Comparative Analysis of Numerical Methods for Simulating N-Heptane Combustion with Steam Additive
Abstract
:1. Introduction
2. Problem Statement and Research Methods
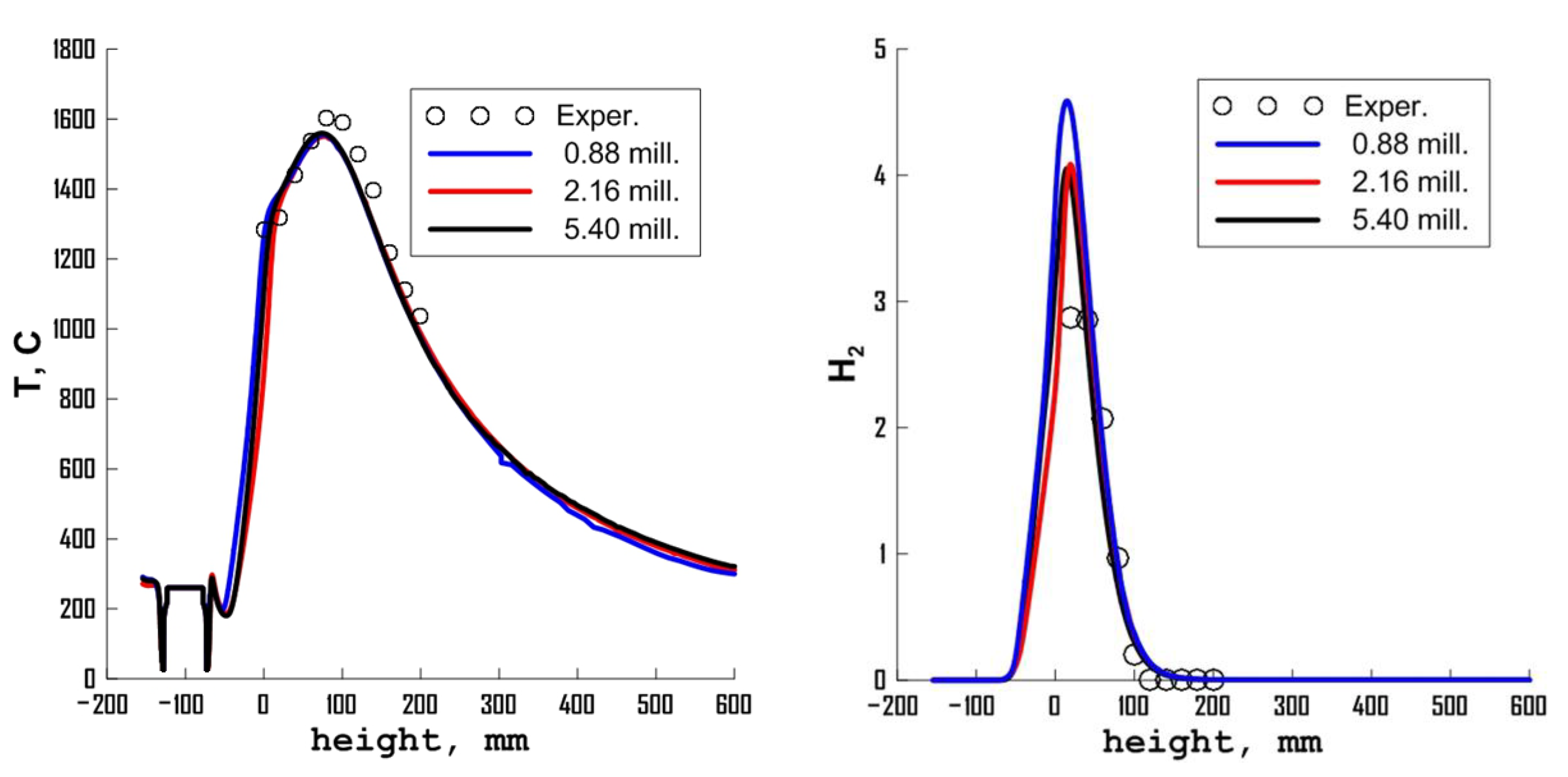
3. Results
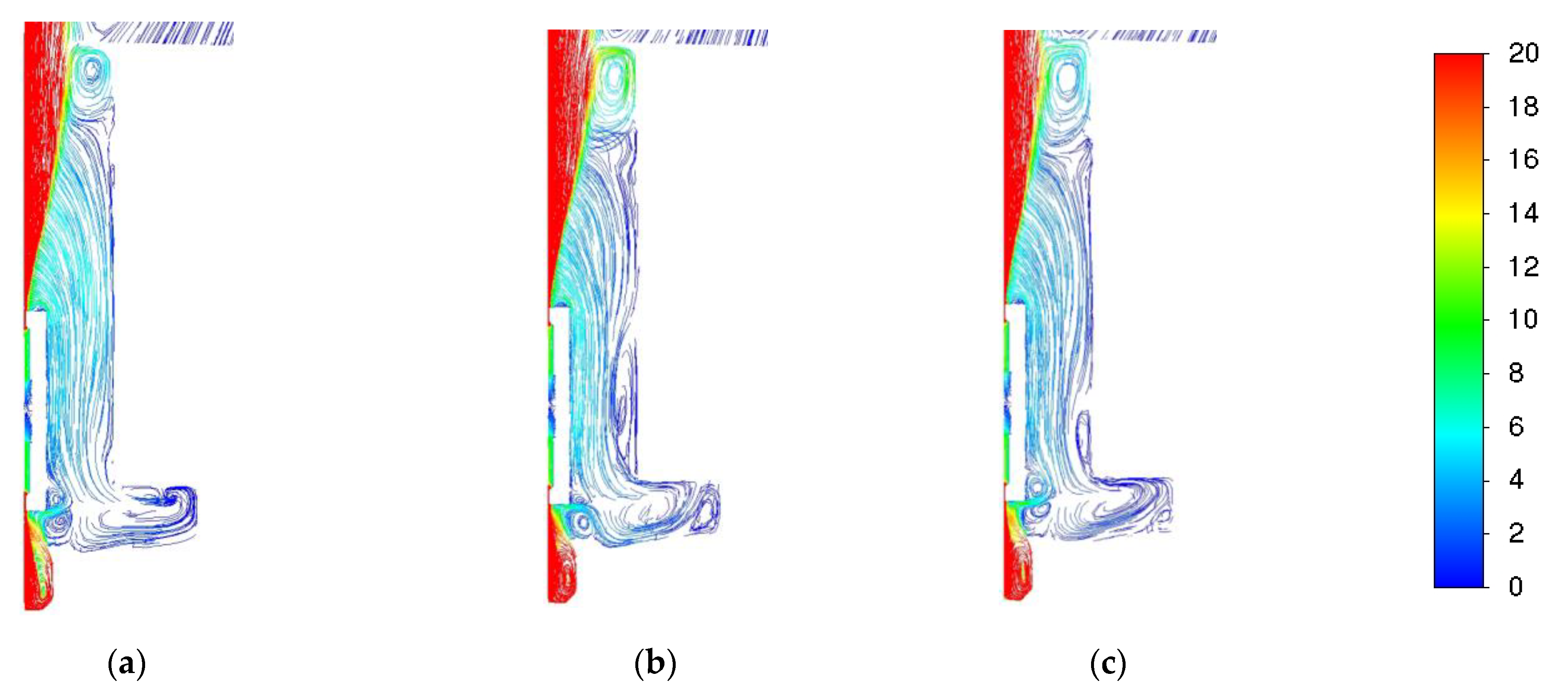
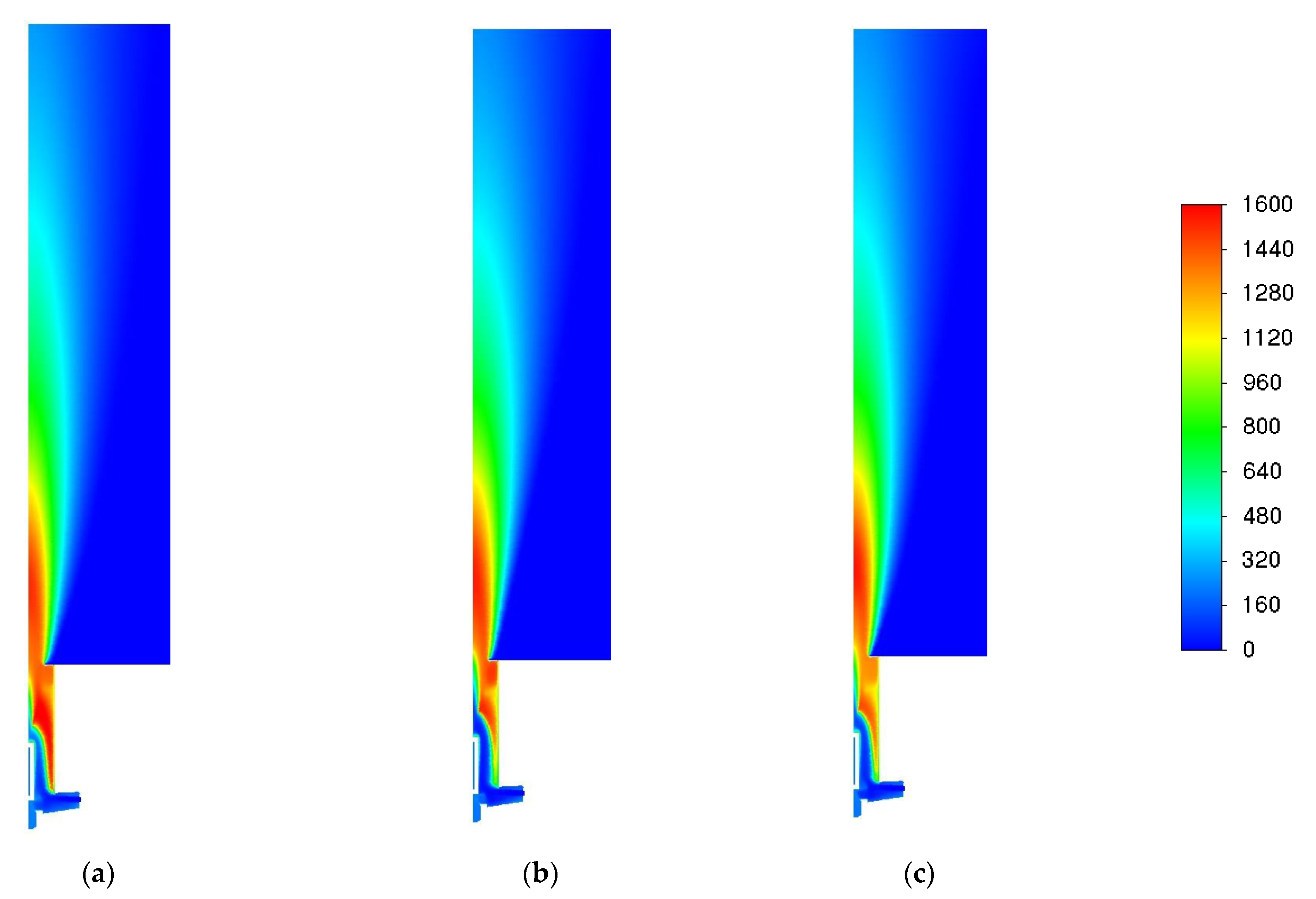
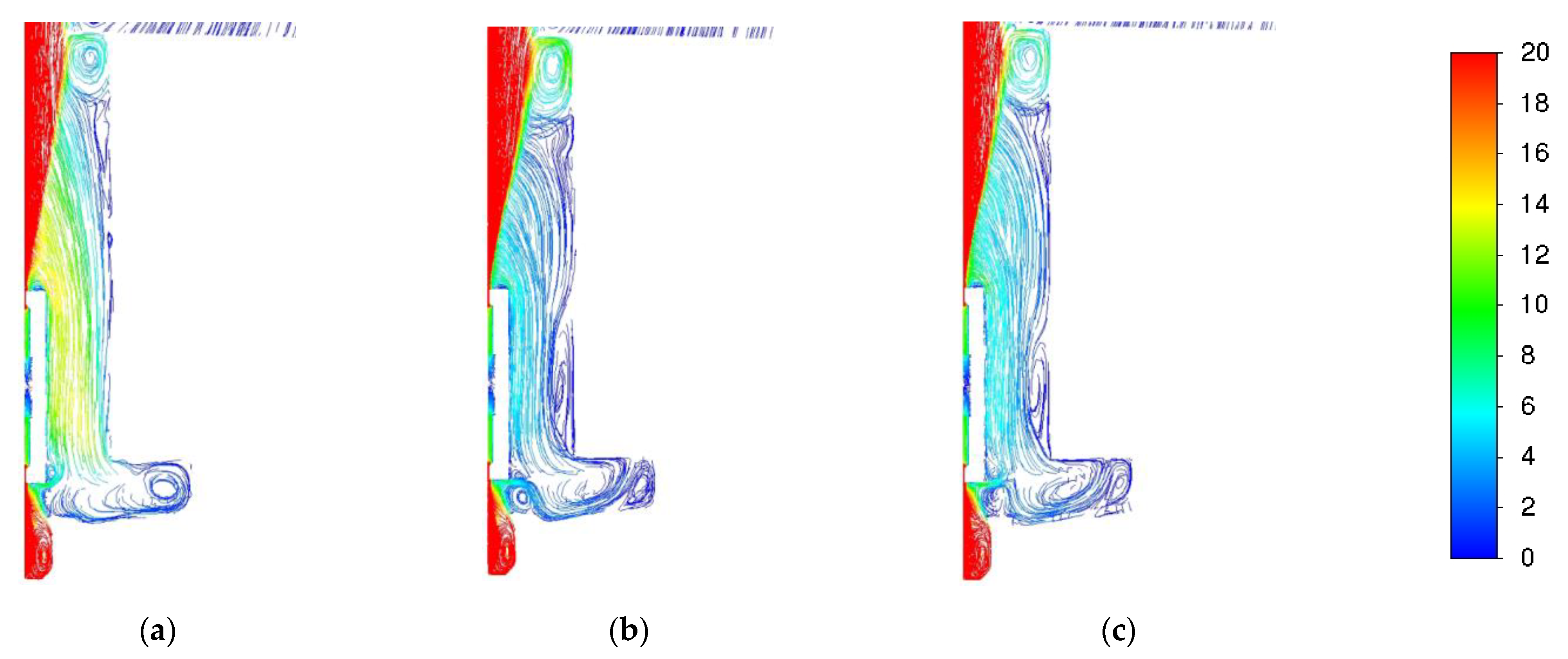
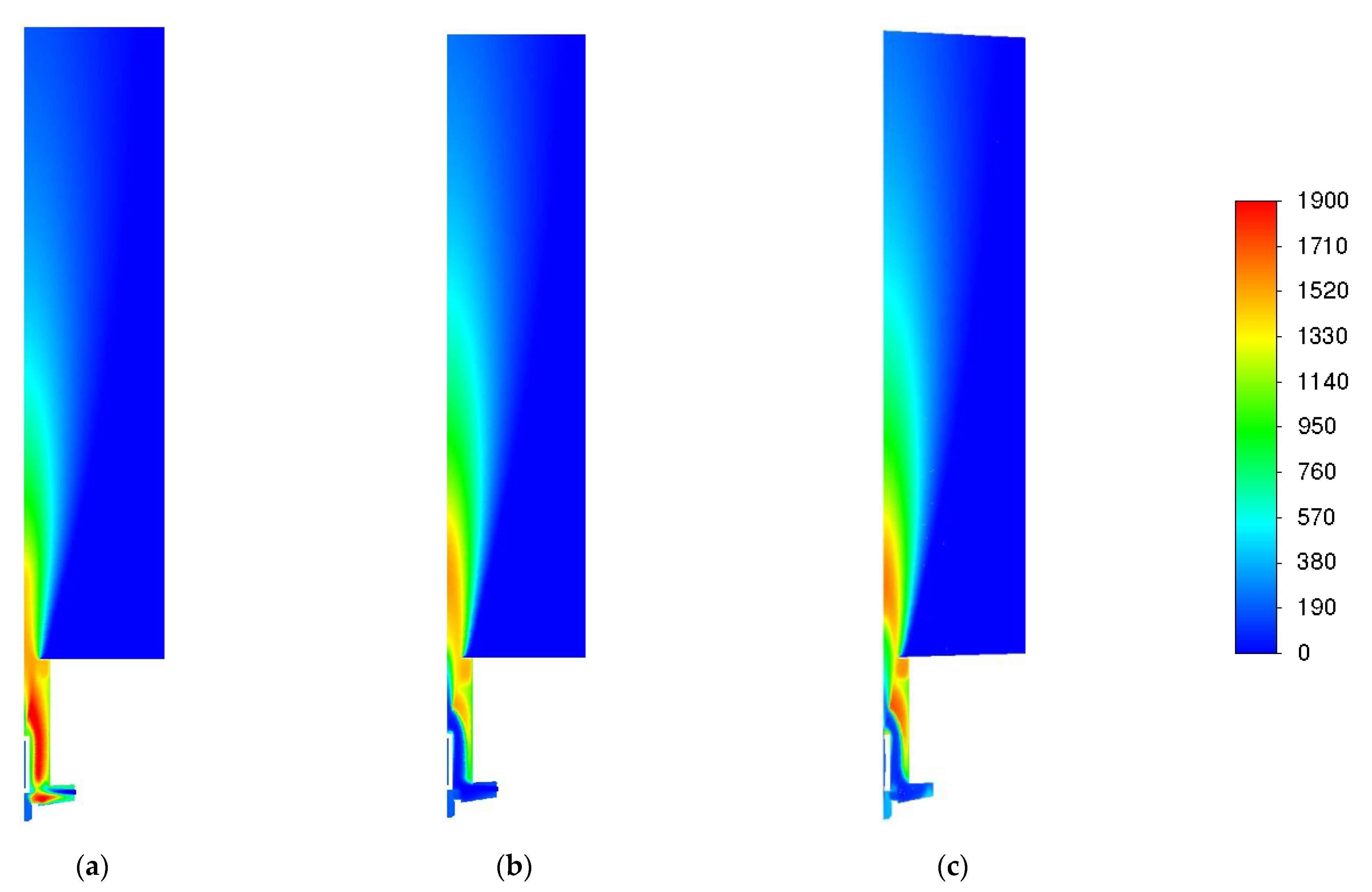
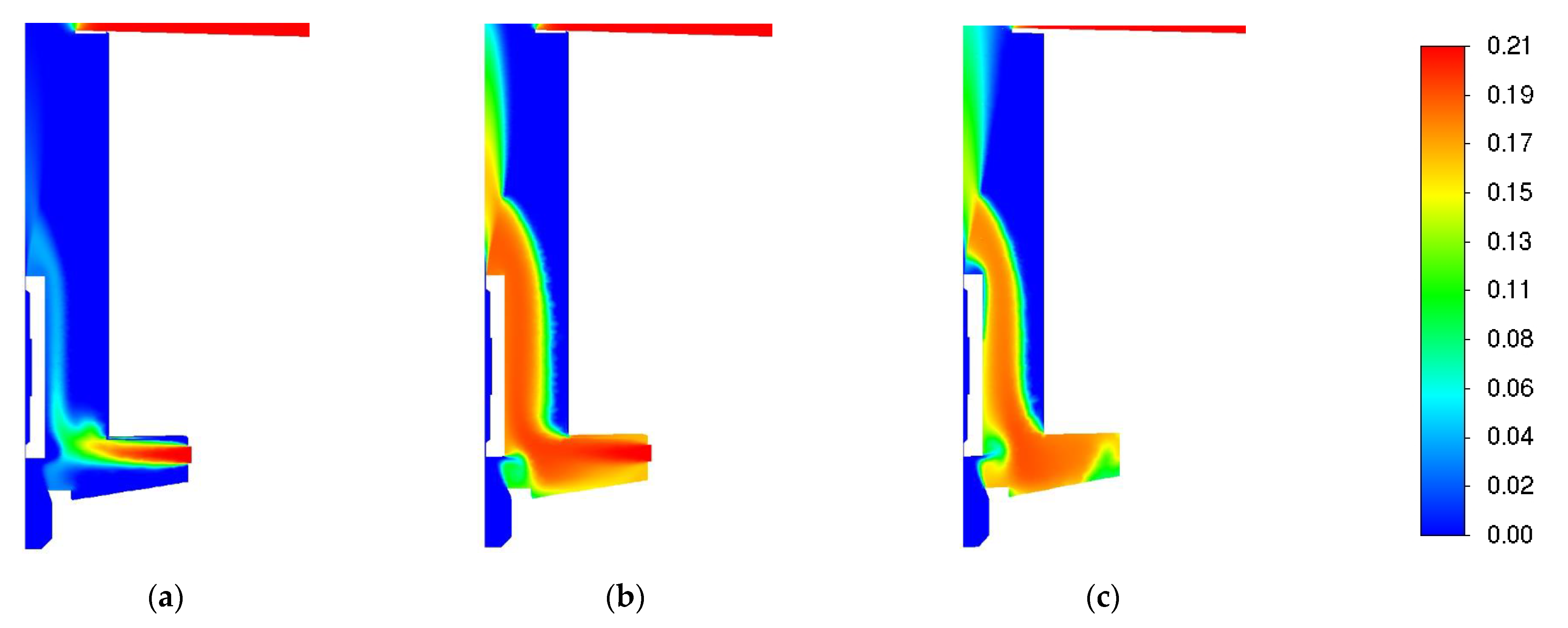
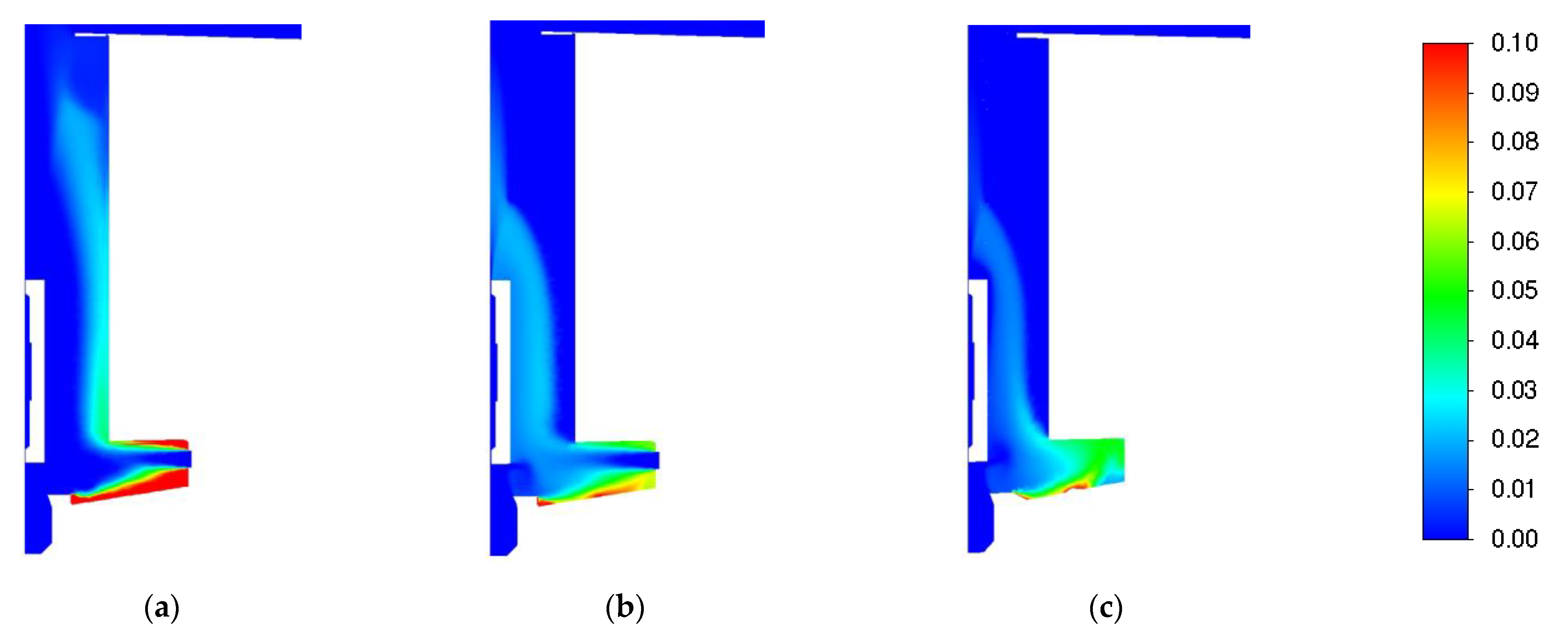
3.1. Influence of Turbulence Models
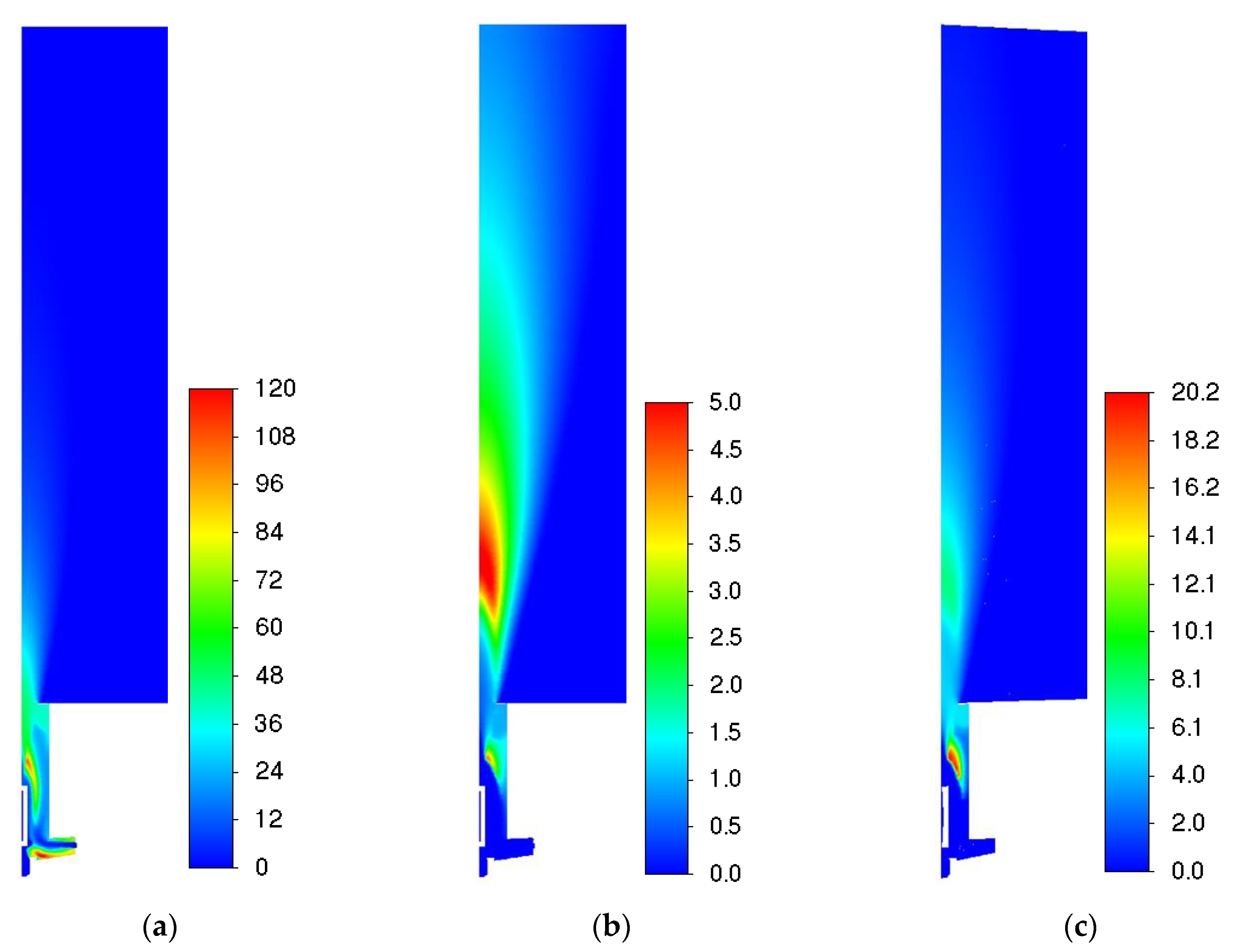
3.2. Influence of the Combustion Model
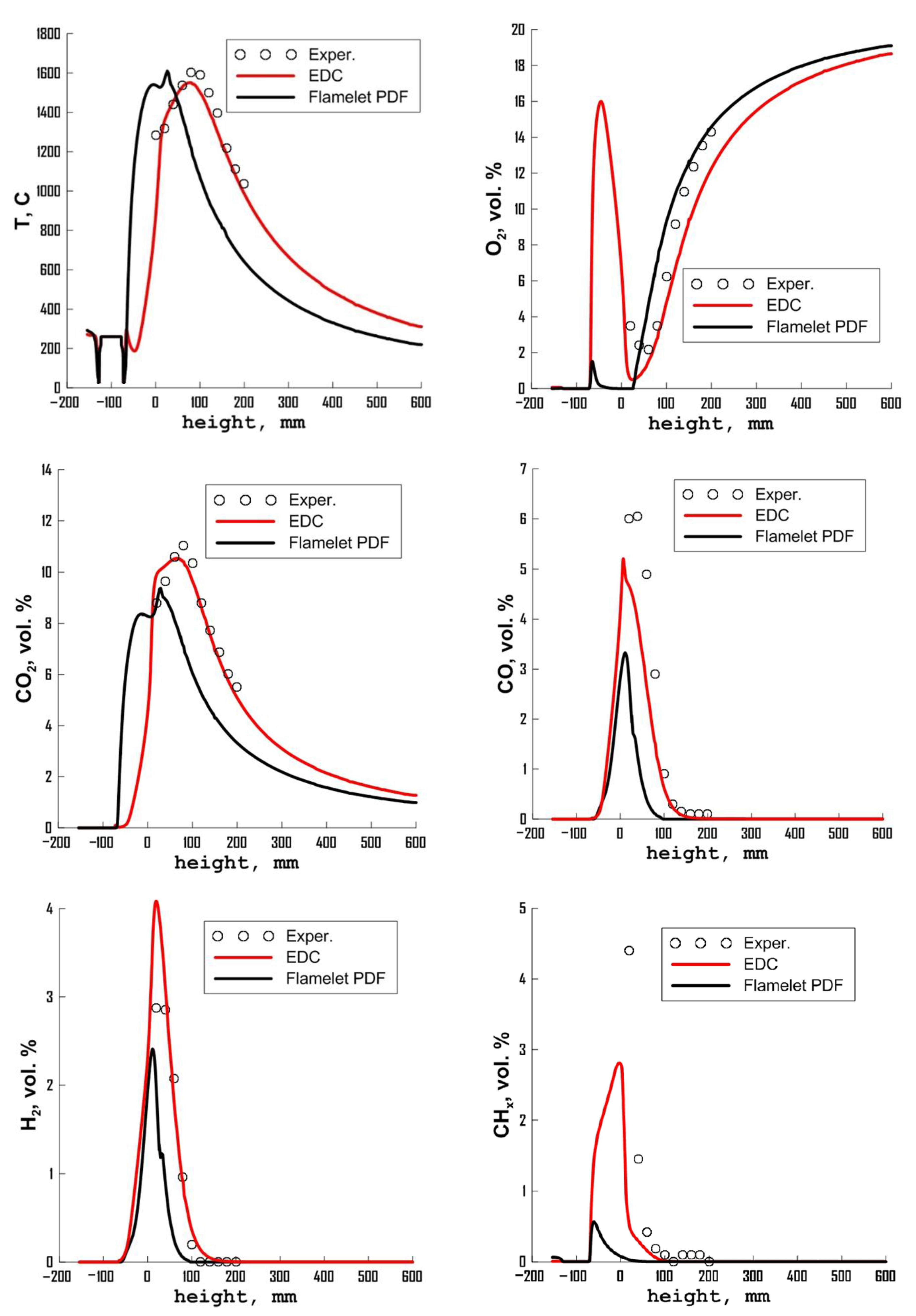
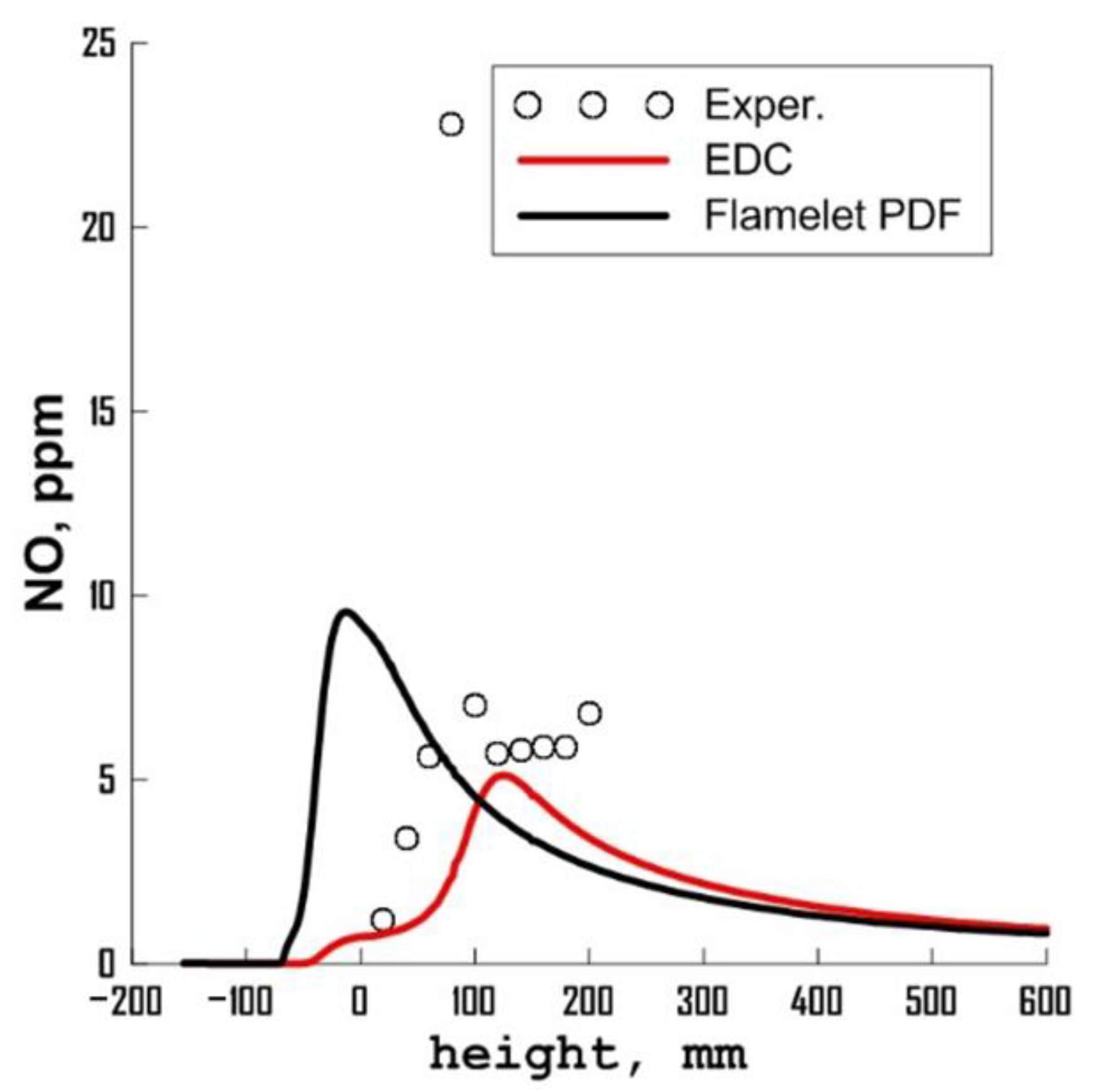
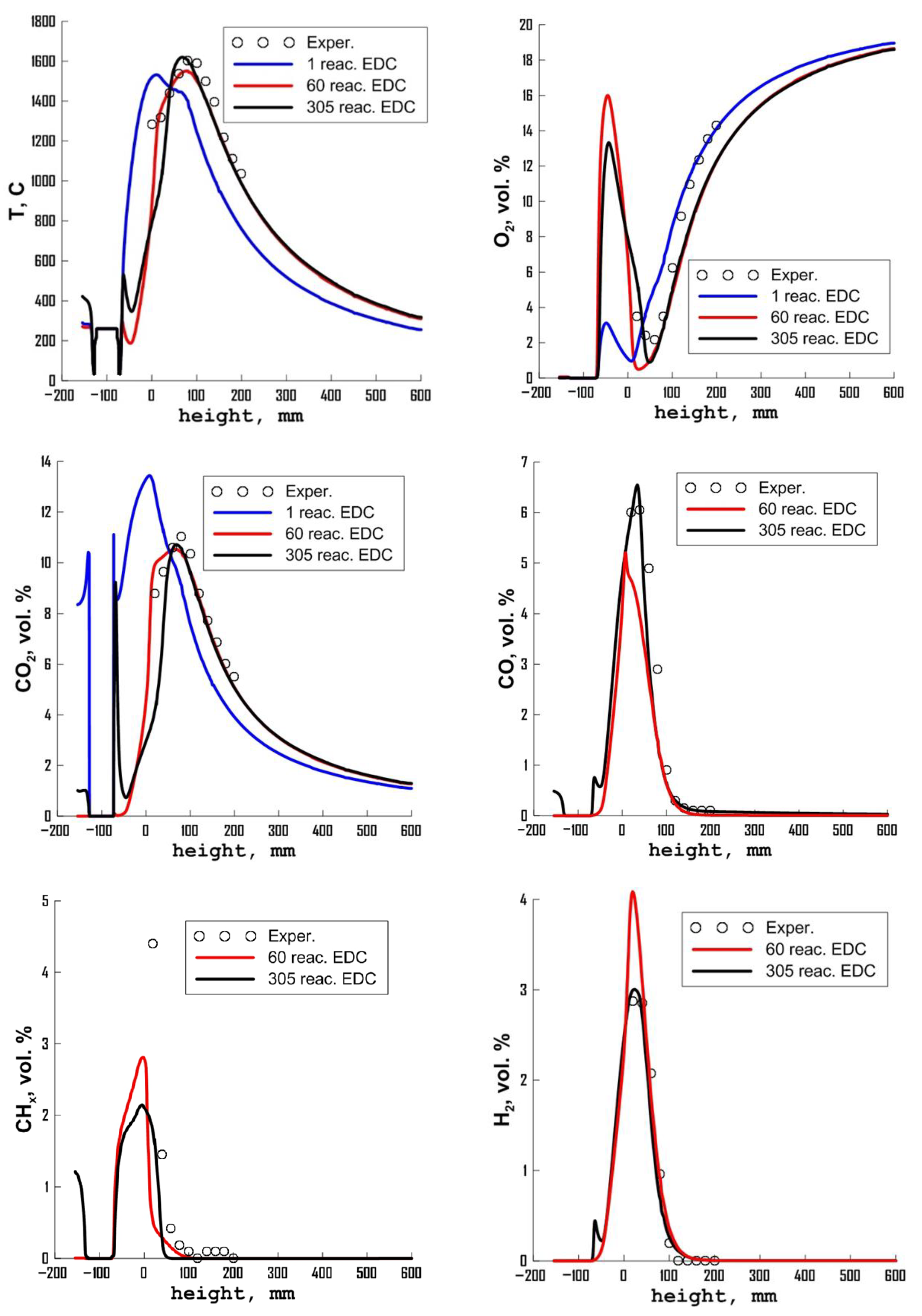
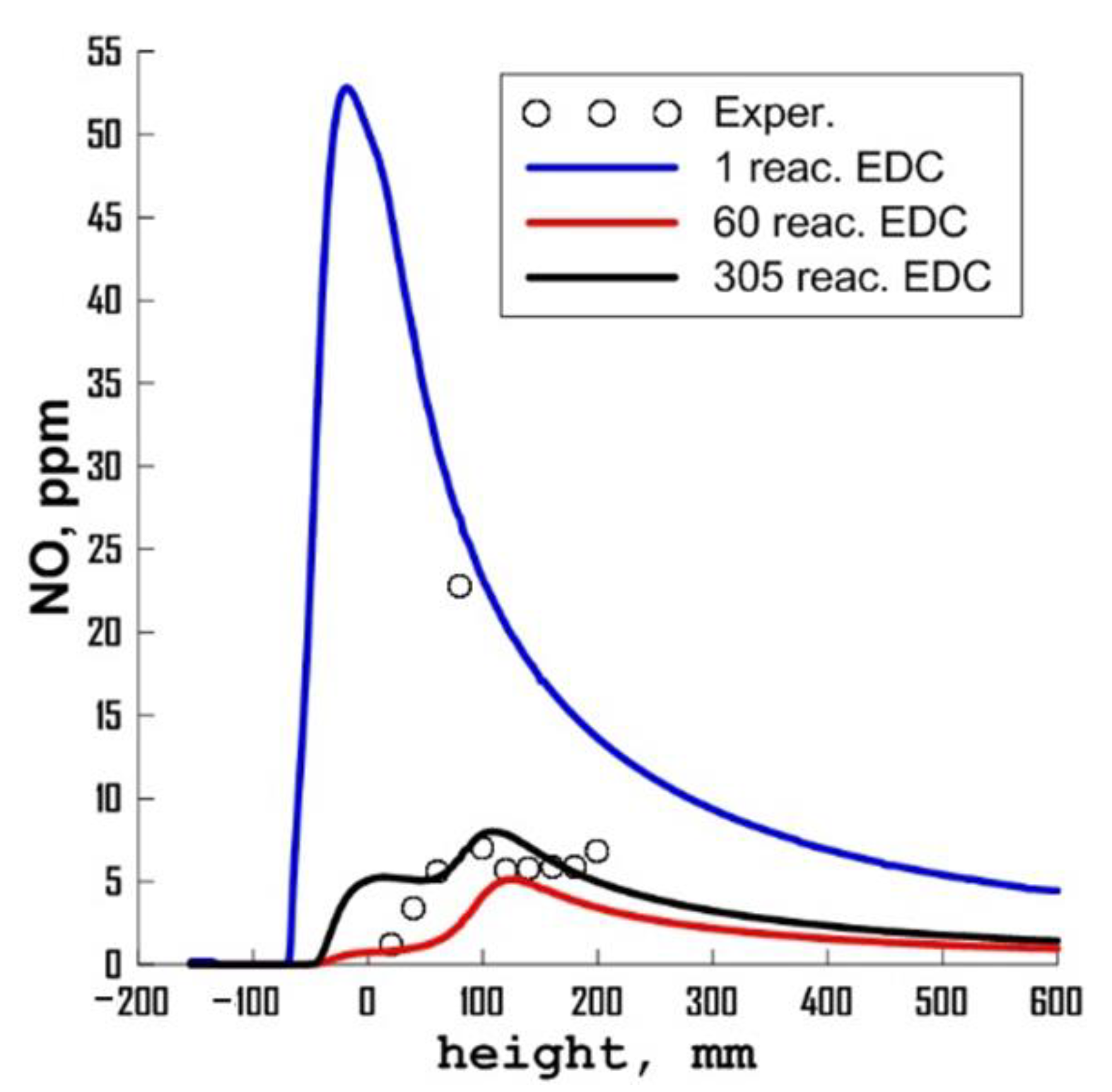
3.3. Influence of Chemical Kinetics Mechanisms
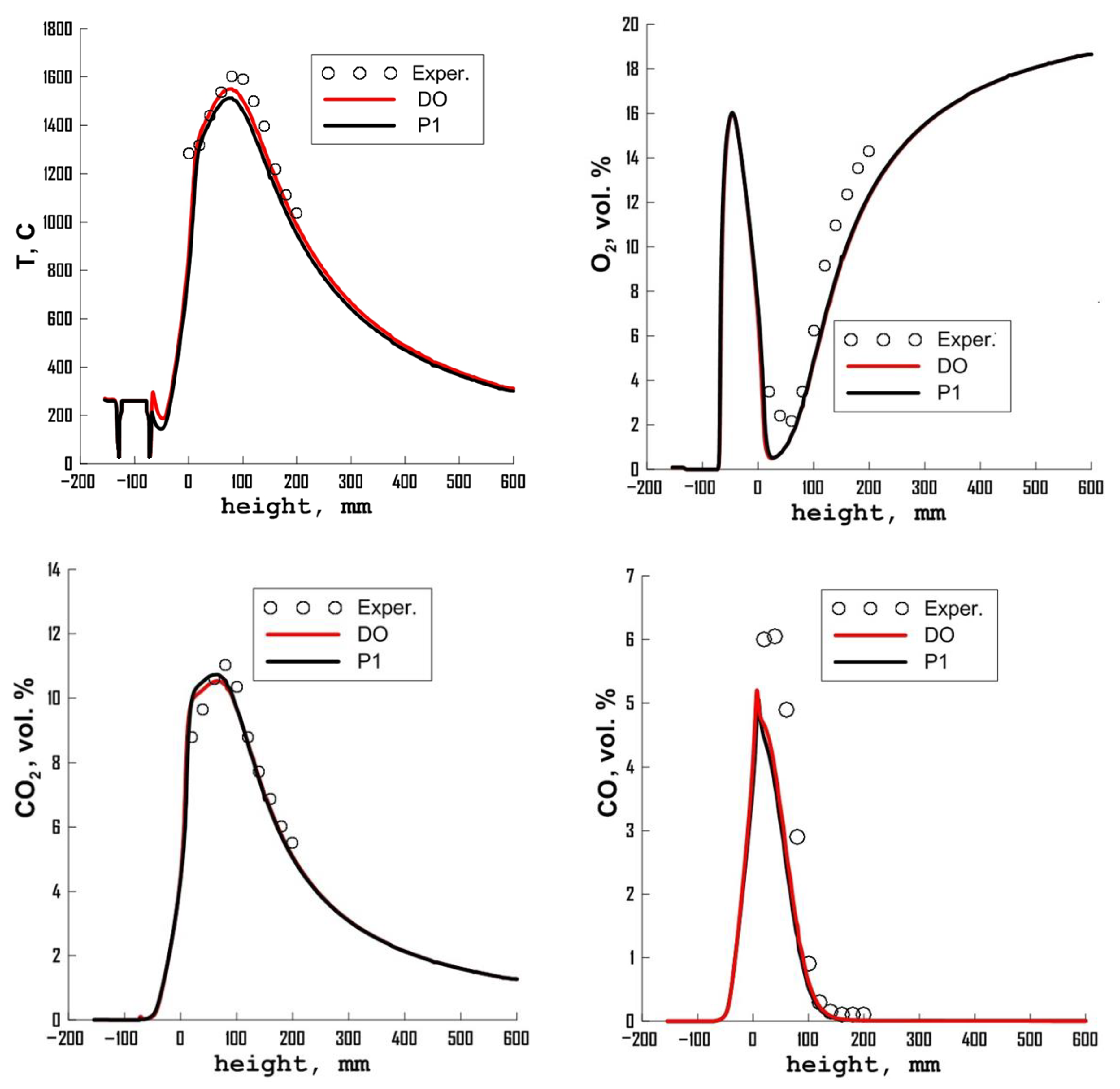
3.4. Influence of Radiation Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fratita, M.; Popescu, F.; Martins, J.; Brito, F.P.; Costa, T. Water Injection as a Way for Pollution Control. Energy Rep. 2021, 7, 543–549. [Google Scholar] [CrossRef]
- Alekseenko, S.; Anufriev, I.; Glavniy, V.; Krasinsky, D.; Rakhmanov, V.; Salomatov, V.; Shadrin, E. Study of 3D Flow Structure and Heat Transfer in a Vortex Furnace. Heat Transf. Res. 2016, 47, 653–667. [Google Scholar] [CrossRef]
- Amani, E.; Akbari, M.R.; Shahpouri, S. Multi-Objective CFD Optimizations of Water Spray Injection in Gas-Turbine Combustors. Fuel 2018, 227, 267–278. [Google Scholar] [CrossRef]
- Da Rocha, D.D.; de Castro Radicchi, F.; Lopes, G.S.; Brunocilla, M.F.; Gomes, P.C.F.; Santos, N.D.S.A.; Malaquias, A.C.T.; Rodrigues Filho, F.A.; Baêta, J.G.C. Study of the Water Injection Control Parameters on Combustion Performance of a Spark-Ignition Engine. Energy 2021, 217, 119346. [Google Scholar] [CrossRef]
- Pavri, R.; Moore, G. Gas Turbine Emissions and Control; GE Power Syst.: Atlanta, GA, USA, 2001. [Google Scholar]
- Gu, J.; Shao, Y.; Zhong, W. Study on Oxy-Fuel Combustion Behaviors in a S-CO2 CFB by 3D CFD Simulation. Chem. Eng. Sci. 2020, 211, 115262. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, T.; Xiao, D.; Xu, H.; Li, X.; Xu, M. Effect of Port Water Injection on the Characteristics of Combustion and Emissions in a Spark Ignition Direct Injection Engine. Fuel 2021, 283, 119271. [Google Scholar] [CrossRef]
- Sehat, A.; Ommi, F.; Saboohi, Z. Effects of Steam Addition and/or Injection on the Combustion Characteristics: A Review. Therm. Sci. 2021, 25, 1625–1652. [Google Scholar] [CrossRef] [Green Version]
- Li, S.C.; Williams, F.A. NOx Formation in Two-Stage Methane–Air Flames. Combust. Flame 1999, 118, 399–414. [Google Scholar] [CrossRef]
