Abstract
Microbial electrolysis cells (MECs) have been explored for various applications, including the removal of industrial pollutants, wastewater treatment chemical synthesis, and biosensing. On the other hand, MEC technology is still in its early stages and faces significant obstacles regarding practical large-scale implementations. MECs are used for energy generation and hydrogen peroxide, methane, hydrogen/biohydrogen production, and pollutant removal. This review aimed to investigate the aforementioned uses in order to better understand the different applications of MECs in the following scenarios: MECs for energy generation and recycling, such as hydrogen, methane, and hydrogen peroxide; contaminant removal, particularly complex organic and inorganic contaminants; and resource recovery. MEC technology was examined in terms of new concepts, configuration optimization, electron transfer pathways in biocathodes, and coupling with other technologies for value-added applications, such as MEC anaerobic digestion, combined MEC–MFC, and others. The goal of the review was to help researchers and engineers understand the most recent developments in MEC technologies and applications.
1. Introduction
Because of the tremendous growth in the world population and the expansion of industries and mining in recent decades, non-renewable energy supplies have been rapidly depleting [1]. In the meantime, ample new substances are causing major environmental damage. According to recent studies, surface water contamination in developing countries has resulted in environmental and possible socioeconomic difficulties and substantial public health hazards [2]. Since the industrial revolution, energy is also a key factor in economic growth. According to estimates, the global energy requirement will rise to 57% more than the current requirements by 2050, assuming a yearly population increase of 1.1% [3]. Natural oil, gas, and coal are currently some of the main renewable energy sources available in the world, and their widespread use produces numerous inorganic and organic pollutants [4]. Renewable energy sources are unstable and intermittent during generation, and thus, these valuable electric energy sources are difficult to apply continuously and stably. This also opens the spatial and temporal gaps between the availability of the energy and its consumption by the end users [5,6]. On the other hand, water pollution and freshwater shortages are among the most crucial worldwide issues.
Everybody believes that we need to move away from using fossil fuels and toward carbon-free energy sources, but it is not clear how that transition can be done. However, all of these resources are rare, demanding appropriate maintenance techniques. Hydrogen generation via water electrolysis is one of the more efficient ways, with tremendous potential for the change of chemical energy from the electrical energy that may be stored, transmitted, and then used or converted back to electricity on command [7]. Water electrolysis technologies of various types have been developed, but more progress is needed before they can be integrated into massive and expensive electrical infrastructure [8]. The kinetics of electrocatalysts, in particular, should be enhanced, along with their cost-effectiveness. Solar-powered electrolysis, for instance, is not yet cost-effective when contrasted with hydrogen synthesis from fossil fuels [9]. The growing need for fossil fuels and the demand to prevent releasing dangerous elements into the environment have necessitated the development of alternative and sustainable energy sources. In this context, bio-electrochemical systems have gained considerable interest from all around the world.
For well over a decade, scientists have been fascinated by microorganisms that can produce a current and microorganisms that can transmit electrons directly without the use of intermediaries or electron shuttles [10]. These systems are in different stages of evolution into diverse technologies, such as microbiological fuel cells (MFCs) for the treatment of wastewater and power generation [11]; MECs, which are devices that use electricity to generate methane or hydrogen gas; and other microbial electrochemical technologies (METs) for desalinating water and producing products, such as hydrogen peroxide (H2O2) [12]. MFCs are devices that oxidize organic and inorganic materials while generating a current using bacteria as catalysts [13]. An MEC is a biohydrogen-producing reactor that combines an MFC and electrolysis. An MEC comprises an anode compartment and cathode compartment, a power supply, and a separator. A separator, typically a cation/anion exchange membrane, separates the cathode electrodes. At the anode, microbial strains colonize the electrode surface to form an active biofilm by producing electrons, protons, and carbon dioxide via biological oxidation using organic materials, such as sewage sludge, wastewater, or sugar solutions, as their energy source. The electrons produced at the anode travel through an external circuit and the solution to the cathode, where they merge with protons to form hydrogen (a schematic representation is given in Figure 1a,b) [14,15,16]. The protons are transferred from the anode to the cathode through the proton exchange membrane (PEM). In the case of the anion exchange membrane (AEM), the OH− ions produced from the cathodic oxygen reduction reaction are transferred to the anode via the AEM.
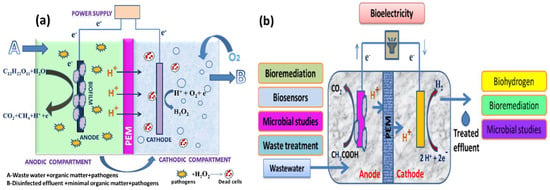
Figure 1.
(a) Anodic effluent containing residual organic matter and pathogens recalculated into the cathodic chamber for disinfection through H2O2 and (b) various important applications of MECs.
Meanwhile, CO2 will react with electrons and protons to produce methane and water. The MEC, in particular, has a lot of potential to become a green and sustainable energy source. Unfortunately, because an MEC’s cathode potential is more significant than its anode potential, the electrons generated cannot easily flow to the cathode. As a result, a low-power source of 0.2–0.8 V is required to activate electron migration [17].
An MEC is used to produce hydrogen peroxide, methane, and hydrogen/biohydrogen to remove pollutants (Figure 2) [18]. This review investigated the earlier uses to better comprehend MEC’s uniqueness. Problems and prospects were also examined to assist academics in understanding the most recent developments in MEC technology and applications. Furthermore, the future scope of research is considered in light of numerous issues related to the system’s representativeness and flexibility, which could lead to a cost-effective and potentially useful technology.
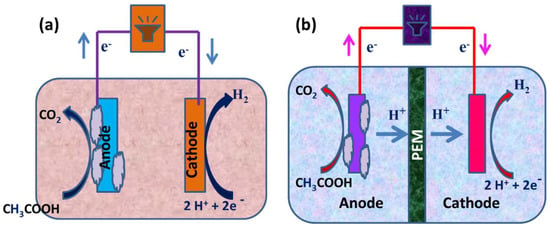
Figure 2.
(a) Construction of a single-chambered MEC and (b) a schematic representation of a dual-chambered MEC.
The goal of this research was to investigate the MEC concept and the developments that were discussed in the literature from a range of technical approaches. We investigated the possibility of MECs as a supplement to more traditional bioremediation in domestic wastewater treatment plants (dWWTPs), emphasizing the significant impact on energy saving. We also examined a few of the constraints and challenges that must be solved in order for MECs to become a commercially viable and technologically and environmentally feasible technology in dWW treatment facilities.
2. MFC and MEC
MFCs are devices that use microorganisms as catalysts to oxidize organic and inorganic substances and generate electricity, whereas an MEC combines an MFC and electrolysis to create biohydrogen. In an MEC, however, an external voltage must be provided to overcome the thermodynamic hindrance. MFCs have provided a long-term solution for generating bioelectricity from carbon [19]. In an MFC, organic substrates are transformed into hydrogen through the biohydrogen production process. Microorganisms aid in the decomposition of the organic substrate at the anode, which leads to the generation of electrons and protons. Electrons are transmitted from the anode to the cathode using an electrical circuit. Through a series of metabolic reactions, the exoelectrogens in the anode chamber catalyze the oxidation of organic molecules to carbon dioxide [20]. These responses send electrons through an outside circuit, where they combine with protons traveling through the proton trade film to form hydrogen on the cathode. Because organic material is used as a proton source for hydrogen generation instead of water, the process is called biocatalyzed electrolysis. A cathode, an anode, and an ion-selecting membrane separate the two electrodes in both MFCs and MECs. Biocathodes are used in specific situations. The majority of MFCs are used to generate energy. By providing an anaerobic environment to both the anode and cathode compartments, MFCs can also be used to produce hydrogen. Because the efficiency of hydrogen production from an MFC is low, MECs have been designed [21]. MECs, like MFCs, have electrochemically active bacteria on the anode surface that help to convert organic matter into protons, electrons, and carbon dioxide. The electrons created are then transmitted to the cathode, resulting in hydrogen generation. The two types of membranes are commonly used for reaction avoidance between produced hydrogen and oxygen. They are cation exchange membranes and anion exchange membranes. An anion exchange membrane is more typically used for hydrogen generation because it has a lower resistance to ion transport through the membrane due to reduced internal resistance [22]. As a result, the use of an MEC accelerates the degradation of the substrate, resulting in hydrogen production.
