Co-Processing Lignocellulosic Biomass and Sewage Digestate by Hydrothermal Carbonisation: Influence of Blending on Product Quality
Abstract
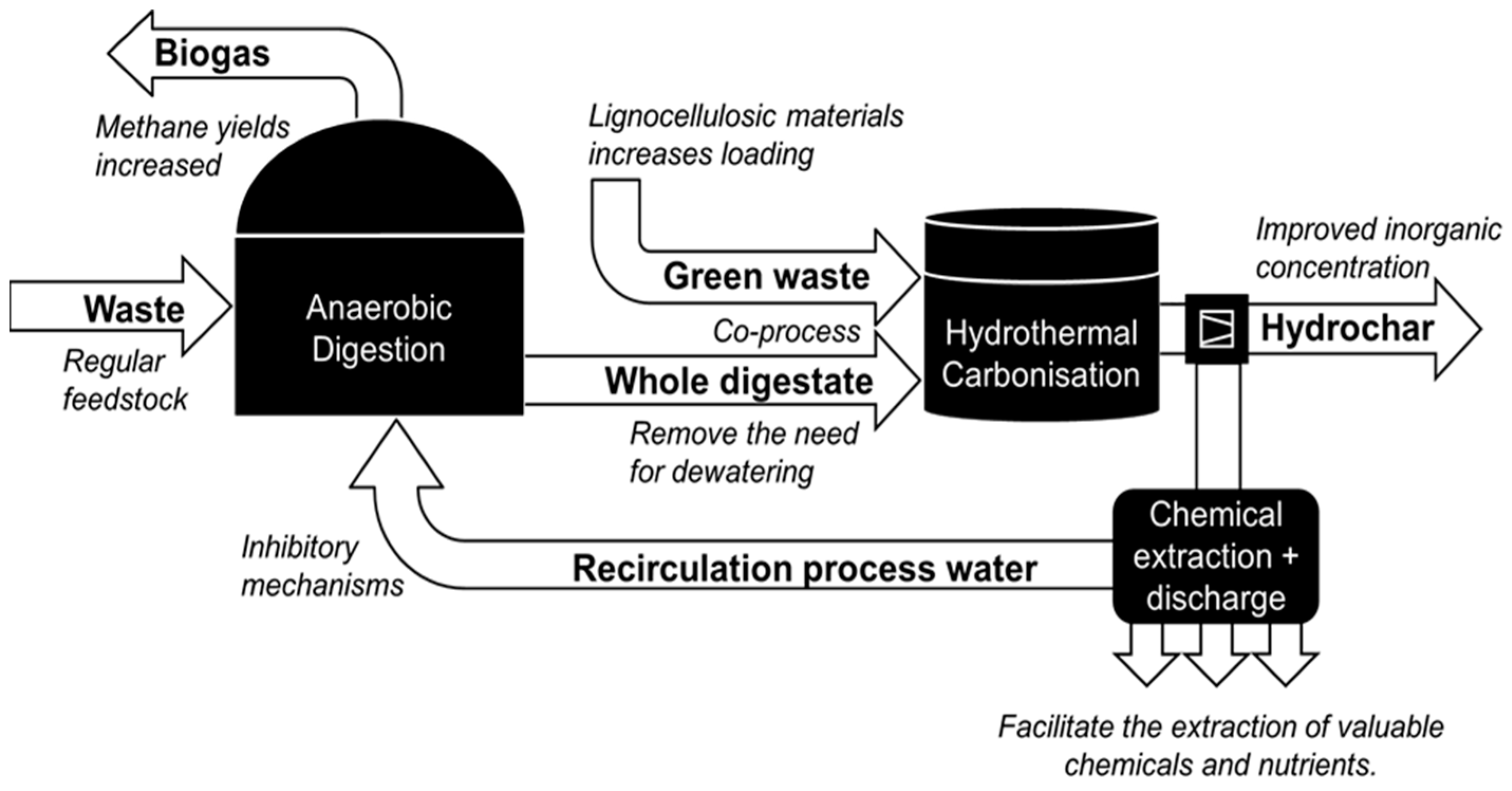
:1. Introduction
2. Materials and Methods
2.1. Source and Preparation of Materials
2.2. Hydrothermal Carbonisation (HTC)
2.3. Feedstock and Hydrochar Analysis
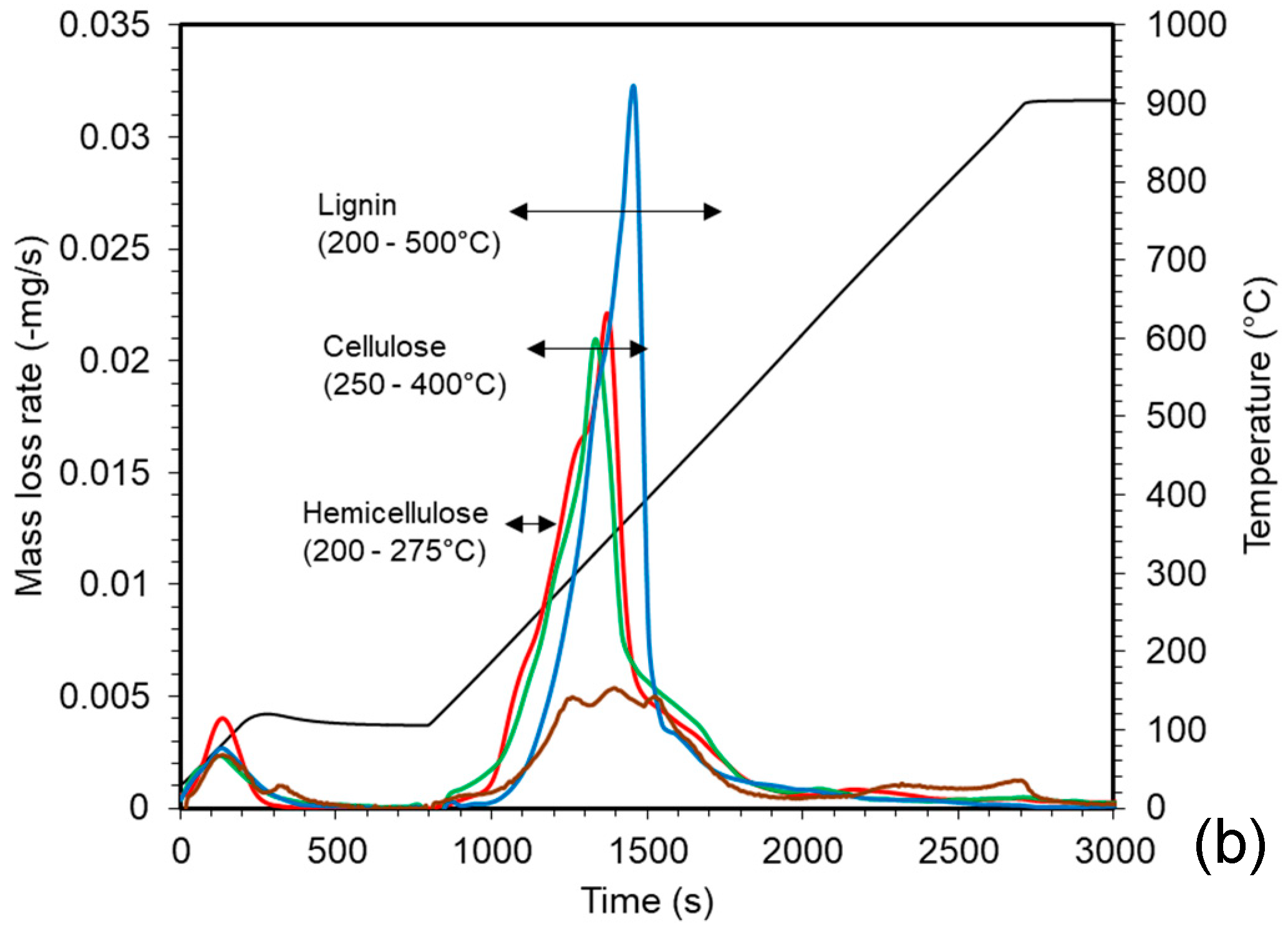
2.3.1. Biochemical Analysis of the Feedstocks
2.3.2. Proximate and Ultimate Analysis and Predictive Higher Heating Value (HHV)
2.3.3. Inorganics Analysis
2.3.4. Predictive Slagging and Fouling Indices
2.4. Process Water Analysis
2.5. Biomethane Production and Biodegradability
2.5.1. Experimental Biochemical Methane Potential (BMPexp)
2.5.2. Biodegradability Calculation
2.6. Assessment of Error and Statistical Analysis
3. Results and Discussion
3.1. Characterisation of Feedstocks
