Hydrogen Production from Catalytic Pyrolysis of Phenol as Tar Model Compound in Magnetic Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Analysis
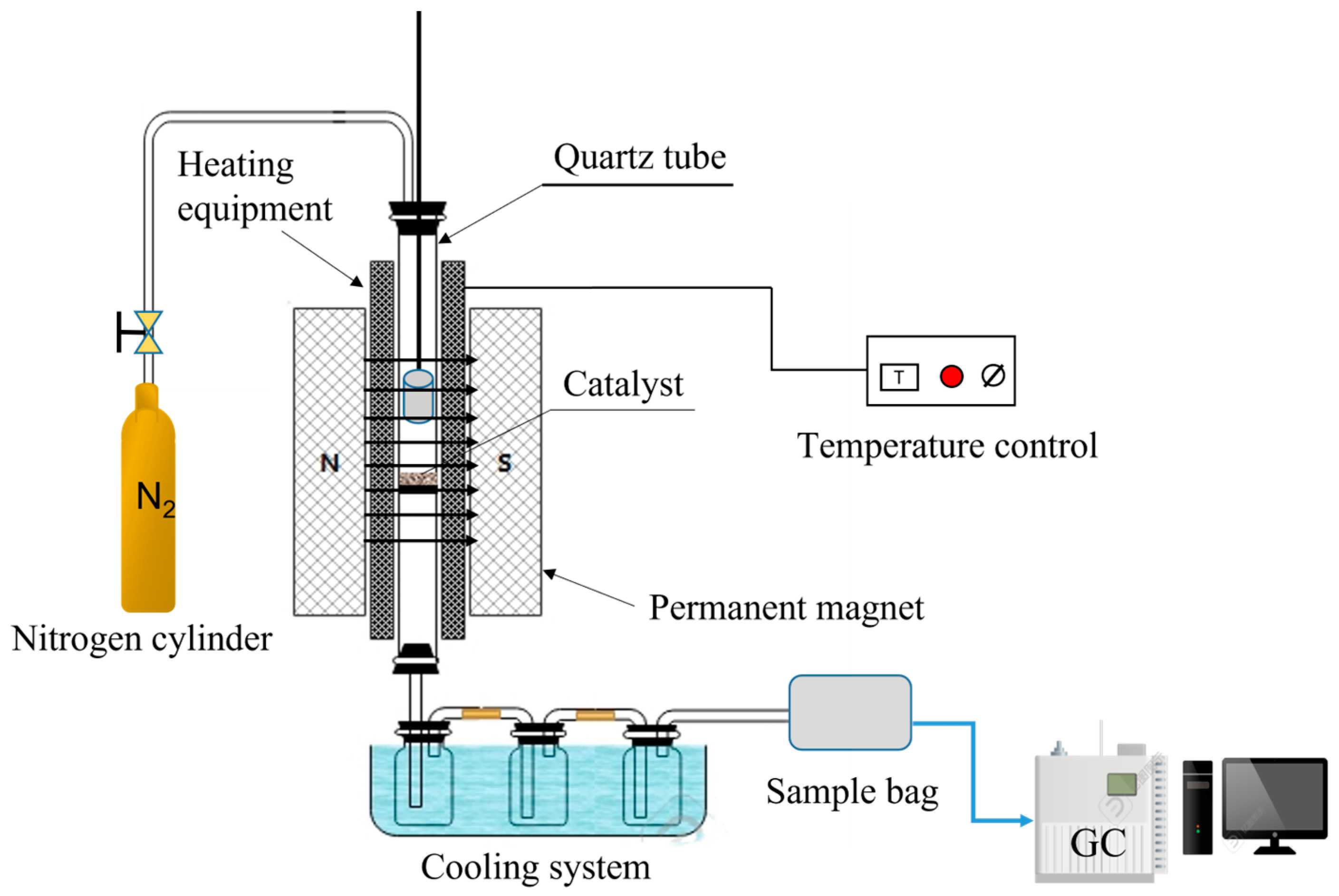
2.3. Experimental Apparatus and Procedures
