Evaporated Alkali Carbonate Effect on an Aluminum Diffusion Coated 253MA Vessel after 4000 h Discontinuous Operation—Lessons Learned
Abstract
:1. Introduction
2. Experimental Layout
2.1. Setup
2.2. Vessel Material and Powder Pack Diffusion Coating
2.3. Alkali Carbonate Salt Melt
2.4. Operation Condition and Individual Experiment Durations
2.5. Vessel Decommissioning and Samples Preparation
2.6. Sample Characterization
3. Results
3.1. Remaining Metal Thickness of the Vessel
3.2. Structural Analysis of the Sample Surfaces
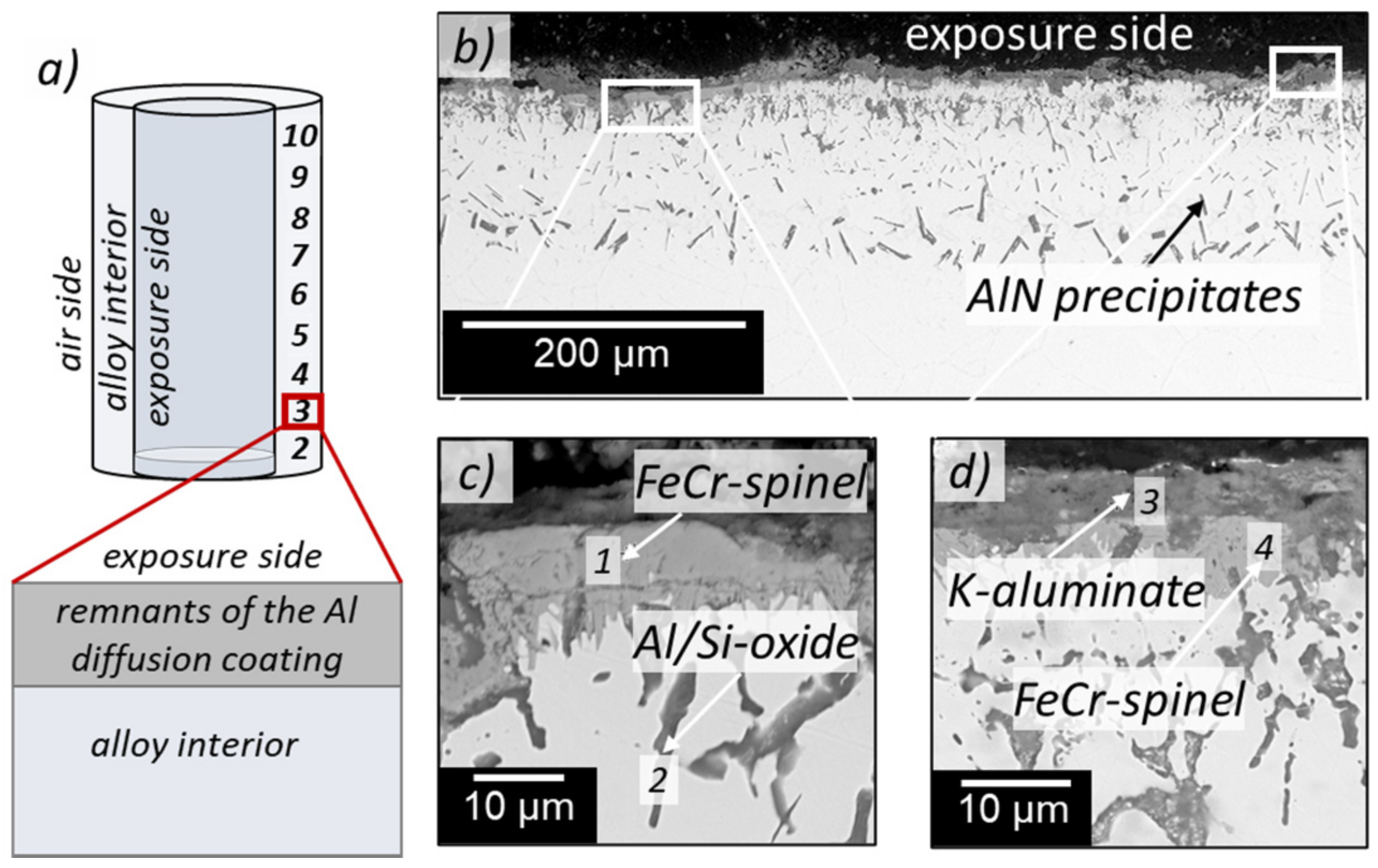
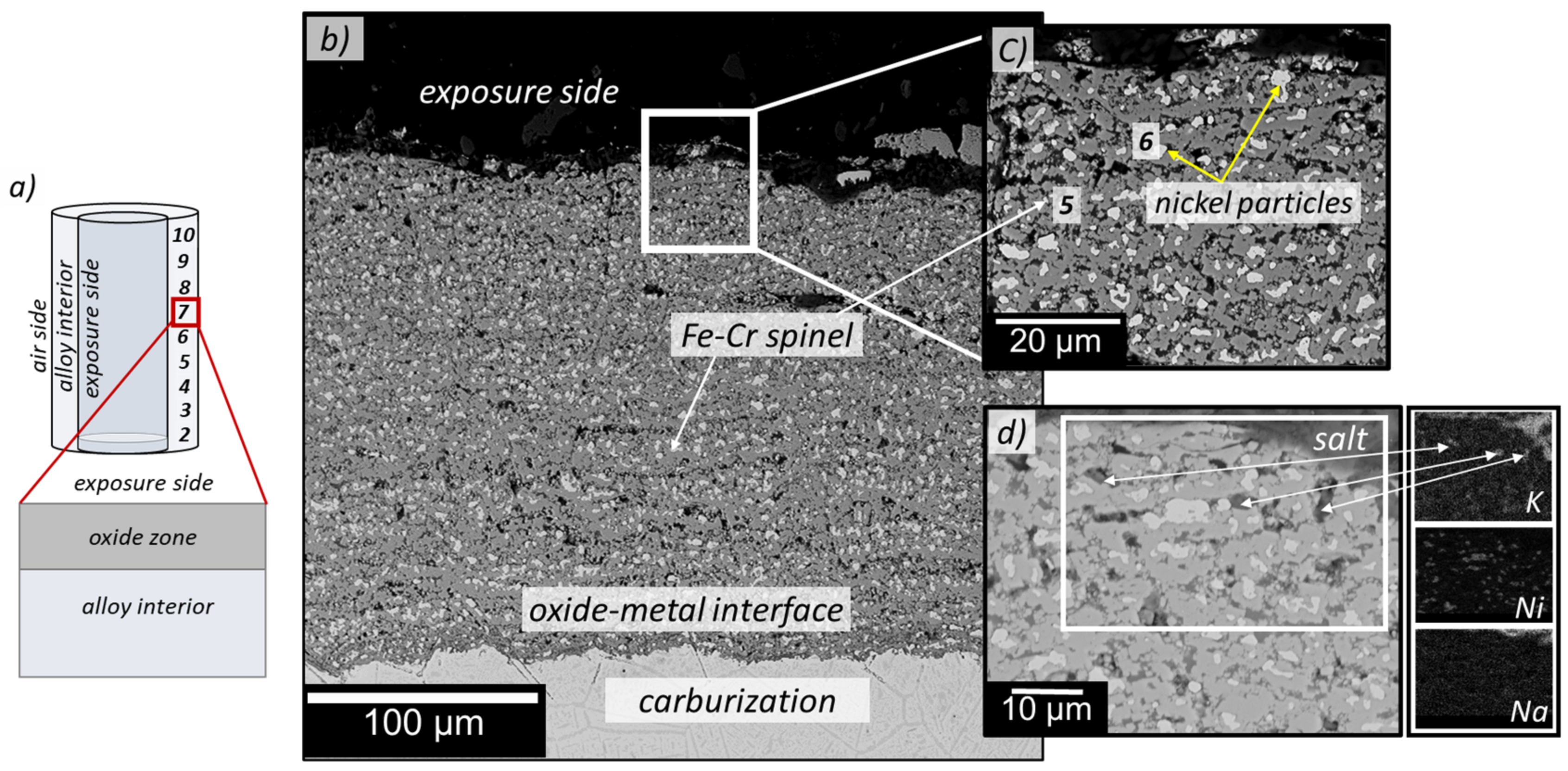
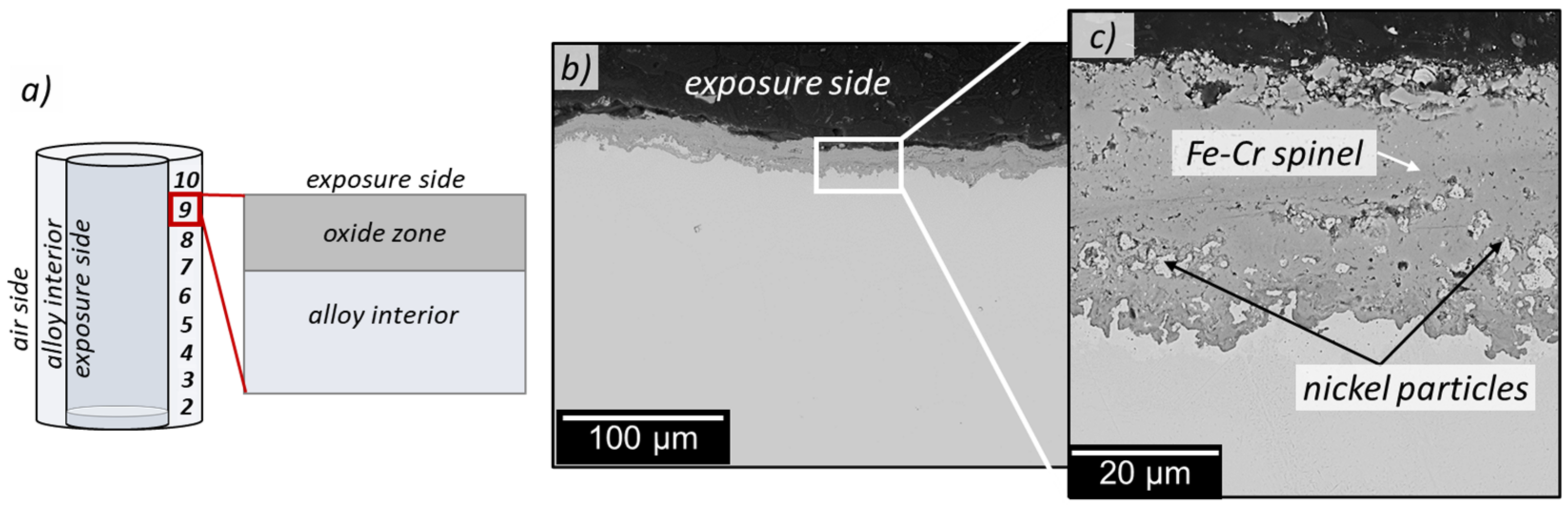
3.3. Cross-Section Analyses
4. Discussion
5. Summary
- -
- More than 4000 h of intermittent operation at high temperature in an alkali carbonate-containing CO2 gas environment significantly damaged a furnace vessel made of alloy 253MA.
- -
- An additional aluminum diffusion coating applied to the 253MA vessel surface before operation, had no lasting effect on the material degradation processes.
- -
- The severity of the corrosion attack does not follow the temperature gradient across the vessel but rather the source of evaporating salt into the gas stream and its flowing direction.
