A Tortuosity Engineered Dual-Microporous Layer Electrode Including Graphene Aerogel Enabling Largely Improved Direct Methanol Fuel Cell Performance with High-Concentration Fuel
Abstract
:1. Introduction
2. Experimental
2.1. Fabrication of GDL
2.2. Fabrication of MEA
2.3. Single Cell Fixture
2.4. Electrochemical Characterization
2.5. Microstructural Characterization
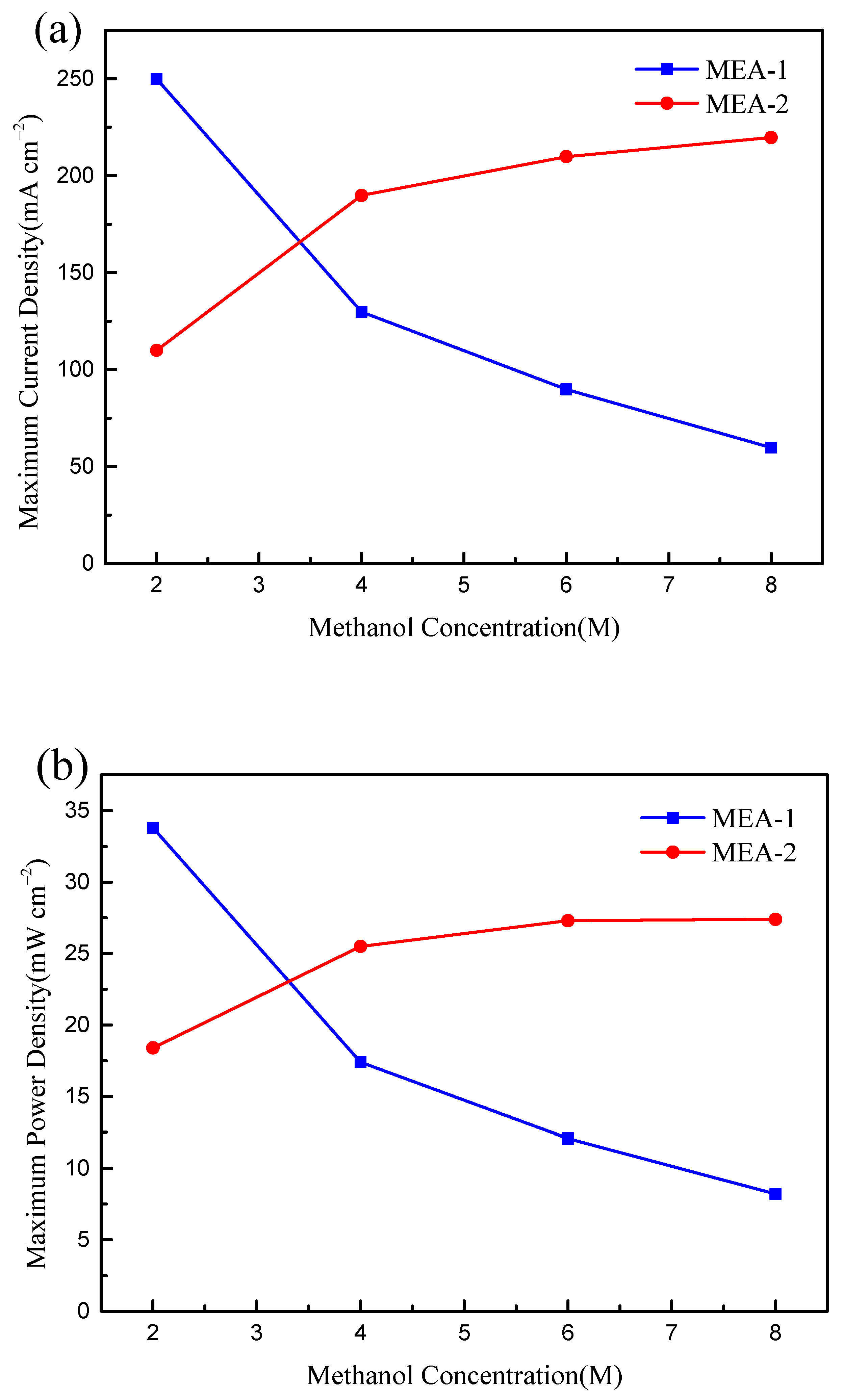
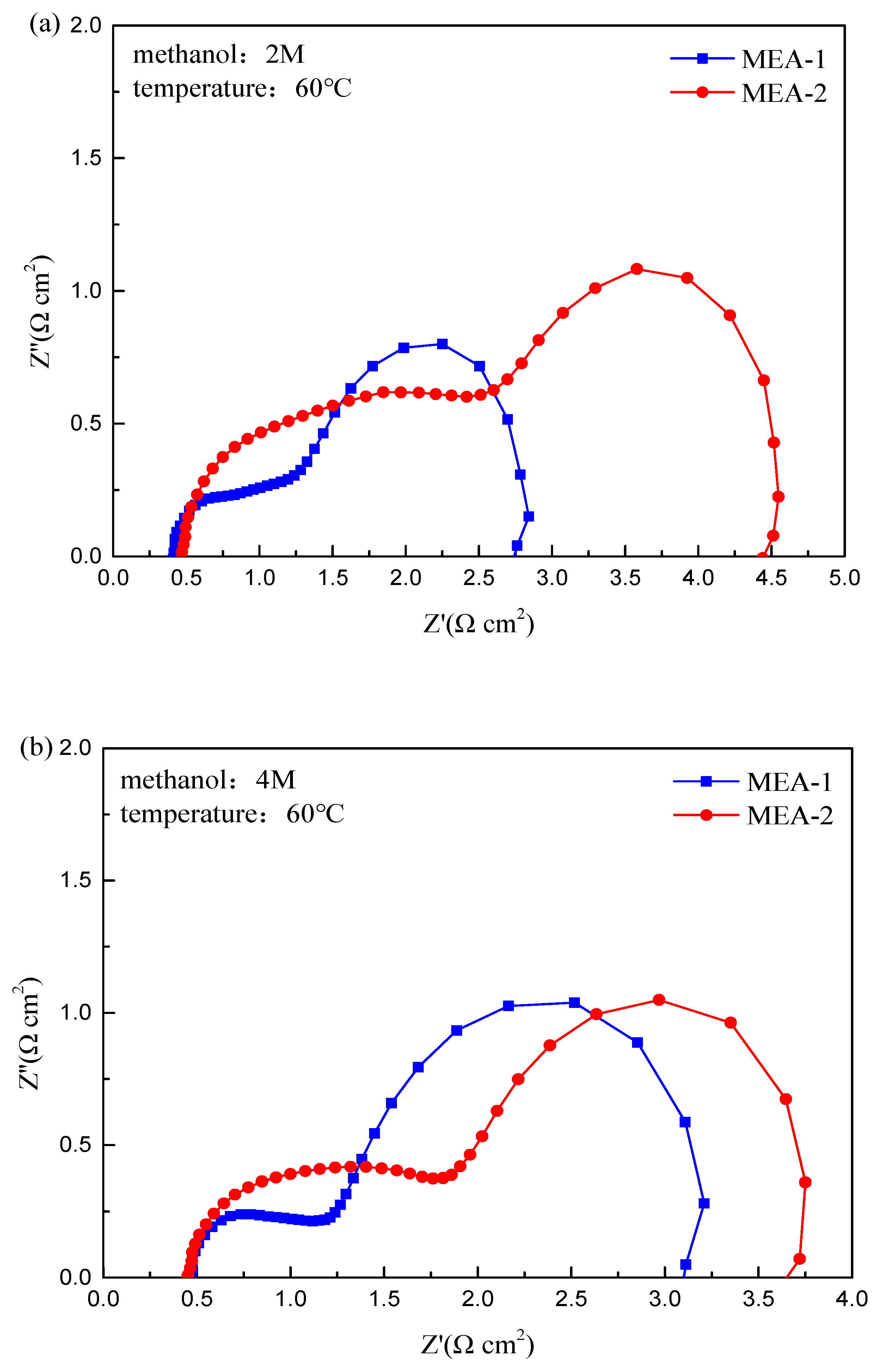
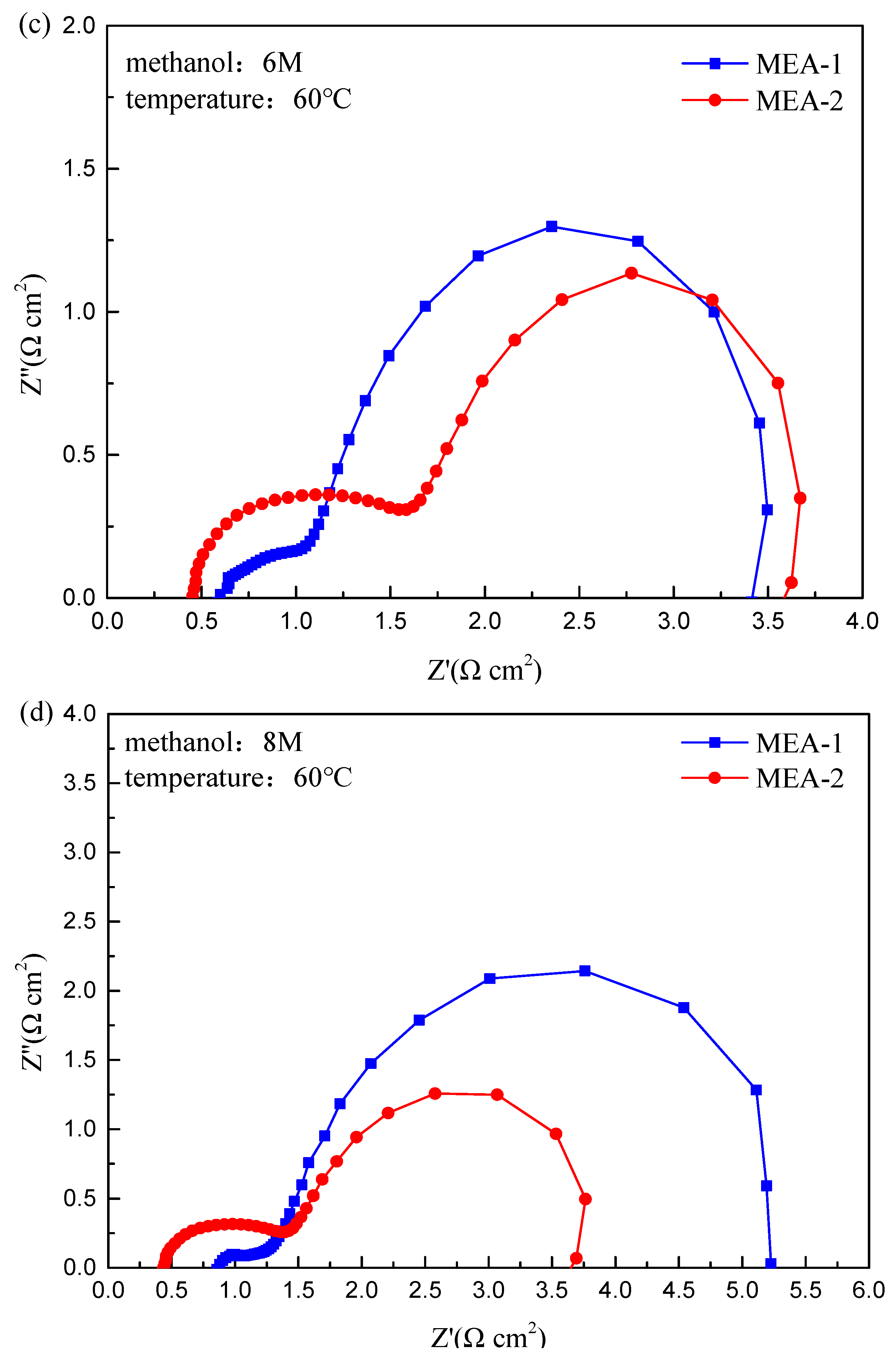
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Q.X.; Zhao, T.S.; Chen, R.; Yang, W.W. Enhancement of water retention in the membrane electrode assembly for direct methanol fuel cells operating with neat methanol. Int. J. Hydrogen Energy 2010, 35, 10547–10555. [Google Scholar] [CrossRef]
- Yang, S.-H.; Chen, C.-Y.; Wang, W.-J. An impedance study for the anode micro-porous layer in an operating direct methanol fuel cell. J. Power Sources 2010, 195, 3536–3545. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Peck, D.-H.; Kim, S.-K.; Jung, D.-H.; Lim, S.; Kim, S.-H. Effects of the microstructure and powder compositions of a micro-porous layer for the anode on the performance of high concentration methanol fuel cell. Int. J. Hydrogen Energy 2013, 38, 7159–7168. [Google Scholar] [CrossRef]
- Wu, Q.X.; Zhao, T.S.; Chen, R.; An, L. A sandwich structured membrane for direct methanol fuel cells operating with neat methanol. Appl. Energy 2013, 106, 301–306. [Google Scholar] [CrossRef]