2.4. Dynamical Methods
3. Result and Discussion
3.1. Catalyst Characterization
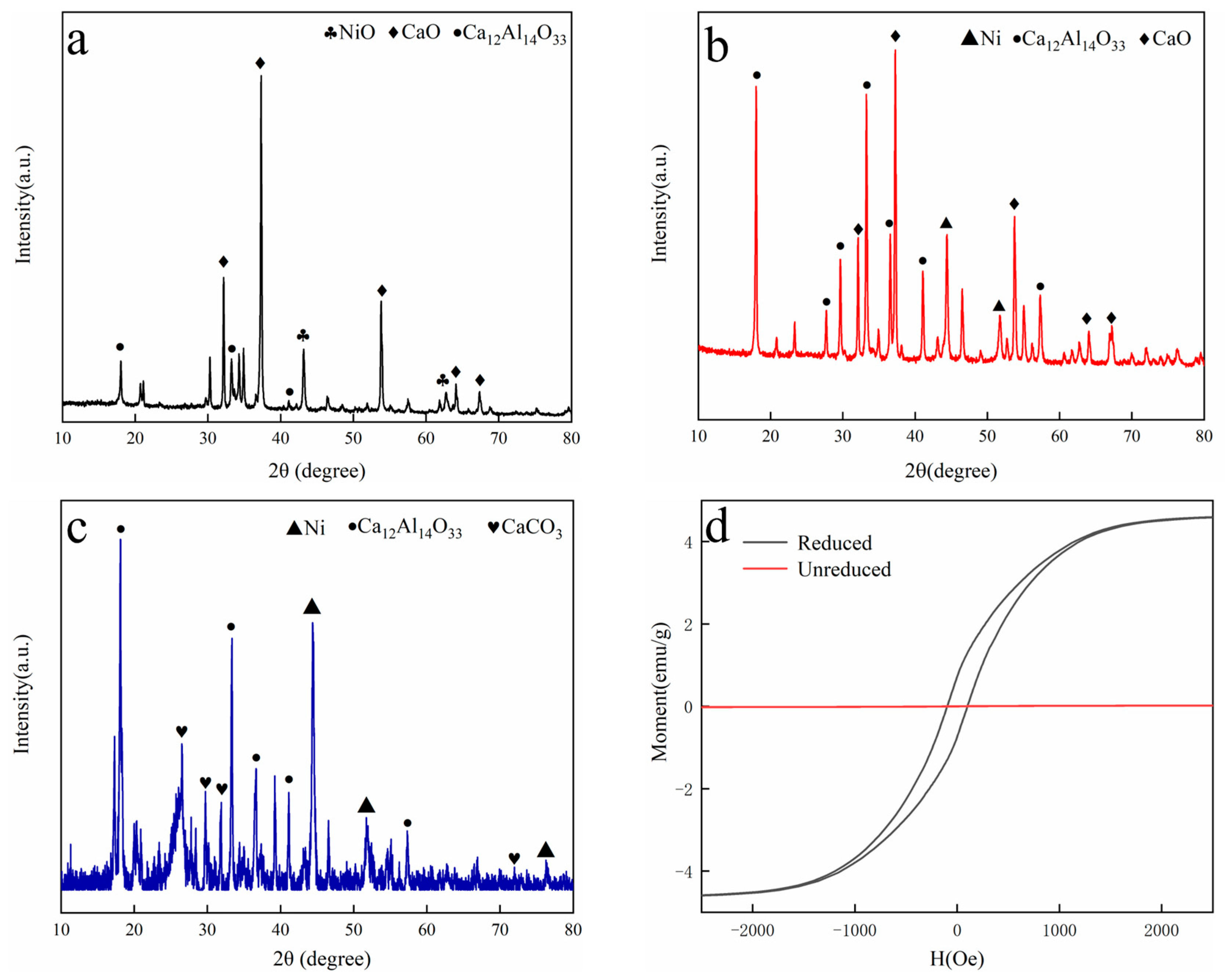
3.1.1. XRD Analysis
3.1.2. Catalyst Magnetic Properties
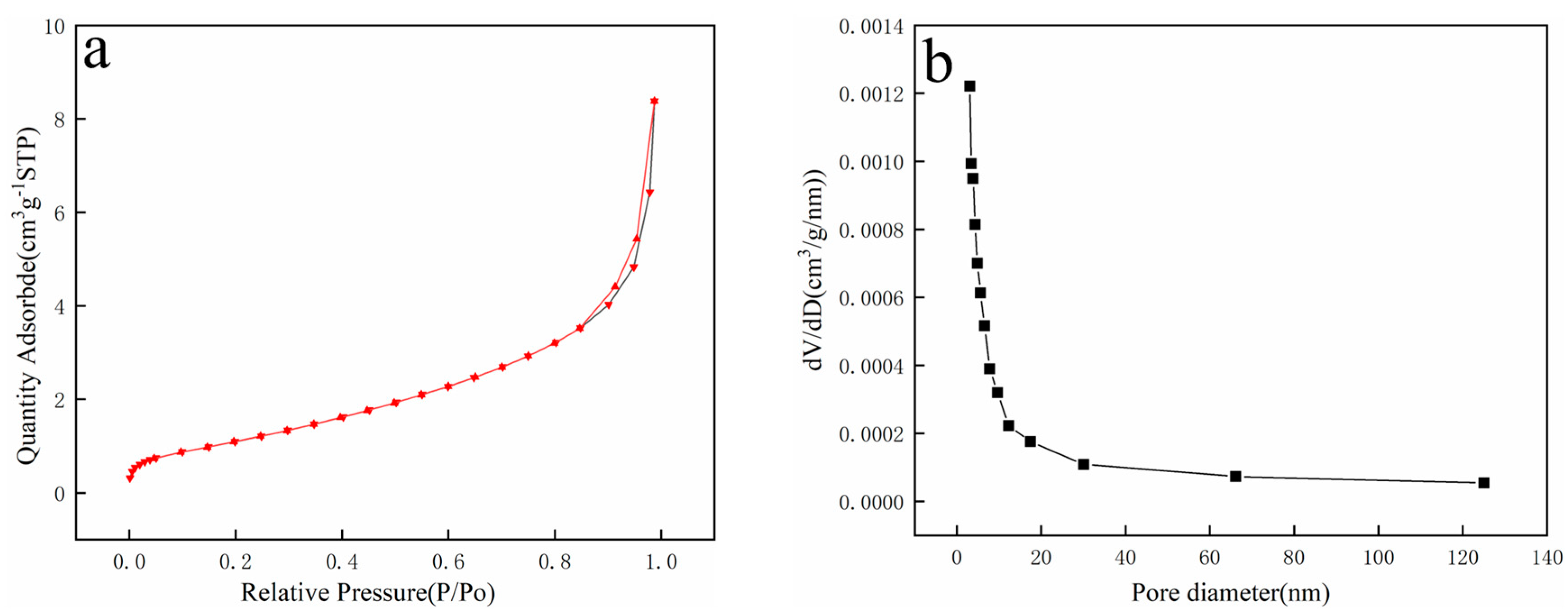
3.1.3. N2 Adsorption/Desorption Analysis
