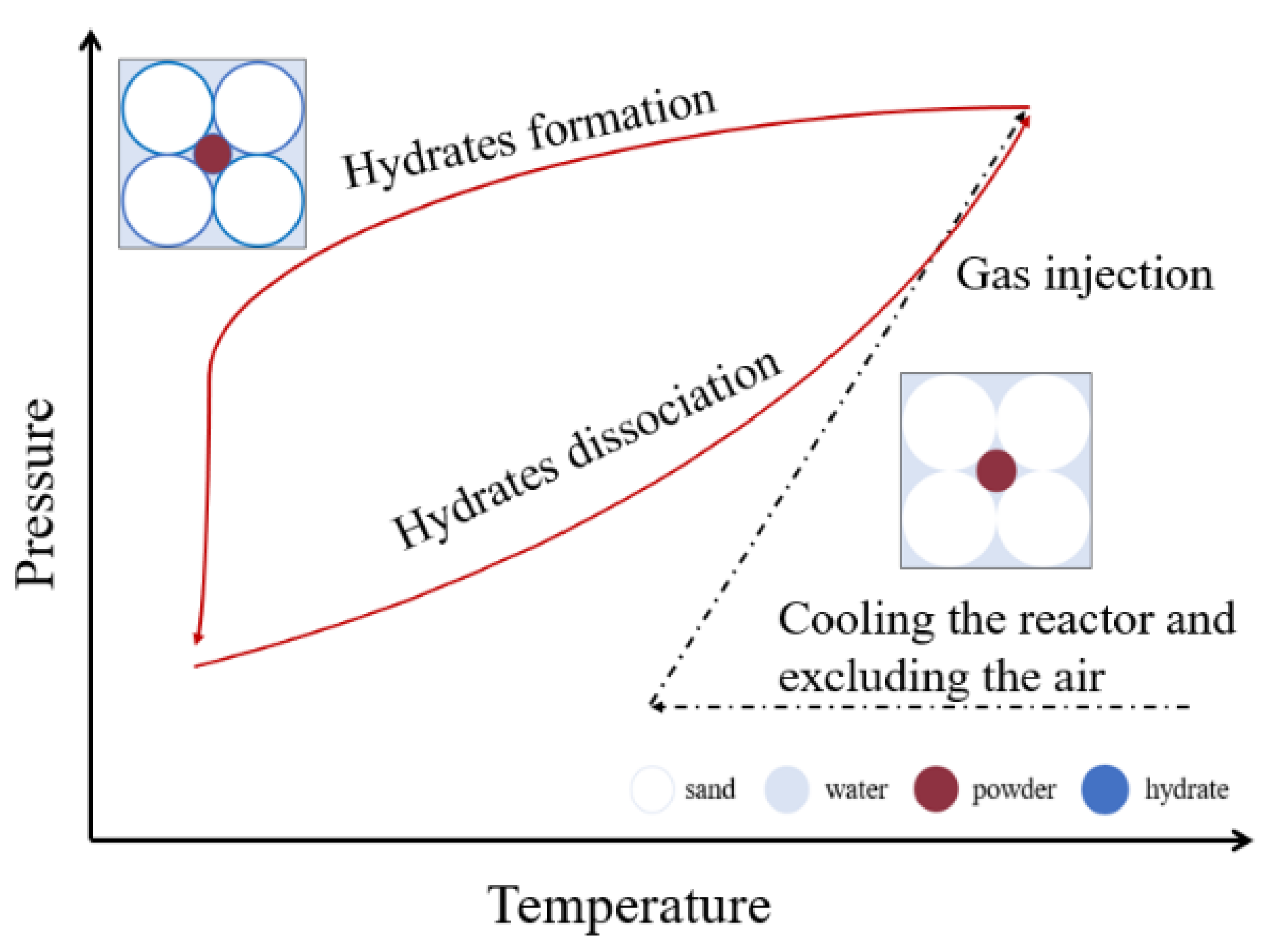
2.3. Procedure
2.4. Data Processing
3. Results and Discussion
3.1. Morphology of CO2 Hydrate Formed in SDS Solution
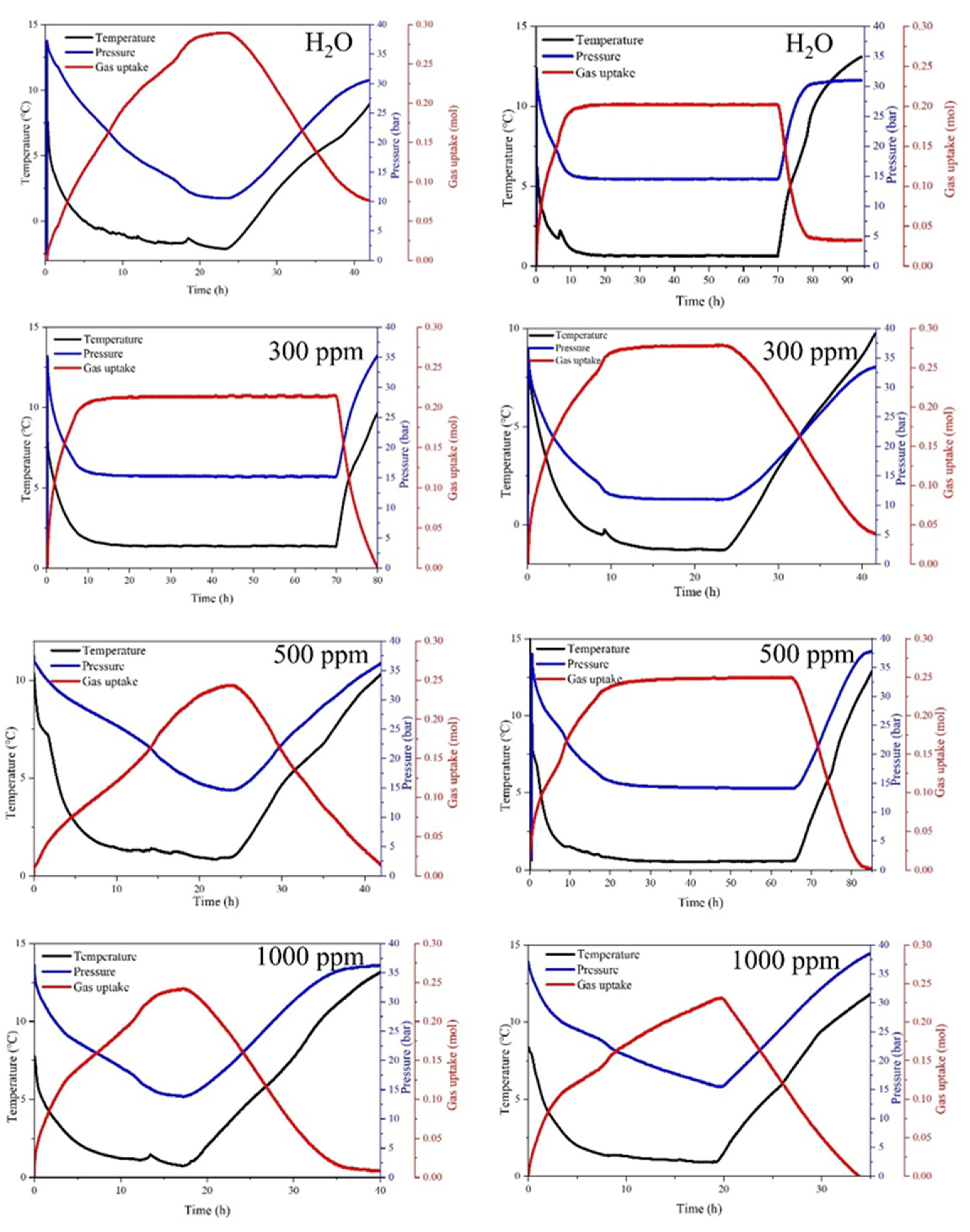
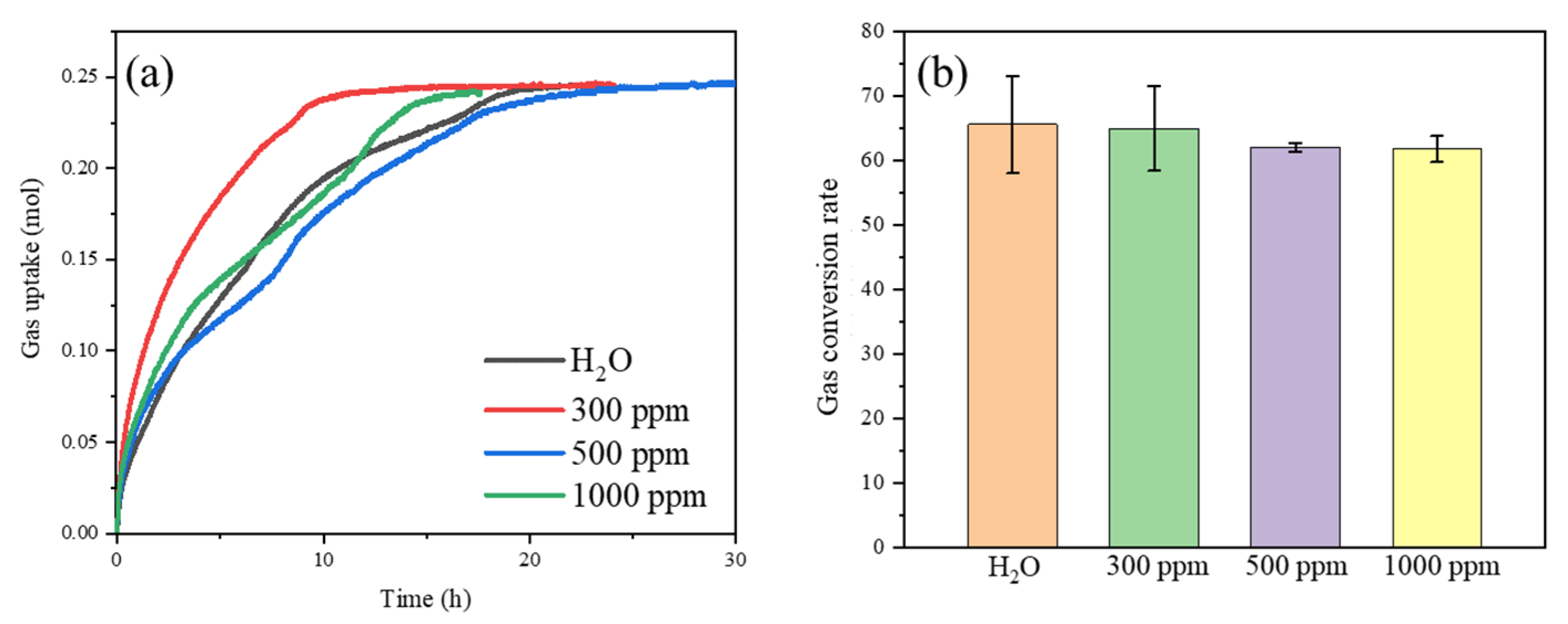
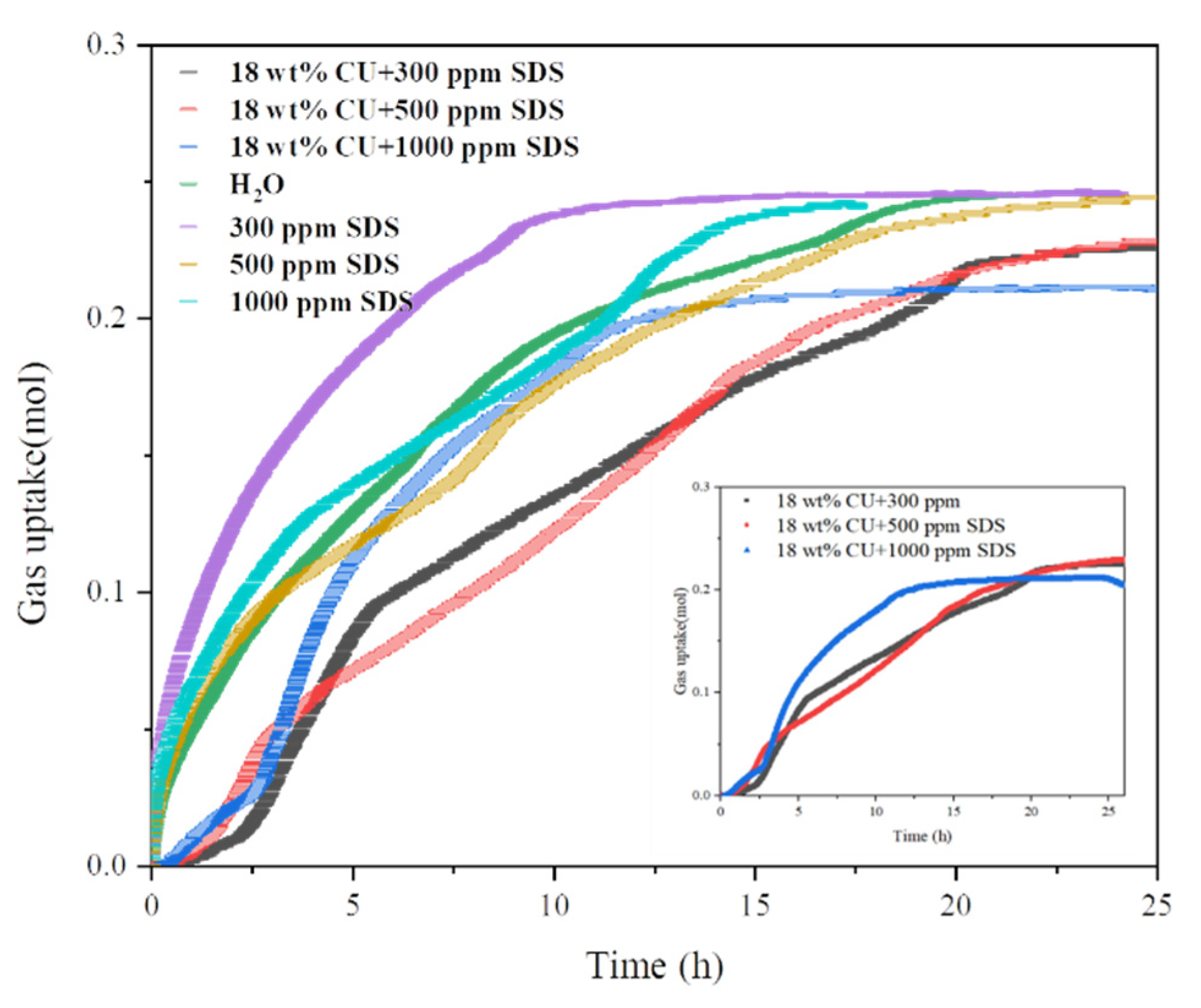
3.2. Effect of SDS on CO2 Hydrate Formation
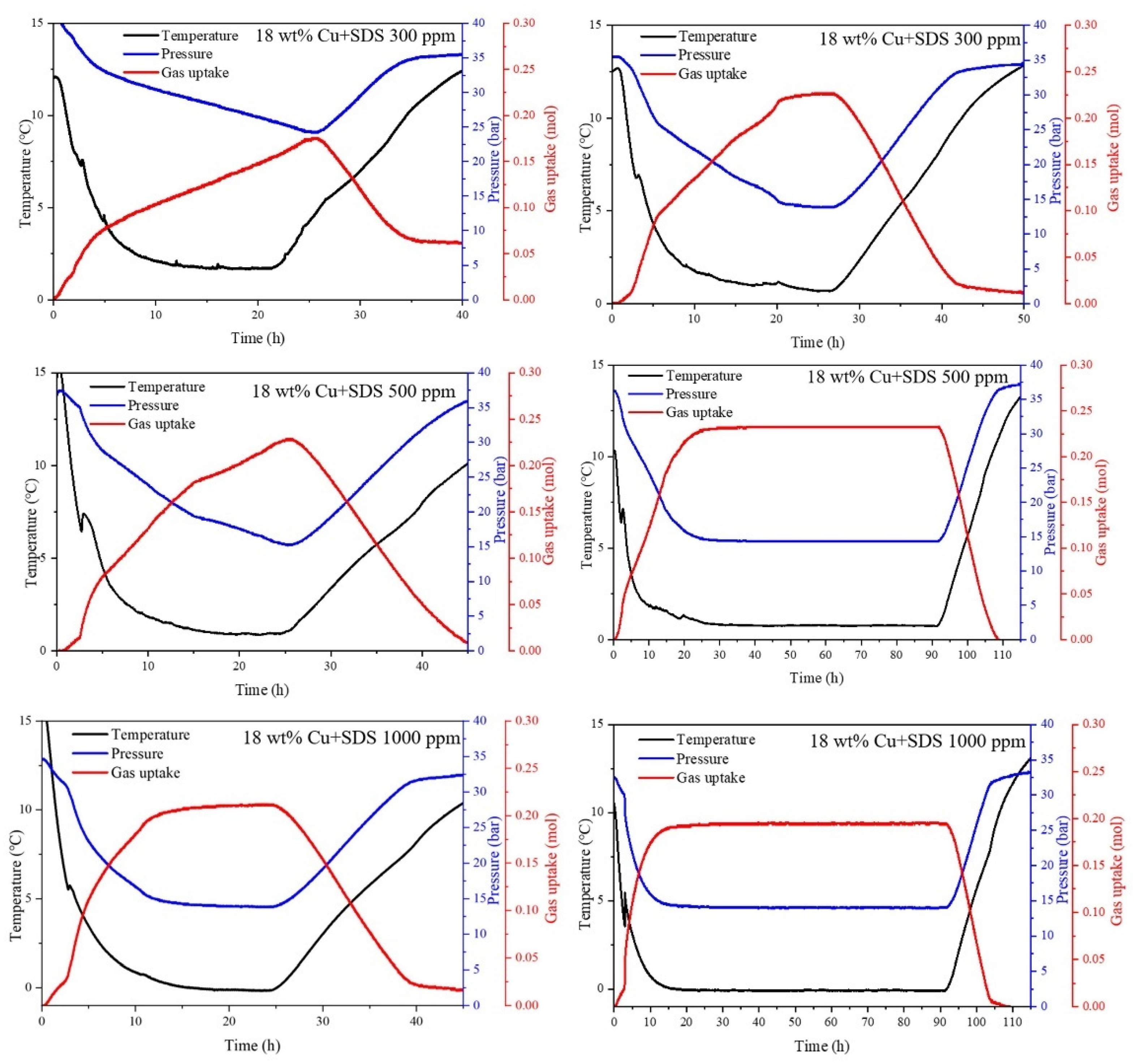
3.3. Effect of SDS + Micron Cu on CO2 Hydrate Formation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrew, R.M. Global CO2 Emissions from Cement Production, 1928–2017. Earth Syst. Sci. Data 2018, 10, 2213–2239. [Google Scholar] [CrossRef]
- Kim, Y.; Tanaka, K.; Matsuoka, S. Environmental and economic effectiveness of the Kyoto Protocol. PLoS ONE 2020, 15, 0236299. [Google Scholar] [CrossRef]
- Jones, C.D.; Friedlingstein, P. Quantifying process-level uncertainty contributions to TCRE and carbon budgets for meeting Paris Agreement climate targets. Environ. Res. Lett. 2020, 15, 074019. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, X.; Zhang, X. The 2 °C Global Temperature Target and the Evolution of the Long-Term Goal of Addressing Climate Change—From the United Nations Framework Convention on Climate Change to the Paris Agreement. Engineering 2017, 3, 272–278. [Google Scholar] [CrossRef]
- Webley, P.A. Adsorption technology for CO2 separation and capture: A perspective. Adsorption 2014, 20, 225–231. [Google Scholar] [CrossRef]
- Arora, V.; Saran, R.K.; Kumar, R.; Yadav, S. Separation and sequestration of CO2 in geological formations. Mater. Sci. Technol. 2019, 2, 647–656. [Google Scholar] [CrossRef]
- Koide, H.; Takahashi, M.; Tsukamoto, H.; Shindo, Y. Self-trapping mechanisms of carbon dioxide in the aquifer disposal. Energy Convers. Manag. 1995, 36, 505–508. [Google Scholar] [CrossRef]
- Nakashiki, N. Lake-type storage concepts for CO2 disposal option. Waste Manag. 1998, 17, 361–367. [Google Scholar] [CrossRef]
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef]
- Englezos, P. Clathrate hydrates. Ind. Eng. Chem. Res. 1993, 32, 1251–1274. [Google Scholar] [CrossRef]
- Qureshi, M.F.; Zheng, J.; Khandelwal, H.; Venkataraman, P.; Usadi, A.; Barckholtz, T.A.; Mhadeshwar, A.B.; Linga, P. Laboratory demonstration of the stability of CO2 hydrates in deep-oceanic sediments. Chem. Eng. J. 2022, 432, 134290. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.-S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Zheng, J.; Chong, Z.R.; Qureshi, M.F.; Linga, P. Carbon dioxide sequestration via gas hydrates: A potential pathway toward decarbonization. Energy Fuels 2020, 34, 10529–10546. [Google Scholar] [CrossRef]