- Lue, S.J.; Pai, Y.-L.; Shih, C.-M.; Wu, M.-C.; Lai, S.-M. Novel bilayer well-aligned Nafion/graphene oxide composite membranes prepared using spin coating method for direct liquid fuel cells. J. Membr. Sci. 2015, 493, 212–223. [Google Scholar] [CrossRef]
- Yuan, T.; Pu, L.; Huang, Q.; Zhang, H.; Li, X.; Yang, H. An effective methanol-blocking membrane modified with graphene oxide nanosheets for passive direct methanol fuel cells. Electrochim. Acta 2014, 117, 393–397. [Google Scholar] [CrossRef]
- Matos, B.R.; Isidoro, R.A.; Santiago, E.I.; Linardi, M.; Ferlauto, A.S.; Tavares, A.C.; Fonseca, F.C. In Situ Fabrication of Nafion–Titanate Hybrid Electrolytes for High-Temperature Direct Ethanol Fuel Cell. J. Phys. Chem. C 2013, 117, 16863–16870. [Google Scholar] [CrossRef]
- Li, X.-Y.; Yang, W.-W.; He, Y.-L.; Zhao, T.-S.; Qu, Z.-G. Effect of anode micro-porous layer on species crossover through the membrane of the liquid-feed direct methanol fuel cells. Appl. Therm. Eng. 2012, 48, 392–401. [Google Scholar] [CrossRef]
- Bresciani, F.; Rabissi, C.; Zago, M.; Marchesi, R.; Casalegno, A. On the effect of gas diffusion layers hydrophobicity on direct methanol fuel cell performance and degradation. J. Power Sources 2015, 273, 680–687. [Google Scholar] [CrossRef]
- Liu, G.; Ding, X.; Zhou, H.; Chen, M.; Wang, M.; Zhao, Z.; Yin, Z.; Wang, X. Structure optimization of cathode microporous layer for direct methanol fuel cells. Appl. Energy 2015, 147, 396–401. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wang, S.Y.; Pei, P.C.; Chen, L.; Li, J.L.; Wang, X.D.; Li, Q.F. The function of microporous layers and the interaction between the anode and cathode in DMFCs. Part I: The double roles of microporous layers in DMFCs. Electrochem. Commun. 2008, 10, 407–410. [Google Scholar] [CrossRef]