MECs are similar to MFCs in that they have two chambers connected by an ion-exchange membrane. Several various combinations of MECs have been modified throughout the decades to increase efficiency and are discussed below. A fundamental H-type cell with gas collection pieces coupled to a cathode compartment was used in prior designs. Subsequently, significant improvements were made to create dual-compartmental MECs that were simple to operate. Following a comprehensive evaluation of numerous configurations, a single-compartmental MEC exhibited larger fabrication/recovery rates and current densities than a dual-compartment MEC. As a result, a lot of time and effort has been put into fine-tuning this grouping for usage in scale-up investigations. Several sorts of reactor upgrades were put together based on the results: single-chambered, dual-chambered, combined, and many others.
2.1. Single-Chambered MECs
The initial configuration used a glass container with an overall capacity of 50 mL. The subsequent setup applied borosilicate glass vials with a total volume of 10 mL; the cells generally contained a mixed culture or pure culture. To keep the cathode and anode, which measured 4 × 5 cm2 and 3.5 × 4 cm2, 2 cm apart, plastic screws were utilized. The anode was constructed of type A carbon, while the cathode was type B carbon with platinum. As illustrated in Figure 2a, single-chambered MECs lack a membrane. Since hydrogen is moderately insoluble in water, when production rates are greater, the microbial conversion of methane from hydrogen is slowed. In membrane-less MECs, energy losses are reduced, and the energy recovery phase is effective.
2.2. Dual-Chambered MECs
The anodic and cathodic chambers of dual-chambered MECs are divided by a membrane, as seen in Figure 2b. Due to their complex structures and high volumes with greater internal resistance, dual-compartmental MECs are difficult to scale up. The application of a membrane serves two purposes. It shortens the transition from the anode compartment to the cathode compartment, preventing short circuits and preserving the quality of the cathode-side product. The proton exchange membrane (PEM) is the most commonly used one because it is designed to allow only freely available protons to pass through while using –SO3-type functional groups. Secondary membranes, including anion-exchange membranes, such as bipolar membranes, AMI7001, and charge-mosaic membranes, have been studied in MECs, along with regular membranes.
2.3. Proton Exchange Membranes
The primary role of a PEM in an MEC-based technique is to separate reactants and transfer protons from the anode to the cathode. A PEM is a semipermeable membrane formed of ionomers that is developed to transfer protons while being impermeable to gases, such as oxygen and hydrogen. Polymeric membranes or mixed membranes, wherein additional materials are embedded in a polymer matrix, can be used to construct PEMs. Nafion is the most popular PEM material, with a hydrophobic Teflon-like backbone (-CF2-CF2-) and hydrophilic side chains terminating with ion-conducting sulfonic acid groups (-SO3-H). However, the Nafion membrane is costly, prone to fuel and gas crossovers, and has limited proton selectivity. By combining with the gases produced in the anode compartment, an MEC decreases hydrogen purity in the cathode compartment. As a result, a variety of new membrane types have been developed that use different proton (or ion) conductors. Therefore, for commercial applications of those technologies, it is important to develop alternative membranes to the expensive Nafion. The alternative choices to Nafion are considered based on a few previous works reported by researchers [23,24]. A nanofiber-reinforced composite proton exchange membrane (NFR–PEM) based on sulfonated polyether ether ketone (SPEEK) as a proton conductor was prepared and studied for microbial electrolysis cells (MECs) [23]. A sulfonated poly(arylene ether sulfone) (SPAES)/polyimide nanofiber (PIN) composite proton exchange membrane was developed for use in microbial electrolysis cells (MECs), where diverse cations that compete with protons coexist in high concentrations [24].
3. Applications of MEC
3.1. Electrosynthesis of Compounds
3.1.1. Hydrogen Peroxide
MFCs and MECs can manufacture H2O2 from wastewater via electron reduction of O2 in the cathodic compartment, overcoming some of the current obstacles in H2O2 synthesis [25,26]. However, MFCs have a lower rate of H2O2 production, which limits their use in wastewater treatment on a large scale [27]. To overcome this challenge, many researchers have worked on MECs to accelerate production by providing external power. Compared to current technologies, MECs’ H2O2 generation is favorable since the process can treat wastewater while also producing H2O2 [28]. Unlike the other approach, this one does not involve using any harmful ingredients or catalysts. Furthermore, the process can be run with little or no energy input, making it compatible with a sustainable future.
An MFC can effectively remove organic matter from wastewater; nevertheless, tertiary treatment is required to remove the remaining contaminants, such as residual organic matter, pathogens, and xenobiotics [29]. By delivering secondary and tertiary treatments in anodic and cathodic chambers, an MEC can satisfy both requirements. Once the majority of the organic matter is removed from the effluent in the anodic compartment, it leads to a cathodic compartment for H2O2 production [30]. The H2O2 produced in an MEC’s cathodic chamber can also remove dyes and other xenobiotic chemicals, providing comprehensive wastewater treatment. Both treatment steps can be provided in a single reactor with such a modular arrangement, lowering both operational and capital expenses. As a result, an MEC is more efficient than an MFC in terms of the H2O2 generation rate [31]. Over the past decade, researchers have adapted many modifications to increase the production of H2O2. Junyoung et al. looked into cathode potential and O2 supply procedures to enhance the synthesis of H2O2. Their study found that decreasing the current density for passive O2 diffusion to the cathode increased H2O2 conversion efficiency by 65%. The MEC was made up of an acetate medium gas diffusion cathode and wastewater. They obtained 141 mg H2O2/Lh using an acetate medium and 6 mg H2O2/Lh using wastewater [32]. Dongwon et al. created an anaerobic energy conversion method for converting primary sludge at the anode using a dual-chambered, flat-plate, energy-efficient microbial peroxide-producing cell (see Figure 3a). H2O2 concentrations and H2O2 production efficiency during batch cathode operation in the MPPC are shown in Figure 3b. By 6 h, the H2O2 content had risen to 230 mg L−1, but by 24 h, it had dropped to 121 mg L−1. Depending on the electrical current generated at the anode, the predicted H2O2 produced rose linearly up to 2300 mg L−1, showing that the PPE decreased over time, from 72% at 1.5 h to 5% at 24 h. [33]. Rusen et al. created a dual-chambered 20 L MEC for in situ and proficient H2O2 electrosynthesis, as represented in Figure 4. Under acidic or alkaline circumstances, freshly generated H2O2 would later undergo reduction to give H2O and OH-, and H2O2 would decompose. As a result, more H2O2 would be decomposed with a longer running time. In 42 h, the H2O2 concentration at the cathode grew monotonically as the aeration rate was increased, indicating that the preferred aeration rate range had no detrimental influence on H2O2 manufacture at the cathode. After 42 h, a 0.6 V input voltage resulted in a greater rate of H2O2 production of 10.82 mg/Lh and a collective H2O2 concentration of 454.44 mg/L. In conclusion, the earlier studies demonstrated the viability of using a graphite plate as the cathode in a scaled-up ORR to create H2O2 [34]. Wang et al. modified carbon nanotubes by doping them with fluorine and used the same method to fabricate the gas diffusion electrode. This modification improves H2O2 selectivity and produced approximately 47.6 mg/L [35,36]. Table 1 gives the details of different studies performed on hydrogen production.
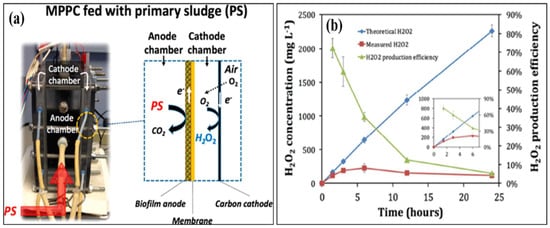
Figure 3.