3.2. Co-Processing of Sewage Sludge Digestate with Lignocellulosic Biomass
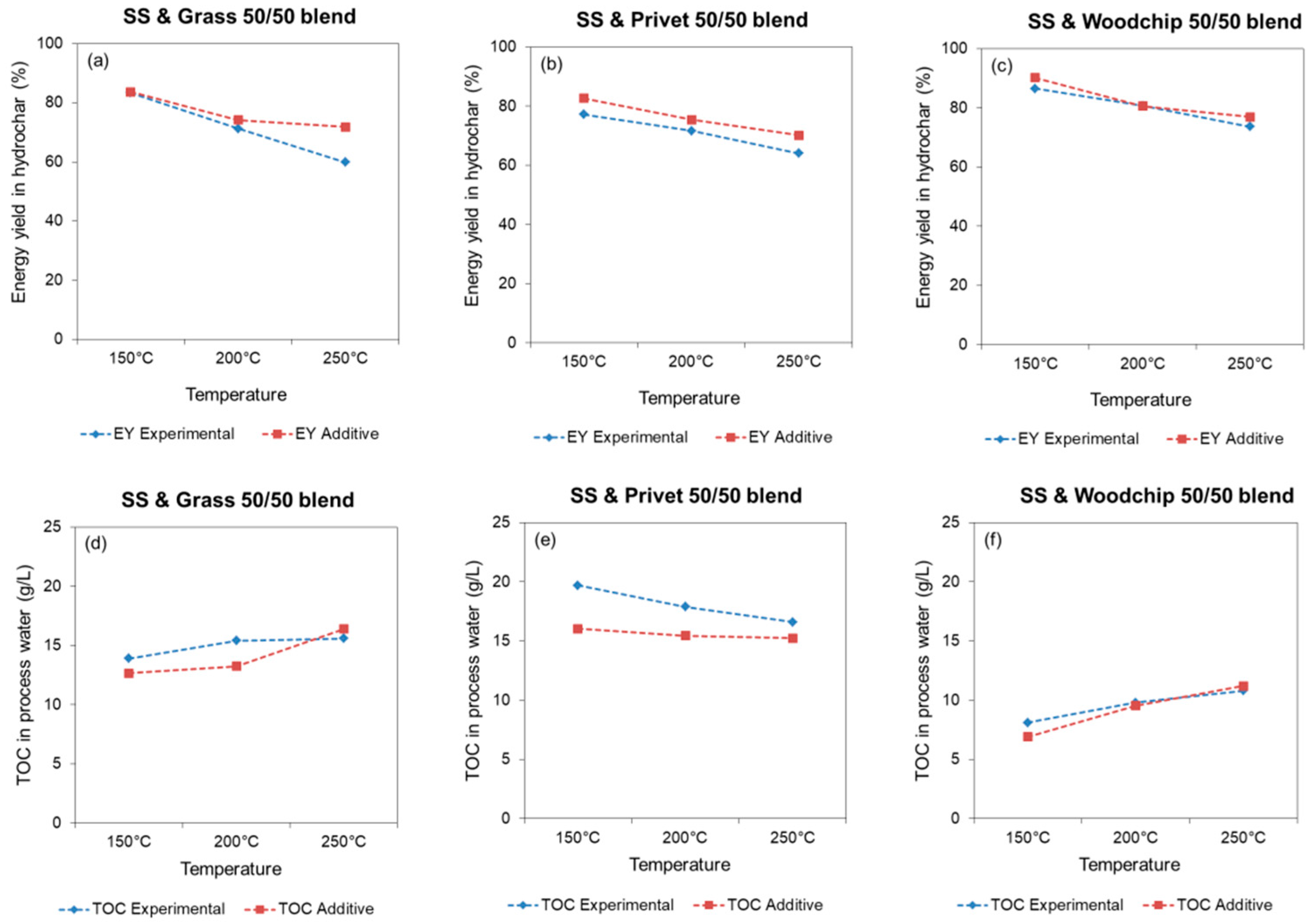
3.2.1. HTC Product Yields
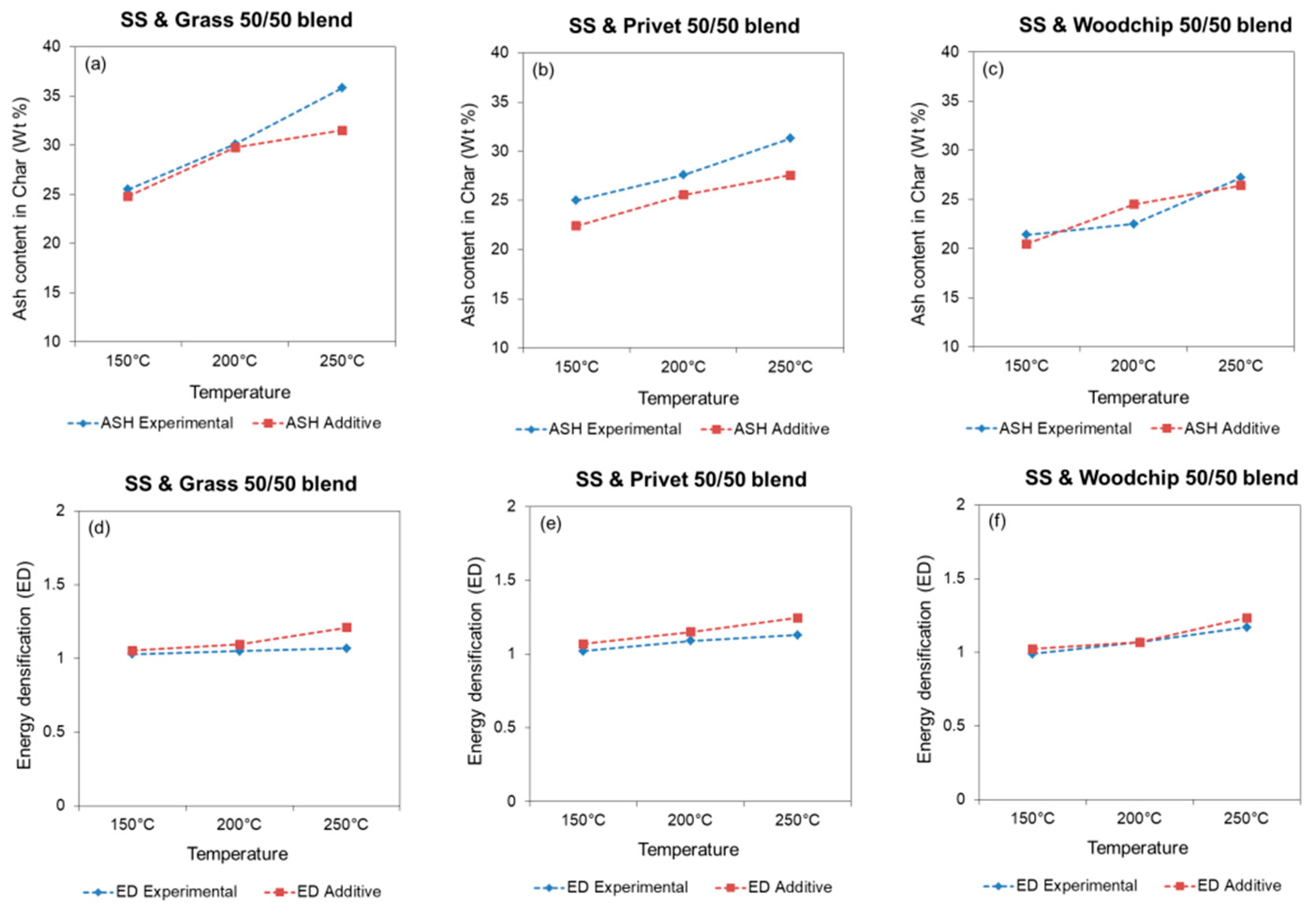
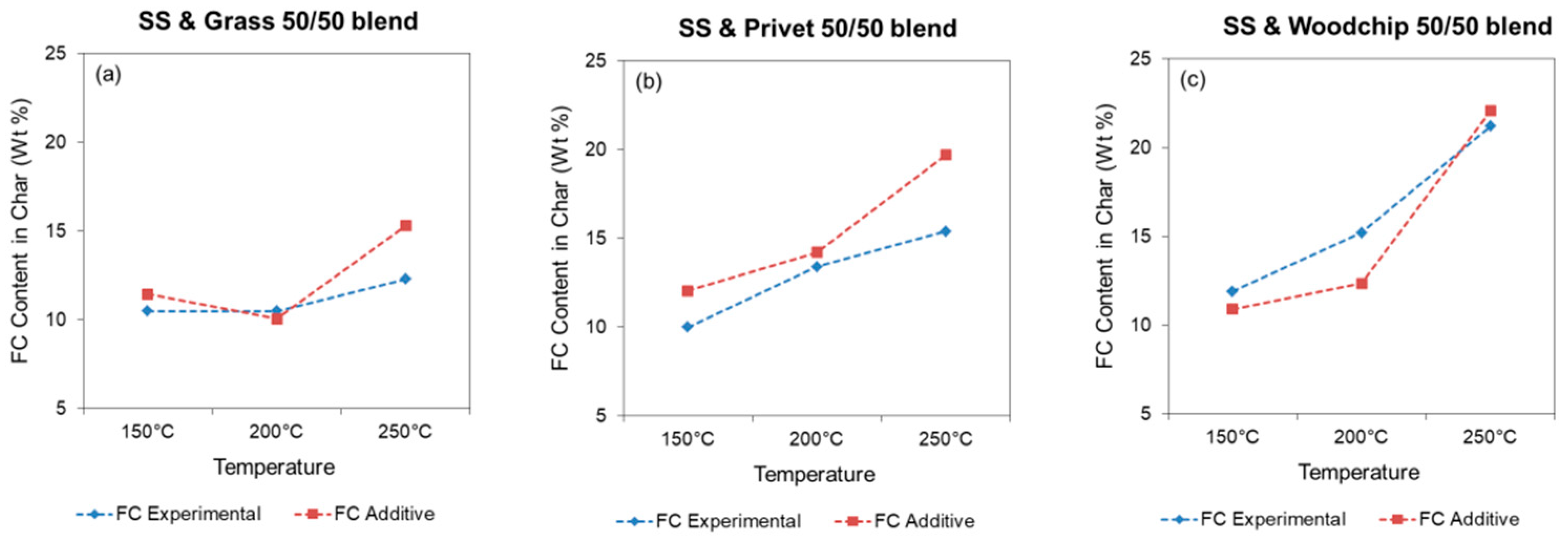
3.2.2. Hydrochar Properties
3.2.3. Inorganic Content and Influence on Combustion Behaviour
3.3. Process Water Composition, Biomethane Yield, and Biodegradability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Environment Agency. Spreading Organic Manure on Agricultural Land: RPS 252; Environment Agency: Bristol, UK, 2021. Available online: https://www.gov.uk/government/publications/spreading-organic-manure-on-agricultural-land-rps-252/spreading-organic-manure-on-agricultural-land-rps-252 (accessed on 13 January 2022).
- Assured Biosolids Limited. Biosolids Recycling to Agricultural Land Position Statement. 2020. Available online: https://assuredbiosolids.co.uk/position-statement-on-recycling-of-biosolids-to-agricultural-land/ (accessed on 13 January 2022).
- Kruse, A.; Dahmen, N. Hydrothermal biomass conversion: Quo vadis? J. Supercrit. Fluids 2018, 134, 114–123. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Parmar, K.R.; Ross, A.B. Integration of Hydrothermal Carbonisation with Anaerobic Digestion; Opportunities for Valorisation of Digestate. Energies 2019, 12, 1586. [Google Scholar] [CrossRef] [Green Version]
- Ipiales, R.P.; de la Rubia, M.A.; Diaz, E.; Mohedano, A.F.; Rodriguez, J.J. Integration of Hydrothermal Carbonization and Anaerobic Digestion for Energy Recovery of Biomass Waste: An Overview. Energy Fuels 2021, 35, 17032–17050. [Google Scholar] [CrossRef]
- Phoenixville New Energy Optimization (PXVNEO). PXVNEO—New Energy Optimization. Available online: https://phoenixville.org/2538/PXVNEO---New-Energy-Optimization (accessed on 13 January 2022).