- Yuan, T.; Yang, J.; Wang, Y.; Ding, H.; Li, X.; Liu, L.; Yang, H. Anodic diffusion layer with graphene-carbon nanotubes composite material for passive direct methanol fuel cell. Electrochim. Acta 2014, 147, 265–270. [Google Scholar] [CrossRef]
- Yuan, T.; Zou, Z.; Chen, M.; Li, Z.; Xia, B.; Yang, H. New anodic diffusive layer for passive micro-direct methanol fuel cell. J. Power Sources 2009, 192, 423–428. [Google Scholar] [CrossRef]
- Fu, X.; Li, T.; Tang, L.; Deng, X.; Zhang, R.; Hu, S.; Zhao, F.; Li, X.; Liu, Q. Reticulated polyaniline nanowires as a cathode microporous layer for high-temperature PEMFCs. Int. J. Hydrogen Energy 2021, 46, 8802–8809. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Song, H.; Kim, D.; Kim, G.H.; Im, D.; Jeong, Y.; Park, T. Carbon nanotube sheet as a microporous layer for proton exchange membrane fuel cells. Energy 2021, 227, 120459. [Google Scholar] [CrossRef]
- Pal, S.; Mondal, R.; Chatterjee, U. Sulfonated polyvinylidene fluoride and functional copolymer based blend proton exchange membrane for fuel cell application and studies on methanol crossover. Renew. Energy 2021, 170, 974–984. [Google Scholar] [CrossRef]
- Li, Y.; Hoorfar, M.; Shen, K.; Fang, J.; Yue, X.; Jiang, Z. Development of a Crosslinked Pore-filling Membrane with an Extremely Low Swelling Ratio and Methanol Crossover for Direct Methanol Fuel Cells. Electrochim. Acta 2017, 232, 226–235. [Google Scholar] [CrossRef]