- -
- The amount of potassium from the evaporating salt species into corrosion products is significantly higher than observed for immersion samples in direct contact with the melt, where lithium was dominating instead.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehos, M.; Turchi, C.; Vidal, J.; Wagner, M.; Ma, Z.; Ho, C.; Kolb, W.; Andraka, C.; Kruizenga, A. Concentrating Solar Power Gen3 Demonstration Roadmap; NREL/TP-5500-67464 United States 10.2172/1338899 NREL English; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2017. Available online: https://www.osti.gov/servlets/purl/1338899 (accessed on 15 August 2021).
- Zhao, Y.; Li, P.; Jin, H. Heat Transfer Performance Comparisons of Supercritical Carbon Dioxide and NaCl–KCl–ZnCl2 Eutectic Salts for Solar s-CO2 Brayton Cycle. Energy Procedia 2017, 142, 680–687. [Google Scholar] [CrossRef]
- Yin, J.-M.; Zheng, Q.Y.; Peng, Z.R.; Zhang, X.R. Review of supercritical CO2 power cycles integrated with CSP. Int. J. Energy Res. 2019, 44, 1337–1369. [Google Scholar] [CrossRef]
- Ho, C.K.; Carlson, M.; Garg, P.; Kumar, P. Technoeconomic Analysis of Alternative Solarized s-CO2 Brayton Cycle Configurations. J. Sol. Energy Eng. 2016, 138, 051008. [Google Scholar] [CrossRef]
- Walczak, M.; Pineda, F.; Fernández, Á.G.; Mata-Torres, C.; Escobar, R.A. Materials corrosion for thermal energy storage systems in concentrated solar power plants. Renew. Sustain. Energy Rev. 2018, 86, 22–44. [Google Scholar] [CrossRef]
- Patel, N.S.; Pavlík, V.; Boča, M. High-Temperature Corrosion Behavior of Superalloys in Molten Salts—A Review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 83–97. [Google Scholar] [CrossRef]
- Kuravi, S.; Trahan, J.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 2013, 39, 285–319. [Google Scholar]
- Frangini, S. Corrosion of structural materials in molten carbonate fuel cells: An overview. Molten Salt Forum 2003, 7, 135–154. [Google Scholar]
- Frangini, S. Corrosion Behavior of AISI 316L Stainless Steel and ODS FeAl Aluminide in Eutectic Li2CO3–K2CO3 Molten Carbonates under Flowing CO2–O2 Gas Mixtures. Oxid. Met. 2000, 53, 139–156. [Google Scholar] [CrossRef]
- Lee, K.S.; Cho, K.; Lim, T.H.; Hong, S.-A.; Kim, H. Corrosion behaviour of sensitized AISI-type 316L stainless steel in molten carbonate fuel cell in cathode-gas environment. J. Power Sources 1999, 83, 32–40. [Google Scholar] [CrossRef]
- Sarvghad, M.; Will, G.; Steinberg, T.A. Corrosion of Inconel 601 in molten salts for thermal energy storage. Sol. Energy Mater. Sol. Cells 2017, 172, 220–229. [Google Scholar] [CrossRef]
- Hamdy, E.; Nockert-Olovsjö, J.; Geers, C. Additional data and experimental setups used for the study on alloys in contact to high temperature eutectic melts for thermal storage. Data Brief 2021, 38, 107446. [Google Scholar] [CrossRef]
- Hamdy, E.; Nockert-Olovsjö, J.; Geers, C. Perspectives on selected alloys in contact with eutectic melts for thermal storage: Nitrates, carbonates and chlorides. Sol. Energy 2021, 224, 1210–1221. [Google Scholar] [CrossRef]
- Hamdy, E.; Strach, M.; Nockert-Olovsjö, J.; Geers, C. Differentiation in corrosion performance of alumina forming alloys in alkali carbonate melts. Corros. Sci. 2021, 192, 109857. [Google Scholar] [CrossRef]
- TNguyen, D.; Zhang, J.; Young, D.J. Effects of cerium and manganese on corrosion of Fe–Cr and Fe–Cr–Ni alloys in Ar–20CO2 and Ar–20CO2–20H2O gases at 650 °C. Corros. Sci. 2015, 100, 448–465. [Google Scholar] [CrossRef]
- Johansson, C.; Lind, M. Evaluation of the η (Eta) Nitride with Three Laboratory Melts. Master’s Thesis, Royal Institute of Technology, Stockholm, Sweden, 2015. [Google Scholar]
- Sandström, R.; Farooq, M.; Lundberg, M. Precipitation during long time ageing in the austenitic stainless steel 310. Mater. High Temp. 2014, 29, 8–16. [Google Scholar] [CrossRef]
- TCFE, version 10.1; Thermo-Calc Software AB: Stockholm, Sweden, 2020.
