Photovoltaic Performance of Dye-Sensitized Solar Cells with a Solid-State Redox Mediator Based on an Ionic Liquid and Hole-Transporting Triphenylamine Compound
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Fabrication of Sandwich-Structured sDSSCs
2.3. Fabrication of Hole-Only Devices
2.4. Measurements
3. Results and Discussion
3.1. Photovoltaic Performance of sDSSCs with m-MTDATA-Solidified MPII
3.2. Hole Conduction Mechanism in sDSSCs with m-MTDATA-Solidified MPII
3.3. Effects of Additives on Performance of sDSSCs with m-MTDATA-Solidified MPII
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energ. Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-sensitized solar cells: Fundamentals and current status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Devadiga, D.; Selvakumar, M.; Shetty, P.; Santosh, M.S. Recent progress in dye sensitized solar cell materials and photo-supercapacitors: A review. J. Power Sources 2021, 493, 229698. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Lee, C.P.; Chen, P.Y.; Vittal, R.; Ho, K.C. Iodine-free high efficient quasi solid-state dye-sensitized solar cell containing ionic liquid and polyaniline-loaded carbon black. J. Mater. Chem. 2010, 20, 2356–2361. [Google Scholar] [CrossRef]
- Benesperi, I.; Michaels, H.; Freitag, M. The researcher’s guide to solid-state dye-sensitized solar cells. J. Mater. Chem. 2018, 6, 11903–11942. [Google Scholar] [CrossRef] [Green Version]
- Chung, I.; Lee, B.; He, J.; Chang, R.P.H.; Kanatzidis, M.G. All-solid-state dye-sensitized solar cells with high efficiency. Nature 2012, 485, 486–489. [Google Scholar] [CrossRef]
- Lee, B.; Stoumpos, C.C.; Zhou, N.; Hao, F.; Malliakas, C.; Yeh, C.Y.; Marks, T.J.; Kanatzidis, M.G.; Chang, R.P.H. Air-stable molecular semiconducting iodosalts for solar cell applications: Cs2SnI6 as a hole conductor. J. Am. Chem. Soc. 2014, 136, 15379–15385. [Google Scholar] [CrossRef]
