Comparative Fuel Yield from Anaerobic Digestion of Emerging Waste in Food and Brewery Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
3. Results
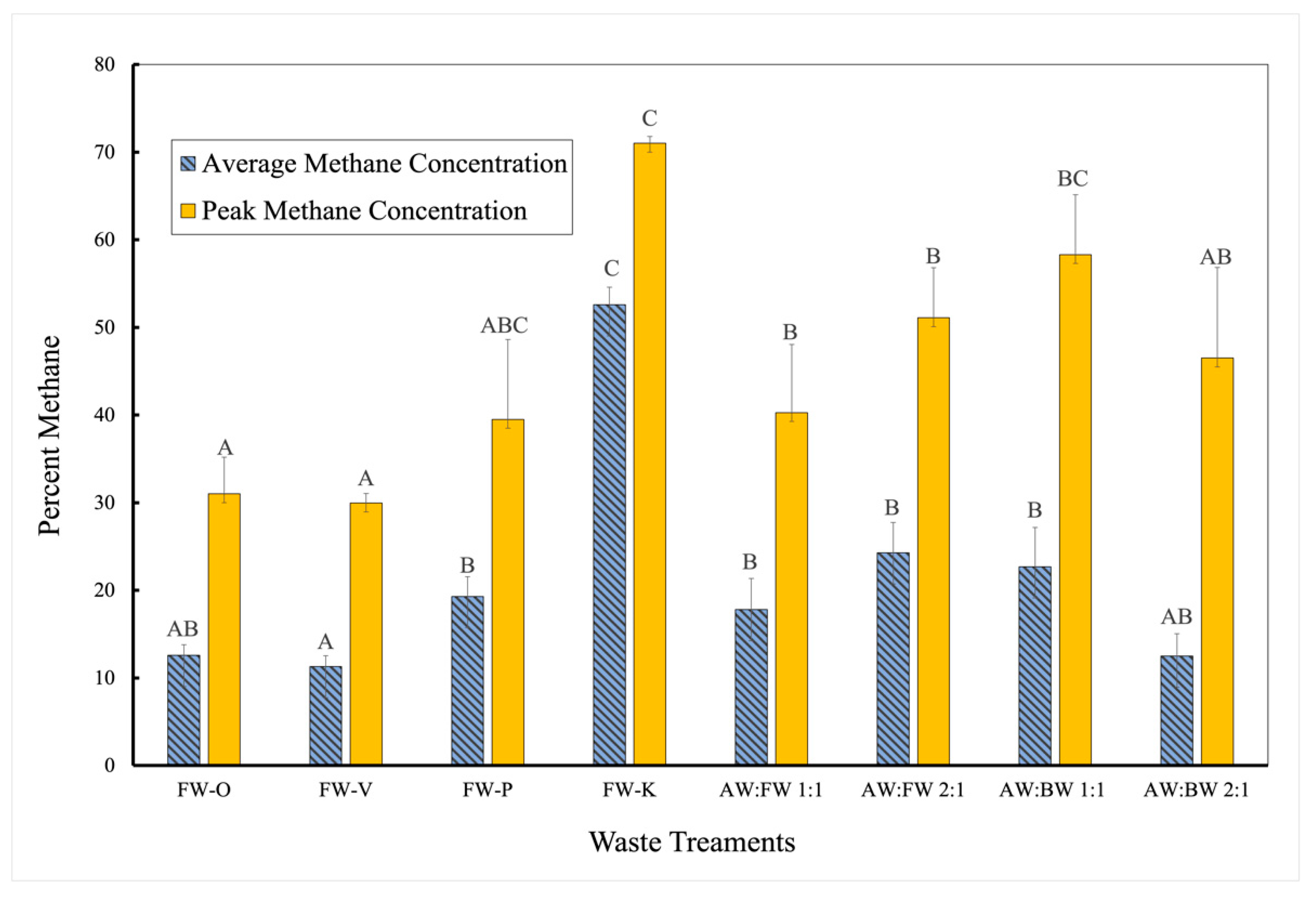
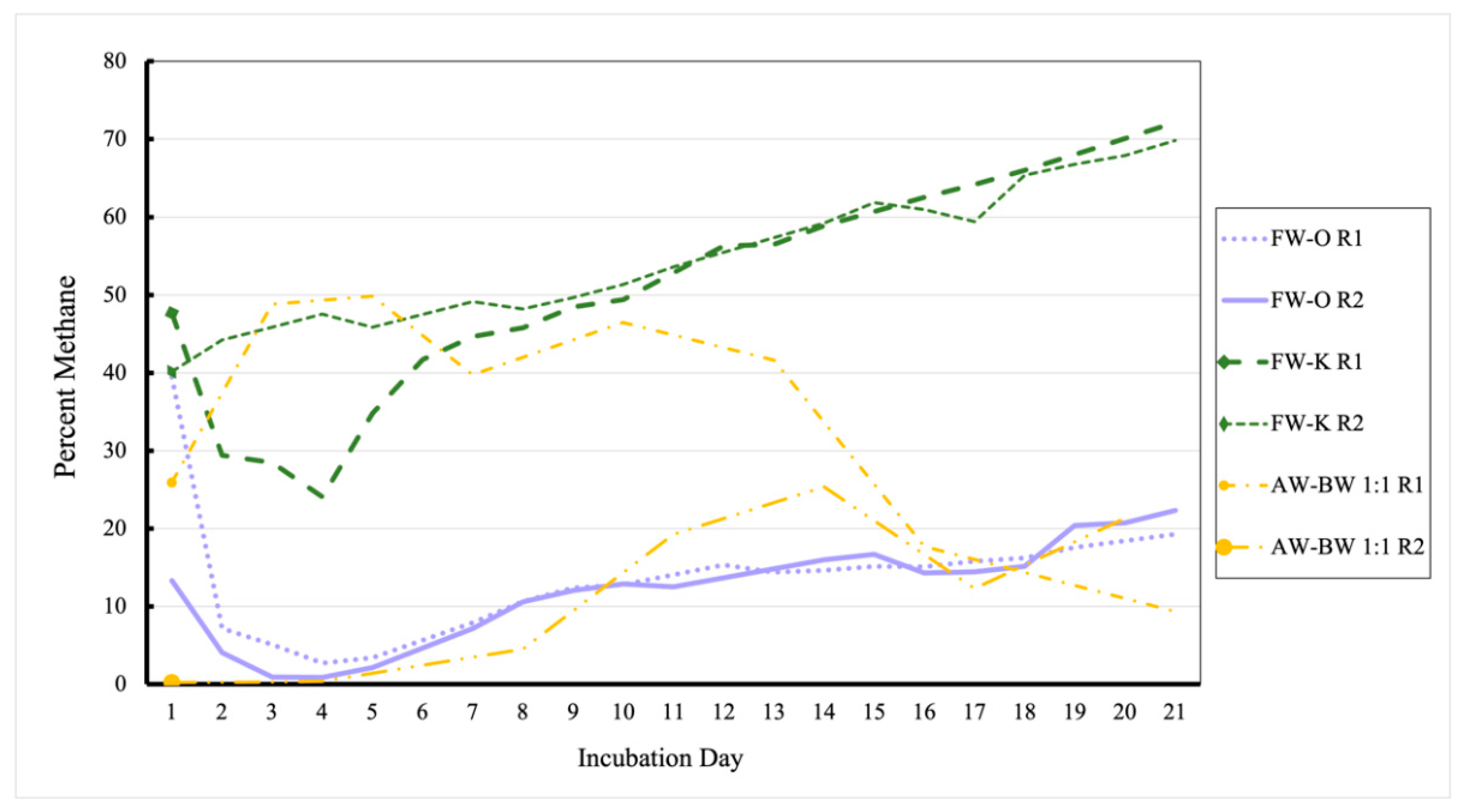
3.1. Methane Outputs of Various Waste Treatments
3.2. Macronutrient Trends
3.3. Digestate Quality Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USEPA. National Overview: Facts and Figures on Materials, Wastes and Recycling. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials#Landfilling (accessed on 1 December 2021).