Results of a microbial peroxide producing cell’s cathode batch operation (MPPC). (a) Theoretical and measured H2O2 concentrations that rely on a 100% transition from cumulative coulombs and a detection method, and H2O2 productivity improvement (PPE) (N = 3 measurements). The inset shows the first six hours of operation in the MPPC. (b) Catholyte pH and alkalinity over a 24 h batch run. The results of a cathode batch operation of a microbial peroxide producing cell are given. (Reprinted with permission from [33]. Copyright 2019, American Chemical Society.)
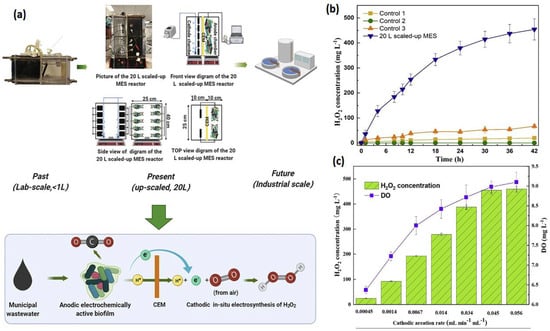
Figure 4.
(a) Verification of this 20 L scaled-up MES reactor’s feasibility for H2O2 generation. (b) Input and operating conditions: the electrolyte nature and concentration were 50 mM Na2SO4, the voltage was 0.6 V, the cathode aeration velocity was 0.045 mL min−1 mL−1, and the original catholyte pH was 3. There was no cathodic aeration in control 1. The circuit for control 2 was broken. Control 3: no voltage input. H2O2 production was affected by cathodic aeration velocity. Input voltage of 0.6 V, initial catholyte pH of 3, and electrolyte nature and concentration of 50 mM Na2SO4 were used as operating conditions [34]. (c) Effect of cathodic aeration velocity on H2O2 production. Operating conditions: input voltage of 0.6 V, initial catholyte pH of 3, and electrolyte nature and concentration of 50 mM Na2SO4, respectively. (Reprinted with permission from [34]. Copyright 2021, Elsevier.)

Table 1.
The details of different studies performed on hydrogen production.
MECs have emerged as a viable technology for eco-friendly H2O2 generation. There are several fundamental and applied characteristics of H2O2 production in MECs that focus on the significance of a variety of operational parameters and potential environmental uses of generated H2O2. A literature search revealed that lab-scale MECs successfully created appropriate H2O2 concentrations for various water treatment systems. Despite its promise, there are still several obstacles that still need to be resolved. Initially, the electrolyte was introduced into this study with the intention of increasing the system current and, thus, H2O2 production, which would increase production costs. Furthermore, the cathodic oxygen use rate was low, as evidenced by the cathodic aeration delivered by the pump, which accounts for the majority of the total energy usage. However, if the reactor architecture is optimized and novel electrode materials with improved oxygen mass transfer efficiency are developed, this issue can be fixed in the future.
3.1.2. Methane
Methane is a major component of natural gas (50–90%) and is used in every application where natural gas is employed. Besides that, methane can be used in the manufacturing of a variety of compounds. According to the Global Energy Statistical Yearbook, global natural gas consumption climbed by 11% between 2010 and 2018. By 2040, the International Energy Agency predicts that this percentage will climb to about 55%, with total gas consumption of 53,330 TWh [44]. Various methods for producing methane from feedstock are being studied; photocatalytic, thermochemical, electrochemical, and biological pathways are among them.
A recently invented MEC uses bioelectrochemical reactions to boost biogas production in a powerplant by allowing supersaturated organic wastes, hazardous chemicals, and non-degradable organic material to degrade quickly. When a low voltage of 0.2 V to 0.8 V is applied to the reactor, exoelectrogenic bacteria break down organic materials and generate electrons at the anode. These electrons then travel to the cathode in a closed circuit, generating CH4, as given in Equations (1) and (2) [45,46].
C2H4O2 + H2O → 2 CO2 + 8 H+ + 8 e−
CO2 + 8H+ + 8e− → CH4 + 2H2O
Seelajaroen et al. found that by applying a constant voltage of 0.4 V to the MEC, which used a modified chitosan carbon felt electrode, 67% of COD could be removed and 87 μmol/day of CH4 could be generated from the wastewater [47]. Park et al. compared the methanogenesis between an anaerobic digestive reactor (AD) and an AD coupled with an MEC, including an acrylic cylinder framework. Each reactor had a total volume of 25 L and a working volume of 20 L. Six sets of 150 mm wide and 300 mm high electrodes were used in the AD–MEC reactor. Each electrode was made of a graphite carbon mesh coated with Ni to improve the electrical conductivity. Though both resulted in almost similar amounts of CH4 yield, where the AD–MEC showed accelerated synthesis, demonstrating the efficiency of MEC [48]. Mieke et al. found that an MEC can transform CO2 to methane and that a biocathode can convert 100% methane for 188 days. The highest achievable energy efficiency throughout the yield test applying water oxidation in this study was 51.3%. When wrapping 10% of a land area with PV cells, a 51.3% energy efficiency increased methane production per hectare of land area by a factor of 1.8 [49]. According to Villano et al. [50], an MEC achieved a high acetate discharge and efficient conversion to methane at a potential of 0.2 V. The methane collected 75% of the electrical energy input, inferring that specific gross energy was exerted on the influent load treatment (0.85 kWh/kg removed COD). Seelajaroen et al [47]. created a system that can degrade organic substances at the anode while converting CO2 and CH4 at the biocathode. Under anaerobic conditions, the bioelectrodes were inoculated with a mixed culture. The removal of COD and CH4 generation in the cathode chamber was investigated using a constant potential of 0.40 V vs. Ag/AgCl (3 M NaCl). Figure 5a–c for MECs 1, 2, and 3 depicts the accumulated COD removal in each running cycle (red circle data point) versus total electrical charge (Q) (blue triangle data point) over the entire running time. The reaction rates in each cycle are shown linearly by the dashed lines. COD removal in MEC 1 increased from 1.0 g L−1 in the first cycle to 1.8 and 2.2 g L−1 in the second and third cycles, respectively, indicating that the oxidation process improved over time. In the first cycle of MECs 2 and 3, considerably increased COD removal results were obtained at 1.6 and 1.9 g L−1. The COD levels in MECs 2 and 3 were increased by 1.0 and 0.5 g L−1 in the second cycle, respectively, compared to the first cycle. The COD levels in MECs 2 and 3 were increased by 1.0 and 0.5 g L−1 in the second cycle, respectively, compared to the first cycle. Figure 5d–f depicts the plots of cumulative CH4 concentration (black square data point) and accumulative Q (blue triangle data point) during each running cycle of MECs 1, 2, and 3. The generated CH4 in MEC 1 increased steadily from 0.8 mmol in the first cycle to 1.3 mmol in the second cycle to 1.8 mmol in the third. The first and second cycles of MEC 2 yielded rather stable amounts of CH4 at 2.6 and 2.7 mmol, respectively. Meanwhile, in the third cycle, the production reduced to 2.2 mmol, while the generated CH4 in MEC 3 climbed from 2.8 mmol in the first cycle to 3.3 mmol in the second cycle before dropping to 2.0 mmol in the third cycle.
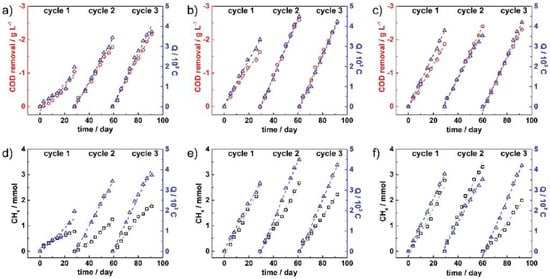
Figure 5.
Plots of gathered COD removal (red circle) of (a) MEC 1, (b) MEC 2, and (c) MEC 3, and CH4 production (black square) of (d) MEC 1, (e) MEC 2, and (f) MEC 3 over the entire running time, along with accumulated charges (blue triangles). Dashed lines represent each data set’s linear fitting curves. (Adopted from [47], open access.)