- Cao, Y.; Saygili, Y.; Ummadisingu, A.; Teuscher, J.; Luo, J.; Pellet, N.; Giordano, F.; Zakeeruddin, S.M.; Moser, J.E.; Freitag, M.; et al. 11% efficiency solid-state dye-sensitized solar cells with copper(II/I) hole transport materials. Nat. Commun. 2016, 8, 15390. [Google Scholar] [CrossRef] [Green Version]
- Freitag, M.; Daniel, Q.; Pazoki, M.; Sveinbjornsson, K.; Zhang, J.; Sun, L.; Hagfeldt, A.; Boschloo, G. High-efficiency dye-sensitized solar cells with molecular copper phenanthroline as solid hole conductor. Energy Environ. Sci. 2015, 8, 2634–2637. [Google Scholar] [CrossRef]
- Kashif, M.K.; Milhuisen, R.A.; Nippe, M.; Hellerstedt, J.; Zee, D.Z.; Duffy, N.W.; Halstead, B.; Angelis, F.D.; Fantacci, S.; Fuhrer, M.S.; et al. Cobalt polypyridyl complexes as transparent solution-processable solid-state charge transport materials. Adv. Energy Mater. 2016, 6, 1600874. [Google Scholar] [CrossRef]
- Koh, J.K.; Kim, J.; Kim, B.; Kim, J.H.; Kim, E. Highly efficient, iodine-free dye-sensitized solar cells with solid-state synthesis of conducting polymers. Adv. Mater. 2011, 23, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Chen, P.Y.; Lee, C.P.; Ho, K.C. Binary room-temperature ionic liquids based electrolytes solidified with SiO2 nanoparticles for dye-sensitized solar cells. J. Power Sources 2009, 190, 573–577. [Google Scholar] [CrossRef]
- Lee, C.P.; Lee, K.M.; Chen, P.Y.; Ho, K.C. On the addition of conducting ceramic nanoparticles in solvent-free ionic liquid electrolyte for dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 1411–1416. [Google Scholar] [CrossRef]
- Katakabe, T.; Kawano, R.; Watanabe, M. Acceleration of redox diffusion and charge-transfer rates in an ionic liquid with nanoparticle addition. Electrochem. Solid-State Lett. 2007, 10, F23–F25. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, H.; Li, X.; Li, F.; Yi, T.; Huang, C. Thermostable succinonitrile-based gel electrolyte for efficient, long-life dye-sensitized solar cells. J. Mater. Chem. 2007, 17, 1602–1607. [Google Scholar] [CrossRef]