- Palodkar, A.V.; Jana, A.K. Modeling recovery of natural gas from hydrate reservoirs with carbon dioxide sequestration: Validation with Iġnik Sikumi field data. Sci. Rep. 2019, 9, 18901. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.F.; Dhamu, V.; Usadi, A.; Barckholtz, T.A.; Mhadeshwar, A.B.; Linga, P. CO2 Hydrate Formation Kinetics and Morphology Observations Using High-Pressure Liquid CO2 Applicable to Sequestration. Energy Fuels 2022. [Google Scholar] [CrossRef]
- Song, Y.; Wang, S.; Cheng, Z.; Huang, M.; Zhang, Y.; Zheng, J.; Jiang, L.; Liu, Y. Dependence of the hydrate-based CO2 storage process on the hydrate reservoir environment in high-efficiency storage methods. Chem. Eng. J. 2021, 415, 128937. [Google Scholar] [CrossRef]
- Kalogerakis, N.; Jamaluddin, A.K.M.; Dholabhai, P.D.; Bishno, P.R. Effect of Surfactants on Hydrate Formation Kinetics. In Proceedings of the SPE International Symposium on Onfield Chemistry, New Orleans, LA, USA, 2–5 March 1993; pp. 375–383. [Google Scholar]
- He, Y.; Sun, M.-T.; Chen, C.; Zhang, G.-D.; Chao, K.; Lin, Y.; Wang, F. Surfactant-based promotion to gas hydrate formation for energy storage. J. Mat. Chem. A 2019, 7, 21634–21661. [Google Scholar] [CrossRef]
- Link, D.D.; Ladner, E.P.; Elsen, H.A.; Taylor, C.E. Formation and dissociation studies for optimizing the uptake of methane by methane hydrates. Fluid Phase Equilibr. 2003, 211, 1–10. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Presciutti, A.; Rossi, F. Review on the characteristics and advantages related to the use of flue-gas as CO2/N2 mixture for gas hydrate production. Fluid Phase Equilibr. 2021, 541, 113077. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, P.; Linga, P. Semiclathrate hydrate process for pre-combustion capture of CO2 at near ambient temperatures. Appl. Energy 2017, 194, 267–278. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Chen, F.-L.; Yu, S.-J.; Wang, F. Biopromoters for gas hydrate formation: A mini review of current status. Front. Chem. 2020, 8, 514. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Lal, B.; Osei, H.; Sabil, K.M.; Mukhtar, H. A review on the role of amino acids in gas hydrate inhibition, CO2 capture and sequestration, and natural gas storage. J. Nat. Gas Sci. Eng. 2019, 64, 52–71. [Google Scholar] [CrossRef]