3.1.3. Hydrogen
Hydrogen is an eco-friendly and sustainable energy carrier that can also be employed in automobiles. Biological procedures for hydrogen production are environmentally favorable, although fermentation has minimal energy retrieval and yield. Biocatalytic processes or pure enzymes can produce higher yields, but neither of these technologies is cost-effective for hydrogen manufacture. There are methods that involve the latest hydrogen production technologies based on studies reported by a few researchers [51,52]. MECs are a new technology that can produce sustainable hydrogen from diverse forms of biomass. At the anode, microbes break down organic matter. Protons in solution and electrons are discharged to the anode to form hydrogen at the cathode. This method has various benefits over other biohydrogen methods, including higher hydrogen outputs and capacity using a variety of substrates, along with fermentable and non-fermentable organics. For MECs to be used in practical applications, hydrogen production rates must be increased.
Samsudeen et al. built an MEC within an anaerobic digester that was compact and easy to install. In this design, the cathode compartment is integrated into the anodic compartment for portability, increased hydrogen production, and water treatment efficacy. The standard and modified MEC systems produced a total of 30.12 ± 0.5 mL and 40.05 ± 0.5 mL of hydrogen, respectively, at current densities of 908.3 ± 25 and 811.7 ± 20 mA/m2. Furthermore, the wastewater COD elimination effectiveness for predictable and tailored MEC designs was found to be 77.5% and 75.6% over 70 h, respectively [53]. The system designed by Chen et al. was based on current pilot plants with a flow rate of 65 L.d1 of urban wastewater and 500 mg COD.L1 of wastewater strength. As the overall hydrogen recovery ratio rose, the emission levels per functional unit and the MEC emissions ratio to overall emissions greatly decreased. They also demonstrated that increasing cathodic gas retrieval, hydrogen generation rate, and COD volume loading rate was the most effective strategy for lowering emissions per kilogram of H2 produced under the following conditions: (i) operated voltage of 0.5 V, (ii) cathodic gas recovery of 90%, (iii) 90% electricity transformation efficiency, and (iv) global warming potential of 18.8-kilogram carbon dioxide-eq/kg hydrogen from the operation phase [54]. Zhang et al. built a dual-chamber MEC with concentric cylinders to investigate H2 production from three separate lignocellulosic materials via simultaneous saccharification and fermentation, as shown in Figure 6a (SSF). The concentrations of reducing sugar and organic acids in the MEC system were measured (Figure 6b,c). Reducing sugar was perhaps the essential outcome of the MEC system. The residual reducing sugar concentrations increased linearly from zero to the peak, then gradually decreased. The mixed substrate had a maximum hydrogen production rate (HPR) of 2.46 mmol/L/D, a total energy conversion efficiency of 11.29%, and a maximum hydrogen volumetric yield of 28.67 L/kg [55]. Fabregat et al. conducted the first experimental assessment of alkaline bioelectrochemical hydrogen production using genuine crude glycerol as the primary ingredient. The results indicate that alkaline glycerol can indeed be decomposed in both MFCs (71.4 A/m3, 2 mA, and 55% CE) and MECs (85% rCAT and 0.46 LH2/L/d). In a MEC, hydrogen production was 85% of rCAT and 0.46 LH2/L/d [56]. Table 2 shows a few more modifications to the MECs under consideration.
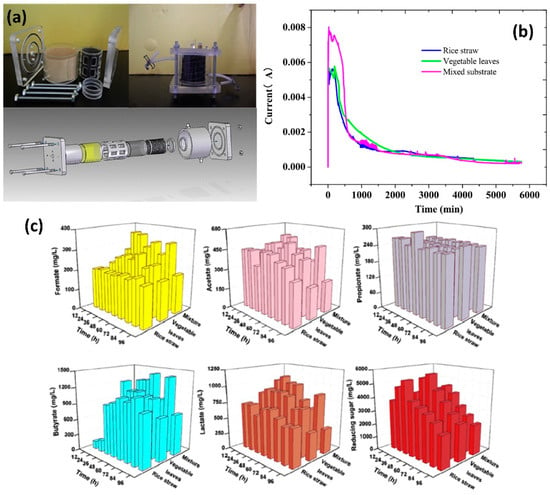
Figure 6.
(a) The configuration of an MEC reactor. (b) The present manufacturing in MEC systems. (c) The consequence of various substrates on the intermediates of an MEC system. (Reprinted with permission from [55]. Copyright 2019, Elsevier.)

Table 2.
Modifications of MECs for hydrogen production.
MECs’ capital costs have risen to the point where they cannot be used in massive wastewater treatments. The extent to which these issues are addressed will ultimately decide how MECs can be implemented. As a result, the economic aspects of hydrogen production using wastewater treatment inventions will need to be considered in the future.
Hassanein [60] et al. compared the effects of energy production and waste treatment in a combined MEC and AD (AD–MEC) system to an AD without MEC (AD only) (see Figure 7a). On day 20 of the digestion process, a single-compartmental MEC (150 mL) was installed within a 10 L digester and ran for 272 h (11 days) to regulate the residual treatment and energy capacity with an MEC incorporated in the AD–MEC system. The AD–MEC manufactured higher H2 and CH4 overall (2.43 L H2 and 23.6 L CH4) than the AD-only MEC (0.00 L H2 and 10.9 L CH4). During the first 24 h after the MEC injection, hydrogen accounted for 20% of the biogas produced, but the H2 concentration fell as the CH4 concentration grew from 50% to 63%. During the MEC-inclusion period (days 20–31), progressive biogas output from the AD–MEC treatment was 93.0% greater than that of the AD-only treatment (Figure 7b). Electrical energy recovery efficiency in the MEC ranged from 73–324%, with a total energy increase of 170% on average compared to the AD-only treatment. Even though many researchers can provide efficient methods for producing methane from MECs, there are only a few steps that must be taken. Future research should look into increasing MEC energy recovery by monitoring lower potentials of the anode without sacrificing substrate percentage removal through a cationic membrane; ammonium was concentrated at the cathode, resulting in very little biomass formation at the anode. According to these research results, a methane-manufacturing MEC could treat low-strength wastewater or refine raw fluid sewage and anaerobic digestion biogas.
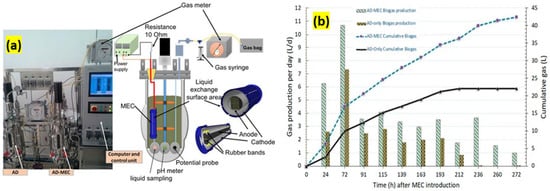
Figure 7.
(a) Photograph and block diagrams of a coupled AD and MEC (AD–MEC) system versus AD treatment without MEC (AD only). (b) Accumulated and everyday biogas manufacturing for AD–MEC and AD-only treatments for days 20–31 (272 cumulative h) after the MEC introduction (days 0–20 are not shown because the treatments were reproduced). (Adopted from [58], open access.)
3.2. Wastewater Treatment
Wastewater is increasingly regarded as a “misplaced resource” that may be used to create quality products and energy. In the case of wastewaters, proper management frequently necessitates energy treatment, adding a significant impact on climate change. Because of their stability and diversity, biological anaerobic treatment methods have become the go-to option for reclaiming most of this energy. MECs have several advantages over MFCs. Nonetheless, the resultant product by an MEC has a significant impact on its performance (hydrogen, methane, ethanol, hydrogen peroxide, etc.), and its tremendous energy production has led to speculation that it would be the future’s power source. In combination with sulfate-reducing bacteria, an MEC was developed by Kai Wang et al. and was utilized to reduce sulfate-rich wastewater that lacked electron donors. The results showed that it might produce a powerful synergy when SRB is combined with an applied current.
The maximum sulfate removal of 14.9% greater than the control reactor was achieved with a 1.5 mA applied current [64]. Yan Li et al. achieved instantaneous elimination of nitrogen in wastewaters, metal in industrial effluents, and salinity in saltwater using a combined microbial desalination cell (MDC)–MEC system. The cathode solution was transferred to the MDC anode when the ammonium content fell below 2 mg L−1 during aerobic nitrification to improve total nitrogen percentage removal so that the accumulated nitrate and nitrite might be reduced via heterotrophic denitrification in an anoxic anode with a carbon supply, as shown in Figure 8a. A test was conducted using wastewater alone without ammonium injection in the cathode to further examine the effect of nitrogen removal on the power output of the MDC handling nitrogen-rich wastewater. During the first 36 h, the voltage output of the MDC was slightly more significant than that of the test, suggesting that ammonium in wastewater had an impact on the voltage output, likely leading to an increase in conductivity to lower internal resistance. MDC displayed removal effectiveness of 62.9% for 48 h, greater than conventional reverse osmosis but significantly lower than RO, showing that MDC could successfully extract salt but required a longer retention period (see Figure 8b). Nitrification oxidized greater than 95.1% of the nitrogen in batch testing, resulting in a complete nitrogen elimination rate of 4.07 mg L−1 h−1.