- Usui, H.; Matsui, H.; Tanabe, N.; Yanagida, S. Improved dye-sensitized solar cells using ionic nanocomposite gel electrolytes. J. Photochem. Photobiol. A Chem. 2004, 164, 97–101. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Comte, P.; Exnar, I.; Grätzel, M. Gelation of ionic liquid-based electrolytes with silica nanoparticles for quasi-solid-state dye-sensitized solar cells. J. Am. Chem. Soc. 2003, 125, 1166–1167. [Google Scholar] [CrossRef]
- Shan, M.; Jiang, H.; Guan, Y.; Sun, D.; Wang, Y.; Hua, J.; Wang, J. Enhanced hole injection in organic light-emitting diodes utilizing a copper iodide-doped hole injection layer. RSC Adv. 2017, 7, 13584–13589. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Kwak, G.; Choi, Y.C.; Kim, D.H.; Han, Y.S. Enhanced performance of perovskite solar cells by incorporation of a triphenylamine derivative into hole-transporting poly(3-hexylthiophene) layers. J. Ind. Eng. Chem. 2019, 73, 175–181. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, D.; Li, Z.; Zhang, X.; Shen, L.; Guo, W. Efficient 4,4′,4″-tris(3-methylphenylphenylamino)triphenylamine (m-MTDATA) hole transport layer in perovskite solar cells enabled by using the nonstoichiometric precursors. Adv. Funct. Mater. 2018, 28, 1803126. [Google Scholar] [CrossRef]
- Baek, G.W.; Kim, Y.J.; Jung, K.H.; Han, Y.S. Enhancement of solar cell performance through the formation of a surface dipole on polyacrylonitrile-treated TiO2 photoelectrodes. J. Ind. Eng. Chem. 2019, 73, 260–267. [Google Scholar] [CrossRef]
- Kong, M.; Kim, K.S.; Nga, N.V.; Lee, Y.; Jeon, Y.S.; Cho, Y.; Kwon, Y.; Han, Y.S. Molecular weight effects of biscarbazole-based hole transport polymers on the performance of solid-state dye-sensitized solar cells. Nanomaterials 2020, 10, 2516. [Google Scholar] [CrossRef]