- Parthiban, V.; Akula, S.; Sahu, A.K. Surfactant templated nanoporous carbon-Nafion hybrid membranes for direct methanol fuel cells with reduced methanol crossover. J. Membr. Sci. 2017, 541, 127–136. [Google Scholar] [CrossRef]
- Sun, X.; Yang, C.; Xia, Z.; Qi, F.; Sun, H.; Sun, G. Molecular sieve as an effective barrier for methanol crossover in direct methanol fuel cells. Int. J. Hydrogen Energy 2020, 45, 8994–9003. [Google Scholar] [CrossRef]
- Sun, H.; Mei, L.; Liang, J.; Zhao, Z.; Lee, C.; Fei, H.; Ding, M.; Lau, J.; Li, M.; Wang, C.; et al. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599–604. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Q.; Yu, Y.; Chen, W.; Hu, H.; Li, H. Naturally Dried Graphene Aerogels with Superelasticity and Tunable Poisson’s Ratio. Adv. Mater. 2016, 28, 9223–9230. [Google Scholar] [CrossRef]
- Zhao, L.; Sui, X.-L.; Li, J.-Z.; Zhang, J.-J.; Zhang, L.-M.; Huang, G.-S.; Wang, Z.-B. Supramolecular assembly promoted synthesis of three-dimensional nitrogen doped graphene frameworks as efficient electrocatalyst for oxygen reduction reaction and methanol electrooxidation. Appl. Catal., B 2018, 231, 224–233. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Q.; Yan, M.; Wei, Y.; Zong, J.; Zhang, J.; Wu, Y.; Huang, H. Three-Dimensional Boron- and Nitrogen-Codoped Graphene Aerogel-Supported Pt Nanoparticles as Highly Active Electrocatalysts for Methanol Oxidation Reaction. ACS Sustain. Chem. Eng. 2018, 6, 6644–6653. [Google Scholar] [CrossRef]