- Xue, R.; Hu, C.; Sethi, V.; Nikolaidis, T.; Pilidis, P. Effect of Steam Addition on Gas Turbine Combustor Design and Performance. Appl. Therm. Eng. 2016, 104, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, S.; Garnayak, S.; Lee, B.J.; Elbaz, A.M.; Roberts, W.L.; Dash, S.K.; Reddy, V.M. Numerical and Chemical Kinetic Analysis to Evaluate the Effect of Steam Dilution and Pressure on Combustion of N-Dodecane in a Swirling Flow Environment. Fuel 2021, 288, 119710. [Google Scholar] [CrossRef]
- Benini, E.; Pandolfo, S.; Zoppellari, S. Reduction of NO Emissions in a Turbojet Combustor by Direct Water/Steam Injection: Numerical and Experimental Assessment. Appl. Therm. Eng. 2009, 29, 3506–3510. [Google Scholar] [CrossRef] [Green Version]
- Alekseenko, S.; Anufriev, I.; Vigriyanov, M.; Kopyev, E.; Sadkin, I.; Sharypov, O. Burning of Heavy Fuel Oil in a Steam Jet in a New Burner. J. Appl. Mech. Tech. Phys. 2020, 61, 324–330. [Google Scholar] [CrossRef]
- Anufriev, I.; Kovyev, E.; Alekseenko, S.; Sharypov, O.; Butakov, E.; Vigriyanov, M.; Sadkin, I. Cleaner Crude Oil Combustion during Superheated Steam Atomization. Therm. Sci. 2021, 25, 331–345. [Google Scholar] [CrossRef]
- Anufriev, I.; Kopyev, E.; Sadkin, I.; Mukhina, M. Diesel and Waste Oil Combustion in a New Steam Burner with Low NOX Emission. Fuel 2021, 290, 120100. [Google Scholar] [CrossRef]
- Anufriev, I.; Kopyev, E. Diesel Fuel Combustion by Spraying in a Superheated Steam Jet. Fuel Process. Technol. 2019, 192, 154–169. [Google Scholar] [CrossRef]
- Anufriev, I.; Alekseenko, S.; Sharypov, O.; Kopyev, E. Diesel Fuel Combustion in a Direct-Flow Evaporative Burner with Superheated Steam Supply. Fuel 2019, 254, 115723. [Google Scholar] [CrossRef]
- Anufriev, I.; Kopyev, E.; Sadkin, I.; Mukhina, M. NOx Reduction by Steam Injection Method during Liquid Fuel and Waste Burning. Process Saf. Environ. Prot. 2021, 152, 240–248. [Google Scholar] [CrossRef]
- Alekseenko, S.; Pashchenko, S.; Salomatov, V. Nanocluster Initiation of Combustion of Off-Grade Hydrocarbon Fuels. J. Eng. Phys. Thermophys. 2010, 83, 729–741. [Google Scholar] [CrossRef]
- Kopyev, E.; Anufriev, I.; Mukhina, M.; Sadkin, I. Combustion of Kerosene Sprayed with a Jet of Superheated Steam. J. Phys. Conf. Ser. 2021, 2119, 012040. [Google Scholar] [CrossRef]
- Alekseenko, S.; Kuznetsov, V.; Mal’tsev, L.; Dekterev, A.; Chernetskii, M. Analysis of Combustion of Coal-Water Fuel in Low-Power Hot-Water Boiler via Numerical Modeling and Experiments. J. Eng. Thermophys. 2019, 28, 177–189. [Google Scholar] [CrossRef]