- Boschloo, G.; Hagfeldt, A. Characteristics of the iodide/triiodide redox mediator in dye-sensitized solar cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Longeaud, C.; Allah, A.F.; Schmidt, J.; Yaakoubi, M.E.; Berson, S.; Lemaitre, N. Determination of diffusion lengths in organic semiconductors: Correlation with solar cell performances. Org. Electron. 2016, 31, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Mikhnenko, O.V.; Blom, P.W.M.; Nguyen, T.Q. Exciton diffusion in organic semiconductors. Energy Environ. Sci. 2015, 8, 1867–1888. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, C.R.; Jeong, W.S.; Im, J.H.; Ryu, T.I.; Park, N.G. Unusual enhancement of photocurrent by incorporation of bronsted base thiourea into electrolyte of dye-sensitized solar cell. J. Phys. Chem. 2010, 114, 19849–19852. [Google Scholar] [CrossRef]
- Bonomo, M.; Carlo, A.D.; Dini, D. Study of the influence of the I-based electrolyte composition on the photoconversion properties of p-type dye-sensitized solar cell. J. Electrochem. Soc. 2018, 165, H889–H896. [Google Scholar] [CrossRef]
- Sakurai, M.; Kabe, R.; Fuki, M.; Lin, Z.; Jinnal, K.; Kobori, Y.; Adachi, C.; Tachikawa, T. Organic photostimulated luminescence associated with persistent spin-correlated radical pairs. Commun. Mater. 2021, 2, 74. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Stanbury, D.M.; Bounds, P.L. Electrode potentials partially reduced oxygen species, from dioxygen to water. Free Radic. Biol. Med. 2010, 49, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Chulkin, P.; Lapkowski, M.; Bryce, M.R.; Santos, J.; Data, P. Determination of standard redox rate constants of OLED active compounds by electrochemical impedance spectroscopy. Electrochim. Acta 2017, 258, 1160–1172. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.K.; Lee, Y.; Kim, W.H.; Lee, S.J.; Sung, S.J.; Kim, D.H.; Han, Y.S. Enhanced power conversion efficiency dye-sensitized solar cells by band edge shift of TiO2 photoanode. Molecules 2020, 25, 1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abate, A.; Leijtens, T.; Pathak, S.; Teusher, J.; Avolio, R.; Errico, M.E.; Kirkpatrik, J.; Ball, J.M.; Docampo, P.; McPherson, I.; et al. Lithium salts as “redox active” p-type dopants for organic semiconductors and their impact in solid-state dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2013, 15, 2575–2579. [Google Scholar] [CrossRef]