- David, A.; Govil, T.; Tripathi, A.K.; McGeary, J.; Farrar, K.; Sani, R.K. Thermophilic Anaerobic Digestion: Enhanced and Sustainable Methane Production from Co-Digestion of Food and Lignocellulosic Wastes. Energies 2018, 11, 2058. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.; Herman, T.; Philipinanto, D.; Davis, S. Anaerobic Digestion of Food Waste, Brewery Waste, and Agricultural Residues in an Off-Grid Continuous Reactor. Sustainability 2021, 13, 6509. [Google Scholar] [CrossRef]
- Hills, R.D., Jr.; Erpenbeck, E. Guide to Popular Diets, Food Choices, and Their Health Outcome. Health Care Curr. Rev. 2018, 6, 1–6. [Google Scholar] [CrossRef]
- Freedman, M.R.; King, J.; Kennedy, E. Executive Summary. Obes. Res. 2001, 9, 1S–5S. [Google Scholar] [CrossRef]
- White, R.; Frank, E. Health effects and prevalence of vegetarianism. West. J. Med. 1994, 160, 465–470. [Google Scholar]
- Bryngelsson, S.; Asp, N.-G. Popular diets, body weight and health. Scand. J. Nutr. 2005, 49, 15–20. [Google Scholar] [CrossRef]
- Statista. America’s Brewery Boom. Available online: https://www.statista.com/chart/10280/americas-brewery-boom/ (accessed on 13 December 2021).
- Mathias, T.S.; de Aguiar, P.F.; de Almeida e Silva, J.B.; de Mello, P.M.; Servulo, E.C. Brewery waste reuse for protease production by lactic acid fermentation. Food Technol. Biotechnol. 2017, 2, 4378. [Google Scholar] [CrossRef]
- Colen, L.; Swinnen, J. Economic Growth, Globalisation and Beer Consumption. J. Agric. Econ. 2015, 67, 186–207. [Google Scholar] [CrossRef]
- Mussatto, S.I. Biotechnological Potential of Brewing Industry By-Products. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Singh Nee Nigam, P., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 1–466. ISBN 9781402099410. [Google Scholar]
- Bocher, B.T.; Agler, M.; Garcia, M.L.; Beers, A.R.; Angenent, L.T. Anaerobic digestion of secondary residuals from an anaerobic bioreactor at a brewery to enhance bioenergy generation. J. Ind. Microbiol. Biotechnol. 2008, 35, 321–329. [Google Scholar] [CrossRef]
- Vitanza, A.; Cortesi, V.; Gallo, V.; Colussi, I. Biovalorization of brewery waste by applying anaerobic digestion. Chem. Biochem. Eng. Q. 2016, 30, 351–357. [Google Scholar] [CrossRef]
- Mainardis, M.; Flaibani, S.; Mazzolini, F.; Peressotti, A.; Goi, D. Techno-economic analysis of anaerobic digestion implementation in small Italian breweries and evaluation of biochar and granular activated carbon addition effect on methane yield. J. Environ. Chem. Eng. 2019, 7, 103184. [Google Scholar] [CrossRef]
- Davis, S.C.; Parton, W.J.; Del Grosso, S.J.; Keough, C.; Marx, E.; Adler, P.R.; DeLucia, E.H. Impact of second-generation biofuel agriculture on greenhouse-gas emissions in the corn-growing regions of the US. Front. Ecol. Environ. 2011, 10, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.; Zhuang, Q.; Zhu, X. Carbon and nitrogen dynamics in bioenergy ecosystems: 2. Potential greenhouse gas emissions and global warming intensity in the conterminous United States. GCB Bioenergy 2013, 7, 25–39. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; DeGruyter: Berlin, Germany, 1984; ISBN 3-11-0084813. [Google Scholar]
- Hendricks, A.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Khalid, H.; Zhu, Z.; Zhang, R.; Liu, G.; Chen, C.; Thorin, E. Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar] [CrossRef]
- Prescott, M.P.; Herritt, C.; Bunning, M.; Cunningham-Sabo, L. Resources, Barriers, and Tradeoffs: A Mixed Methods Analysis of School Pre-Consumer Food Waste. J. Acad. Nutr. Diet. 2019, 119, 1270–1283.e2. [Google Scholar] [CrossRef] [PubMed]
- CalorieKing. Available online: https://www.calorieking.com/us/en/ (accessed on 13 December 2021).