- Lobasov, A.; Dekterev, A.; Minakov, A. Numerical Simulation of Premixed Methane/Air and Synthesis Gas/Air Flames for Turbulence Swirling Jet. J. Phys. Conf. Ser. 2019, 1382, 012060. [Google Scholar] [CrossRef]
- Dekterev, A.; Kuznetsov, V. Investigation of Pulverized Coal Flaring Based on Eddy-Resolving Turbulence Models. J. Phys. Conf. Ser. 2020, 1677, 012104. [Google Scholar] [CrossRef]
- Dulin, V.; Markovich, D.; Minakov, A.; Hanjalic, K.; Chikishev, L. Experimental and Numerical Simulation for Swirl Flow in a Combustor. Therm. Eng. 2013, 60, 990–997. [Google Scholar] [CrossRef]
- Nooren, P.A.; Wouters, H.A.; Peeters, T.W.J.; Roekaerts, D.; Maas, U.; Schmidt, D. Monte Carlo PDF Modelling of a Turbulent Natural-Gas Diffusion Flame. Combust. Theory Model. 2010, 1, 79–96. [Google Scholar] [CrossRef]
- Celis, C.; Figueira Da Silva, L.F. Lagrangian Mixing Models for Turbulent Combustion: Review and Prospects. Flow, Turbul. Combust. 2015 943 2015, 94, 643–689. [Google Scholar] [CrossRef]
- Lysenko, D.A.; Ertesvåg, I.S.; Kjell, R.E.; Rian, E.; Lysenko, D.A.; Ertesvåg, I.S.; Rian, K.E. Numerical Simulations of the Sandia Flame D Using the Eddy Dissipation Concept. Flow Turbul. Combust. 2014, 93, 665–687. [Google Scholar] [CrossRef]
- Trisjono, P.; Kleinheinz, K.; Pitsch, H.; Kang, S. Large Eddy Simulation of Stratified and Sheared Flames of a Premixed Turbulent Stratified Flame Burner Using a Flamelet Model with Heat Loss. Flow Turbul. Combust. 2013, 92, 201–235. [Google Scholar] [CrossRef]
- Wang, G.; Boileau, M.; Veynante, D. Implementation of a Dynamic Thickened Flame Model for Large Eddy Simulations of Turbulent Premixed Combustion. Combust. Flame 2011, 158, 2199–2213. [Google Scholar] [CrossRef]
- Schmitt, T.; Sadiki, A.; Fiorina, B.; Veynante, D. Impact of Dynamic Wrinkling Model on the Prediction Accuracy Using the F-TACLES Combustion Model in Swirling Premixed Turbulent Flames. Proc. Combust. Inst. 2013, 34, 1261–1268. [Google Scholar] [CrossRef]
- Kuenne, G.; Seffrin, F.; Fuest, F.; Stahler, T.; Ketelheun, A.; Geyer, D.; Janicka, J.; Dreizler, A. Experimental and Numerical Analysis of a Lean Premixed Stratified Burner Using 1D Raman/Rayleigh Scattering and Large Eddy Simulation. Combust. Flame 2012, 159, 2669–2689. [Google Scholar] [CrossRef]
- Mercier, R.; Auzillon, P.; Moureau, V.; Darabiha, N.; Gicquel, O.; Veynante, D.; Fiorina, B. LES Modeling of the Impact of Heat Losses and Differential Diffusion on Turbulent Stratified Flame Propagation: Application to the TU Darmstadt Stratified Flame. Flow Turbul. Combust. 2014, 93, 349–381. [Google Scholar] [CrossRef]