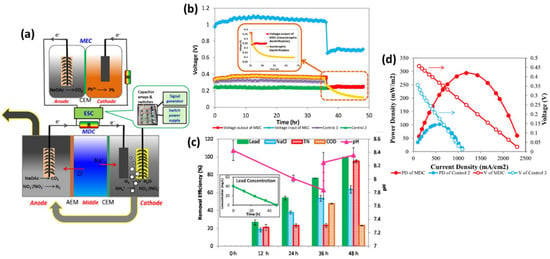
Figure 8.
(a) The integrated MDC–MEC system diagram (b) Voltage changes of MDC output and MEC input through ESC. (Inserted figure: Voltage output of autotrophic denitrification and heterotrophic denitrification) (c) Removal efficiencies of NaCl, total nitrogen, and COD in the MDC and lead (II) in the MEC and pH variation at different operational periods (Inserted figure: lead (II) concentration over time) (d) Power densities and voltages of the MDC and Control 2 at the Rext. of 14-4580 Ω (V: voltage; PD: power density). (Reprinted with permission from [65]. Copyright 2017, Elsevier.)
Merging denitrification and water recycling in MDC solved the pH variation issue in the cathode and anode, resulting in 63.7% desalination and 99.5% lead (II) removal over 48 h [65]. The study’s goal was to improve and test new microbial electrolysis aided by an anaerobic flow reactor for beer wastewater treatment and the subsequent methane yield. Under a 0.8 V operating voltage and 24 h HRT, the reactor with Ni cathode had an 85% maximum COD removal, methane production of 142.8 mL/g COD, 83% TOC elimination, 97% carbohydrate elimination, 62% protein elimination, and 8.6 mA current production [66]. Escapa et al. investigated MEC performance in energy consumption, chemical oxygen demand elimination, and hydrogen production rate using widely different organic loading rates and applied voltages in domestic wastewater treatment. COD removal was enhanced at low organic loading rates. The amount of electrical energy needed to take out 1 g of COD increased substantially as the organic loading rates were reduced. As a function of organic loading rates, hydrogen generation followed a Monod-type trend, with a maximum rate of 0.30 L/(L/d) [67]. Hongbo et al. studied a combination anaerobic baffled reactor MFC–MEC for power generation and fecal wastewater process. The single-chamber MEC experimental procedure is shown here, with a dual compartment setup available in the electronic supplement.
The reactors were made of Plexiglass that had a thickness of 5 mm. The arrangement consisted of three parts: MFC (cathode–anode) unit, ABR1–4 unit, and an MEC (cathode–anode) unit, as shown in Figure 8a. Figure 8b shows the results of analyzing constituents of the collected biogas. N= and CH4 are the main components of biogas, accounting for about 45% of the overall volume. Compared to a standard anaerobic reactor (CH4 55–65%, CO2 30–45%), the gas composition was slightly lower for CH4 but substantially greater for N2 owing to the micro-aeration that delivered nitrogen into the system (see Figure 8b). COD and ammonia nitrogen removal rates to their maximum potential COD and ammonia nitrogen removal rates to their maximum potential (NH4+-N) were 85.0 ± 0.4 g NH4+ -N/m+/day and 1.35 ± 0.05 kg COD/m3/day, respectively, and the collected gas phase contained 9% carbon dioxide (CO2), 45% methane (CH4), and 45% nitrogen gas (N2). When the initial COD concentration was 1500.0 ± 20.0 mg/L and the preliminary NH4+-N concentration was 110.0 ± 5.0 mg/L, the integrated system produced an average excess output voltage of 452.5 ± 10.5 mV. In contrast, raw sewage COD could attain 50.0 mg/L with a 48 h HRT [68].
3.3. Nutrient Recovery
With the world’s population growing at a faster rate, there is a greater demand for food and, as a result, a greater requirement for fertilizers. The Food and Agriculture Organization (FAO) of the United Nations reported a 1.8% annual increase in total fertilizer demand from 2014 to 2018. Demand for nitrogen (N)- and phosphorus (P)-based fertilizers is projected to increase by 5.8% and 2.1%, including both, over the same period. Both N and P are two of the essential elements in commercial fertilizers, and they are also abundant in wastewater. In an ideal world, these nutrients would be extracted from wastewater to fulfill discharge limitations while also being reused to make fertilizers.
MEC with 10 electrode pairs placed in primary clarifier effluent from a municipal wastewater treatment facility designed by Hui Guo et al. [69] MEC-1 (which contains only one electrode pair), MEC-5 (which contains five electrode pairs), and MEC-10 (which contains 10 electrode pairs) all had liquid volumes of 28, 35, and 40 mL correspondingly. The anode was made of activated carbon cloth pre-treated with a surfactant. There were no precious metal catalysts in the cathode, which was made of stainless-steel mesh. Each electrode pair, which consisted of one carbon cloth piece and one stainless-steel mesh piece, was sandwiched and divided by two rubber gaskets and one plastic mesh. The two gaskets measured 2.8 mm in thickness. Each electrode pair had its own set of controls. As demonstrated in Figure 9a, the feed wastewater normally flowed to the sandwiched electrodes. To allow for sufficient wastewater flow and biogas collection through the stacked electrode pairs, each electrode’s upper and lower parts (both the anode and cathode) were cut into small pieces 0.3 cm from the circular edge, resulting in 5.42 cm2 of total surface area per electrode. A plastic tube was placed on the polypropylene block to collect biogas (Figure 9b,c). The wide range of the Coulomb efficiency can be discussed by using real wastewater, whose composition, particularly biodegradable COD, changed regularly. The flow rate was increased from 0.1 to 0.2 mL/min, which resulted in a significant decrease in CE. The rise in COD (Figure 9d) and the comparable electric current output at 0.2 mL/min were responsible for the considerable drop.
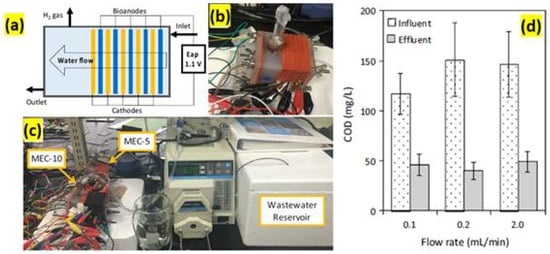
Figure 9.
Sandwiched electrode stack system: (a) schematic of the sandwiched electrode stack, (b) MEC-5, (c) continuous-flow system with MEC-10 and MEC-5 in sequence, and (d) plot of the increase in the COD vs. flow rate. Wastewater treatability of the MEC system: COD removal. (Adopted from Ref [69], open access.)