- Yan, M.; Jiang, Q.; Zhang, T.; Wang, J.; Yang, L.; Lu, Z.; He, H.; Fu, Y.; Wang, X.; Huang, H. Three-dimensional low-defect carbon nanotube/nitrogen-doped graphene hybrid aerogel-supported Pt nanoparticles as efficient electrocatalysts toward the methanol oxidation reaction. J. Mater. Chem. A 2018, 6, 18165–18172. [Google Scholar] [CrossRef]
- Gao, Z.; Li, M.; Wang, J.; Zhu, J.; Zhao, X.; Huang, H.; Zhang, J.; Wu, Y.; Fu, Y.; Wang, X. Pt nanocrystals grown on three-dimensional architectures made from graphene and MoS2 nanosheets: Highly efficient multifunctional electrocatalysts toward hydrogen evolution and methanol oxidation reactions. Carbon 2018, 139, 369–377. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Zhu, Y.; Lan, L.; Zhang, H.; Liu, C.; Tao, K.; Li, J. Fe3C/C nanoparticles encapsulated in N-doped graphene aerogel: An advanced oxygen reduction reaction catalyst for fiber-shaped fuel cells. Int. J. Hydrogen Energy 2019, 44, 18393–18402. [Google Scholar] [CrossRef]
- Guo, Q.; Lu, X.; Fei, G.; Wang, Z.; Xia, H. Nitrogen-Doped Graphene Aerogel Microspheres Used as Electrocatalyst Supports for Methanol Oxidation. Ind. Eng. Chem. Res. 2022, 61, 1398–1407. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Sun, H.; Xiang, D.; Jiang, Q.; Lu, Z.; He, H.; Huang, H. Rh-Decorated Three-Dimensional Graphene Aerogel Networks as Highly-Efficient Electrocatalysts for Direct Methanol Fuel Cells. Front. Energy Res. 2020, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Zha, M.; Sun, P.; Hu, G.; Feng, L. PdNi/N-doped graphene aerogel with over wide potential activity for formic acid electrooxidation. J. Energy Chem. 2021, 59, 748–754. [Google Scholar] [CrossRef]