- Xi, X.; Kong, C.; Zhang, J. Effect of Cyclic Reaction on Corrosion Behavior of Chromium-Containing Alloys in CO2 Gas at 650 °C. Oxid. Met. 2020, 93, 131–157. [Google Scholar] [CrossRef]
- Oskay, C.; Meißner, T.M.; Dobler, C.; Grégoire, B.; Galetz, M.C. Scale Formation and Degradation of Diffusion Coatings Deposited on 9% Cr Steel in Molten Solar Salt. Coatings 2019, 9, 687. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-T.; Ren, N.; Wang, T.; Ma, C.-F. Experimental study on optimized composition of mixed carbonate salt for sensible heat storage in solar thermal power plant. Sol. Energy 2011, 85, 1957–1966. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Arvay, A.; Hsu, K.; Kannan, A.M. Heat transfer fluids for concentrating solar power systems—A review. Appl. Energy 2015, 146, 383–396. [Google Scholar] [CrossRef]
- Simmons, L.; Lowden, L.F.; Ehlert, T.C. A mass spectrometric study of potassium carbonate and potassium oxide. J. Phys. Chem. 1977, 81, 706–709. [Google Scholar] [CrossRef]
- Tetsuo, K. Measurements of the Vapor and Dissociation Pressures of Potassium Sulfate and Carbonate at High Temperatures. Bull. Chem. Soc. Jpn. 1972, 45, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Motzfeldt, K. The Thermal Decomposition.of Sodium Carbonate by the Effusion Method. J. Phys. Chem. 1955, 59, 139–147. [Google Scholar] [CrossRef]
- Sergeev, D.; Yazhenskikh, E.; Kobertz, D.; Müller, M. Vaporization behavior of Na2CO3 and K2CO3. Calphad 2019, 65, 42–49. [Google Scholar] [CrossRef]
- Maru, H.C. Alkali Metal Carbonates. In Molten Salt Techniques; Lovering, D.G., Gale, R.J., Eds.; Plenum Press: New York, NY, USA; Plenum Publishing Corp.: New York, NY, USA, 1983; Volume 1, p. 272. [Google Scholar]
- Atkins, P.W. Chemical Principles: The Quest for Insight; W.H. Freeman: New York, NY, USA, 2000. (In English) [Google Scholar]
- Sah, S.P.; Tada, E.; Nishikata, A. Corrosion behaviour of austenitic stainless steels in carbonate melt at 923 K under controlled CO2-O2 environment. Corros. Sci. 2018, 133, 310–317. [Google Scholar] [CrossRef]
- TCNI, version 10.0; Thermo-Calc Software AB: Stockholm, Sweden, 2020.