- Sabelnikov, V.; Fureby, C. Extended LES-PaSR Model for Simulation of Turbulent Combustion. Prog. Propuls. Phys. 2013, 4, 539–568. [Google Scholar] [CrossRef] [Green Version]
- Moureau, V.; Domingo, P.; Vervisch, L. Design of a Massively Parallel CFD Code for Complex Geometries. Comptes Rendus Mécanique 2011, 339, 141–148. [Google Scholar] [CrossRef]
- Ge, H.W.; Duwel, I.; Kronemayer, H.; Dibble, R.W.; Gutheil, E.; Schulz, C.; Wolfrum, J. Laser-Based Experimental and Monte Carlo PDF Numerical Investigation of an Ethanol/Air Spray Flame. Combust. Sci. Technol. 2008, 180, 1529–1547. [Google Scholar] [CrossRef]
- Peters, N. Laminar Diffusion Flamelet Models in Non-Premixed Turbulent Combustion. Prog. Energy Combust. Sci. 1984, 10, 319–339. [Google Scholar] [CrossRef]
- Lemaire, R.; Faccinetto, A.; Therssen, E.; Ziskind, M.; Focsa, C.; Desgroux, P. Experimental Comparison of Soot Formation in Turbulent Flames of Diesel and Surrogate Diesel Fuels. Proc. Combust. Inst. 2009, 32, 737–744. [Google Scholar] [CrossRef]
- Zhang, K.; Xin, Q.; Mu, Z.; Niu, Z.; Wang, Z. Numerical Simulation of Diesel Combustion Based on N-Heptane and Toluene. Propuls. Power Res. 2019, 8, 121–127. [Google Scholar] [CrossRef]
- Pitz, W.J.; Mueller, C.J. Recent Progress in the Development of Diesel Surrogate Fuels. Prog. Energy Combust. Sci. 2011, 37, 330–350. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Ju, D.; Han, D. Numerical Study on the Physical and Chemical Processes in N-Decane Spray Ignition. Chem. Eng. Sci. 2021, 241, 116716. [Google Scholar] [CrossRef]
- Kolaitis, D.I.; Founti, M.A. On the Assumption of Using N-Heptane as a “Surrogate Fuel” for the Description of the Cool Flame Oxidation of Diesel Oil. Proc. Combust. Inst. 2009, 32, 3197–3205. [Google Scholar] [CrossRef]
- Minakov, A.; Anufriev, I.; Kuznetsov, V.; Dekterev, A.; Kopyev, E.; Sharypov, O. Combustion of Liquid Hydrocarbon Fuel in an Evaporative Burner with Forced Supply of Superheated Steam and Air to the Reaction Zone. Fuel 2022, 309, 122181. [Google Scholar] [CrossRef]
- Alekseenko, S.; Anufriev, I.; Vigriyanov, M.; Kopyev, E.; Sharypov, O. Characteristics of Diesel Fuel Combustion in a Burner with Injection of a Superheated Steam Jet. Combust. Explos. Shock Waves 2016, 52, 286–293. [Google Scholar] [CrossRef]
- Alekseenko, S.; Anufriev, I.; Arsentyev, S.; Vigriyanov, M.; Kopyev, E.; Sharypov, O. Influence of Parameters of Superheated Steam on Combustion of Liquid Hydrocarbons. Thermophys. Aeromech. 2019, 26, 103–107. [Google Scholar] [CrossRef]