- Cirne, D.; Paloumet, X.; Björnsson, L.; Alves, M.; Mattiasson, B. Anaerobic digestion of lipid-rich waste—Effects of lipid concentration. Renew. Energy 2007, 32, 965–975. [Google Scholar] [CrossRef] [Green Version]
- Asri, O.; Afilal, M.E. Comparison of the experimental and theoretical production of biogas by monosaccharides, disaccharides, and amino acids. Int. J. Environ. Sci. Technol. 2017, 15, 1957–1966. [Google Scholar] [CrossRef]
- Oliveira, J.V.; Alves, M.M.; Costa, J.C. Biochemical methane potential of brewery by-products. Clean Technol. Environ. Policy 2018, 20, 435–440. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Lopez, V.M.; De la Cruz, F.; Barlaz, M.A. Chemical composition and methane potential of commercial food wastes. Waste Manag. 2016, 56, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Wang, Y.; Lyu, X.; Zhao, N.; Song, J.; Wang, X.; Yang, G. Interactive effects of carbohydrate, lipid, protein composition and carbon/nitrogen ratio on biogas production of different food wastes. Bioresour. Technol. 2020, 312, 123566. [Google Scholar] [CrossRef] [PubMed]
- Das Neves, L.C.M.; Converti, A.; Penna, T.C.V. Biogas Production: New Trends for Alternative Energy Sources in Rural and Urban Zones. Chem. Eng. Technol. 2009, 32, 1147–1153. [Google Scholar] [CrossRef]
- Wang, X.; Ren, G.; Han, X.; Yang, G.; Feng, Y. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 12073, 83. [Google Scholar] [CrossRef]
- Choi, Y.; Ryu, J.; Lee, S.R. Influence of carbon type and carbon to nitrogen ratio on the biochemical methane potential, pH, and ammonia nitrogen in anaerobic digestion. J. Anim. Sci. Technol. 2020, 62, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Delgenés, J.P.; Penaud, V.; Moletta, R. Pretreatments for the enhancement of anaerobic digestion of solid wastes: Chapter 8. In Biomethanization of the Organic Fraction of Municipal Solid Wastes 2002; IWA Publishing: London, UK; pp. 201–228.