- Cappel, U.B.; Daeneke, T.; Bach, U. Oxygen-induced doping of spiro-MeOTAD in solid-state dye-sensitized solar cells and its impact on device performance. Nano Lett. 2012, 12, 4925–4931. [Google Scholar] [CrossRef]
- Xu, B.; Gabrielsson, E.; Safdari, M.; Cheng, M.; Hua, Y.; Tian, H.; Gardner, J.M.; Kloo, L.; Sun, L. 1,1,2,2-Tetrachloroethane (TeCA) as a solvent additive for organic hole transport materials and its application in highly efficient solid-state dye-sensitized solar cells. Adv. Energy Mater. 2015, 5, 1402340. [Google Scholar] [CrossRef]
- Quan, V.A. Degradation of the Solar Cell Dye Sensitizer N719 Preliminary Building of Dye-Sensitized Solar Cell. Master’s Thesis, Roskide University, Roskide, Denmark, June 2006. [Google Scholar]
- Schafferhans, J.; Baumann, A.; Wagernpfahl, A.; Deibel, C.; Dyakonov, V. Oxygen doping of P3HT:PCBM blends: Influence on trap states, charge carrier mobility and solar cell performance. Org. Electron. 2010, 11, 1693–1700. [Google Scholar] [CrossRef] [Green Version]
- Kusama, H.; Arakawa, H. Influence of aminotriazole additives in electrolytic solution on dye-sensitized solar cell performance. J. Photochem. Photobio. A Chem. 2004, 164, 103–110. [Google Scholar] [CrossRef]
- Shaikh, S.F.; Mane, R.S.; Min, B.K.; Hwang, Y.J.; Joo, O.S. D-sorbitol-induced phase control of TiO2 nanoparticles and its application for dye-sensitized solar cells. Sci. Rep. 2016, 6, 20103. [Google Scholar] [CrossRef]
- Xu, J.; Fan, K.; Shi, W.; Li, K.; Peng, T. Application of ZnO micro-flowers as scattering layer for ZnO-based dye-sensitized solar cells with enhanced conversion efficiency. Sol. Energy 2014, 101, 150–159. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.Y.; Kim, J.H.; Choi, C.J.; Kim, H.; Sung, Y.E.; Ahn, K.S. Enhanced efficiency of dye-sensitized solar cells through TiCl4-treated, nanoporous-layer-covered TiO2 nanotube arrays. J. Power Sources 2011, 196, 8904–8908. [Google Scholar] [CrossRef]