- Liu, X.; Ren, J.; Chen, D.; Yin, Z. Comparison of SDS and L-Methionine in promoting CO2 hydrate kinetics: Implication for hydrate-based CO2 storage. Chem. Eng. J. 2022, 438, 135504. [Google Scholar] [CrossRef]
- Mohammadi, A.; Manteghian, M.; Haghtalab, A.; Mohammadi, A.H.; Rahmati-Abkenar, M. Kinetic study of carbon dioxide hydrate formation in presence of silver nanoparticles and SDS. Chem. Eng. J. 2014, 237, 387–395. [Google Scholar] [CrossRef]
- Najibi, H.; Mirzaee Shayegan, M.; Heidary, H. Experimental investigation of methane hydrate formation in the presence of copper oxide nanoparticles and SDS. J. Nat. Gas Sci. Eng. 2015, 23, 315–323. [Google Scholar] [CrossRef]
- Nesterov, A.N.; Reshetnikov, A.M.; Manakov, A.Y.; Rodionova, T.V.; Paukshtis, E.A.; Asanov, I.P.; Bardakhanov, S.P.; Bulavchenko, A.I. Promotion and inhibition of gas hydrate formation by oxide powders. J. Mol. Liq. 2015, 204, 118–125. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Stornelli, G.; Di Schino, A.; Rossi, F. Methane and Carbon Dioxide Hydrate Formation and Dissociation in Presence of a Pure Quartz Porous Framework Impregnated with CuSn12 Metallic Powder: An Experimental Report. Materials 2021, 14, 5115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, D.; Guo, K.; Wang, R.; Fan, S. Formation and dissociation of HFC134a gas hydrate in nano-copper suspension. Energy Conv. Manag. 2006, 47, 201–210. [Google Scholar] [CrossRef]
- Nashed, O.; Partoon, B.; Lal, B.; Sabil, K.M.; Shariff, A.M. Review the impact of nanoparticles on the thermodynamics and kinetics of gas hydrate formation. J. Nat. Gas Sci. Eng. 2018, 55, 452–465. [Google Scholar] [CrossRef]
- Morimoto, T.; Koga, Y.; Suzuki, R.; Takeya, S.; Inada, T.; Kumano, H. Effect of metal particles on promoting the nucleation of tetra-n-butylammonium semiclathrate hydrate. J. Int. Acad. Refrig. 2021, 121, 136–142. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, H.; Zhou, J.; Yang, L.; Liu, D. Molecular dynamics simulations on formation of CO2 hydrate in the presence of metal particles. J. Mol. Liq. 2021, 331, 113793. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Stornelli, G.; Di Schino, A.; Rossi, F. Formation and Dissociation of CH4 and CO2 Hydrates in Presence of a Sediment Composed by Pure Quartz Mixed with Ti23 Particles. Materials 2022, 15, 1470. [Google Scholar] [CrossRef] [PubMed]