- Fisgativa, H.; Tremier, A.; Dabert, P. Characterizing the variability of food waste quality: A need for efficient valorisation through anaerobic digestion. Waste Manag. 2016, 50, 264–274. [Google Scholar] [CrossRef]
- Sogn, T.A.; Dragicevic, I.; Linjordet, R.; Krogstad, T.; Eijsink, V.G.H.; Eich-Greatorex, S. Recycling of biogas digestates in plant production: NPK fertilizer value and risk of leaching. Int. J. Recycl. Org. Waste Agric. 2018, 7, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.E.; Grossman, E.; Stuart, B.J.; Davis, S.C. Pilot-scale biogas production in a temperate climate using variable food waste. Biomass Bioenergy 2020, 138, 105568. [Google Scholar] [CrossRef]
- Conrad, Z. Food Waste, Healthy Diets, and Environmental Sustainability. Nutr. Today 2020, 55, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Cohen, E.; Cragg, M.; Defonseka, J.; Hite, A.; Rosenberg, M.; Zhou, B. Statistical review of US macronutrient consumption data, 1965–2011: Americans have been following dietary guidelines, coincident with the rise in obesity. Nutrition 2015, 31, 727–732. [Google Scholar] [CrossRef]

| Paleolithic | Omnivorous | Ketogenic | Vegetarian | AW:FW 1:1 | AW:FW 2:1 | AW:BW 1:1 | AW:BW 2:1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feedstock Component | Feedstock Component According to Diet [7] | Included? | Mass Used in Experiment (g) ** | Included? | Mass Used in Experiment (g) ** | Included? | Mass Used in Experiment (g) ** | Included? | Mass Used in Experiment (g) ** | Included? | Mass Used in Experiment (g) ** | Included? | Mass Used in Experiment (g) ** | Included? | Mass Used in Experiment (g) ** | Included? | Mass Used in Experiment (g) ** |
| Sweets | Sugar | No | - | Yes | 19.76 | No | - | Yes | 22.90 | Yes | - | Yes | - | No | - | No | - |
| Starch | Bread | Whole grain * | 95.22 | Yes | 33.33 | No | - | Yes | 38.40 | Yes | - | Yes | - | No | - | No | - |
| Pasta | No | - | Yes | 33.13 | No | - | Yes | 37.70 | Yes | - | Yes | - | No | - | No | - | |
| Breakfast Cereals | No | - | Yes | 33.79 | No | - | Yes | 36.60 | Yes | - | Yes | - | No | - | No | - | |
| Rice | No | - | Yes | 35.50 | No | - | Yes | 37.40 | Yes | - | Yes | - | No | - | No | - | |
| Vegetables | Potatoes | No | - | Yes | 39.35 | No | - | Yes | 41.60 | Yes | - | Yes | - | No | - | No | - |
| Root vegetables | Yes | 103.04 | Yes | 38.82 | No | - | Yes | 43.30 | Yes | - | Yes | - | No | - | No | - | |
| Vegetables | Yes | 102.55 | Yes | 38.90 | Low carb | 106.60 | Yes | 42.80 | Yes | - | Yes | - | No | - | No | - | |
| Fruits | Fruits, berries | Yes | 54.24 | Yes | 19.26 | Low carb | 56.17 | Yes | 23.20 | Yes | - | Yes | - | No | - | No | - |
| Animal-based protein | Milk, yogurt | No | - | Yes | 8.90 | Full-fat | 26.70 | Yes | 8.90 | Yes | - | Yes | - | No | - | No | - |
| Cheese | No | - | Yes | 7.50 | Full-fat | 26.40 | Yes | 8.00 | Yes | - | Yes | - | No | - | No | - | |
| Plant-based protein | Fish, meat, seafood, chicken | Grass-fed | 69.28 | Yes | 50.60 | Yes | 174.57 | No | - | Yes | - | Yes | - | No | - | No | - |