- Minakov, A.; Kuznetsov, V.; Anufriev, I.; Kopyev, E. Numerical Analysis of a Pre-Chamber Vortex Burner with a Steam Blast Atomizer. Fuel 2022, 323, 124375. [Google Scholar] [CrossRef]
- Launder, B.E.; Spalding, D.B. Lectures in Mathematical Models of Turbulence; Academic Press: London, UK, 1972. [Google Scholar]
- Smirnov, P.E.; Menter, F.R. Sensitization of the SST Turbulence Model to Rotation and Curvature by Applying the Spalart-Shur Correction Term. J. Turbomach. 2009, 131, 1–8. [Google Scholar] [CrossRef]
- Menter, F. Zonal Two Equation K-w Turbulence Models For Aerodynamic Flows. In Proceedings of the 23rd Fluid Dynamics, Plasmadynamics, and Lasers Conference; American Institute of Aeronautics and Astronautics: Reston, Virigina, Orlando, FL, USA, 6–9 July 1993; p. 2906. [Google Scholar]
- Daly, B.J.; Harlow, F.H. Transport Equations in Turbulence. Phys. Fluids 2003, 13, 2634. [Google Scholar] [CrossRef]
- Launder, B.E.; Reece, G.J.; Rodi, W. Progress in the Development of a Reynolds-Stress Turbulence Closure. J. Fluid Mech. 1975, 68, 537–566. [Google Scholar] [CrossRef] [Green Version]
- Spalding, D.B. Mixing and Chemical Reaction in Steady Confined Turbulent Flames. Symp. Combust. 1971, 13, 649–657. [Google Scholar] [CrossRef]
- Magnussen, B.F.; Hjertager, B.H. On Mathematical Modeling of Turbulent Combustion with Special Emphasis on Soot Formation and Combustion. Symp. Combust. 1977, 16, 719–729. [Google Scholar] [CrossRef]
- Magnussen, B. On the Structure of Turbulence and a Generalized Eddy Dissipation Concept for Chemical Reaction in Turbulent Flow. In Proceedings of the 19th Aerospace Sciences Meeting; American Institute of Aeronautics and Astronautics, Reston, VA, USA, 12 January 1981. [Google Scholar] [CrossRef]
- Raithby, G.D.; Chui, E.H. A Finite-Volume Method for Predicting a Radiant Heat Transfer in Enclosures With Participating Media. J. Heat Transfer 1990, 112, 415–423. [Google Scholar] [CrossRef]
- Cheng, P. Two-Dimensional Radiating Gas Flow by a Moment Method. AIAA J. 1964, 2, 1662–1664. [Google Scholar] [CrossRef]
- Howell, J.R.; Menguc, M.P.; Siegel, R. Thermal Radiation Heat Transfer. Therm. Radiat. Heat Transf. 2010, 93, 17522. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Kong, S.C.; Reitz, R.D. Development and Validation of a Reduced Reaction Mechanism for HCCI Engine Simulations. In Proceedings of the SAE 2004 World Congress & Exhibition, Detroit, MI, USA, 8–11 March 2004. [Google Scholar] [CrossRef]
- UC San Diego Website. Chemical-Kinetic Mechanisms for Combustion Applications: Heptane Chemistry. Available online: https://web.eng.ucsd.edu/mae/groups/combustion/mechanism.html (accessed on 17 December 2022).
