Pool Fire Suppression Using CO2 Hydrate
Abstract
:1. Introduction
2. Experimental Technique
3. Results and Discussion
3.1. Patterns of Pool Fire Suppression
3.2. Quantitative Characteristics of Fire Containment and Suppression
4. Effective Conditions for Extinguishing Burning Liquids during the Dissociation of Carbon Dioxide Hydrate
5. Conclusions
- (i)
- The aim of this research was achieved through several series of experiments with different flammable liquids. When heated, gas hydrates exhibit accelerated dissociation into ice and gas. Dissociation is also accompanied by ice melting, water evaporation, and gas release. This complex set of phase transitions makes it possible to activate any of the liquid combustion suppression mechanisms: reduction in the flammable liquid temperature due to melting and evaporation, access of powder granules to the lower layers of fire, and blocking the oxidizer access by a large amount of carbon dioxide released in the process. A series of experiments demonstrated how the dominating mechanism of liquid pool fire suppression changes with varying mass and type of hydrate (powder, tablets, and spheres).
- (ii)
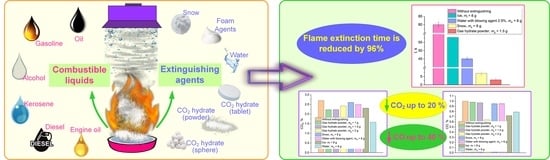
- The experiments determined the optimal CO2 hydrate mass-to-surface area ratio for the containment and suppression of fires involving the most widespread and fire-hazardous flammable liquids: gasoline, kerosene, diesel fuel, oil, and alcohol. CO2 hydrate powder is found to be more effective than ice, snow, and water/foaming agent emulsion as a fire suppressant in terms of flammable liquid extinction time. The use of CO2 hydrate in the form of powder for extinguishing flammable liquid pool fires provides a 90–96% shorter extinction time.
- (iii)
- Fire suppression by CO2 hydrate powder reduces the CO2 and CO concentrations by 40% for different flammable liquids due to the additional water vapor from the dissociating gas hydrate joining the reaction.
- (iv)
- The extinction of a laboratory-scale flammable-liquid pool fire with a fuel film thickness of up to 2 mm requires a CO2 hydrate discharge density of at least 1.3 kg/m2. The specific discharge density required for extinguishing a laboratory-scale pool fire must exceed 0.2 kg/(m2∙s).
- (v)
- The CO2 hydrate dissociation modeling shows that the suppression of a flame using powder with a granule size of about 3 mm will require 20 times less carbon dioxide hydrate than in the case of pressed tablets. The suppression time using powder granules is 1 s.
- (vi)
- The experimental findings can be used as a database for the development of flammable liquid fire suppression in industrial buildings and warehouses within a short period of time using carbon dioxide hydrate spheres, tablets, and granules.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ding, F.; Kang, W.; Yan, L.; Xu, Z.; Guo, X. Influence of gas–liquid ratio on the fire-extinguishing efficiency of compressed gas protein foam in diesel pool fire. J. Therm. Anal. Calorim. 2021, 146, 1465–1472. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, X.; Zhao, Z.; Jiang, X. Dynamic Fire Risk Classification Prediction of Stadiums: Multi-Dimensional Machine Learning Analysis Based On Intelligent Perception. Appl. Sci. 2022, 12, 6607. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Dongxu, O.; Cao, S.; Wang, Z.; Wang, J. A simplified analysis to predict the fire hazard of primary lithium battery. Appl. Sci. 2018, 8, 2329. [Google Scholar] [CrossRef] [Green Version]
- Argyropoulos, C.D.; Christolis, M.N.; Nivolianitou, Z.; Markatos, N.C. A hazards assessment methodology for large liquid hydrocarbon fuel tanks. J. Loss Prev. Process Ind. 2012, 25, 329–335. [Google Scholar] [CrossRef]