| Egg | Yes | 69.10 | Yes | 50.40 | Yes | 174.72 | Yes | 56.60 | Yes | - | Yes | - | No | - | No | - | |
| Plant-based protein | Beans, lentils | No | - | Yes | 5.10 | No | - | Yes | 5.65 | Yes | - | Yes | - | No | - | No | - |
| Nuts, seeds | Yes | 13.50 | Yes | 5.00 | Yes | 17.34 | Yes | 5.90 | Yes | - | Yes | - | No | - | No | - | |
| Cooking fats | No | - | Yes | - | Yes | - | Yes | - | Yes | - | Yes | - | No | - | No | - | |
| Total Food Waste | Yes | 79.22 | Yes | 52.9 | |||||||||||||
| Beer Waste | - | No | - | No | - | No | - | No | - | No | - | No | - | Yes | 310.91 | Yes | 212.8 |
| Miscanthus | - | No | - | No | - | No | - | No | - | Yes | 30.83 | Yes | 41.11 | Yes | 30.83 | Yes | 41.11 |
| FW-O | FW-P | FW-K | FW-V | AW:FW 1:1 | AW:FW 2:1 | AW:BW 1:1 | AW:BW 2:1 | |
|---|---|---|---|---|---|---|---|---|
| Fat | 13.8% | 18.2% | 36.1% | 11.1% | 6.9% | 4.9% | 5% | 3.3% |
| Protein | 19.8% | 31.1% | 49.3% | 13.9% | 9.9% | 6.6% | 12.3% | 8.2% |
| Soluble Carbohydrate | 62.4% | 40.1% | 12.1% | 70.5% | 34.7% | 25.4% | 3.9% | 4.9% |
| Fiber | 4% | 10.6% | 2.4% | 4.5% | 2.0% | 1.3% | - | - |
| Ash | - | - | - | - | 1.4% | 1.8% | 3.1% | 3.0% |
| Cellulose | - | - | - | - | 16.2% | 21.6% | 25.5% | 27.8% |
| Hemicellulose | - | - | - | - | 12.3% | 16.4% | 23.5% | 23.9% |
| Lignin | - | - | - | - | 9.7% | 13.0% | 19.8% | 19.7% |
| Unresolved Portion | - | - | - | - | 7.0% | 9.4% | 7.0% | 9.4% |
| C:N ratio | 14.2 | 10.0 | 6.9 | 19.4 | 19.2 | 27.3 | 12.0 | 13.9 |
| N | DF 1 | DF 2 | F-Value | Sig. | Null | |
|---|---|---|---|---|---|---|
| Daily Average CH4 | 212 | 7 | 204 | 45.703 | <0.001 | Rejected |
| Peak CH4 | 50 | 7 | 42 | 3.487 | 0.005 | Rejected |
| Diet (Jars) | C:N Pre- Digestion (SE) | C:N Post- Digestion (SE) | % C Post- Digestion (SE) | % N Post- Digestion (SE) | % P Post- Digestion (SE) | TS Pre- Digestion (%) | TS Post- Digestion (%) |
|---|---|---|---|---|---|---|---|
| Paleo (N = 5) | 8.94 (0.09) | 8.65 (0.18) | 42.11 (0.49) | 4.87 (0.11) | 0.16 (0.04) | 10 | 7 |
| Omni (N = 6) | 11.09 (0.45) | 8.17 (0.14) | 41.23 (1.46) | 5.01 (0.14) | 0.05 (0.01) | 10 | 7 |
| Keto (N = 6) | 7.12 (0.03) | 12.26 (0.13) | 43.62 (0.55) | 3.56 (0.04) | 0.12 (0.05) | 9 | 6 |
| Veg (N = 4) | 13.60 (0.09) | 8.72 (0.08) | 41.97 (0.40) | 4.81 (0.05) | 0.03 (0.01) | 11 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herman, T.; Nungesser, E.; Miller, K.E.; Davis, S.C. Comparative Fuel Yield from Anaerobic Digestion of Emerging Waste in Food and Brewery Systems. Energies 2022, 15, 1538. https://doi.org/10.3390/en15041538
Herman T, Nungesser E, Miller KE, Davis SC. Comparative Fuel Yield from Anaerobic Digestion of Emerging Waste in Food and Brewery Systems. Energies. 2022; 15(4):1538. https://doi.org/10.3390/en15041538
Chicago/Turabian StyleHerman, Tess, Emily Nungesser, Kimberley E. Miller, and Sarah C. Davis. 2022. "Comparative Fuel Yield from Anaerobic Digestion of Emerging Waste in Food and Brewery Systems" Energies 15, no. 4: 1538. https://doi.org/10.3390/en15041538