- Hiroyasu, H.; Kadota, T. Models for Combustion and Formation of Nitric Oxide and Soot in Direct Injection Diesel Engines. In Proceedings of the 1976 Automotive Engineering Congress and Exposition, Detroit, MI, USA, 23–27 February 1976; p. 760129. [Google Scholar] [CrossRef]
- Nagle, J.; Strickland-Constable, R.F. Oxidation Of Carbon between 1000–2000 °C. In Proceedings of the Fifth Conference on Carbon; Macmillan: New York, NY, USA, 1962; pp. 154–164. [Google Scholar] [CrossRef]
- Zeldovich, Y.A.; Frank-Kamenetskii, D.; Sadovnikov, P. Oxidation of Nitrogen in Combustion; Publishing House of the Acad of Sciences of USSR: Moscow, Russia, 1947. [Google Scholar]
- De Soete, G.G. Overall Reaction Rates of NO and N2 Formation from Fuel Nitrogen. Symp. Combust. 1975, 15, 1093–1102. [Google Scholar] [CrossRef]


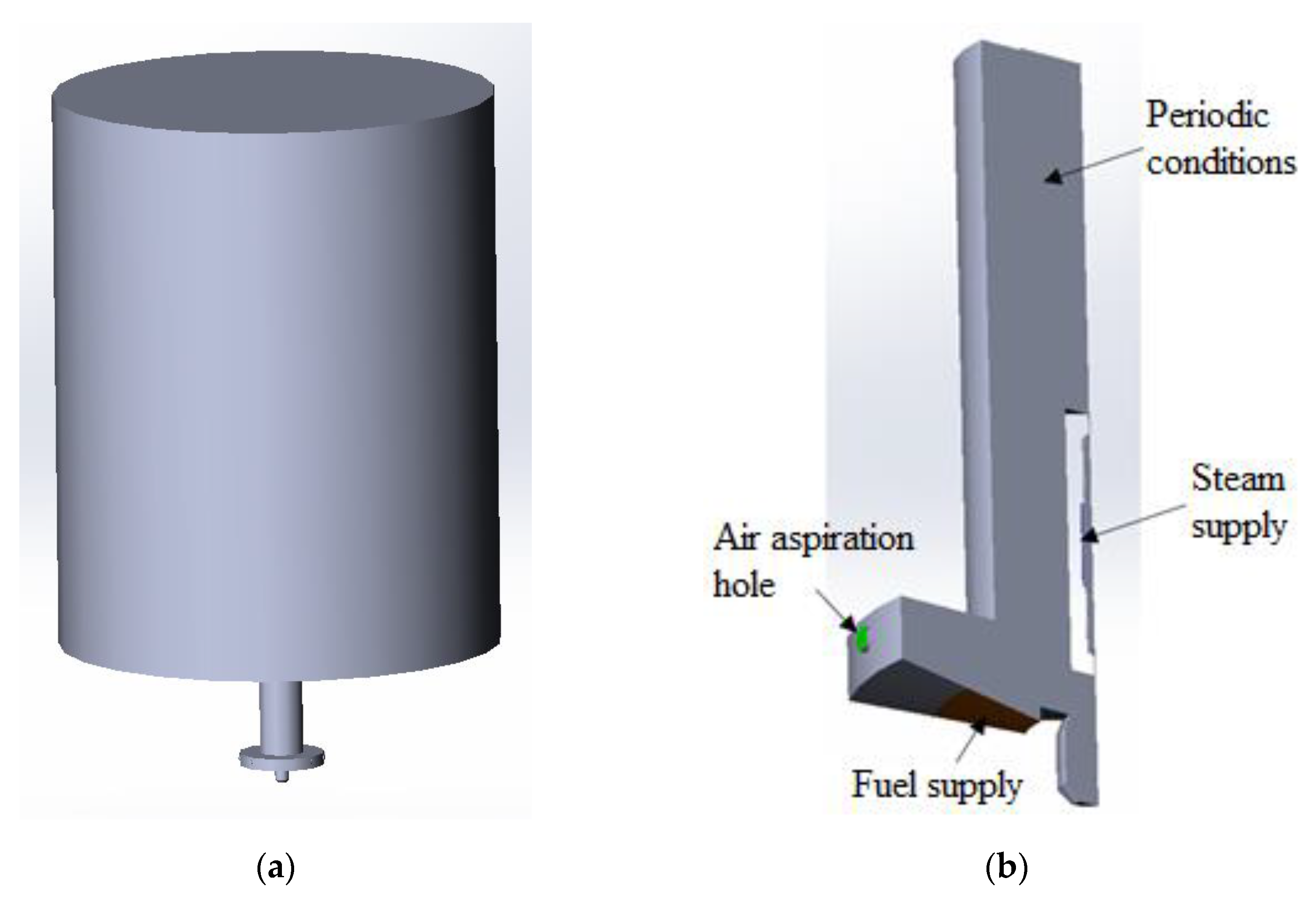
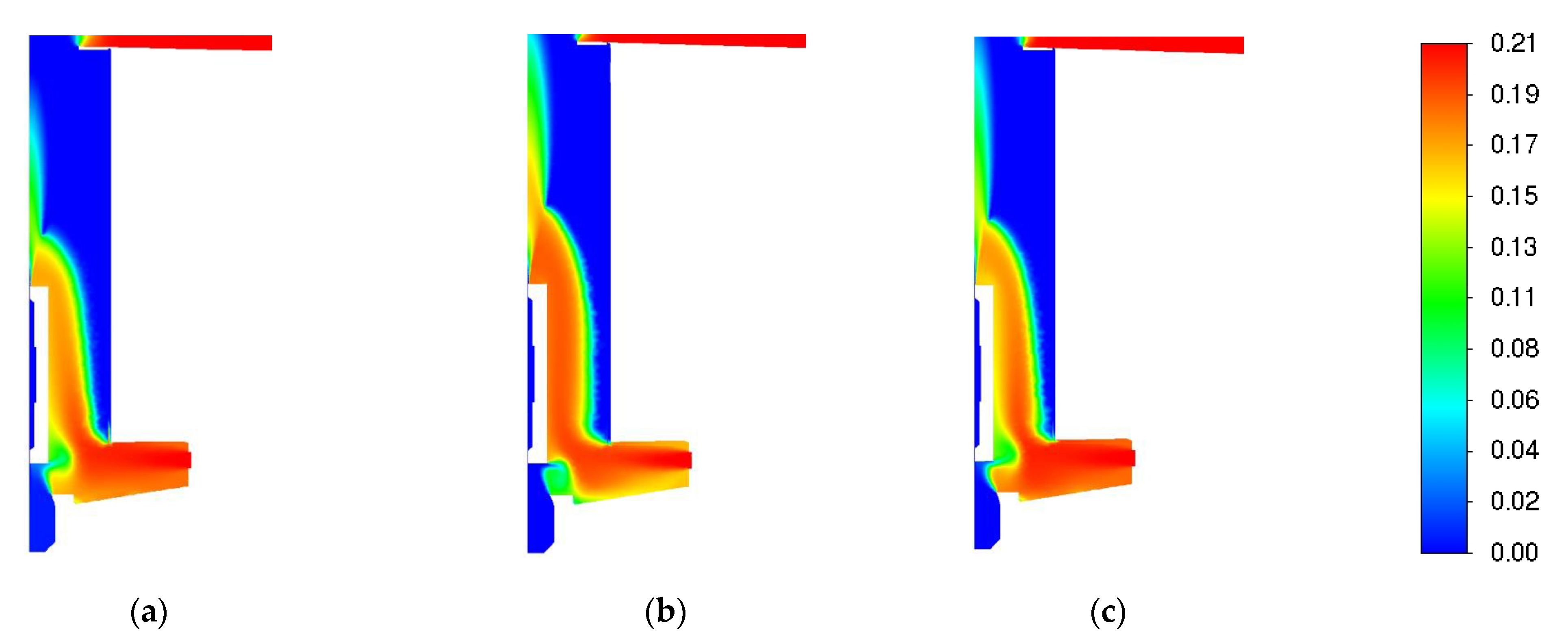