- Gambelli, A.M.; Stornelli, G.; Di Schino, A.; Rossi, F. Methane and carbon dioxide hydrates properties in presence of Inconel 718 particles: Analyses on its potential application in gas separation processes to perform efficiency improvement. J. Env. Chem. Eng. 2021, 9, 106571. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Di Schino, A.; Rossi, F. The effect of FeSi3 particles on methane and carbon dioxide hydrates: Experimental evidences and comparison with the current theory. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management (SGEM), Albena, Bulgaria, 16–22 August 2021; Volume 21, pp. 19–31. [Google Scholar]
- Gambelli, A.M.; Di Schino, A.; Rossi, F. Experimental characterization of CH4 and CO2 hydrates formation in presence of porous quartz and Cu gas-atomized particles: Thermodynamic analyses and evidences about the feasibility of CH4/CO2 reverse replacement. Chem. Eng. Res. Des. 2022, 186, 511–524. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Rezaei, S.; Khanlarkhani, M.; Manteghian, M.; Mohammadi, A.H. Kinetic study of methane hydrate formation in the presence of copper nanoparticles and CTAB. J. Nat. Gas Sci. Eng. 2016, 34, 803–810. [Google Scholar] [CrossRef]
- Nasir, Q.; Suleman, H.; Elsheikh, Y.A. A review on the role and impact of various additives as promoters/inhibitors for gas hydrate formation. J. Nat. Gas Sci. Eng. 2020, 76, 103211. [Google Scholar] [CrossRef]
- Aliabadi, M.; Rasoolzadeh, A.; Esmailzadeh, F.; Alamdari, A. Experimental study of using CuO nanoparticles as a methane hydrate promoter. J. Nat. Gas Sci. Eng. 2015, 27, 1518–1522. [Google Scholar] [CrossRef]
- Rossi, F.; Gambelli, A.M. Thermodynamic phase equilibrium of single-guest hydrate and formation data of hydrate in presence of chemical additives: A review. Fluid Phase Equilibr. 2021, 536, 112958. [Google Scholar] [CrossRef]
- Mech, D.; Pandey, G.; Sangway, J.S. Effect of NaCl, methanol and ethylene glycol on the phase equilibrium of methane hydrate in aqueous solutions of tetrahydrofuran (THF) and tetra-n-butyl ammonium bromide (TBAB). Fluid Phase Equilibr. 2015, 402, 9–17. [Google Scholar] [CrossRef]