- Aragón-Briceño, C.; Ross, A.B.; Camargo-Valero, M.A. Evaluation and comparison of product yields and bio-methane potential in sewage digestate following hydrothermal treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Smith, A.M.; Singh, S.; Ross, A.B. Fate of inorganic material during hydrothermal carbonisation of biomass: Influence of feedstock on combustion behaviour of hydrochar. Fuel 2016, 169, 135–145. [Google Scholar] [CrossRef]
- Smith, A.M.; Whittaker, C.; Shield, I.; Ross, A.B. The potential for production of high quality bio-coal from early harvested Miscanthus by hydrothermal carbonisation. Fuel 2018, 220, 546–557. [Google Scholar] [CrossRef]
- Smith, A.M.; Ekpo, U.; Ross, A.B. The Influence of pH on the Combustion Properties of Bio-Coal Following Hydrothermal Treatment of Swine Manure. Energies 2020, 13, 331. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G.; Zhu, Y. Hydrothermal carbonisation of sewage sludge for char production with different waste biomass: Effects of reaction temperature and energy recycling. Energy 2017, 127, 167–174. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Li, A. Hydrothermal co-carbonization of sewage sludge and pinewood sawdust for nutrient-rich hydrochar production: Synergistic effects and products characterization. J. Environ. Manag. 2017, 201, 52–62. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, X.; Yao, Z.; Chen, X. The properties and combustion behaviors of hydrochars derived from co-hydrothermal carbonization of sewage sludge and food waste. Bioresour. Technol. 2019, 285, 121347. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Guo, Y.; Zheng, Q.; Liu, Z.; Gai, C. Co-hydrothermal carbonization of lignocellulosic biomass and swine manure: Hydrochar properties and heavy metal transformation behavior. Bioresour. Technol. 2018, 266, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Zhang, B.; Liu, Z.; Chen, Z.; Xia, Y.; Li, D.; Ma, J.; Gai, C. Co-hydrothermal carbonization of corn stalk and swine manure: Combustion behavior of hydrochar by thermogravimetric analysis. Bioresour. Technol. 2019, 271, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Li, H.; Zhu, Y.; Li, S.; Peng, C.; Wang, B.; Wang, Z.; Xi, Y.; Wang, S.; et al. Co-hydrothermal carbonization of food waste-woody biomass blend towards biofuel pellets production. Bioresour. Technol. 2018, 267, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, D.; Turley, D. Lignocellulosic Feedstock in the UK; NNFCC: York, UK, 2014. [Google Scholar]
- Phillips, D.; Mitchell, E.J.S.; Lea-Langton, A.R.; Parmar, K.R.; Jones, J.M.; Williams, A. The use of conservation biomass feedstocks as potential bioenergy resources in the United Kingdom. Bioresour. Technol. 2016, 212, 271–279. [Google Scholar] [CrossRef]
- Hindrichsen, I.K.; Kreuzer, M.; Madsen, J.; Knudsen, K.E.B. Fiber and lignin analysis in concentrate, forage, and feces: Detergent versus enzymatic-chemical method. J. Dairy Sci. 2006, 89, 2168–2176. [Google Scholar] [CrossRef]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Xing, P.; Mason, P.E.; Chilton, S.; Lloyd, S.; Jones, J.M.; Williams, A.; Nimmo, W.; Pourkashanian, M. A comparative assessment of biomass ash preparation methods using X-ray fluorescence and wet chemical analysis. Fuel 2016, 182, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Mayala, T.S.; Ngavouka, M.D.; Douma, D.H.; Hammerton, J.M.; Ross, A.B.; Brown, A.E.; M’Passi-mabiala, B.; Lovett, J.C. Characterisation of Congolese Aquatic Biomass and Their Potential as a Source of Bioenergy. Biomass 2022, 2, 1. [Google Scholar] [CrossRef]
- Sommersacher, P.; Brunner, T.; Obernberger, I. Fuel indexes: A novel method for the evaluation of relevant combustion properties of new biomass fuels. Energy Fuels 2012, 26, 380–390. [Google Scholar] [CrossRef]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Demirbaş, A. Relationships between lignin contents and fixed carbon contents of biomass samples. Energy Convers. Manag. 2003, 44, 1481–1486. [Google Scholar] [CrossRef]