Cusick et al. discovered an effective technique of simultaneous hydrogen gas and struvite (MgNH4PO46H2O) synthesis based on bio-electrochemically induced struvite crystallization at the cathode of a single-MEC system. The phosphate elimination ranged from 20–40%, with mesh cathodes achieving higher eliminations than flat plates. The overall energy efficiency, including substrate and electricity, was high (73%) and unaffected by the applied voltage [70]. Fe2+ salt-modified biochar was created by Xiaoyu et al. that significantly increased the electrochemical performance of MECs (Figure 10a). Compared to a standard anaerobic reactor (CH4 55–65%, CO2 30–45%), the gas composition was marginally lower for CH4 but substantially greater for N2 owing to the micro-aeration that delivered nitrogen into the system. When compared to the pristine charcoal cathode, the MEC’s phosphate removal increased from 28.8 ± 1 to 62.4 ± 3.5% and the current density increased from 16.8 ± 0.2 A/m3 to 20.7 ± 0.8 A/m3. In general, biochar can help plants develop by adjusting soil pH and increasing water accessibility in the soil. This study discovered that Pakchoi grew better in biochar enriched soil than in plain soil in terms of dry weight, germination rate, and stem length (Figure 10b). It was also discovered that adding P-rich biochar to the soil improved Pakchoi agriculture [71]. Isabel et al. used a phosphate-buffered solution or a NaCl solution as the catholyte to test MEC sizes of 1000, 500, and 100 mL at applied voltages of 1.4, 1, and 0.6 V. The recovery efficiency of ammonia, dropped from 47 to 42% when the reactor capacity was increased from 500 to 1000 mL. [72]. Li et al. removed organics and salinity from municipal wastewater while recovering nutrients in an MEDC system. At a voltage of 2 V, the energy utilization for nutrient separation and revival was 0.12 kWh/m3. The MEDC system removed 75.5 ± 1.4% COD and had a Coulomb efficiency of 8.5 ± 1.1%. Furthermore, the nitrogen and phosphate recovery efficiencies were 66.7 ± 4.7% and 66.3%, respectively [73]. All this research shows that MEC can also be used efficiently to recover nutrients, metals, and other chemicals while removing and reducing toxic chemicals.
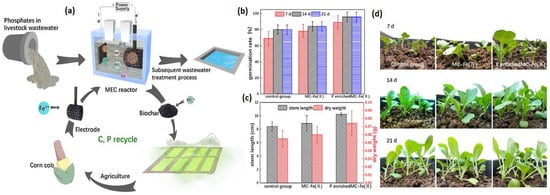
Figure 10.
(a) Using a biochar-based cathode, MECs recovered phosphorus from wastewater and produced fertilizer. (b) On the 7th, 14th, and 21st days, the survival rate of Pakchoi varied by group. (c) On the 21st day, the stem length and dry weight of Pakchoi were measured in various groups. (d) Pakchoi photos from the 7th, 14th, and 21st days. (Reprinted with permission from [71]. Copyright 2022, Elsevier.)
4. Summary and Outlook
Although a few initiatives to conduct large-scale MFC tests have taken place, MEC is still a relatively new idea. Critical obstacles, particularly techno-economic concerns, remain unanswered for the actual use of this technique.
To summarize, MEC technology has yielded positive outcomes, primarily in lowering the overall budget for treating wastewater and energy generation. Simultaneously, it provides a major benefit by producing enhanced fuels, such as hydrogen. Furthermore, MEC innovation is still in its infancy due to several challenges, such as mass transfer limits, energy loss, and other issues that must be researched extensively on pilot and commercial scales with real-world industrial effluents.
However, with regards to the practical implementations of MEC innovative technology, it should be highlighted that to maximize the technology’s cost-effectiveness, establishing remarkable specifications should be prioritized in both anode and cathode structural design, as well as membrane structural design. However, reports have arisen in recent years stating that the prospects for technology are good, as evidenced by the fortunate building of multiple pilot-scale MEC reactors, implying that the technology’s first commercial encounter is on the way. Nevertheless, the literature results and the truth about optimized research are vastly different, and the main problem nowadays is to investigate the reliability of pilot-scale investigations. Furthermore, there is no cost estimation comparison between conventional and MEC technologies, which could aid commercialization. Consequently, the creation of integrated MECs using hydrolysis can boost the overall effectiveness by speeding up the decomposition of non-biodegradable complex organic products.
For future research, the following critical issues must be resolved: (i) reduce methanogen H2 intake; (ii) improve the performance of bio-anode sensors for real-time, in situ, and self-sustaining water management; (iii) on a large scale, create novel MEC configurations; (iv) incorporate MEC with other subjects, such as computing science, materials engineering, and sensory perception; (v) efforts need to be taken to reduce the materials cost in order to realize the practical applications of MECs, such as developing cheaper electro materials, PEMs (AEMs), and MEC designs. MEC technology was shown to be a suitable tool for treating wastewater, pollution control, and energy generation.
Author Contributions
Conceptualization and methodology, writing—original draft: D.R.; validation: A.S.; formal analysis: D.R.K.; review and editing: R.K.; review and editing: S.G.P. All authors read and agreed to the published version of the manuscript.
Funding
The authors thank the National Research Foundation of Korea (NRF) funded by the Korean government, Ministry of Science and ICT (MSIT) (No. 2021R1F1A1046648), Republic of Korea. This work was also supported by the Department of Basic Sciences (Chemistry), FET, Jain Deemed-to-be University. Dr. Devi Radhika gratefully acknowledges the VGST grant number KSTePS/VGST/2020-21/RGS/F/GRD-981/83/2021-22/964.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abbasi, K.R.; Hussain, K.; Redulescu, M.; Ozturk, I. Does natural resources depletion and economic growth achieve the carbon neutrality target of the UK? A way forward towards sustainable development. Resour. Policy 2021, 74, 102341. [Google Scholar] [CrossRef]
- Ite, A.E.; Harry, T.A.; Obadimu, C.O.; Asuaiko, E.R.; Inim, I.J. Petroleum hydrocarbons contamination of surface water and groundwater in the niger delta region of Nigeria. J. Environ. Pollut. Hum. Health 2018, 6, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Junne, T.; Wulff, N.; Breyer, C.; Naegler, T. Critical materials in global low-carbon energy scenarios: The case for neodymium, dysprosium, lithium, and cobalt. Energy 2020, 211, 118532. [Google Scholar] [CrossRef]
- Kang, K.; Klinghoffer, N.B.; ElGhamrawy, I.; Berruti, F. Thermochemical conversion of agroforestry biomass and solid waste using decentralized and mobile systems for renewable energy and products. Renew. Sustain. Energy Rev. 2021, 149, 111372. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Review of the development of first-generation redox flow batteries: Iron-chromium system. ChemSusChem 2021, 15, e202101798. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagota, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; di Notoa, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Peera, S.G.; Koutavarapu, R.; Liu, C.; Rajeshkhanna, G.; Asokan, A.; Reddy, C.V. Cobalt nanoparticle-embedded nitrogen-doped carbon catalyst derived from a solid-state metal-organic framework complex for OER and HER electrocatalysis. Energies 2021, 14, 1320. [Google Scholar] [CrossRef]
- Grigoriev, S.; Fateev, V.; Bessarabov, D.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Ifkovits, Z.P.; Evans, J.M.; Meier, M.C.; Papadantonakis, K.M.; Lewis, N.S. Decoupled electrochemical water-splitting systems: A review and perspective. Energy Environ. Sci. 2021, 14, 4740–4759. [Google Scholar] [CrossRef]
- Kadier, A.; Wang, J.; Chandrasekhar, K.; Abdeshahian, P.; Islam, M.A.; Ghanbari, F.; Bajpai, M.; Katoch, S.S.; Bhagawati, P.B.; Li, H.; et al. Performance optimization of microbial electrolysis cell (MEC) for palm oil mill effluent (POME) wastewater treatment and sustainable Bio-H2 production using response surface methodology (RSM). Int. J. Hydrogen Energy 2021, in press. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Kumar, R.; Malyan, S.K.; Pugazhendhi, A. Microbial fuel cells as a sustainable platform technology for bioenergy, biosensing, environmental monitoring, and other low power device applications. Fuel 2019, 255, 115682. [Google Scholar] [CrossRef]
- Segundo-Aguilar, A.; González-Gutiérrez, L.V.; Payá, V.C.; Feliu, J.; Buitrón, G.; Cercado, B. Energy and economic advantages of simultaneous hydrogen and biogas production in microbial electrolysis cells as a function of the applied voltage and biomass content. Sustain. Energy Fuels 2021, 5, 2003–2017. [Google Scholar] [CrossRef]
- Nandikes, G.; Peera, S.G.; Singh, L. Perovskite-based nanocomposite electrocatalysts: An alternative to platinum ORR catalyst in microbial fuel cell cathodes. Energies 2022, 15, 272. [Google Scholar] [CrossRef]
- Koffi, N.J.; Okabe, S. Bioelectrochemical anoxic ammonium nitrogen removal by an MFC driven single chamber microbial electrolysis cell. Chemosphere 2021, 274, 129715. [Google Scholar] [CrossRef] [PubMed]
- Peera, S.G.; Maiyalagan, T.; Liu, C.; Ashmath, S.; Lee, T.G.; Jiang, Z.; Mao, S. A review on carbon and non-precious metal-based cathode catalysts in microbial fuel cells. Int. J. Hydrog. Energy 2021, 46, 3056–3089. [Google Scholar] [CrossRef]
- Afify, A.H.; Gwad, A.A.E.; Rahman, N.A.E. Effect of power supply and bacteria on bio-hydrogen production using microbial electrolysis cells (MECs). J. Agric. Chem. Biotechn. 2017, 8, 221–224. Available online: https://www.researchgate.net/publication/352933973 (accessed on 26 November 2021).