- Ambade, S.B.; Ambade, R.B.; Mane, R.S.; Lee, G.W.; Shaikh, S.F.; Patil, S.A.; Joo, O.S.; Han, S.H.; Lee, S.H. Low temperature chemically synthesized rutile TiO2 photoanodes with high electron lifetime for organic dye-sensitized solar cells. Chem. Commun. 2013, 49, 2921–2923. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, Z.; Wang, X.; Li, Y.; Gunther, M.; Valenzuela, S.; Parikh, P.; Cabreros, A.; Xiong, W.; Meng, Y.S. Unveiling the role of tBP−LiTFSI complexes in perovskite solar cells. J. Am. Chem. Soc. 2018, 140, 16720–16730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
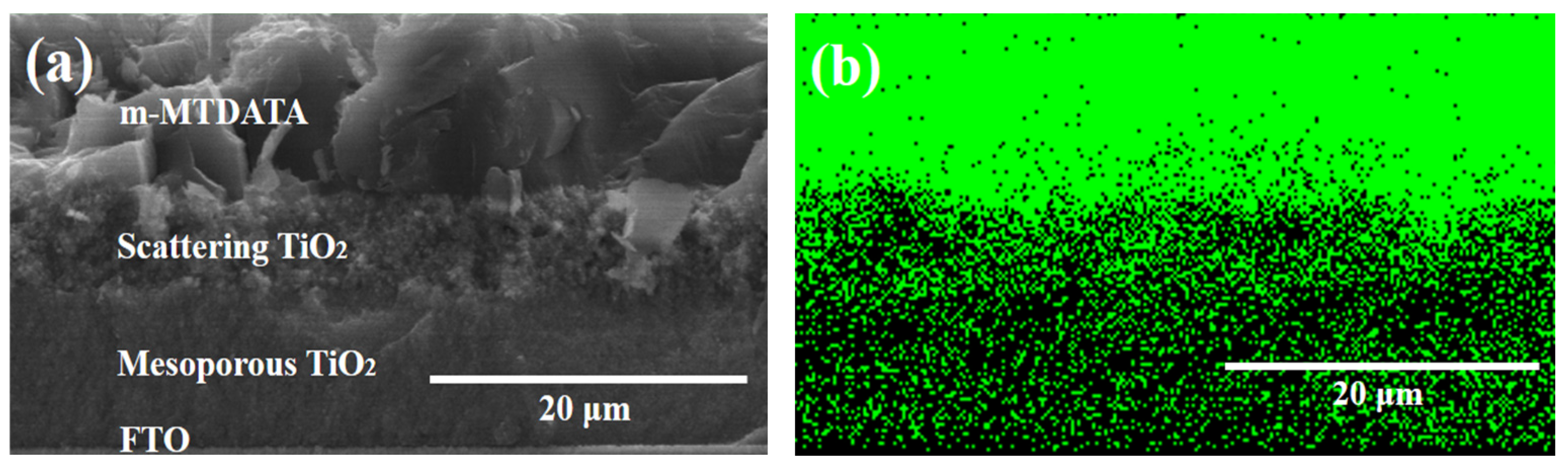



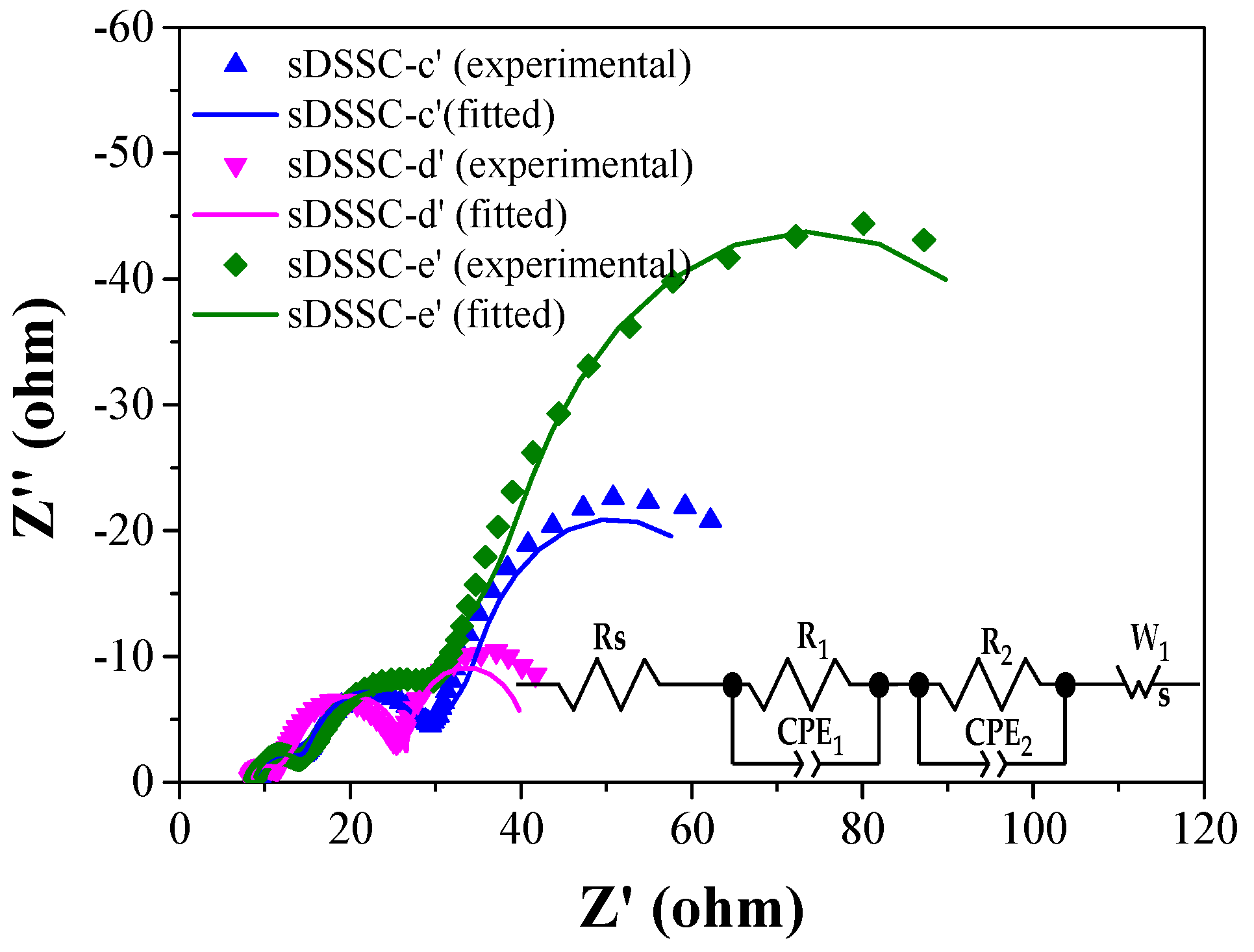

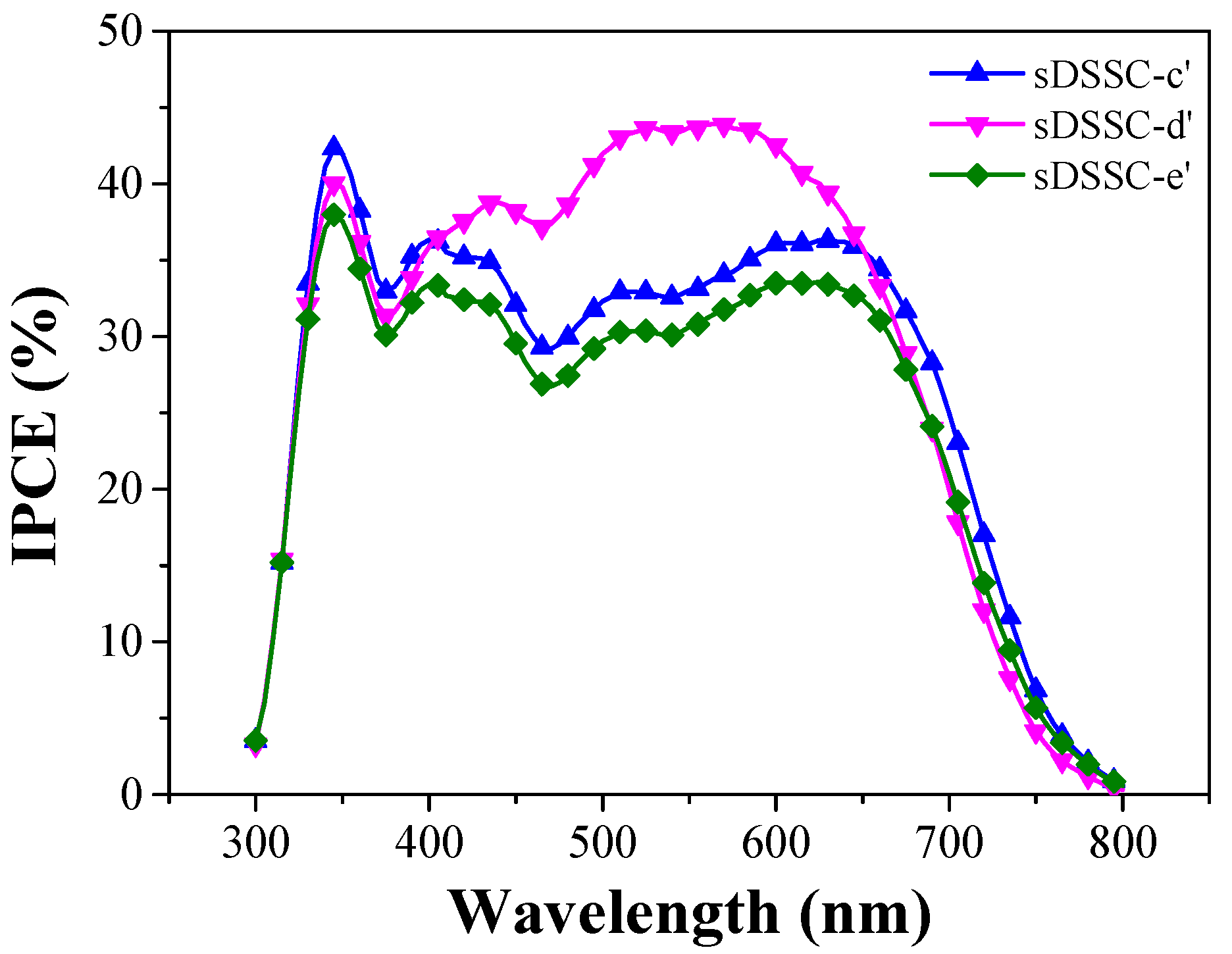
| Ionic Liquids | Solidifying Agents | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| MPII (without I2) | P3HT | 14.2 | 0.64 | 60 | 5.4 | [13] |
| MPII (without I2) | polyaniline-loaded carbon black | 12.20 | 0.737 | 65 | 5.81 | [6] |
| MPII (without I2) | m-MTDATA | 9.43 | 0.610 | 73.0 | 4.20 | This study |
| BMII + BMISO3CF3 (with I2) | SiO2 | 11.3 | - | - | 4.83 | [14] |
| BMII (with I2) | TiC | 3.40 | 0.6686 | 74 | 1.68 | [15] |
| EMITFSI + EMII (with I2) | SiO2 | 10.4 | 0.592 | 62 | 3.7 | [16] |
| BMIBF4 (with I2) | Silica | 8.60 | 0.621 | 69.9 | 4.98 | [17] |
| EMITFSI + EMII (with I2) | TiO2 | 11.45 | 0.675 | 65 | 5.00 | [18] |
| EMITFSI + EMII (with I2) | Carbon fiber | 11.11 | 0.688 | 65 | 4.97 | [18] |
| MPII (with I2) | Silica | 13.67 | 0.700 | 73.1 | 7.0 | [19] |
| Solar Cells | Components of RM | Phase of RM |
|---|---|---|
| DSSC-a | MPII | Viscous liquid |
| sDSSC-b | m-MTDATA | Solid powder |
| sDSSC-c | MPII, m-MTDATA | Solid-state (nonfluidic composite) |
| sDSSC-d | MPII, m-MTDATA, TBP | Solid-state (nonfluidic composite) |
| sDSSC-e | MPII, m-MTDATA, TBP, LiTFSI | Solid-state (nonfluidic composite) |
| Solar Cells | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| DSSC-a | 0.54 ± 0.13 | 0.722 ± 0.052 | 57.38 ± 5.25 | 0.22 ± 0.05 |