- Kwon, K.; Kim, Y.; Kwon, Y.; Koseki, H. Study on accidental fire at a large-scale floating-roof gasoline storage tank. J. Loss Prev. Process Ind. 2021, 73, 104613. [Google Scholar] [CrossRef]
- Zhou, R.; Dou, X.; Lang, X.; He, L.; Liu, J.; Mu, S. Foaming ability and stability of silica nanoparticle-based triple-phase foam for oil fire extinguishing: Experimental. Soft Mater. 2018, 16, 327–338. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, X.; Wu, Z.; Ju, X.; Peng, Y.; Yang, L. Ignition and burning behaviors of automobile oil in engine compartment. J. Therm. Anal. Calorim. 2018, 132, 305–316. [Google Scholar] [CrossRef]
- Tianwei, Z.; Cunwei, Z.; Hao, L.; Zhiyue, H. Experimental investigation of novel dry liquids with aqueous potassium Solution@Nano-SiO2 for the suppression of liquid fuel fires: Preparation, application, and stability. Fire Saf. J. 2020, 115, 103144. [Google Scholar] [CrossRef]
- Von Blottnitz, H.; Curran, M.A. A review of assessments conducted on bio-ethanol as a transportation fuel from a net energy, greenhouse gas, and environmental life cycle perspective. J. Clean. Prod. 2007, 15, 607–619. [Google Scholar] [CrossRef]
- Kim, D.; Sakimoto, K.K.; Hong, D.; Yang, P. Artificial Photosynthesis for Sustainable Fuel and Chemical Production. Angew. Chem. Int. Ed. 2015, 54, 3259–3266. [Google Scholar] [CrossRef]
- Shinoda, K.; Nomura, T. Miscibility of fluorocarbon and hydrocarbon surfactants in micelles and liquid mixtures. Basic studies of oil repellent and fire extinguishing agents. J. Phys. Chem. 1980, 84, 365–369. [Google Scholar] [CrossRef]
- Khoat, H.T.; Kim, J.T.; Quoc, T.D.; Kwark, J.H.; Ryou, H.S. A numerical analysis of the fire characteristics after sprinkler activation in the compartment fire. Energies 2020, 13, 3099. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, M.; Hwang, I.J.; Kim, Y.J. Noise reduction of an extinguishing nozzle using the response surface method. Energies 2019, 12, 4346. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Guo, X.; Yan, L.; Kang, W. Fire-extinguishing performance and mechanism of aqueous film-forming foam in diesel pool fire. Case Stud. Therm. Eng. 2020, 17, 100578. [Google Scholar] [CrossRef]
- Wang, H.; Du, Z.; Zhang, T.; Wang, Q.; Li, Y.; Kang, Q. Performance of Foam Agents on Pool Fires at High Altitudes. Fire Technol. 2022, 58, 1285–1304. [Google Scholar] [CrossRef]
- Rie, D.H.; Lee, J.W.; Kim, S. Class B fire-extinguishing performance evaluation of a compressed air foam system at different air-to-aqueous foam solution mixing ratios. Appl. Sci. 2016, 6, 191. [Google Scholar] [CrossRef] [Green Version]
- Lv, D.; Tan, W.; Zhu, G.; Liu, L. Gasoline fire extinguishing by 0.7 MPa water mist with multicomponent additives driven by CO2. Process Saf. Environ. Prot. 2019, 129, 168–175. [Google Scholar] [CrossRef]
- Harding, B.; Zhang, B.; Liu, Y.; Chen, H.; Mannan, M.S. Improved research-scale foam generator design and performance characterization. J. Loss Prev. Process Ind. 2016, 39, 173–180. [Google Scholar] [CrossRef]
- Koshiba, Y.; Okazaki, S.; Ohtani, H. Experimental investigation of the fire extinguishing capability of ferrocene-containing water mist. Fire Saf. J. 2016, 83, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Rumminger, M.D.; Linteris, G.T. Inhibition of premixed carbon monoxide–hydrogen–oxygen–nitrogen flames by iron pentacarbonyl. Combust. Flame 2000, 120, 451–464. [Google Scholar] [CrossRef]
- Liu, C. Fire fighting of wind extinguisher with CO2 gas assisted. Appl. Mech. Mater. 2012, 130–134, 1054–1057. [Google Scholar] [CrossRef]
- Sloan, E.D., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar] [CrossRef]