- Kumar, A.; Vedula, S.S.; Kumar, R.; Linga, P. Hydrate phase equilibrium data of mixed methane-tetrahydrofuran hydrates in saline water. J. Chem. Thermodyn. 2018, 117, 2–8. [Google Scholar] [CrossRef]
- Sangway, J.S.; Oellrich, L. Phase equilibrium of semiclathrate hydrates of methane in aqueous solutions of tetra-n-butyl ammonium bromide (TBAB) and TBAB-NaCl. Fluid Phase Equilibr. 2014, 367, 95–102. [Google Scholar] [CrossRef]
- Kumar, A.; Sakpal, T.; Linga, P.; Kumar, R. Influence of contact medium and surfactants on carbon dioxide clathrate hydrate kinetics. Fuel 2013, 105, 664–671. [Google Scholar] [CrossRef]
- Torré, J.P.; Dicharry, C.; Ricaurte, M.; Daniel-David, D.; Broseta, D. CO2 capture by hydrate formation in quiescent conditions: In search of efficient kinetic additives. Energy Proc. 2011, 4, 621–628. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.-C.; Li, X.-S.; Zhang, Y. Experimental investigation of optimization of well spacing for gas recovery from methane hydrate reservoir in sandy sediment by heat stimulation. Appl. Energy 2017, 207, 562–572. [Google Scholar] [CrossRef]
- Yuan, Q.; Sun, C.-Y.; Yang, X.; Ma, P.-C.; Ma, Z.-W.; Liu, B.; Ma, Q.-L.; Yang, L.-Y.; Chen, G.-J. Recovery of methane from hydrate reservoir with gaseous carbon dioxide using a three-dimensional middle-size reactor. Energy 2012, 40, 47–58. [Google Scholar] [CrossRef]
- Yin, Z.; Huang, L.; Linga, P. Effect of wellbore design on the production behaviour of methane hydrate-bearing sediments induced by depressurization. Appl. Energy 2019, 254, 113635. [Google Scholar] [CrossRef]
- Sun, J.; Chou, I.M.; Jiang, L.; Lin, J.; Sun, R. Crystallization Behavior of the Hydrogen Sulfide Hydrate Formed in Microcapillaries. ACS Omega 2021, 6, 14288–14297. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Gambelli, A.M.; Zhao, X.; Gao, Y.; Rossi, F.; Mei, S. In situ experimental study on the effect of mixed inhibitors on the phase equilibrium of carbon dioxide hydrate. Chem. Eng. Sci. 2022, 248, 117230. [Google Scholar] [CrossRef]
- Chou, I.-M.; Buruss, R.C.; Lu, W. A new optical capillary cell for spectroscopic studies of geologic fluids at pressures up to 100 MPa-Chapter 24. In Advances in High-Pressure Technology for Geophysical Applications; Elsevier: Amsterdam, The Netherlands, 2005; pp. 475–485. [Google Scholar]