| sDSSC-b | 0.001 ± 0.002 | 0.275 ± 0.082 | 0 | 0 |
| sDSSC-c | 3.48 ± 1.20 | 0.553 ± 0.017 | 74.90 ± 2.30 | 1.42 ± 0.42 |
| sDSSC-d | 8.38 ± 1.08 | 0.603 ± 0.006 | 74.34 ± 1.19 | 3.75 ± 0.43 |
| sDSSC-e | 3.45 ± 0.19 | 0.553 ± 0.007 | 78.82 ± 0.91 | 1.50 ± 0.12 |
| Champion Cells | Jsc (mA/cm2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| DSSC-a’ | 0.72 | 0.708 | 56.86 | 0.29 |
| sDSSC-b’ | 0.004 | 0.304 | 0 | 0 |
| sDSSC-c’ | 4.61 | 0.544 | 71.81 | 1.80 |
| sDSSC-d’ | 9.43 | 0.610 | 73.03 | 4.20 |
| sDSSC-e’ | 3.28 | 0.546 | 77.53 | 1.39 |
| Champion Cells | Additive | Rs (Ω) | R1 (Ω) | R2 (Ω) | w1 (Ω) |
|---|---|---|---|---|---|
| sDSSC-c’ | None | 8.36 | 7.43 | 19.78 | 46.98 |
| sDSSC-d’ | TBP | 8.39 | 4.41 | 15.00 | 22.02 |
| sDSSC-e’ | TBP and LiTFSI | 8.89 | 6.53 | 29.39 | 103.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, M.; Oh, D.H.; Choi, B.; Han, Y.S. Photovoltaic Performance of Dye-Sensitized Solar Cells with a Solid-State Redox Mediator Based on an Ionic Liquid and Hole-Transporting Triphenylamine Compound. Energies 2022, 15, 2765. https://doi.org/10.3390/en15082765
Kong M, Oh DH, Choi B, Han YS. Photovoltaic Performance of Dye-Sensitized Solar Cells with a Solid-State Redox Mediator Based on an Ionic Liquid and Hole-Transporting Triphenylamine Compound. Energies. 2022; 15(8):2765. https://doi.org/10.3390/en15082765
Chicago/Turabian StyleKong, Minseon, Da Hyeon Oh, Baekseo Choi, and Yoon Soo Han. 2022. "Photovoltaic Performance of Dye-Sensitized Solar Cells with a Solid-State Redox Mediator Based on an Ionic Liquid and Hole-Transporting Triphenylamine Compound" Energies 15, no. 8: 2765. https://doi.org/10.3390/en15082765
APA StyleKong, M., Oh, D. H., Choi, B., & Han, Y. S. (2022). Photovoltaic Performance of Dye-Sensitized Solar Cells with a Solid-State Redox Mediator Based on an Ionic Liquid and Hole-Transporting Triphenylamine Compound. Energies, 15(8), 2765. https://doi.org/10.3390/en15082765