- Forootan Fard, H.; Khodaverdi, M.; Pourfayaz, F.; Ahmadi, M.H. Application of N-doped carbon nanotube-supported Pt-Ru as electrocatalyst layer in passive direct methanol fuel cell. Int. J. Hydrogen Energy 2020, 45, 25307–25316. [Google Scholar] [CrossRef]
- Ramli, Z.A.C.; Kamarudin, S.K.; Basri, S.; Zainoodin, A.M. The potential of novel carbon nanocages as a carbon support for an enhanced methanol electro-oxidation reaction in a direct methanol fuel cell. Int. J. Energy Res. 2020, 44, 10071–10086. [Google Scholar] [CrossRef]
- Ju, Z.; Zhang, X.; Wu, J.; King, S.T.; Chang, C.-C.; Yan, S.; Xue, Y.; Takeuchi, K.J.; Marschilok, A.C.; Wang, L.; et al. Tortuosity Engineering for Improved Charge Storage Kinetics in High-Areal-Capacity Battery Electrodes. Nano Lett. 2022, 22, 6700–6708. [Google Scholar] [CrossRef]
- Kang, K.; Lee, G.; Gwak, G.; Choi, Y.; Ju, H. Development of an advanced MEA to use high-concentration methanol fuel in a direct methanol fuel cell system. Int. J. Hydrogen Energy 2012, 37, 6285–6291. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, X.; Zhang, Y.; Liu, X. Improved Anode Two-Phase Mass Transfer Management of Direct Methanol Fuel Cell by the Application of Graphene Aerogel. ACS Sustain. Chem. Eng. 2019, 7, 11653–11661. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, X.; Hou, C.; Zhang, Y.; Wang, H.; Liu, X. Enhanced water management via the optimization of cathode microporous layer using 3D graphene frameworks for direct methanol fuel cell. J. Power Sources 2020, 451, 227800. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, W.; Su, H.; Leung, P.; Xing, L.; Xu, L.; Yang, C.; Xu, Q. Improving cell performance and alleviating performance degradation by constructing a novel structure of membrane electrode assembly (MEA) of DMFCs. Int. J. Hydrogen Energy 2019, 44, 32231–32239. [Google Scholar] [CrossRef]