- Zhen, G.; Lu, X.; Kumar, G.; Bakonyi, P.; Xu, K.; Zhao, Y. Microbial electrolysis cell platform for simultaneous waste biorefinery and clean electrofuels generation: Current situation, challenges and future perspectives. Prog. Energy Combust. Sci. 2017, 63, 119–145. [Google Scholar] [CrossRef]
- Anwer, A.H.; Khan, M.D.; Joshi, R. Microbial electrochemical cell: An emerging technology for waste water treatment and carbon sequestration. In Modern Age Waste Water Problems; Springer: Cham, Switzerland, 2020; pp. 339–360. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Adhami, A.; Darvari, S.; Zirehpour, A.; Oh, S.-E. Microbial fuel cell as new technology for bioelectricity generation: A review. Alex Eng. J. 2015, 54, 745–756. [Google Scholar] [CrossRef] [Green Version]
- Logan, B.E.; Rossi, R.; Ragab, A.I.; Saikaly, P.E. Electroactive microorganisms in bioelectrochemical systems. Nat. Rev. Microbiol. 2019, 17, 307–319. [Google Scholar] [CrossRef]
- Liu, H.; Hu, H.; Chignell, J.; Fan, Y. Microbial electrolysis: Novel technology for hydrogen production from biomass. Biofuels 2010, 1, 129–142. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sust. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Chae, K.-J.; Kim, K.-Y.; Choi, M.-J.; Yang, E.; Kim, I.S.; Ren, X.; Lee, M. Sulfonated polyether ether ketone (SPEEK)-based composite proton exchange membrane reinforced with nanofibers for microbial electrolysis cells. Chem. Eng. J. 2014, 254, 393–398. [Google Scholar] [CrossRef]
- Park, S.-G.; Chae, K.-J.; Lee, M. A sulfonated poly(arylene ether sulfone)/polyimide nanofiber composite proton exchange membrane for microbial electrolysis cell application under the coexistence of diverse competitive cations and protons. J. Membr. Sci. 2017, 540, 165–173. [Google Scholar] [CrossRef]
- Fu, L.; You, S.-J.; Yang, F.-L.; Gao, M.-M.; Fang, X.-H.; Zhang, G.-Q. Synthesis of hydrogen peroxide inmicrobial fuel cell. J. Chem. Technol. Biotechnol. 2010, 85, 715–719. [Google Scholar] [CrossRef]
- Khan, W.; Nam, J.-Y.; Woo, H.; Ryu, H.; Kim, S.; Maeng, S.K.; Kim, H.-C. A proof of concept study for wastewater reuse using bioelectrochemical processes combined with complementary post-treatment technologies. Environ. Sci. Water Res. Technol. 2019, 5, 1489. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total Environ. 2021, 754, 142429. [Google Scholar] [CrossRef]
- Chung, T.H.; Meshref, M.N.; Hai, F.I.; Al-Mamun, A.; Dhar, B.R. Microbial electrochemical systems for hydrogen peroxide synthesis: Critical review of process optimization, prospective environmental applications, and challenges. Bioresour. Technol. 2020, 313, 123727. [Google Scholar] [CrossRef]
- Ghangrekar, M.M.; Chakraborty, I. Exploiting bioelectrochemical systems for wastewater treatment and value-added product recovery. In Post Treatments of Anaerobically Treated Effluents; IWA Publishing: London, UK, 2019; pp. 389–408. [Google Scholar] [CrossRef]
- Das, S.; Mishra, A.; Ghangrekar, M.M. Production of hydrogen peroxide using various metal-based catalysts in electrochemical and bioelectrochemical systems: Mini review. J. Hazardous Toxic Radioact. Waste 2020, 24, 06020001. [Google Scholar] [CrossRef]
- Hua, T.; Li, S.; Li, F.; Zhou, Q.; Ondon, B.S. Microbial electrolysis cell as an emerging versatile technology: A review on its potential application, advance and challenge. J. Chem. Technol. Biotechnol. 2019, 94, 1697–1711. [Google Scholar] [CrossRef]
- Sim, J.; An, J.; Elbeshbishy, E.; Ryu, H.; Lee, H.-S. Characterization and optimization of cathodic conditions for H2O2 synthesis in microbial electrochemical cells. Bioresour. Technol. 2015, 195, 31–36. [Google Scholar] [CrossRef]
- Ki, D.; Popat, S.C.; Rittmann, B.E.; Torres, C.I. H2O2 production in microbial electrochemical cells fed with primary sludge. Environ. Sci. Technol. 2017, 51, 6139–6145. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Hasanzadeh, A.; Khataee, A.; Yang, X.; Xu, M.; Angelidaki, I.; Zhang, Y. General rights Scaling-up of microbial electrosynthesis with multiple electrodes for in-situ production of hydrogen peroxide. IScience 2021, 24, 102094. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, X.; Su, P.; Li, Y.; Cai, J.; Zhang, Q.; Zhou, M.; Arotiba, O. Enhancement of hydrogen peroxide production by electrochemical reduction of oxygen on carbon nanotubes modified with fluorine. Chemosphere 2020, 259, 127423. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; An, J.; Zhou, L.; Li, T.; Li, J.; Feng, C.; Wang, X. A novel carbon black graphite hybrid air-cathode for efficient hydrogen peroxide production in bioelectrochemical systems. J. Power Sources 2016, 306, 495–502. [Google Scholar] [CrossRef]
- Modin, O.; Fukushi, K. Development and testing of bioelectrochemical reactors converting wastewater organics into hydrogen peroxide. Water Sci. Technol. 2012, 66, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Young, M.N.; Links, M.J.; Popat, S.C.; Rittmann, B.E.; Torres, C.I. Tailoring microbial electrochemical cells for production of hydrogen peroxide at high concentrations and efficiencies. ChemSusChem 2016, 9, 3345–3352. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Zhao, L.; Li, N.; Liu, H. A microbial fuel cell with the three-dimensional electrode applied an external voltage for synthesis of hydrogen peroxide from organic matter. J. Power Sources 2015, 287, 291–296. [Google Scholar] [CrossRef]
- Zhao, Q.; An, J.; Wang, S.; Wang, C.; Liu, J.; Li, N. Heterotopic formaldehyde biodegradation through UV/H2O2 system with biosynthetic H2O2. Water Environ. Res. 2019, 91, 598–605. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Leone, E.; Keller, J.; Rabaey, K. Efficient hydrogen peroxide generation from organic matter in a bioelectrochemical system. Electrochem. Commun. 2019, 11, 1752–1755. [Google Scholar] [CrossRef]
- Arends, J.; Van Denhouwe, S.; Verstraete, W.; Boon, N.; Rabaey, K. Enhanced disinfection of wastewater by combining wetland treatment with bioelectrochemical H2O2 production. Bioresour. Technol. 2014, 155, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Rozendal, R.A.; Jeremiasse, A.W.; Hamelers, H.V.M.; Buisman, C.J.N. Buisman, hydrogen production with a microbial biocathode. Environ. Sci. Technol. 2007, 42, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Palhares, D.D.D.F.; Vieira, L.; Damasceno, J.J.R. Hydrogen production by a low-cost electrolyzer developed through the combination of alkaline water electrolysis and solar energy use. Int. J. Hydrogen Energy 2018, 43, 4265–4275. [Google Scholar] [CrossRef]
- Capuano, L. International Energy Outlook 2018 (IEO2018), U.S. Energy Inf. Adm. 2018. Available online: www.eia.gov (accessed on 1 November 2021).