- Dieguez-Alonso, A.; Funke, A.; Anca-Couce, A.; Rombolà, A.G.; Ojeda, G.; Bachmann, J.; Behrendt, F. Towards biochar and hydrochar engineering—Influence of process conditions on surface physical and chemical properties, thermal stability, nutrient availability, toxicity and wettability. Energies 2018, 11, 496. [Google Scholar] [CrossRef] [Green Version]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Roberts, L.J. Additives to Mitigate against Slagging and Fouling in Biomass Combustion. Ph.D. Thesis, University of Leeds, Leeds, UK, 2018. [Google Scholar]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Kwapinski, W.; Leahy, J.J. Hydrothermal carbonisation of poultry litter: Effects of initial pH on yields and chemical properties of hydrochars. Bioresour. Technol. 2017, 238, 78–85. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]




| Key | Interpretation | Slagging and Fouling Indices | |||||
|---|---|---|---|---|---|---|---|
| AI | BAI | Rb/a | SI | FI | 2S/Cl | ||
| Low/safe | <0.17 | >0.15 | <0.5 | <0.6 | <0.6 | >4 | |
| Medium/likely | >0.17<0.34 | <0.15 | >0.5<0.7 | >0.6<2.0 | >0.6<40 | - | |
| High/certain | >0.34 | - | >0.7 | >2.0 | >40 | <4 | |
| SS Digestate | Grass Clippings | Privet Hedge | Woodchip | |
|---|---|---|---|---|
| % Protein (ar) | 19.9 ± 0.7 | 21.1 ± 0.6 | 6.7 ± 0.6 | 2.2 ± 0.2 |
| % Cellulose (ar) | 5.8 | 23.2 | 20.5 | 32.8 |
| % Hemicellulose (ar) | 4.9 | 22.6 | 9.4 | 13.9 |
| % Lignin (ar) | 17.3 | 3.5 | 10.2 | 21.7 |
| % C (db) | 28.6 ± 0.3 | 45.6 ± 0.2 | 46.7 ± 0.0 | 49.6 ± 0.4 |
| % H (db) | 3.1 ± 0.1 | 5.3 ± 0.2 | 5.6 ± 0.1 | 6.4 ± 0.9 |
| % N (db) | 3.4 ± 0.0 | 3.5 ± 0.0 | 1.1 ± 0.0 | 0.4 ± 0.0 |
| % S (db) | 1.5 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| % O a (db) | 16.4 ± 0.3 | 33.2 ± 0.9 | 39.4 ± 0.5 | 36.9 ± 0.7 |
| H/C (daf) | 1.30 | 1.37 | 1.42 | 1.55 |
| O/C (daf) | 0.43 | 0.55 | 0.63 | 0.60 |
| % VM (db) | 51.0 ± 0.1 | 71.1 ± 0.1 | 76.7 ± 0.1 | 76.1 ± 0.2 |
| % FC (db) | 2.1 ± 0.1 | 16.6 ± 0.3 | 16.1 ± 0.2 | 17.1 ± 0.4 |
| % Ash a (db) | 46.9 ± 0.0 | 12.4 ± 0.4 | 7.2 ± 0.3 | 6.8 ± 0.6 |
| HHV (MJ/kg db) b | 14.9 | 18.4 | 18.6 | 19.9 |
| Feedstock and HTC Process Conditions | Yields (wt.% db) | ||
|---|---|---|---|
| Solid | Liquid | Gas | |
| SS digestate | |||
| 150 °C | 90.6 ± 2.3 | 9.0 ± 2.2 | 0.4 ± 0.0 |
| 200 °C | 77.0 ± 0.8 | 22.2 ± 0.7 | 0.8 ± 0.2 |
| 250 °C | 70.5 ± 0.3 | 28.0 ± 0.0 | 1.5 ± 0.3 |
| Grass clippings | |||
| 150 °C | 68.7 ± 2.4 | 30.7 ± 2.4 | 0.5 ± 0.0 |
| 200 °C | 59.8 ± 0.5 | 39.0 ± 0.4 | 1.1 ± 0.1 |
| 250 °C | 51.4 ± 0.3 | 46.1 ± 0.4 | 2.4 ± 0.0 |
| SS + grass 50/50 | |||
| 150 °C | 81.0 ± 0.4 | 18.4 ± 0.1 | 0.6 ± 0.2 |
| 200 °C | 67.9 ± 0.1 | 30.8 ± 0.2 | 1.3 ± 0.3 |
| 250 °C | 56.0 ± 1.4 | 42.3 ± 1.6 | 1.7 ± 0.2 |
| Privet hedge | |||
| 150 °C | 65.2 ± 0.2 | 33.8 ± 0.2 | 0.9 ± 0.0 |
| 200 °C | 56.5 ± 1.2 | 41.8 ± 1.2 | 1.8 ± 0.1 |
| 250 °C | 46.6 ± 0.6 | 51.0 ± 0.6 | 2.5 ± 0.1 |
| SS + Privet 50/50 | |||
| 150 °C | 75.7 ± 0.2 | 23.7 ± 0.3 | 0.6 ± 0.2 |
| 200 °C | 65.8 ± 0.1 | 32.5 ± 0.1 | 1.7 ± 0.0 |
| 250 °C | 56.8 ± 0.5 | 41.3 ± 0.5 | 1.9 ± 0.0 |
| Wood chips | |||
| 150 °C | 85.5 ± 1.2 | 14.1 ± 1.1 | 0.3 ± 0.1 |
| 200 °C | 73.8 ± 0.6 | 25.7 ± 0.3 | 0.5 ± 0.3 |
| 250 °C | 56.5 ± 0.5 | 41.8 ± 0.4 | 1.7 ± 0.0 |
| SS + wood 50/50 | |||
| 150 °C | 87.4 ± 0.3 | 11.8 ± 0.7 | 0.8 ± 0.4 |
| 200 °C | 75.4 ± 0.1 | 23.5 ± 0.4 | 1.1 ± 0.4 |
| 250 °C | 62.9 ± 1.4 | 35.5 ± 1.5 | 1.5 ± 0.1 |
| Hydrochar (20% Solid Loading) | Ultimate Analysis (wt% db) | Proximate Analysis (wt% db) | HHV (MJ/kg, db) | H/C (daf) | O/C (daf) | ED | EY (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O a | VM | FC | Ash a | ||||||
| SS digestate | |||||||||||||