| Measured Composition of Alloy 253MA (wt%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | C | Si | Mn | P | S | Cr | Ni | N | Others |
| Bal. | ~0.08 | ≤1.97 | ≤0.55 | ≤0.02 | ≤0.16 | ~20.9 | ~10.6 | ≤0.14 | Ce; V |
 | Microstructure of 253MA alloy before exposure; only Ce-containing precipitates are observed. | ||||||||
| TCFE single point calculation considering the nominal alloy composition and for Mn: 0.55%; P: 0.002%; S: 0.0016% | |||||||||
| 253MA at 1050 °C | 253MA at 800 °C | ||||||||
| Phase | Mass fraction [%] | Phase | Mass fraction [%] | ||||||
| FCC | 99.29 | FCC | 92.14 | ||||||
| (Cr,V)2N | 0.61 | (Cr,V)2N | 0.18 | ||||||
| M23C6 | 0.04 | M23C6 | 1.31 | ||||||
| (Mn,Cr)S | 0.02 | (Cr,Fe)3P | 0.01 | ||||||
| Ce2S3 | 0.04 | Ce2S3 | 0.04 | ||||||
| η-phase | 2.65 | ||||||||
| (Cr,Mn)S | 0.02 | ||||||||
| σ-phase | 3.65 | ||||||||
| Operation Conditions | Duration | Reheating Cycle (Every 336 h) | Duration of the Vessel Opening (Pouring the Melt Off, Air, 500 °C) | |
|---|---|---|---|---|
| Process | ||||
| Aluminum-diffusion coating | ||||
| Step I (at 500 °C), Ar | 1 h | - | - | |
| Step II (at 900 °C), Ar | 3 h | - | - | |
| Pre-oxidation step (at 900 °C), air | 24 h | - | - | |
| Exposures to alkali carbonate melts (CO2, 800 °C) | ||||
| Experiment no. 1 | 72 h | - | 75 min | |
| Experiment no. 2 | 500 h | Once | 75 min | |
| Experiment no. 3 | 500 h | Once | 60 min | |
| Experiment no. 4 | 168 h | - | 45 min | |
| Experiment no. 5 | 1000 h | Thrice | 45 min | |
| Experiment no. 6 | 72 h | - | 50 min | |
| Experiment no. 7 | 1000 h | Thrice | 45 min | |
| Experiment no. 8 | 168 h | - | 60 min | |
| Experiment no. 9 | 72 h | - | 60 min | |
| Experiment no. 10 | 72 h | - | 55 min | |
| Experiment no. 11 | 500 h | Once | 60 min | |
| Element | Input I [mol%] | Phases | Input II [mol%] | Phases | Input III [mol%] | Phases | Input IV [mol%] | Phases |
|---|---|---|---|---|---|---|---|---|
| C | 0.08 | Ni FCC; CO2 | 0.08 | CO2/CO (55/45); Ni-Fe FCC; FeO | 0.08 | Corundum Cr2O3; Ni-Cr FCC; M7C3 | 0.08 | Corundum Cr2O3; Ni-Fe FCC; M3C2; Graphite |
| O | 0.16 | 0.16 | 0.16 | 0.16 | ||||
| Ni | 0.76 | 0.38 | 0.38 | 0.38 | ||||
| Fe | 0.38 | 0.19 | ||||||
| Cr | 0.38 | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamdy, E.; Wagné, A.; Geers, C. Evaporated Alkali Carbonate Effect on an Aluminum Diffusion Coated 253MA Vessel after 4000 h Discontinuous Operation—Lessons Learned. Energies 2022, 15, 3241. https://doi.org/10.3390/en15093241
Hamdy E, Wagné A, Geers C. Evaporated Alkali Carbonate Effect on an Aluminum Diffusion Coated 253MA Vessel after 4000 h Discontinuous Operation—Lessons Learned. Energies. 2022; 15(9):3241. https://doi.org/10.3390/en15093241
Chicago/Turabian StyleHamdy, Esraa, Angelina Wagné, and Christine Geers. 2022. "Evaporated Alkali Carbonate Effect on an Aluminum Diffusion Coated 253MA Vessel after 4000 h Discontinuous Operation—Lessons Learned" Energies 15, no. 9: 3241. https://doi.org/10.3390/en15093241
APA StyleHamdy, E., Wagné, A., & Geers, C. (2022). Evaporated Alkali Carbonate Effect on an Aluminum Diffusion Coated 253MA Vessel after 4000 h Discontinuous Operation—Lessons Learned. Energies, 15(9), 3241. https://doi.org/10.3390/en15093241