- Kang, S.; Bae, G.; Kim, S.-K.; Jung, D.-H.; Shul, Y.-G.; Peck, D.-H. Performance of a MEA using patterned membrane with a directly coated electrode by the bar-coating method in a direct methanol fuel cell. Int. J. Hydrogen Energy 2018, 43, 11386–11396. [Google Scholar] [CrossRef]
- Ghosh, T.; Biswas, C.; Oh, J.; Arabale, G.; Hwang, T.; Luong, N.D.; Jin, M.; Lee, Y.H.; Nam, J.-D. Solution-Processed Graphite Membrane from Reassembled Graphene Oxide. Chem. Mater. 2012, 24, 594–599. [Google Scholar] [CrossRef]
- Xu, C.; He, Y.L.; Zhao, T.S.; Chen, R.; Ye, Q. Analysis of Mass Transport of Methanol at the Anode of a Direct Methanol Fuel Cell. J. Electrochem. Soc. 2006, 153, A1358. [Google Scholar] [CrossRef]
- Zhao, T.S.; Xu, C.; Chen, R.; Yang, W.W. Mass transport phenomena in direct methanol fuel cells. Prog. Energy Combust. Sci. 2009, 35, 275–292. [Google Scholar] [CrossRef]
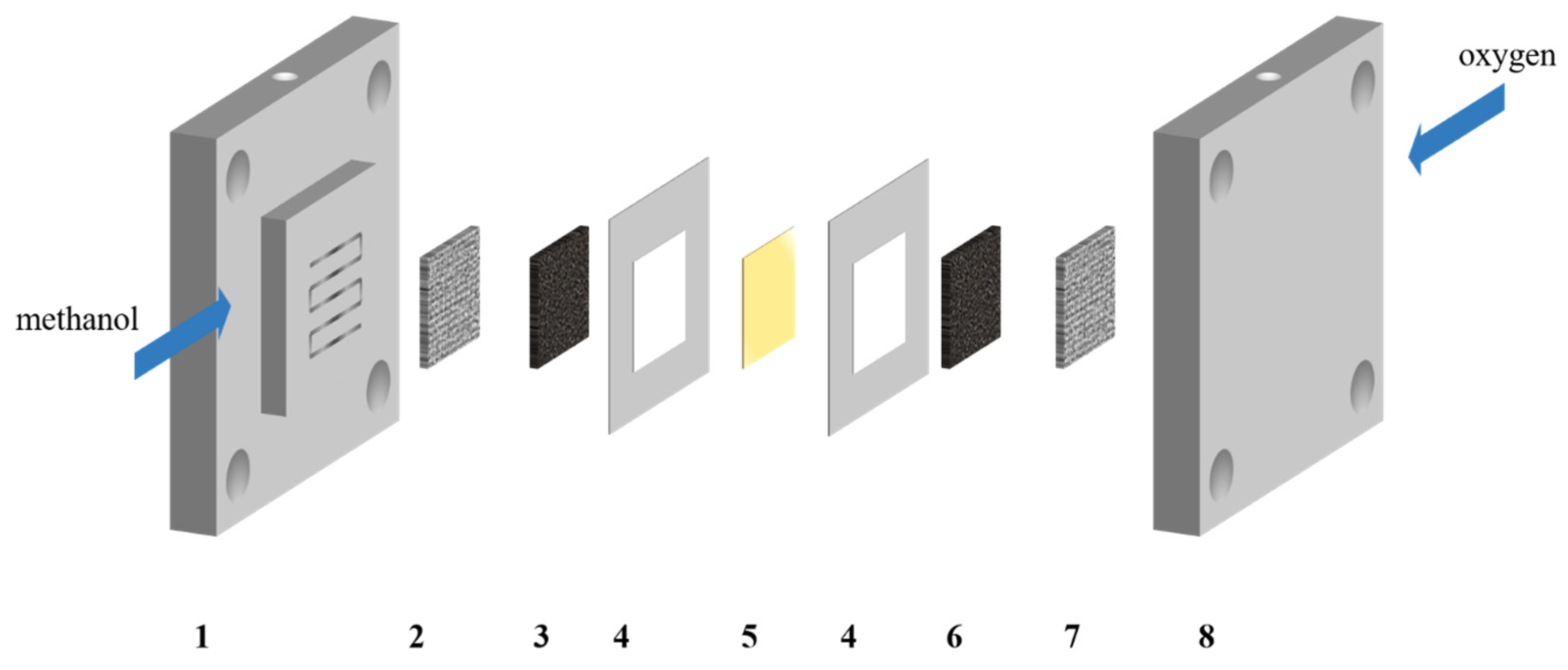


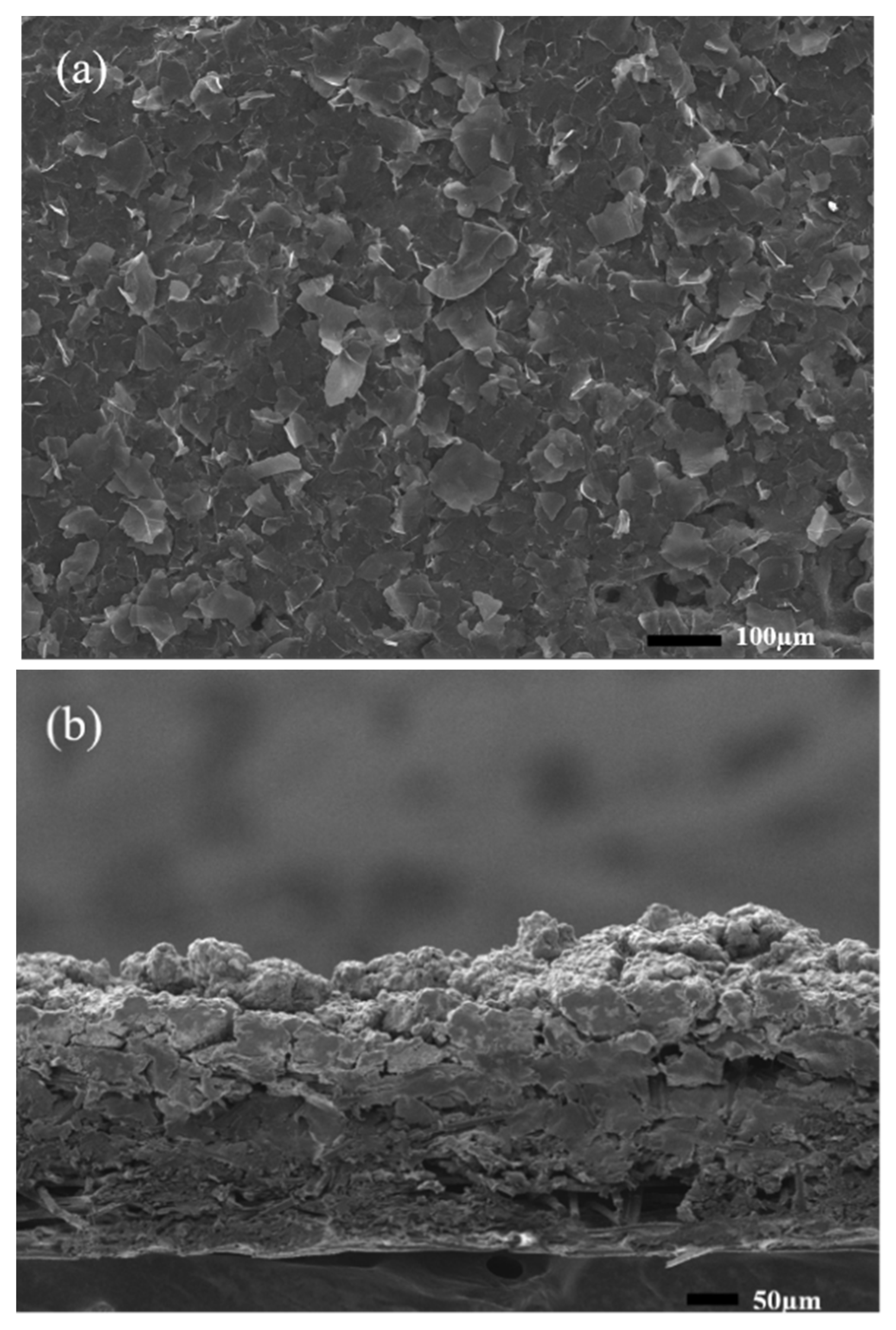
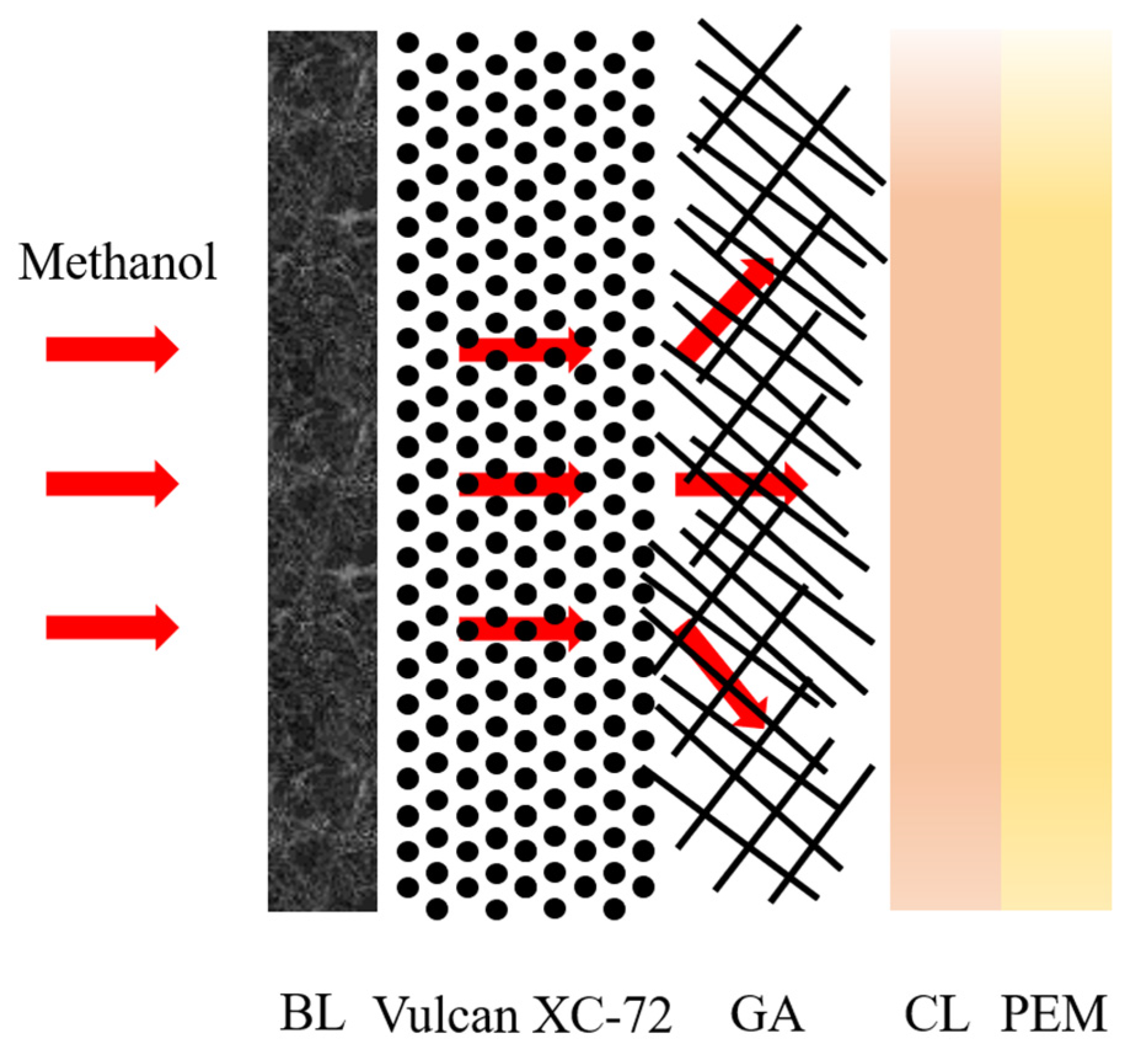



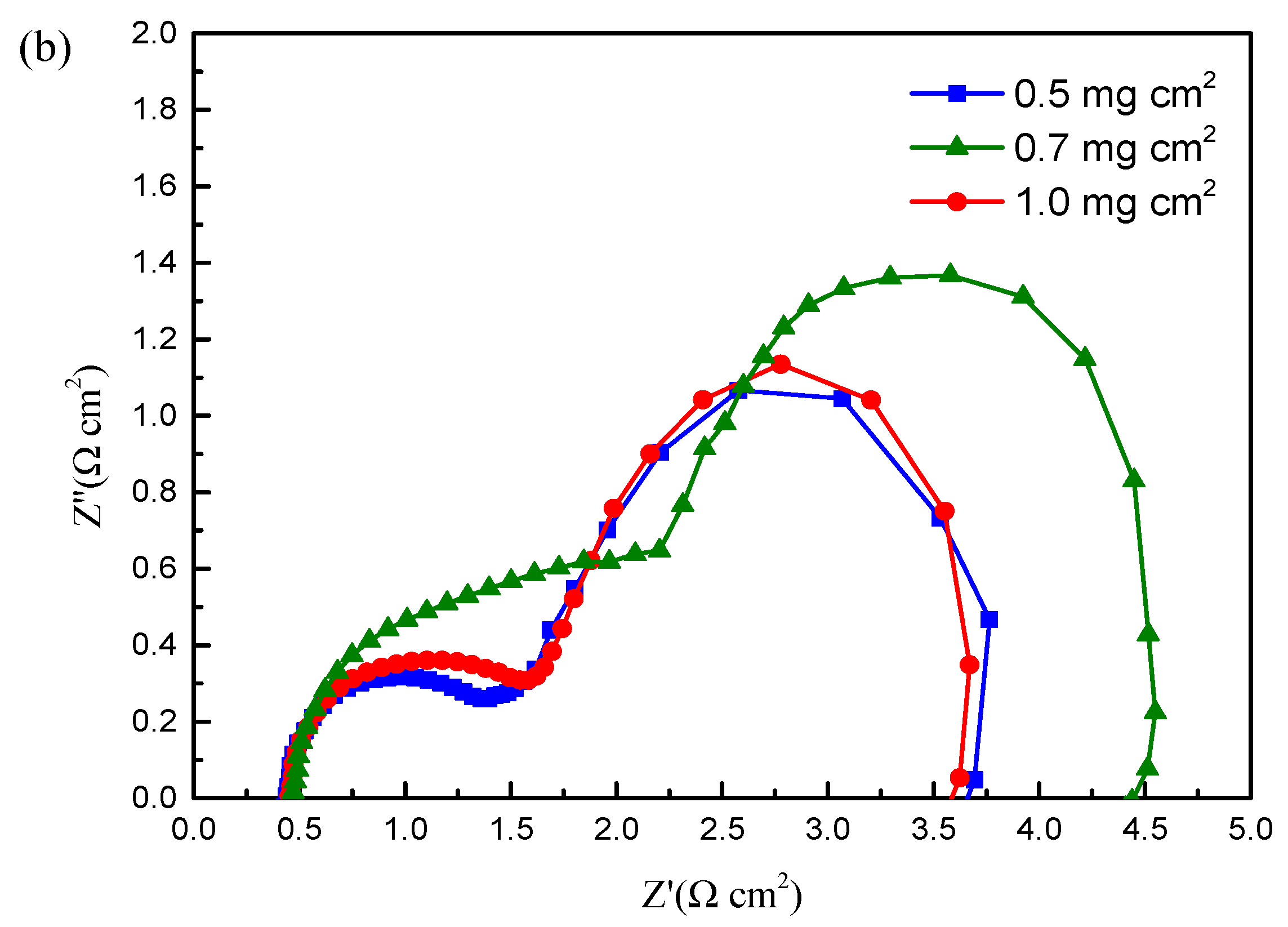
| Material Name | Property | Application | Ref. |
|---|---|---|---|
| Graphene aerogel (GA) | High porosity Three-dimensional porous structure. | Supported Pt nanocrystals as MOR catalysts | [25] |
| Nitrogen-doped carbon nanotubes (N-CNTs) | Larger in diameter than carbon nanotubes. | Supported PtRu as MOR catalysts | [30] |