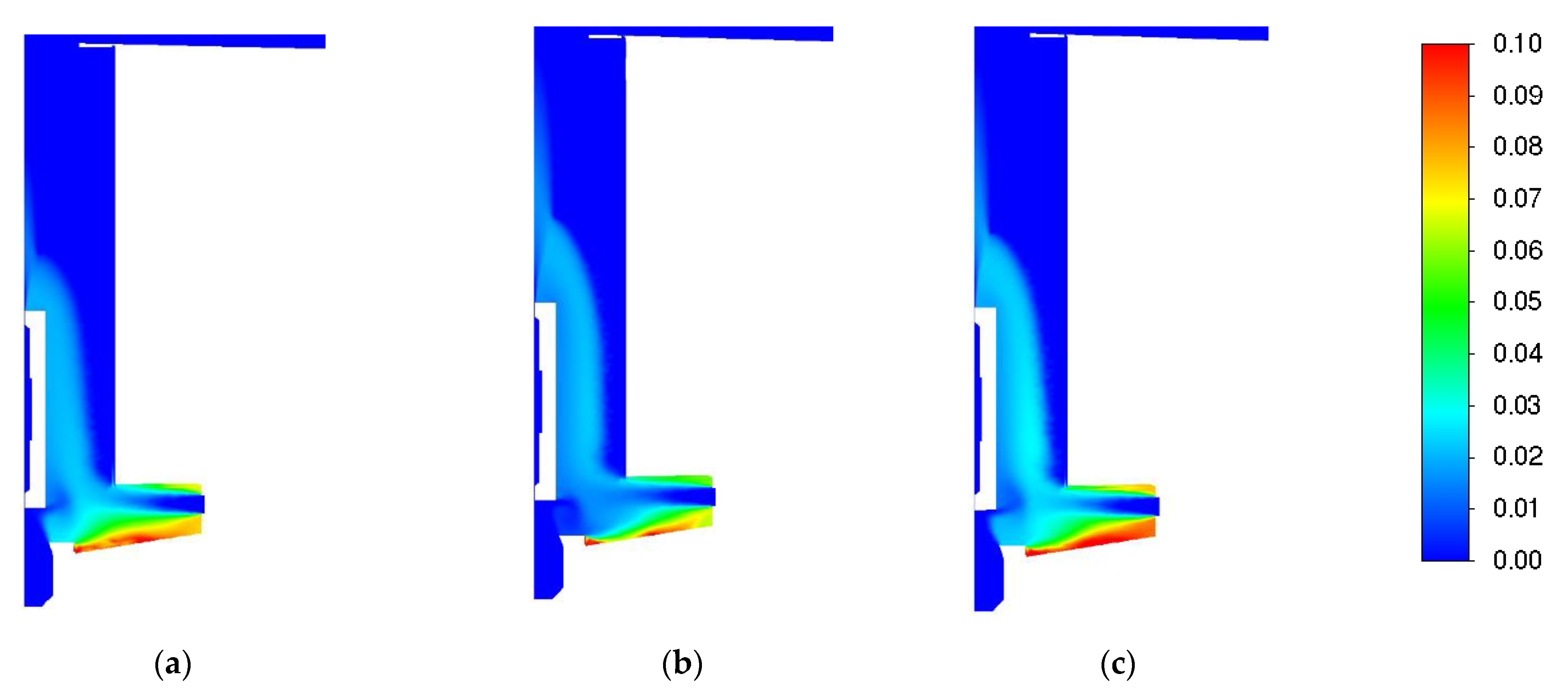
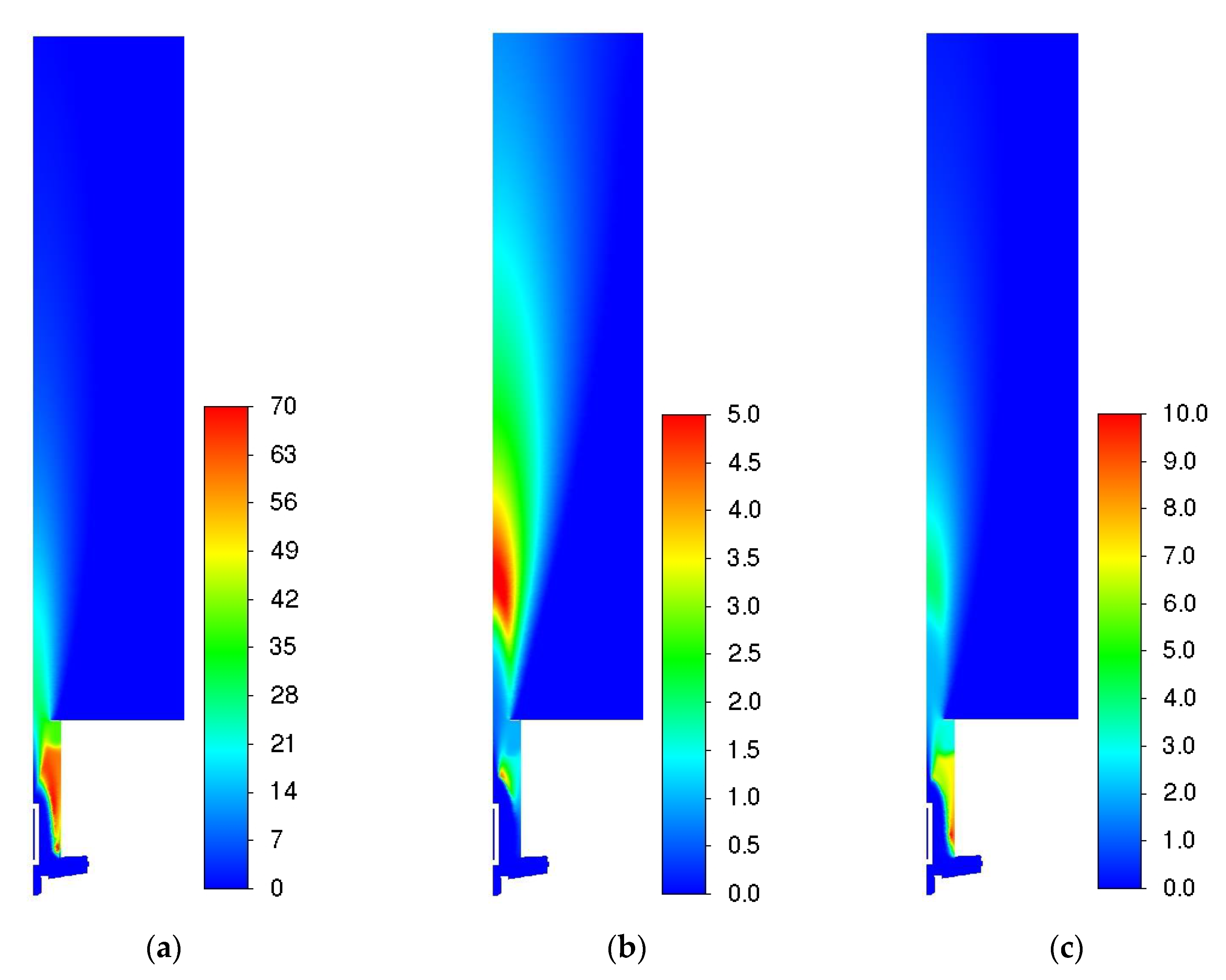

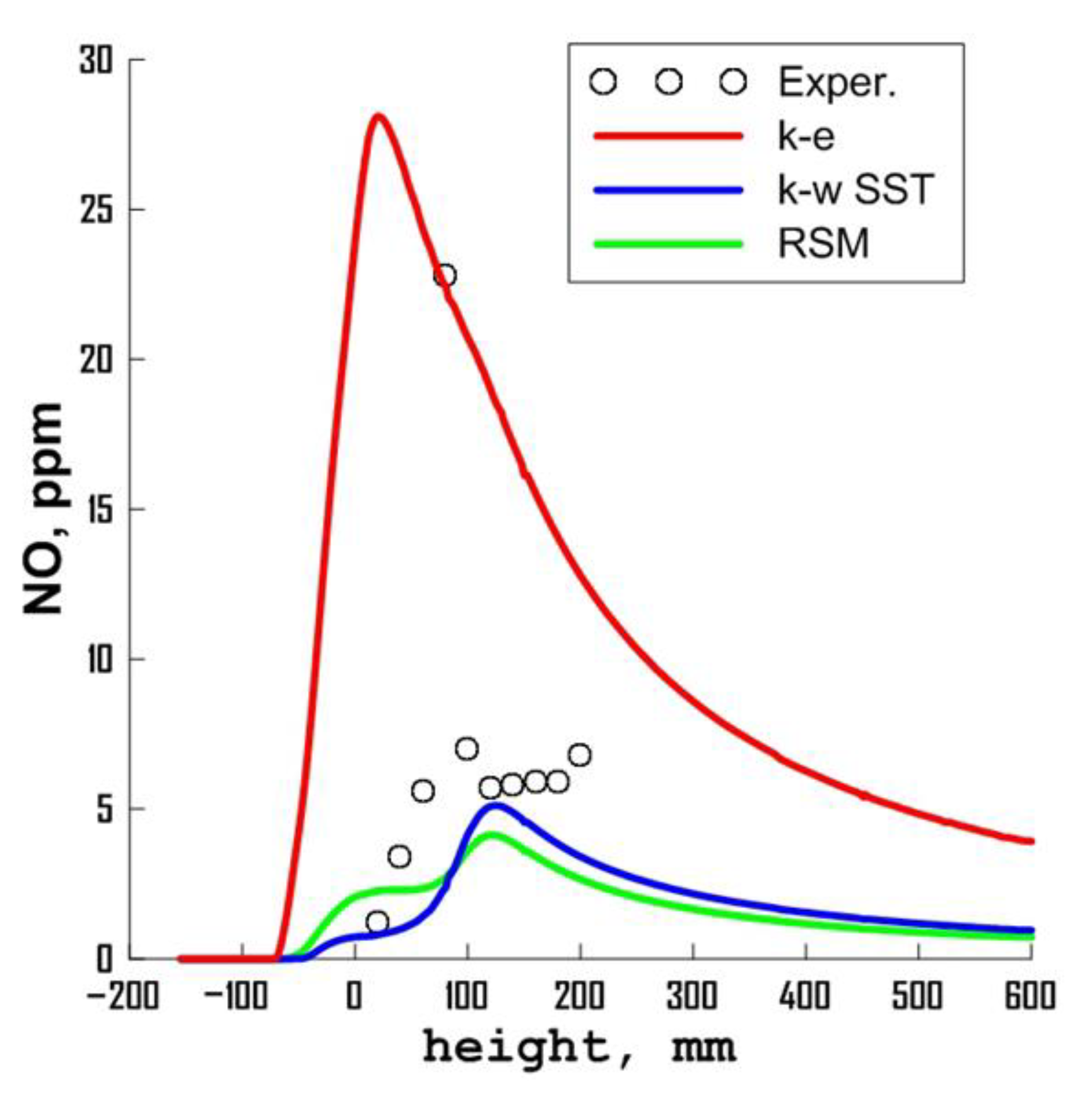
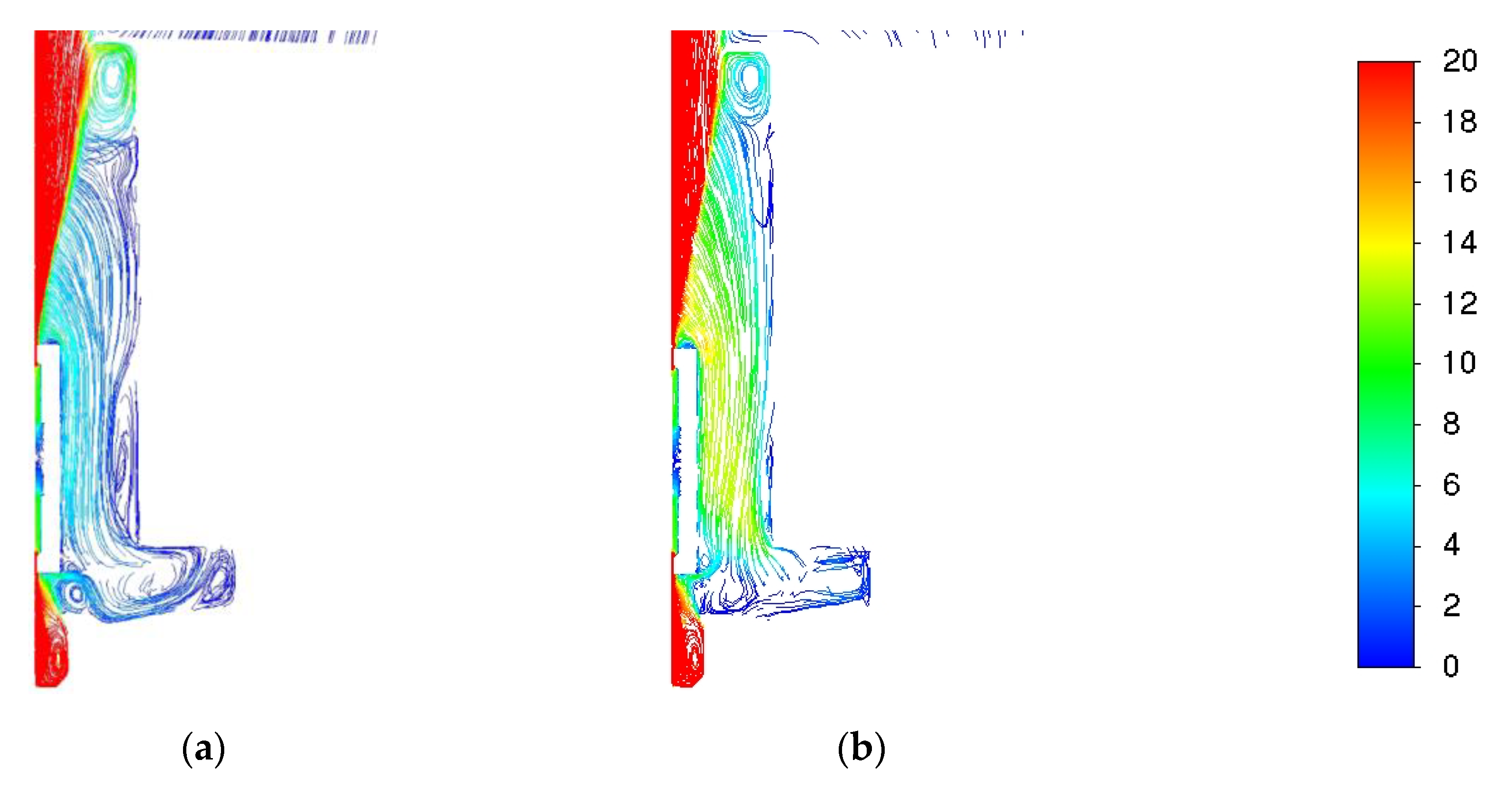
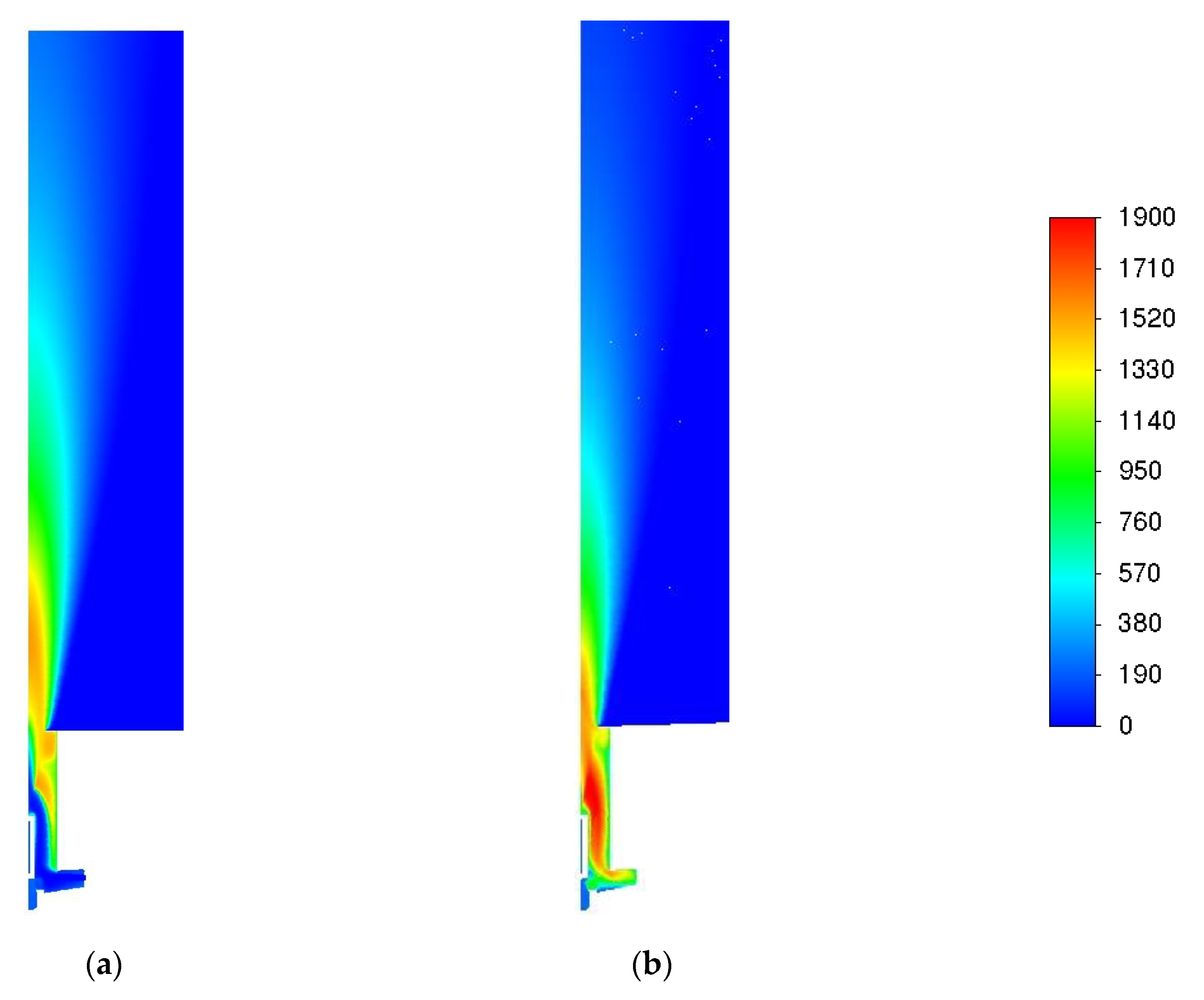
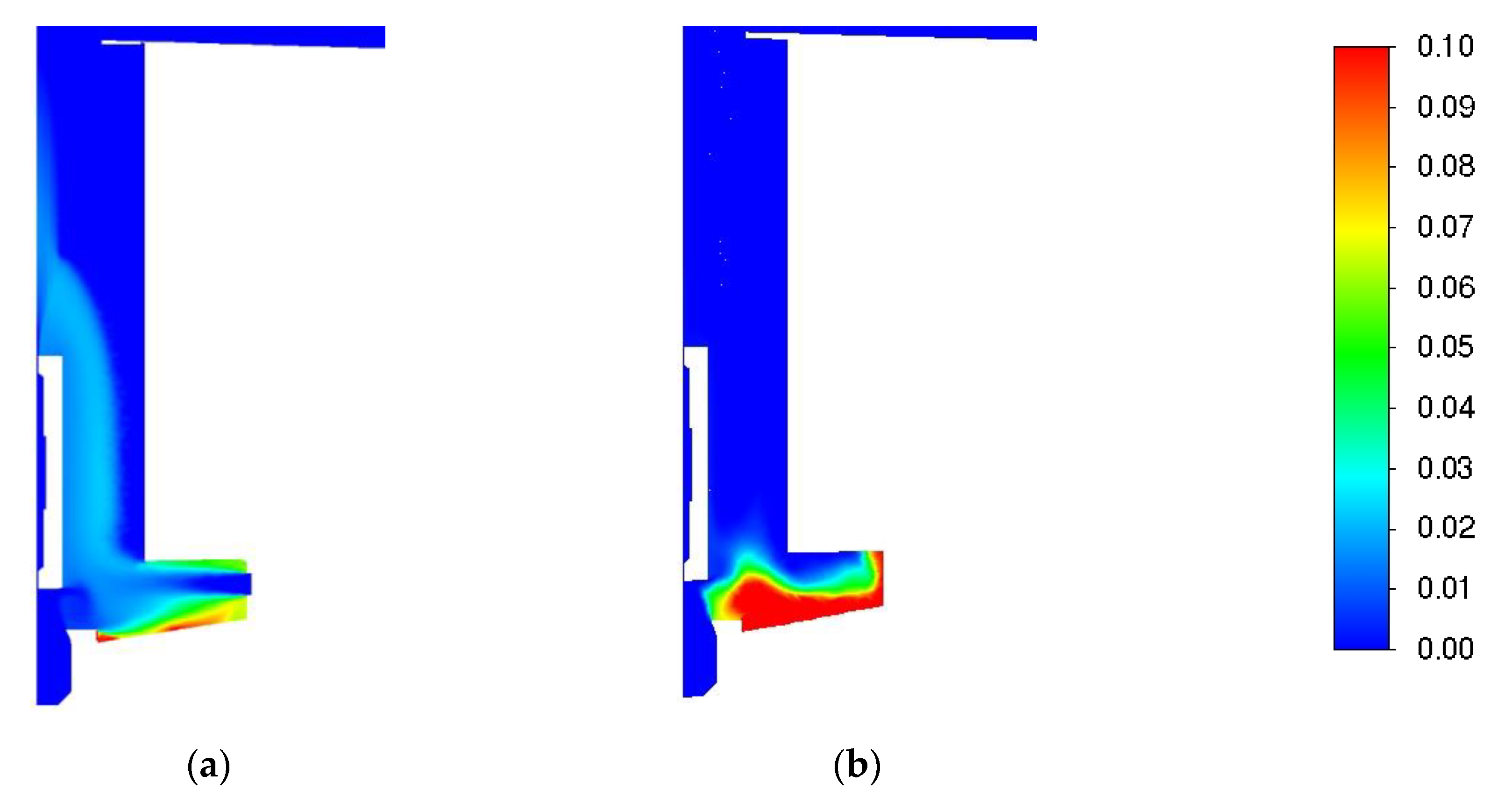
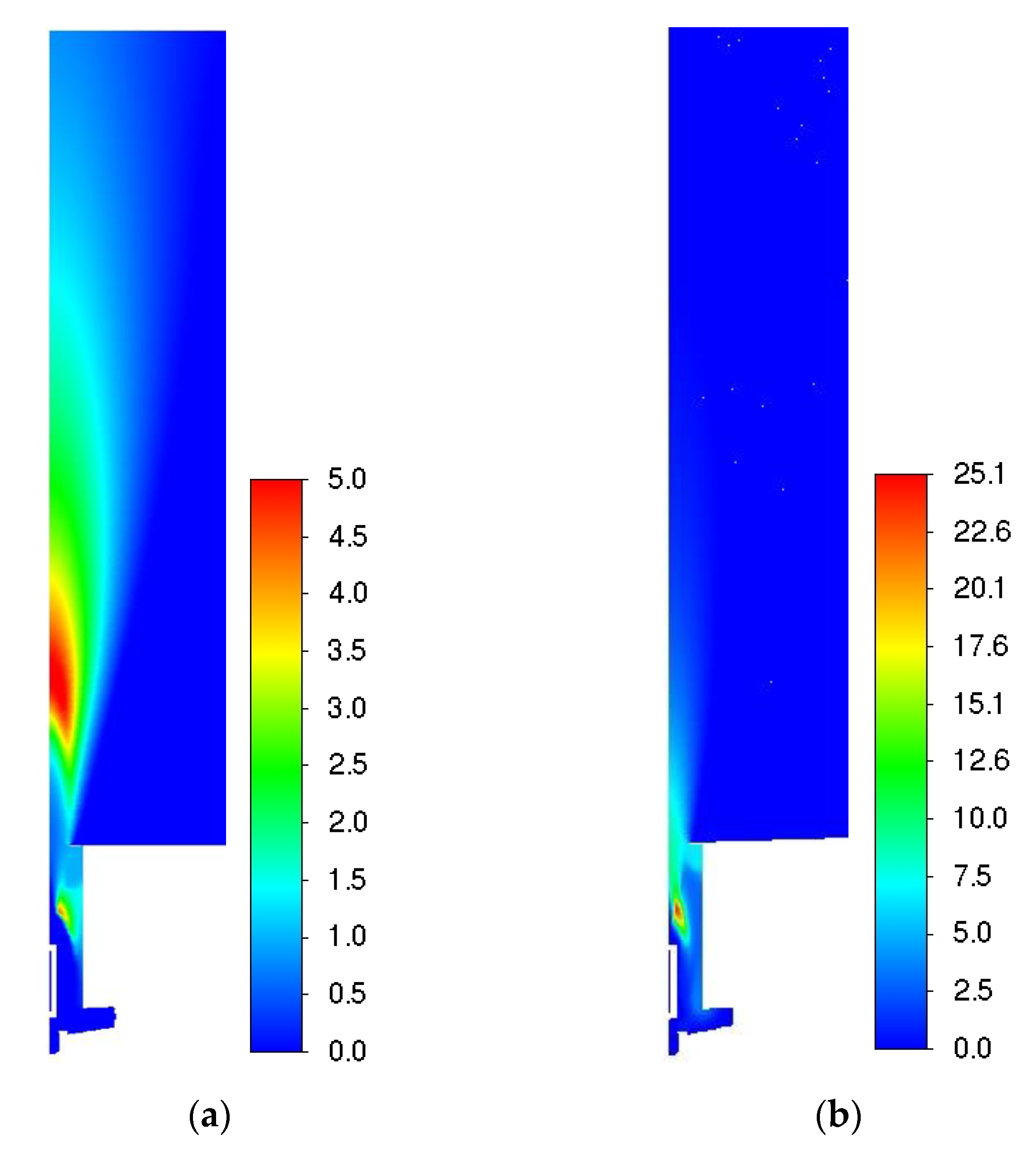

| Parameter | Value |
|---|---|
| Density (kg/m3) | 684 |
| Viscosity (cSt) | 0.6 |
| Low heat value (MJ/kg) | 48.6 |
| High heat value qf (MJ/kg) | 45.0 |
| C (% w/w) | 84 |
| H (% w/w) | 16 |
| Reaction | A [kg/(m2sPa)] | E, J/kmol | β | Components Degree γ |
|---|---|---|---|---|
| nC7H16+11O2 = 7CO2+8H2O | 2.86∙109 | 1.256∙108 | 0 | γCH4 = 0.25, γO2 = 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minakov, A.V.; Kuznetsov, V.A.; Dekterev, A.A.; Anufriev, I.S.; Kopyev, E.P.; Alekseenko, S.V. Comparative Analysis of Numerical Methods for Simulating N-Heptane Combustion with Steam Additive. Energies 2023, 16, 25. https://doi.org/10.3390/en16010025
Minakov AV, Kuznetsov VA, Dekterev AA, Anufriev IS, Kopyev EP, Alekseenko SV. Comparative Analysis of Numerical Methods for Simulating N-Heptane Combustion with Steam Additive. Energies. 2023; 16(1):25. https://doi.org/10.3390/en16010025
Chicago/Turabian StyleMinakov, Andrey V., Viktor A. Kuznetsov, Artem A. Dekterev, Igor S. Anufriev, Evgeny P. Kopyev, and Sergey V. Alekseenko. 2023. "Comparative Analysis of Numerical Methods for Simulating N-Heptane Combustion with Steam Additive" Energies 16, no. 1: 25. https://doi.org/10.3390/en16010025
APA StyleMinakov, A. V., Kuznetsov, V. A., Dekterev, A. A., Anufriev, I. S., Kopyev, E. P., & Alekseenko, S. V. (2023). Comparative Analysis of Numerical Methods for Simulating N-Heptane Combustion with Steam Additive. Energies, 16(1), 25. https://doi.org/10.3390/en16010025