- Sun, J.; Xin, Y.; Chou, I.M.; Sun, R.; Jiang, L. Hydrate Stability in the H2S–H2O system—Visual Observations and Measurements in a High-Pressure Optical Cell and Thermodynamic Models. J. Chem. Eng. Data 2020, 65, 3884–3892. [Google Scholar] [CrossRef]
- Li, Y.; Gambelli, A.M.; Rossi, F.; Mei, S. Effect of promoters on CO2 hydrate formation: Thermodynamic assessment and microscale Raman spectroscopy/hydrate crystal morphology characterization analysis. Fluid Phase Equilibr. 2021, 550, 113218. [Google Scholar] [CrossRef]
- Stornelli, G.; Ridolfi, M.; Folgarait, P.; De Nisi, J.; Corapi, D.; Repitsch, C.; Di Schino, A. Feasibility assessment of magnetic cores through additive manufacturing techniques. Metall. Ital. 2021, 113, 50–61. [Google Scholar]
- Stornelli, G.; Gaggia, G.; Rallini, M.; Di Schino, A. Heat treatment effect on managing steel manufactured by laser powder bed fusion technology: Microstructure and mechanical properties. Acta Metall. Slovaca 2021, 27, 122–126. [Google Scholar] [CrossRef]
- Gambelli, A.M.; Rossi, F. Natural gas hydrates: Comparison between two different applications of thermal stimulation for performing CO2 replacement. Energy 2019, 172, 423–434. [Google Scholar] [CrossRef]
- Gambelli, A.M. Variations in terms of CO2 capture and CH4 recovery during replacement processes in gas hydrate reservoirs, associated to the “memory effect”. J. Clean. Prod. 2022, 360, 132154. [Google Scholar] [CrossRef]
- Fitzgerald, G.C.; Castaldi, M.J.; Zhou, Y. Large scale reactor details and results for the formation and decomposition of methane hydrates via thermal stimulation dissociation. J. Pet. Sci. Eng. 2012, 94–95, 19–27. [Google Scholar] [CrossRef]
- Peng, D.-Y.; Robinson, D.B. A New Two-Constant Equation of State. Ind. Eng. Chem. Fund. 1976, 15, 59–64. [Google Scholar] [CrossRef]
- Aregba, A.G. Gas Hydrate—Properties, Formation and Benefits. Open J. Yangtze Oil Gas 2017, 2, 27–44. [Google Scholar] [CrossRef]
- Takeya, S.; Kida, M.; Minami, H.; Sakagami, H.; Hachikubo, A.; Takahashi, N.; Shoji, H.; Soloviev, V.; Wallmann, K.; Biebow, N. Structure and thermal expansion of natural gas clathrate hydrates. Chem. Eng. Sci. 2006, 61, 2670–2674. [Google Scholar] [CrossRef]
- Touil, A.; Broseta, D.; Desmedt, A. Gas Hydrate Crystallization in Thin Glass Capillaries: Roles of Supercooling and Wettability. Langmuir 2019, 35, 12569–12581. [Google Scholar] [CrossRef]