3.1.4. XRF Characterization
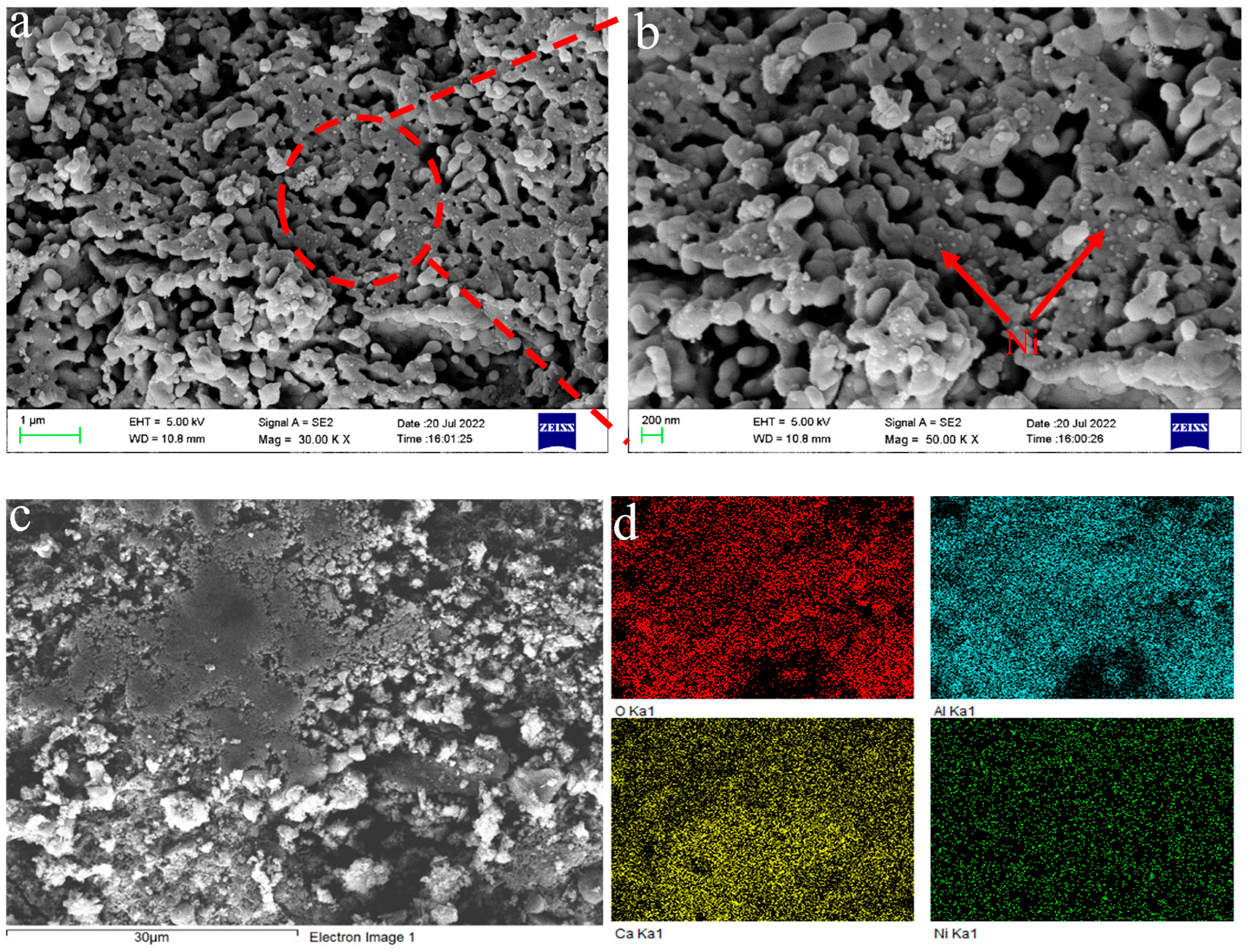
3.1.5. SEM-EDS Characterization and Analysis
3.2. Catalytic Pyrolysis Experimentation
3.2.1. Effect of Temperature
3.2.2. Effect of Carrier Gas Velocity