| Carbon nanocage (CNC) | Large specific surface area Multi-mesoporous structure. | Supported PtRu, PtNi, PtCo and PtFe as MOR catalysts | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, L.; Balakrishnan, P.; Liu, H.; Zhang, W.; Deng, Y.; Su, H.; Xing, L.; Penga, Ž.; Xu, Q. A Tortuosity Engineered Dual-Microporous Layer Electrode Including Graphene Aerogel Enabling Largely Improved Direct Methanol Fuel Cell Performance with High-Concentration Fuel. Energies 2022, 15, 9388. https://doi.org/10.3390/en15249388
Guan L, Balakrishnan P, Liu H, Zhang W, Deng Y, Su H, Xing L, Penga Ž, Xu Q. A Tortuosity Engineered Dual-Microporous Layer Electrode Including Graphene Aerogel Enabling Largely Improved Direct Methanol Fuel Cell Performance with High-Concentration Fuel. Energies. 2022; 15(24):9388. https://doi.org/10.3390/en15249388
Chicago/Turabian StyleGuan, Li, Prabhuraj Balakrishnan, Huiyuan Liu, Weiqi Zhang, Yilin Deng, Huaneng Su, Lei Xing, Željko Penga, and Qian Xu. 2022. "A Tortuosity Engineered Dual-Microporous Layer Electrode Including Graphene Aerogel Enabling Largely Improved Direct Methanol Fuel Cell Performance with High-Concentration Fuel" Energies 15, no. 24: 9388. https://doi.org/10.3390/en15249388
APA StyleGuan, L., Balakrishnan, P., Liu, H., Zhang, W., Deng, Y., Su, H., Xing, L., Penga, Ž., & Xu, Q. (2022). A Tortuosity Engineered Dual-Microporous Layer Electrode Including Graphene Aerogel Enabling Largely Improved Direct Methanol Fuel Cell Performance with High-Concentration Fuel. Energies, 15(24), 9388. https://doi.org/10.3390/en15249388