| 150 °C | 34.2 ± 0.0 | 4.5 ± 0.0 | 3.7 ± 0.0 | 0.9 ± 0.0 | 19.9 ± 0.1 | 55.0 ± 0.0 | 5.9 ± 0.1 | 39.1 ± 0.1 | 15.2 | 1.56 | 0.28 | 1.02 | 92.4 |
| 200 °C | 34.5 ± 0.0 | 4.3 ± 0.0 | 2.6 ± 0.0 | 1.1 ± 0.0 | 13.5 ± 0.7 | 51.2 ± 1.0 | 3.5 ± 1.6 | 45.3 ± 0.6 | 15.2 | 1.47 | 0.20 | 1.02 | 78.5 |
| 250 °C | 34.7 ± 0.4 | 4.3 ± 0.0 | 2.4 ± 0.1 | 0.9 ± 0.1 | 9.0 ± 0.4 | 44.9 ± 0.2 | 5.6 ± 0.1 | 49.5 ± 0.0 | 15.2 | 1.46 | 0.13 | 1.02 | 71.9 |
| Grass clippings | |||||||||||||
| 150 °C | 49.4 ± 0.1 | 6.1 ± 0.0 | 3.1 ± 0.0 | 0.0 ± 0.0 | 33.9 ± 0.2 | 72.5 ± 0.2 | 17.0 ± 0.2 | 10.5 ± 0.1 | 20.0 | 1.48 | 0.35 | 1.09 | 74.9 |
| 200 °C | 53.1 ± 0.4 | 6.0 ± 0.0 | 3.2 ± 0.0 | 0.0 ± 0.0 | 25.9 ± 0.0 | 69.2 ± 0.3 | 16.6 ± 0.2 | 14.2 ± 0.5 | 21.6 | 1.34 | 0.27 | 1.17 | 70.0 |
| 250 °C | 61.4 ± 0.0 | 6.1 ± 0.1 | 3.1 ± 0.0 | 0.1 ± 0.0 | 17.3 ± 0.0 | 61.5 ± 0.0 | 25.0 ± 0.1 | 13.5 ± 0.1 | 25.7 | 1.18 | 0.18 | 1.40 | 71.9 |
| SS + grass 50/50 | |||||||||||||
| 150 °C | 41.9 ± 0.1 | 5.2 ± 0.0 | 3.6 ± 0.0 | 0.6 ± 0.1 | 25.7 ± 0.2 | 64.0 ± 0.1 | 10.5 ± 0.2 | 25.5 ± 0.3 | 17.2 | 1.48 | 0.31 | 1.03 | 83.4 |
| 200 °C | 43.4 ± 0.2 | 4.9 ± 0.0 | 2.9 ± 0.0 | 0.7 ± 0.0 | 19.5 ± 0.9 | 59.4 ± 0.8 | 10.5 ± 0.2 | 30.1 ± 0.6 | 17.6 | 1.35 | 0.25 | 1.05 | 71.3 |
| 250 °C | 44.0 ± 0.0 | 4.5 ± 0.0 | 3.0 ± 0.1 | 0.7 ± 0.0 | 12.8 ± 0.1 | 51.8 ± 0.3 | 12.3 ± 0.0 | 35.8 ± 0.2 | 17.8 | 1.23 | 0.18 | 1.07 | 59.9 |
| Privet hedge | |||||||||||||
| 150 °C | 51.7 ± 0.4 | 6.1 ± 0.1 | 2.0 ± 0.1 | 0.0 ± 0.0 | 37.3 ± 0.3 | 76.1 ± 0.3 | 18.2 ± 0.0 | 5.7 ± 0.4 | 20.9 | 1.41 | 0.38 | 1.12 | 73.0 |
| 200 °C | 58.4 ± 0.2 | 5.9 ± 0.0 | 1.7 ± 0.0 | 0.0 ± 0.0 | 30.2 ± 0.3 | 69.3 ± 0.1 | 24.9 ± 0.0 | 5.8 ± 0.0 | 23.8 | 1.20 | 0.32 | 1.28 | 72.3 |
| 250 °C | 65.2 ± 0.2 | 5.9 ± 0.0 | 2.2 ± 0.1 | 0.1 ± 0.0 | 22.3 ± 0.9 | 60.5 ± 0.4 | 33.8 ± 0.1 | 5.6 ± 0.5 | 27.4 | 1.08 | 0.24 | 1.47 | 68.5 |
| SS + hedge 50/50 | |||||||||||||
| 150 °C | 42.0 ± 0.4 | 5.1 ± 0.1 | 2.7 ± 0.0 | 0.5 ± 0.0 | 27.0 ± 0.7 | 65.0 ± 0.1 | 10.0 ± 0.1 | 25.0 ± 0.2 | 17.1 | 1.44 | 0.33 | 1.02 | 77.2 |
| 200 °C | 45.7 ± 0.1 | 5.0 ± 0.0 | 2.4 ± 0.0 | 0.5 ± 0.0 | 20.3 ± 0.0 | 59.1 ± 0.1 | 13.4 ± 0.0 | 27.6 ± 0.1 | 18.3 | 1.31 | 0.26 | 1.09 | 71.7 |
| 250 °C | 47.5 ± 0.4 | 4.7 ± 0.0 | 2.6 ± 0.0 | 0.6 ± 0.0 | 14.4 ± 0.5 | 53.3 ± 0.0 | 15.4 ± 0.1 | 31.3 ± 0.1 | 18.9 | 1.19 | 0.19 | 1.13 | 64.1 |
| Wood chips | |||||||||||||
| 150 °C | 51.2 ± 1.7 | 6.0 ± 0.1 | 0.3 ± 0.0 | 0.0 ± 0.0 | 43.9 ± 1.5 | 82.3 ± 0.3 | 15.9 ± 0.3 | 1.8 ± 0.0 | 20.5 | 1.41 | 0.46 | 1.03 | 88.0 |
| 200 °C | 55.8 ± 0.8 | 5.7 ± 0.1 | 0.3 ± 0.0 | 0.0 ± 0.0 | 36.5 ± 0.7 | 75.2 ± 0.3 | 21.2 ± 0.2 | 3.7 ± 0.1 | 22.3 | 1.22 | 0.40 | 1.12 | 82.7 |
| 250 °C | 69.2 ± 0.4 | 5.5 ± 0.1 | 0.5 ± 0.0 | 0.0 ± 0.0 | 23.1 ± 0.4 | 58.2 ± 0.9 | 38.5 ± 1.0 | 3.3 ± 0.1 | 28.8 | 0.95 | 0.26 | 1.45 | 81.9 |
| SS + wood 50/50 | |||||||||||||
| 150 °C | 42.9 ± 0.6 | 5.7 ± 0.3 | 2.0 ± 0.1 | 0.4 ± 0.0 | 30.6 ± 0.9 | 66.7 ± 0.1 | 11.9 ± 0.7 | 21.4 ± 0.7 | 17.2 | 1.57 | 0.34 | 0.99 | 86.5 |
| 200 °C | 46.9 ± 0.1 | 5.1 ± 0.1 | 1.8 ± 0.0 | 0.5 ± 0.0 | 24.9 ± 0.3 | 62.3 ± 0.2 | 15.2 ± 0.4 | 22.5 ± 0.2 | 18.7 | 1.29 | 0.31 | 1.07 | 80.7 |
| 250 °C | 51.3 ± 0.0 | 4.8 ± 0.1 | 2.3 ± 0.0 | 0.5 ± 0.0 | 15.2 ± 0.2 | 51.6 ± 0.3 | 21.2 ± 0.1 | 27.2 ± 0.2 | 20.3 | 1.12 | 0.20 | 1.17 | 73.6 |
| Feedstock and Hydrochar | Inorganic Analysis (wt.% db) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | Mg | Al | Si | P | Cl | K | Ca | Fe | |
| SS digestate | 0.49 | 1.00 | 3.02 | 8.76 | 2.48 | 0.05 | 0.98 | 3.46 | 2.82 |
| 150 °C | 0.31 | 0.83 | 2.67 | 7.58 | 1.95 | 0.03 | 0.70 | 2.78 | 2.37 |
| 200 °C | 0.42 | 0.98 | 3.11 | 8.62 | 2.23 | 0.04 | 0.75 | 3.38 | 2.70 |
| 250 °C | 0.47 | 1.09 | 3.26 | 9.09 | 2.58 | 0.04 | 0.93 | 3.78 | 3.03 |
| Grass clippings | 0.26 | 0.39 | 0.15 | 1.76 | 0.58 | 0.07 | 1.42 | 1.93 | 0.28 |
| 150 °C | 0.18 | 0.25 | 0.08 | 1.78 | 0.55 | 0.05 | 1.21 | 1.83 | 0.06 |