- Guo, Z.; Yuan, Y.; Jiang, M.; Liu, J.; Wang, X.; Wang, B. Sensitivity and Resolution of Controlled-Source Electromagnetic Method for Gas Hydrate Stable Zone. Energies 2021, 14, 8318. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Li, Q.; Fan, Q.; Chen, G.; Sun, C. Study on the growth kinetics and morphology of methane hydrate film in a porous glass microfluidic device. Energies 2021, 14, 6814. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Wang, Y.; Qin, C.; Li, B.; Luo, Y.; Feng, J. Investigating the interaction effects between reservoir deformation and hydrate dissociation in hydrate-bearing sediment by depressurization method. Energies 2021, 14, 548. [Google Scholar] [CrossRef]
- Sahith, S.J.K.; Pedapati, S.R.; Lal, B. Investigation on gas hydrates formation and dissociation in multiphase gas dominant transmission pipelines. Appl. Sci. 2020, 10, 5052. [Google Scholar] [CrossRef]
- Lv, X.; Shi, B.; Zhou, S.; Wang, S.; Huang, W.; Sun, X. Study on the decomposition mechanism of natural gas hydrate particles and its microscopic agglomeration characteristics. Appl. Sci. 2018, 8, 2464. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Gambelli, A.M.; Sharma, D.K.; Castellani, B.; Nicolini, A.; Castaldi, M.J. Experiments on methane hydrates formation in seabed deposits and gas recovery adopting carbon dioxide replacement strategies. Appl. Therm. Eng. 2019, 148, 371–381. [Google Scholar] [CrossRef]
- Kang, K.C.; Linga, P.; Park, K.-N.; Choi, S.J.; Lee, J.D. Seawater desalination by gas hydrate process and removal characteristics of dissolved ions (Na+, K+, Mg2+, Ca2+, B3+, Cl−, SO42−). Desalination 2014, 353, 84–90. [Google Scholar] [CrossRef]
- Liu, N.; Meng, F.; Chen, L.; Yang, L.; Liu, D. Investigating the effects of MWCNT-HB on gas storage performance of CO2 hydrate. Fuel 2022, 316, 123289. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Yang, H.; Li, J.; Li, Y.; Wu, Q. Formation and storage characteristics of CO2 hydrate in porous media: Effect of liquefaction amount on the formation rate, accumulation amount. Appl. Therm. Eng. 2022, 214, 118747. [Google Scholar] [CrossRef]
- Prah, B.; Yun, R. CO2 hydrate slurry transportation in carbon capture and storage. Appl. Therm. Eng. 2018, 128, 653–661. [Google Scholar] [CrossRef]
- Sato, T.; Takeya, S.; Nagashima, H.D.; Ohmura, R. Preservation of carbon dioxide clathrate hydrate coexisting with sucrose under domestic freezer conditions. J. Food Eng. 2014, 120, 69–74. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, S.; Kang, Y.T. CO2 hydrate cooling system and LCC analysis for energy transportation application. Appl. Therm. Eng. 2015, 91, 11–18. [Google Scholar] [CrossRef]
- Liu, R.; Gao, F.; Liang, K.; Yuan, Z.; Ruan, C.; Wang, L.; Yang, S. Experimental study on the correlation between rapid formation of gas hydrate and diffusion of guest molecules. Appl. Therm. Eng. 2019, 154, 393–399. [Google Scholar] [CrossRef]
- Wei, J.; Cheng, Y.; Yan, C.; Li, Q.; Han, S.; Ansari, U. Decomposition prevention through thermal sensitivity of hydrate formations around wellbore. Appl. Therm. Eng. 2019, 159, 113921. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Aida, E.; Yokomori, T.; Ohmura, R.; Ueda, T. Fire extinction using carbon dioxide hydrate. Ind. Eng. Chem. Res. 2009, 48, 4083–4087. [Google Scholar] [CrossRef]
- Sugahara, A.; Nakano, H.; Yokomori, T.; Ohmura, R.; Ueda, T. Effect of fuel boiling point of pool flame for the flame extinction by CO2 hydrate. In Proceedings of the ASPACC 2015—10th Asia-Pacific Conference on Combustion, Beijing, China, 19–22 July 2015. [Google Scholar]
- GOST 32513-2013; Automotive Fuels. Unleaded Petrol. Specifications. Federal Agency for Technical Regulation and Metrology: Moskow, Russia, 2015.