- Touil, A.; Broseta, D.; Hobeika, N.; Brown, R. Roles of Wettability and Supercooling in the Spreading of Cyclopentane Hydrate over a Substrate. Langmuir 2017, 33, 10965–10977. [Google Scholar] [CrossRef]
- Yoslim, J.; Linga, P.; Englezos, P. Enhanced growth of methane–propane clathrate hydrate crystals with sodium dodecyl sulfate, sodium tetradecyl sulfate, and sodium hexadecyl sulfate surfactants. J. Cryst. Growth 2010, 313, 68–80. [Google Scholar]
- Watanabe, S.; Saito, K.; Ohmura, R. Crystal Growth of Clathrate Hydrate in Liquid Water Saturated with a Simulated Natural Gas. Cryst. Growth Des. 2011, 11, 3235–3242. [Google Scholar]
- Kumar, A.; Bhattacharjee, G.; Kulkarni, B.D.; Kumar, R. Role of Surfactants in Promoting Gas Hydrate Formation. Ind. Eng. Chem. Res. 2015, 54, 12217–12232. [Google Scholar] [CrossRef]
- Tang, J.; Zeng, D.; Wang, C.; Chen, Y.; He, L.; Cai, N. Study on the influence of SDS and THF on hydrate-based gas separation performance. Chem. Eng. Res. Des. 2013, 91, 1777–1782. [Google Scholar] [CrossRef]
- Molokitina, N.S.; Nesterov, A.N.; Podenko, L.S.; Reshetnikov, A.M. Carbon dioxide hydrate formation with SDS: Further insights into mechanism of gas hydrate growth in the presence of surfactant. Fuel 2019, 235, 1400–1411. [Google Scholar] [CrossRef]
- Zhong, D.-L.; Ding, K.; Lu, Y.-Y.; Yan, J.; Zhao, W.-L. Methane recovery from coal mine gas using hydrate formation in water-in-oil emulsions. Appl. Energy 2016, 162, 1619–1626. [Google Scholar] [CrossRef]
- Liu, G.-Q.; Wang, F.; Luo, S.-J.; Xu, D.-Y.; Guo, R.-B. Enhanced methane hydrate formation with SDS-coated Fe3O4 nanoparticles as promoters. J. Mol. Liq. 2017, 230, 315–321. [Google Scholar]
- Rong, W.; Zhang, Y.; Luo, S.; Guo, R. Promotion Effect of Copper Nanowire-Doped SDS in Methane Hydrate Formation. Energy Fuels 2022, 36, 4880–4887. [Google Scholar] [CrossRef]
- Wang, H. Dispersing carbon nanotubes using surfactants. Curr. Opin. Colloid Interface Sci. 2009, 14, 364–371. [Google Scholar] [CrossRef]
- Said, S.; Govindaraj, V.; Herri, J.M.; Ouabbas, Y.; Khodja, M.; Belloum, M.; Sangway, J.S.; Nagarajan, R. A study on the influence of nanofluids on gas hydrate formation kinetics and their potential: Application to the CO2 capture process. J. Nat. Gas Sci. Eng. 2016, 32, 95–108. [Google Scholar]