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.; Sleutels, T.; Jeremiasse, A.W.; Rozendal, R.A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef] [PubMed]
- Seelajaroen, H.; Spiess, S.; Haberbauer, M.; Hassel, M.M.; Aljabour, A.; Thallner, S.; Guebitz, G.M.; Sariciftci, N.S. Enhanced methane producing microbial electrolysis cells for wastewater treatment using poly(neutral red) and chitosan modified electrodes. Sustain. Energy Fuels 2020, 4, 4238–4248. [Google Scholar] [CrossRef]
- Park, J.; Lee, B.; Tian, D.; Jun, H. Bioelectrochemical enhancement of methane production from highly concentrated food waste in a combined anaerobic digester and microbial electrolysis cell. Bioresour. Technol. 2018, 247, 226–233. [Google Scholar] [CrossRef]
- Van Eerten-Jansen, M.C.A.A.; Ter Heijne, A.; Buisman, C.J.N.; Hamelers, H.V.M. Microbial electrolysis cells for production of methane from CO2: Long-term performance and perspectives. Int. J. Energy Res. 2011, 36, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Villano, M.; Scardala, S.; Aulenta, F.; Majone, M. Carbon and nitrogen removal and enhanced methane production in a microbial electrolysis cell. Bioresour. Technol. 2013, 130, 366–371. [Google Scholar] [CrossRef]
- Wan, C.; Zhou, L.; Xu, S.; Jin, B.; Ge, X.; Qian, X.; Xu, L.; Chen, F.; Zhan, X.; Yang, Y.; et al. Defect engineered mesoporous graphitic carbon nitride modified with AgPd nanoparticles for enhanced photocatalytic hydrogen evolution from formic acid. Chem. Eng. J. 2022, 429, 132388. [Google Scholar] [CrossRef]
- Yajie, F.; Youyu, D.; Hanjun, Z.; Jiangping, M.; Kai, X.Z.; Xiaoyuan, Z. research status of single atom catalyst in hydrogen production by photocatalytic water splitting. Chin. J. Rare Metals 2021, 45, 551–568. [Google Scholar]
- Nainamohamed, S.; Spurgeon, J.; Matheswaran, M.; Satyavolu, J. Simultaneous biohydrogen production with distillery wastewater treatment using modified microbial electrolysis cell. Int. J. Hydrogen Energy 2020, 45, 18266–18274. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Wu, X.; Jiaqiang, E.; Lu, N.; Wang, T.; Zuo, H. System development and environmental performance analysis of a pilot scale microbial electrolysis cell for hydrogen production using urban wastewater. Energy Convers. Manag. 2019, 193, 52–63. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.-Z.; Zhao, T.; Xu, T. Hydrogen production from simultaneous saccharification and fermentation of lignocellulosic materials in a dual-chamber microbial electrolysis cell. Int. J. Hydrogen Energy 2019, 44, 30024–30030. [Google Scholar] [CrossRef]
- Badia-Fabregat, M.; Rago, L.; Baeza, J.A.; Guisasola, A. Hydrogen production from crude glycerol in an alkaline microbial electrolysis cell. Int. J. Hydrogen Energy 2019, 44, 17204–17213. [Google Scholar] [CrossRef]
- Jeremiasse, A.W.; Hamelers, H.V.; Saakes, M.; Buisman, C.J. Ni foam cathode enables high volumetric H2 production in a microbial electrolysis cell. Int. J. Hydrogen Energy 2010, 35, 12716–12723. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Shen, N.; Zeng, R.J. H2 production by the thermoelectric microconverter coupled with microbial electrolysis cell. Int. J. Hydrogen Energy 2016, 41, 22760–22768. [Google Scholar] [CrossRef]
- Kadier, A.; Kalil, M.S.; Abdeshahian, P.; Chandrasekhar, K.; Mohamed, A.; Azman, N.F.; Logroño, W.; Simayi, Y.; Hamid, A.A. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sustain. Energy Rev. 2016, 61, 501–525. [Google Scholar] [CrossRef]
- Hassanein, A.; Witarsa, F.; Lansing, S.; Qiu, L.; Liang, Y. Bio-electrochemical enhancement of hydrogen and methane production in a combined anaerobic digester (AD) and microbial electrolysis cell (MEC) from dairy manure. Sustainability 2020, 12, 8491. [Google Scholar] [CrossRef]
- Luo, H.; Liu, G.; Zhang, R.; Bai, Y.; Fu, S.; Hou, Y. Heavy metal recovery combined with H2 production from artificial acid mine drainage using the microbial electrolysis cell. J. Hazard. Mater. 2014, 270, 153–159. [Google Scholar] [CrossRef]
- Carmona-Martínez, A.A.; Trably, E.; Milferstedt, K.; Lacroix, R.; Etcheverry, L.; Bernet, N. Long-term continuous production of H2 in a microbial electrolysis cell (MEC) treating saline wastewater. Water Res. 2015, 81, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Montpart, N.; Rago, L.; Baeza, J.A.; Guisasola, A. Hydrogen production in single chamber microbial electrolysis cells with different complex substrates. Water Res. 2015, 68, 601–615. [Google Scholar] [CrossRef]
- Wang, K.; Sheng, Y.; Cao, H.; Yan, K.; Zhang, Y. Impact of applied current on sulfate-rich wastewater treatment and microbial biodiversity in the cathode chamber of microbial electrolysis cell (MEC) reactor. Chem. Eng. J. 2017, 307, 150–158. [Google Scholar] [CrossRef]
- Li, Y.; Styczynski, J.; Huang, Y.; Xu, Z.; McCutcheon, J.; Li, B. Energy-positive wastewater treatment and desalination in an integrated microbial desalination cell (MDC)-microbial electrolysis cell (MEC). J. Power Sources 2017, 356, 529–538. [Google Scholar] [CrossRef]
- Sangeetha, T.; Guo, Z.; Liu, W.; Cui, M.; Yang, C.; Wang, L.; Wang, A. Cathode material as an influencing factor on beer wastewater treatment and methane production in a novel integrated upflow microbial electrolysis cell (Upflow-MEC). Int. J. Hydrogen Energy 2016, 41, 2189–2196. [Google Scholar] [CrossRef] [Green Version]
- Escapa, A.; Gil-Carrera, L.; García, V.; Morán, A. Performance of a continuous flow microbial electrolysis cell (MEC) fed with domestic wastewater. Bioresour. Technol. 2012, 117, 55–62. [Google Scholar] [CrossRef]
- Liu, H.; Leng, F.; Guan, Y.; Yao, Y.; Li, Y.; Xu, S. Simultaneous pollutant removal and electricity generation in a combined ABR-MFC-MEC system treating fecal wastewater. Water Air Soil Pollut. 2017, 228, 179. [Google Scholar] [CrossRef]
- Guo, H.; Kim, Y. Stacked multi-electrode design of microbial electrolysis cells for rapid and low-sludge treatment of municipal wastewater. Biotechnol. Biofuels 2019, 12, 23. [Google Scholar] [CrossRef]
- Cusick, R.D.; Logan, B.E. Phosphate recovery as struvite within a single chamber microbial electrolysis cell. Bioresour. Technol. 2012, 107, 110–115. [Google Scholar] [CrossRef]
- Ji, X.; Liu, X.; Yang, W.; Xu, T.; Wang, X.; Zhang, X.; Wang, L.; Mao, X.; Wang, X. Sustainable phosphorus recovery from wastewater and fertilizer production in microbial electrolysis cells using the biochar-based cathode. Sci. Total Environ. 2022, 807, 150881. [Google Scholar] [CrossRef]
- San-Martín, M.I.; Mateos, R.; Escapa, A.; Morán, A. Understanding nitrogen recovery from wastewater with a high nitrogen concentration using microbial electrolysis cells. J. Environ. Sci. Health 2019, 54, 472–477. [Google Scholar] [CrossRef]
- Li, J.; Liu, R.; Zhao, S.; Wang, S.; Wang, Y. Simultaneous desalination and nutrient recovery during municipal wastewater treatment using microbial electrolysis desalination cell. J. Clean. Prod. 2020, 261, 121248. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).