- GOST 10227-86; Jetfuels. Specifications. Federal Agency for Technical Regulation and Metrology: Moskow, Russia, 1987.
- GOST 305-82; Diesel Fuel. Specifications. Federal Agency for Technical Regulation and Metrology: Moskow, Russia, 1982.
- GOST 5962-2013; Rectified Ethyl Alcohol from Edible Raw Material. Specifications. Federal Agency for Technical Regulation and Metrology: Moskow, Russia, 2013.
- Jayasuriya, J.; Moser, I.; de Mel, R. An automated water dispensing system for controlling fires in coal yards. Int. J. Coal Sci. Technol. 2022, 9, 23. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Z.; Zheng, G.; Chen, P. Study on the effect of mist flux on water mist fire extinguishing. Fire Saf. J. 2022, 130, 103601. [Google Scholar] [CrossRef]
- Qin, Y.; Guo, W.; Xu, H.; Song, Y.; Chen, Y.; Ma, L. A comprehensive method to prevent top-coal spontaneous combustion utilizing dry ice as a fire extinguishing medium: Test apparatus development and field application. Environ. Sci. Pollut. Res. 2022, 29, 19741–19751. [Google Scholar] [CrossRef]
- Tupsakhare, S.S.; Castaldi, M.J. Efficiency enhancements in methane recovery from natural gas hydrates using injection of CO2/N2 gas mixture simulating in-situ combustion. Appl. Energy 2019, 236, 825–836. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Manakov, A.Y.; Morozov, V.S.; Nyashina, G.S.; Gaidukova, O.S.; Skiba, S.S.; Volkov, R.S.; Voytkov, I.S. The influence of key parameters on combustion of double gas hydrate. J. Nat. Gas Sci. Eng. 2020, 80, 103396. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Donskoy, I.G. Improving the efficiency of storage of natural and artificial methane hydrates. J. Nat. Gas Sci. Eng. 2022, 97, 104324. [Google Scholar] [CrossRef]
- Kuhs, W.F.; Genov, G.; Staykova, D.K.; Hansen, T. Ice perfection and onset of anomalous preservation of gas hydrates. Phys. Chem. Chem. Phys. 2004, 6, 4917–4920. [Google Scholar] [CrossRef]
- Takeya, S.; Yoneyama, A.; Ueda, K.; Mimachi, H.; Takahashi, M.; Sano, K.; Hyodo, K.; Takeda, T.; Gotoh, Y. Anomalously preserved clathrate hydrate of natural gas in pellet form at 253 K. J. Phys. Chem. C 2012, 116, 13842–13848. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Donskoy, I.G.; Manakov, A.Y.; Morozov, V.S.; Strizhak, P.A.; Skiba, S.S.; Sagidullin, A.K. Studying the influence of key parameters on the methane hydrate dissociation in order to improve the storage efficiency. J. Energy Storage 2021, 44, 103288. [Google Scholar] [CrossRef]
- Cui, G.; Dong, Z.; Wang, S.; Xing, X.; Shan, T.; Li, Z. Effect of the water on the flame characteristics of methane hydrate combustion. Appl. Energy 2020, 259, 114205. [Google Scholar] [CrossRef]