| 200 °C | 0.20 | 0.28 | 0.11 | 2.71 | 0.82 | 0.09 | 1.18 | 2.35 | 0.08 |
| 250 °C | 0.21 | 0.30 | 0.11 | 2.37 | 0.90 | 0.07 | 0.65 | 2.69 | 0.08 |
| SS + grass 50/50 | 0.38 | 0.70 | 1.59 | 5.26 | 1.53 | 0.06 | 1.20 | 2.70 | 1.55 |
| 150 °C | 0.46 | 0.55 | 1.46 | 4.54 | 1.26 | 0.06 | 1.13 | 2.23 | 1.28 |
| 200 °C | 0.27 | 0.64 | 1.71 | 5.44 | 1.50 | 0.02 | 1.11 | 2.81 | 1.47 |
| 250 °C | 0.26 | 0.81 | 2.07 | 6.54 | 1.96 | 0.02 | 0.90 | 3.27 | 1.89 |
| Privet hedge | 0.20 | 0.13 | 0.03 | 0.36 | 0.30 | 0.01 | 1.46 | 1.65 | 0.01 |
| 150 °C | 0.14 | 0.09 | 0.03 | 0.30 | 0.29 | 0.00 | 0.77 | 1.80 | 0.02 |
| 200 °C | 0.07 | 0.07 | 0.03 | 0.22 | 0.40 | 0.01 | 0.73 | 1.87 | 0.02 |
| 250 °C | 0.07 | 0.08 | 0.03 | 0.12 | 0.50 | 0.01 | 0.39 | 1.92 | 0.02 |
| SS + hedge 50/50 | 0.35 | 0.57 | 1.53 | 4.56 | 1.39 | 0.03 | 1.22 | 2.56 | 1.42 |
| 150 °C | 0.23 | 0.45 | 1.60 | 4.62 | 1.04 | 0.02 | 1.72 | 1.95 | 1.19 |
| 200 °C | 0.18 | 0.53 | 1.81 | 4.92 | 1.29 | 0.02 | 1.6 | 2.52 | 1.40 |
| 250 °C | 0.21 | 0.63 | 2.06 | 5.68 | 1.47 | 0.00 | 1.20 | 2.63 | 1.60 |
| Wood chips | 0.05 | 0.02 | 0.04 | 0.16 | 0.01 | 0.05 | 0.02 | 0.26 | 0.03 |
| 150 °C | 0.04 | 0.03 | 0.07 | 0.31 | 0.01 | 0.00 | 0.02 | 0.39 | 0.05 |
| 200 °C | 0.04 | 0.05 | 0.18 | 0.69 | 0.02 | 0.00 | 0.05 | 0.63 | 0.13 |
| 250 °C | 0.18 | 0.04 | 0.13 | 0.63 | 0.02 | 0.03 | 0.13 | 0.39 | 0.09 |
| SS + wood 50/50 | 0.27 | 0.51 | 1.53 | 4.46 | 1.25 | 0.00 | 0.50 | 1.86 | 1.43 |
| 150 °C | 0.21 | 0.44 | 1.45 | 4.12 | 1.03 | 0.02 | 0.54 | 1.57 | 1.29 |
| 200 °C | 0.18 | 0.46 | 1.54 | 4.36 | 0.98 | 0.01 | 0.65 | 1.63 | 1.24 |
| 250 °C | 0.34 | 0.56 | 1.96 | 5.33 | 1.17 | 0.04 | 0.68 | 1.79 | 1.43 |
| Feedstock and Resultant Hydrochars | Slagging and Fouling Indices | |||||
|---|---|---|---|---|---|---|
| AI | BAI | Rb/a | SI | FI | 2S/Cl | |
| SS digestate | 1.23 | 2.19 | 0.47 | 0.7 | 1.8 | 16.7 |
| 150 °C | 0.83 | 2.69 | 0.43 | 0.4 | 1.4 | 16.0 |
| 200 °C | 0.97 | 2.63 | 0.44 | 0.5 | 1.4 | 13.9 |
| 250 °C | 1.15 | 2.47 | 0.48 | 0.4 | 1.7 | 13.0 |
| Grass clippings | 1.13 | 0.19 | 1.42 | 0.0 | 24.4 | 0.2 |
| 150 °C | 0.84 | 0.05 | 1.20 | 0.1 | 19.3 | 0.1 |
| 200 °C | 0.78 | 0.07 | 0.93 | 0.0 | 11.0 | 0.1 |
| 250 °C | 0.42 | 0.11 | 1.03 | 0.1 | 8.1 | 0.8 |
| SS and grass 50/50 | 1.87 | 0.56 | 0.83 | 0.6 | 8.7 | 9.4 |
| 150 °C | 1.15 | 0.93 | 0.58 | 0.3 | 4.5 | 5.6 |
| 200 °C | 0.97 | 1.23 | 0.55 | 0.4 | 3.1 | 16.2 |
| 250 °C | 0.81 | 1.89 | 0.52 | 0.4 | 2.1 | 15.5 |
| Privet hedge | 1.10 | 0.01 | 5.52 | 0.0 | 163.8 | 0.4 |
| 150 °C | 0.53 | 0.02 | 5.49 | 0.0 | 107.1 | 1.6 |
| 200 °C | 0.41 | 0.03 | 7.28 | 0.0 | 121.6 | 1.0 |
| 250 °C | 0.20 | 0.06 | 11.00 | 0.8 | 109.0 | 4.7 |
| SS and hedge 50/50 | 2.70 | 0.26 | 1.36 | 1.0 | 22.8 | 13.0 |
| 150 °C | 1.40 | 0.71 | 0.55 | 0.3 | 5.2 | 13.8 |
| 200 °C | 0.67 | 1.64 | 0.50 | 0.3 | 2.2 | 18.1 |
| 250 °C | 0.91 | 1.32 | 0.51 | 0.3 | 2.8 | 60.0 |
| Woodchip | 0.04 | 0.49 | 1.27 | 0.0 | 9.9 | 0.1 |
| 150 °C | 0.04 | 0.86 | 0.92 | 0.0 | 4.2 | 1.2 |
| 200 °C | 0.05 | 1.72 | 0.68 | 0.0 | 2.0 | 1.1 |
| 250 °C | 0.14 | 0.30 | 0.71 | 0.0 | 8.7 | 0.2 |
| SS and woodchip 50/50 | 0.80 | 1.06 | 0.79 | 0.6 | 4.6 | 8.2 |
| 150 °C | 0.54 | 1.98 | 0.45 | 0.2 | 2.0 | 13.3 |
| 200 °C | 0.55 | 1.74 | 0.44 | 0.2 | 2.0 | 19.7 |
| 250 °C | 0.63 | 1.61 | 0.41 | 0.2 | 1.9 | 6.5 |
| SS, Lignocellulosic Biomass and Blends Process Waters | pH | C:N | TN (g/L) | TOC (g/L) | COD (g/L) | Total VFA (g/L) | Total Phenols (g/L) |
|---|---|---|---|---|---|---|---|
| SS digestate | |||||||
| 150 °C | 6.5 ± 0.0 | 5.7 | 1.0 ± 0.4 | 5.7 ± 0.0 | 18.8 ± 0.0 | 0.2 | 0.2 ± 0.0 |
| 200 °C | 5.1 ± 0.0 | 6.1 | 1.4 ± 0.0 | 8.5 ± 0.1 | 36.5 ± 0.1 | 1.8 | 0.5 ± 0.0 |
| 250 °C | 8.0 ± 0.0 | 6.5 | 1.5 ± 0.0 | 9.8 ± 0.0 | 37.8 ± 0.4 | 4.0 | 0.2 ± 0.0 |
| Grass clippings | |||||||
| 150 °C | 5.8 ± 0.0 | 12.2 | 1.6 ± 0.0 | 19.6 ± 0.1 | 54.3 ± 0.4 | 1.5 | 0.3 ± 0.0 |
| 200 °C | 4.6 ± 0.0 | 6.4 | 2.8 ± 0.0 | 18.0 ± 0.2 | 49.3 ± 0.2 | 3.6 | 1.3 ± 0.0 |