- Song, Y.; Wang, F.; Liu, G.; Luo, S.; Guo, R. Promotion effect of carbon nanotubes-doped SDS on methane hydrate formation. Energy Fuels 2017, 31, 1850–1857. [Google Scholar] [CrossRef]
- Mohammadi, M.; Haghtalab, A.; Fakhroueian, Z. Experimental study and thermodynamic modeling of CO2 gas hydrate formation in presence of zinc oxide nanoparticles. J. Chem. Thermodyn. 2016, 96, 24–33. [Google Scholar] [CrossRef]





| Detailed Information about the Reactor | |||
|---|---|---|---|
| Board height | 21 cm | Internal pipe volume | 1 cm3 |
| Internal height | 22.1 cm | Volume of intake pipes | 19 cm3 |
| Depth from the edge | 18.5 cm | Gas volume from the high edge | 90 cm3 |
| Depth edge to the network | 18.3 cm | Total volume of free gas | 109 cm3 |
| Weld thickness | 1.1 cm | Water volume | 236 cm3 |
| Thickness from the edge | 1 cm | Sand pore volume | 253 cm3 |
| Internal diameter | 7.4 cm | Internal reactor volume | 949 cm3 |
| Materials | Purities | Suppliers |
|---|---|---|
| Carbon dioxide | 99.9 mol% | Hainan Jiateng Gas Co., Ltd. (Haikou, China) |
| Ultrapure water | 18.25 Ω·m | Sichuan Ulupure Technology Co., Ltd. (Chengdu, China) |
| Dodecyl sulfate (SDS) | >99.0% | Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China) |
| Resolution | 1.7 nm (15 kV) and 3.5 nm (1 kV) |
| Magnification | 12–500× |
| Acceleration Voltage | 0.5–20 kV |
| Probe Current | 4 pA–10 nA |
| Std Detectors | High efficiency In-lens detector |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Gambelli, A.M.; Rossi, F. Experimental Study on the Effect of SDS and Micron Copper Particles Mixture on Carbon Dioxide Hydrates Formation. Energies 2022, 15, 6540. https://doi.org/10.3390/en15186540
Li Y, Gambelli AM, Rossi F. Experimental Study on the Effect of SDS and Micron Copper Particles Mixture on Carbon Dioxide Hydrates Formation. Energies. 2022; 15(18):6540. https://doi.org/10.3390/en15186540
Chicago/Turabian StyleLi, Yan, Alberto Maria Gambelli, and Federico Rossi. 2022. "Experimental Study on the Effect of SDS and Micron Copper Particles Mixture on Carbon Dioxide Hydrates Formation" Energies 15, no. 18: 6540. https://doi.org/10.3390/en15186540
APA StyleLi, Y., Gambelli, A. M., & Rossi, F. (2022). Experimental Study on the Effect of SDS and Micron Copper Particles Mixture on Carbon Dioxide Hydrates Formation. Energies, 15(18), 6540. https://doi.org/10.3390/en15186540