- Wu, F.H.; Padilla, R.E.; Dunn-Rankin, D.; Chen, G.B.; Chao, Y.C. Thermal structure of methane hydrate fueled flames. Proc. Combust. Inst. 2017, 36, 4391–4398. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Donskoy, I.G. Co-modeling of methane hydrate dissociation and combustion in a boundary layer. Combust. Flame 2022, 238, 111912. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Donskoy, I.G. Dissociation of a powder layer of methane gas hydrate in a wide range of temperatures and heat fluxes. Powder Technol. 2022, 397, 117017. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Donskoy, I.G. Dissociation kinetics of methane hydrate and CO2 hydrate for different granular composition. Fuel 2020, 262, 116614. [Google Scholar] [CrossRef]




















| AI-92 gasoline | Octane number | 91 |
| Lead content, g/dm3 | 0.010 | |
| Manganese content, mg/dm3 | 18 | |
| Oxidation stability of gasoline, min | 360 | |
| Existent gum content, mg/100 cm3 | 5 | |
| Mass fraction of sulfur, % | 0.05 | |
| TS-1 kerosene | Density at 20 °C, g/cm3 | 0.780 |
| Kinematic viscosity, mm2/s at 20 °C | 1.3 | |
| Lower heating value, kJ/kg | 43120 | |
| Mass fraction of total sulfur, % | 0.2 | |
| Diesel fuel | Cetane number | 45 |
| Kinematic viscosity, mm2/s at 20 °C | 4 | |
| Ash content, % | 0.01 | |
| Existent gum content, mg/100 cm3 of fuel | 30 | |
| Alcohol | Volume fraction of ethyl alcohol, % | 96 |
| Oxidation test, min | 15 | |
| Mass concentration of dry residue, mg/dm3 | 2 | |
| Mass concentration of sulfur, mg/dm3 | no | |
| Lukoil Genesis Armortech 5W30 used motor oil | Density at 15 °C, kg/m³ | 844.8 |
| Kinematic viscosity at 100 °C, mm2/s | 9.7 | |
| Viscosity index | 173 | |
| Sulfate ash content, % | 1.05 | |
| Flash temperature in open crucible, °C | 223 |
| Crude Oil | Separated Oil | ||
|---|---|---|---|
| Name of Indicator | Unit of Measurement | Test Result | Test Result |
| Mass fraction of water | wt% | 2.37 | no |
| Density at 15 °C | kg/m3 | 852.7 | 797.5 |
| Density at 20 °C | kg/m3 | 849.2 | 792.5 |
| Kinematic viscosity at 15 °C | mm2/s | – | 4.391 |
| Kinematic viscosity at 20 °C | mm2/s | 7.567 | 1.741 |
| Kinematic viscosity at 50 °C | mm2/s | 3.943 | – |
| Mass fraction of mechanical impurities | wt% | 3.943 | 0.020 |
| Freezing point | °C | −25.6 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaidukova, O.; Misyura, S.; Donskoy, I.; Morozov, V.; Volkov, R. Pool Fire Suppression Using CO2 Hydrate. Energies 2022, 15, 9585. https://doi.org/10.3390/en15249585
Gaidukova O, Misyura S, Donskoy I, Morozov V, Volkov R. Pool Fire Suppression Using CO2 Hydrate. Energies. 2022; 15(24):9585. https://doi.org/10.3390/en15249585
Chicago/Turabian StyleGaidukova, Olga, Sergey Misyura, Igor Donskoy, Vladimir Morozov, and Roman Volkov. 2022. "Pool Fire Suppression Using CO2 Hydrate" Energies 15, no. 24: 9585. https://doi.org/10.3390/en15249585
APA StyleGaidukova, O., Misyura, S., Donskoy, I., Morozov, V., & Volkov, R. (2022). Pool Fire Suppression Using CO2 Hydrate. Energies, 15(24), 9585. https://doi.org/10.3390/en15249585