| 250 °C | 6.2 ± 0.2 | 2.7 | 8.6 ± 0.1 | 23.0 ± 0.1 | 68.0 ± 0.0 | 4.7 | 0.1 ± 0.0 |
| SS & grass 50/50 | |||||||
| 150 °C | 5.8 ± 0.0 | 7.7 | 1.8 ± 0.0 | 13.9 ± 0.0 | 39.0 ± 0.1 | 1.5 | 0.2 ± 0.0 |
| 200 °C | 4.7 ± 0.0 | 7.7 | 2.0 ± 1.1 | 15.4 ± 0.0 | 44.7 ± 0.0 | 2.9 | 0.3 ± 0.0 |
| 250 °C | 6.9 ± 0.0 | 4.6 | 3.4 ± 0.0 | 15.6 ± 0.1 | 45.1 ± 0.2 | 4.1 | 0.4 ± 0.0 |
| Privet hedge | |||||||
| 150 °C | 5.2 ± 0.0 | 44 | 0.6 ± 0.0 | 26.4 ± 0.9 | 94.4 ± 0.4 | 2.2 | 0.5 ± 0.0 |
| 200 °C | 3.8 ± 0.0 | 10.7 | 2.1 ± 1.0 | 22.4 ± 0.1 | 66.3 ± 0.2 | 7.1 | 0.5 ± 0.0 |
| 250 °C | 5.4 ± 0.0 | 25.9 | 0.8 ± 0.0 | 20.7 ± 0.1 | 62.2 ± 0.0 | 8.2 | 0.9 ± 0.0 |
| SS & hedge 50/50 | |||||||
| 150 °C | 5.5 ± 0.0 | 11.6 | 1.7 ± 0.0 | 19.7 ± 0.0 | 58.7 ± 0.1 | 1.2 | 0.5 ± 0.0 |
| 200 °C | 4.3 ± 0.0 | 8.1 | 2.2 ± 0.0 | 17.9 ± 0.0 | 49.8 ± 0.1 | 3.9 | 0.4 ± 0.0 |
| 250 °C | 5.9 ± 0.0 | 8.3 | 2.0 ± 0.2 | 16.6 ± 0.1 | 46.0 ± 1.1 | 1.5 | 0.7 ± 0.0 |
| Woodchip | |||||||
| 150 °C | 4.0 ± 0.0 | 2.7 | 3.0 ± 0.0 | 8.1 ± 0.0 | 20.3 ± 0.1 | 0.9 | 0.4 ± 0.0 |
| 200 °C | 4.0 ± 0.0 | 3.7 | 2.9 ± 0.0 | 10.6 ± 0.0 | 30.0 ± 0.2 | 3.2 | 0.2 ± 0.0 |
| 250 °C | 4.0 ± 0.0 | 63.0 | 0.2 ± 0.0 | 12.6 ± 0.0 | 32.6 ± 0.0 | 4.8 | 0.3 ± 0.0 |
| SS & woodchip 50/50 | |||||||
| 150 °C | 5.6 ± 0.0 | 6.9 | 1.2 ± 0.0 | 8.1 ± 0.0 | 22.8 ± 0.4 | 0.9 | 0.1 ± 0.0 |
| 200 °C | 4.1 ± 0.0 | 2.2 | 4.4 ± 0.0 | 9.8 ± 0.0 | 26.0 ± 0.1 | 2.1 | 0.7 ± 0.0 |
| 250 °C | 5.1 ± 0.0 | 8.0 | 1.4 ± 0.0 | 10.8 ± 0.1 | 30.1 ± 0.1 | 3.7 | 0.3 ± 0.0 |
| SS, Lignocellulosic Biomass and Blends Process Waters | BMPexp (NmLCH4/gCOD) | COD Removed (per 2g) | Biodegradability (BICOD%) |
|---|---|---|---|
| SS digestate | |||
| 150 °C | 98.2 ± 8.2 | 0.74 | 37 |
| 200 °C | 159.2 ± 5.6 | 1.16 | 58 |
| 250 °C | 141.8 ± 0.4 | 1.11 | 55 |
| Grass clippings | |||
| 150 °C | 165.0 ± 3.7 | 1.49 | 74 |
| 200 °C | 188.5 ± 0.1 | 1.41 | 71 |
| 250 °C | 45.5 ± 7.0 | 0.36 | 19 |
| SS & grass 50/50 | |||
| 150 °C | 165.6 ± 0.5 | 1.48 | 74 |
| 200 °C | 184.4 ± 9.8 | 1.66 | 83 |
| 250 °C | 129.7 ± 2.7 | 1.36 | 68 |
| Privet hedge | |||
| 150 °C | 61.7 ± 4.2 | 0.63 | 32 |
| 200 °C | 112.4 ± 0.1 | 0.74 | 37 |
| 250 °C | 96.9 ± 0.5 | 1.22 | 61 |
| SS & Privet 50/50 | |||
| 150 °C | 116.1 ± 14.7 | 1.28 | 64 |
| 200 °C | 39.2 ± 3.1 | 0.31 | 15 |
| 250 °C | 122.0 ± 3.9 | 1.22 | 61 |
| Woodchip | |||
| 150 °C | 97.8 ± 7.7 | 0.59 | 30 |
| 200 °C | 16.2 ± 0.3 | 0.45 | 22 |
| 250 °C | 18.6 ± 2.1 | 0.26 | 13 |
| SS & woodchip 50/50 | |||
| 150 °C | 142.9 ± 1.1 | 1.44 | 72 |
| 200 °C | 172.4 ± 0.9 | 0.98 | 49 |
| 250 °C | 92.5 ± 17.0 | 0.52 | 26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmar, K.R.; Brown, A.E.; Hammerton, J.M.; Camargo-Valero, M.A.; Fletcher, L.A.; Ross, A.B. Co-Processing Lignocellulosic Biomass and Sewage Digestate by Hydrothermal Carbonisation: Influence of Blending on Product Quality. Energies 2022, 15, 1418. https://doi.org/10.3390/en15041418
Parmar KR, Brown AE, Hammerton JM, Camargo-Valero MA, Fletcher LA, Ross AB. Co-Processing Lignocellulosic Biomass and Sewage Digestate by Hydrothermal Carbonisation: Influence of Blending on Product Quality. Energies. 2022; 15(4):1418. https://doi.org/10.3390/en15041418
Chicago/Turabian StyleParmar, Kiran R., Aaron E. Brown, James M. Hammerton, Miller Alonso Camargo-Valero, Louise A. Fletcher, and Andrew B. Ross. 2022. "Co-Processing Lignocellulosic Biomass and Sewage Digestate by Hydrothermal Carbonisation: Influence of Blending on Product Quality" Energies 15, no. 4: 1418. https://doi.org/10.3390/en15041418
APA StyleParmar, K. R., Brown, A. E., Hammerton, J. M., Camargo-Valero, M. A., Fletcher, L. A., & Ross, A. B. (2022). Co-Processing Lignocellulosic Biomass and Sewage Digestate by Hydrothermal Carbonisation: Influence of Blending on Product Quality. Energies, 15(4), 1418. https://doi.org/10.3390/en15041418