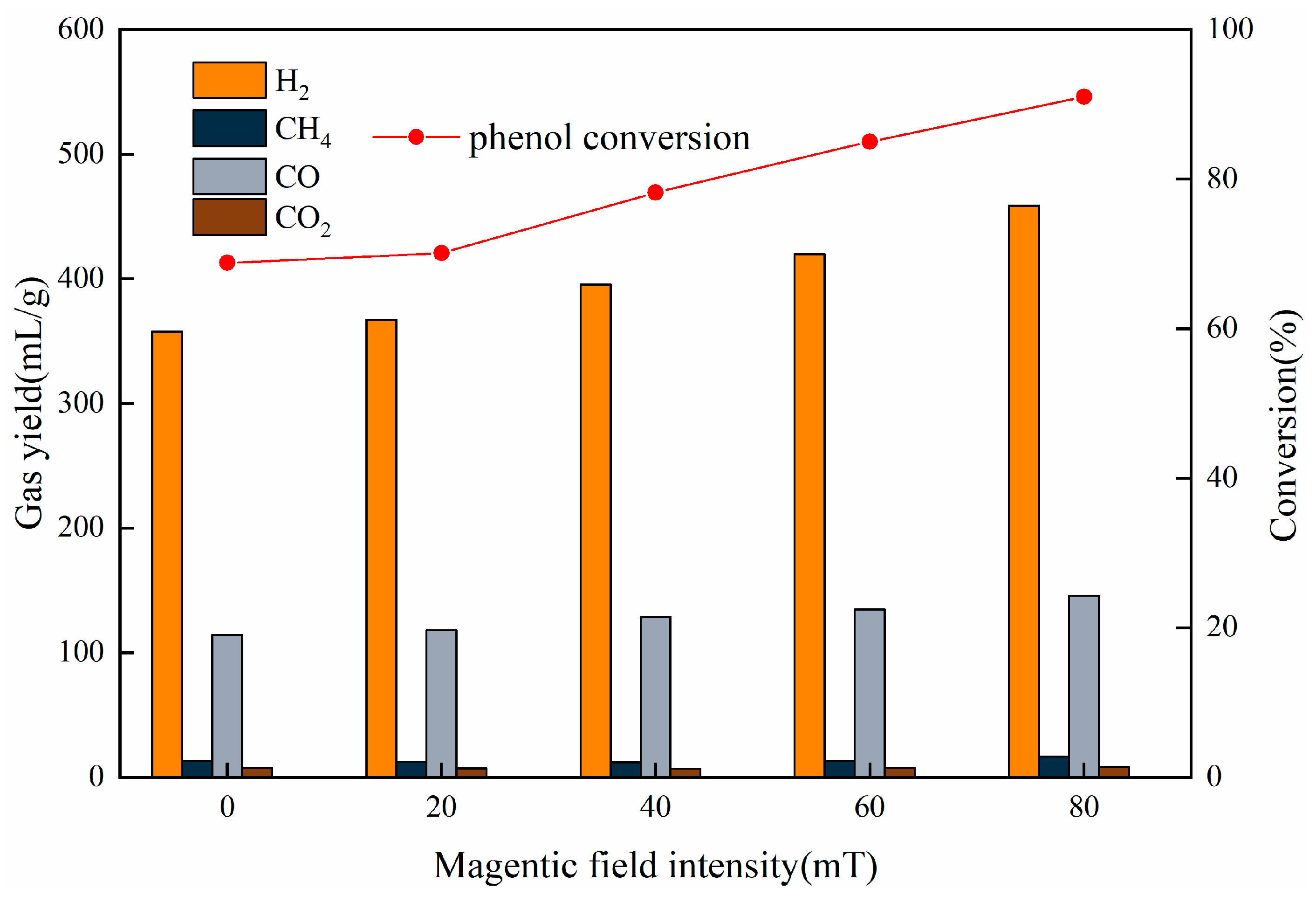
3.2.3. Effect of Magnetic Field
3.3. Kinetic Characterization
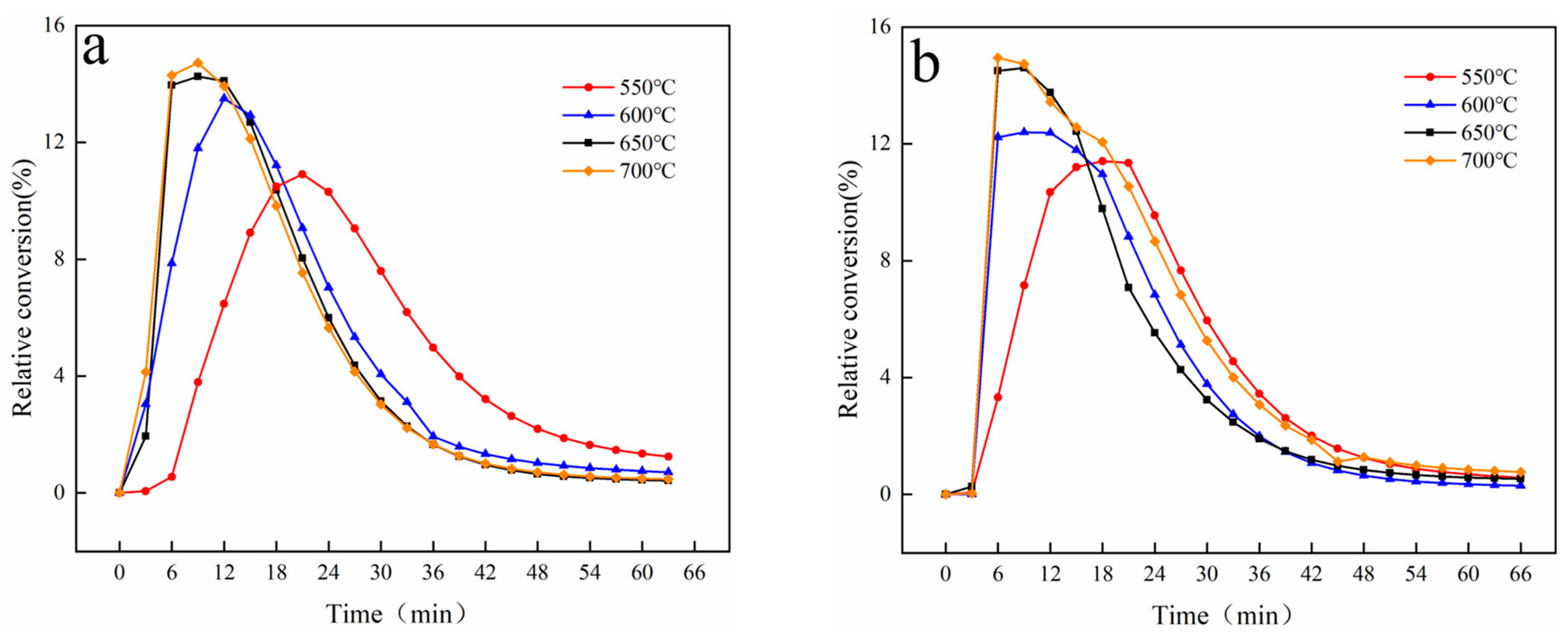
3.3.1. Pyrolysis Gas Production Characteristics
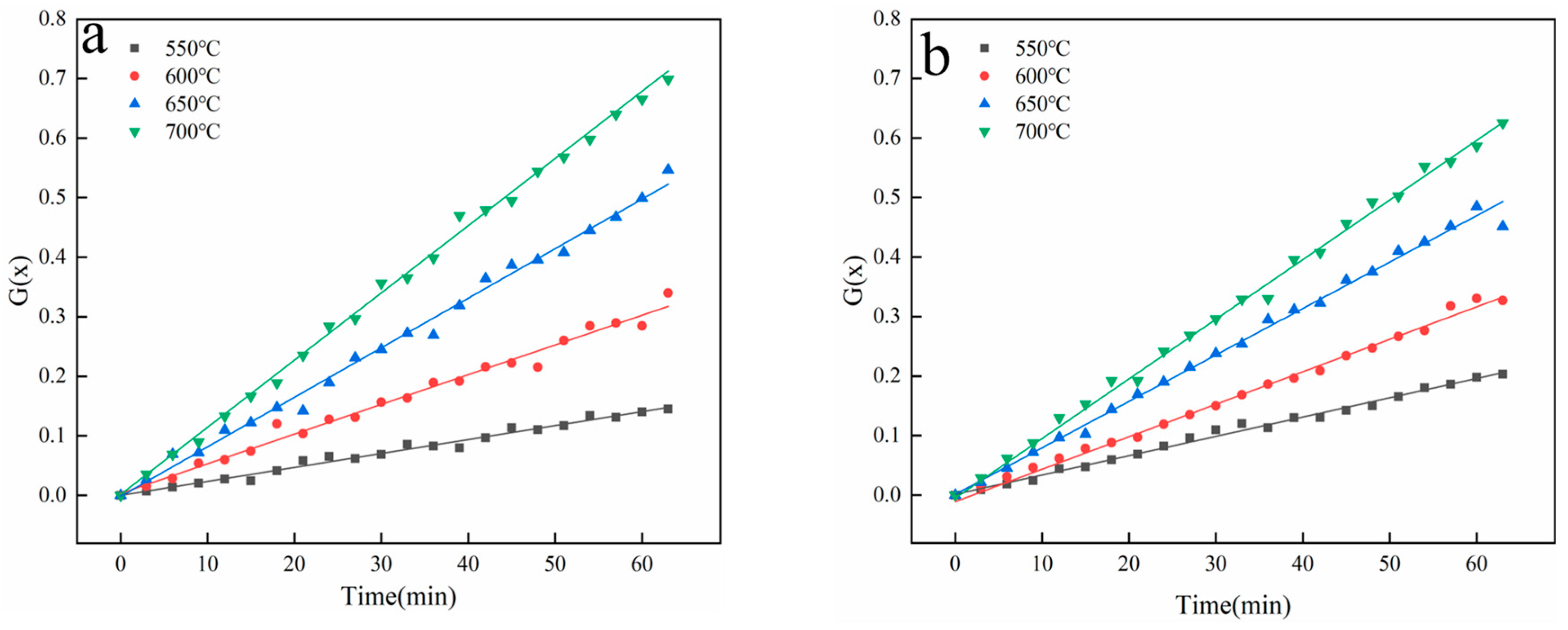
3.3.2. Kinetic Parameter Solving
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, M.; Memon, M.Z.; Ji, G.; Yang, X.; Vuppaladadiyam, A.K.; Song, Y.; Raheem, A.; Li, J.; Wang, W.; Zhou, H. Alkali metal bifunctional catalyst-sorbents enabled biomass pyrolysis for enhanced hydrogen production. Renew. Energy 2020, 148, 168–175. [Google Scholar] [CrossRef]
- Kostyniuk, A.; Bajec, D.; Prašnikar, A.; Likozar, B. Catalytic hydrocracking, hydrogenation, and isomerization reactions of model biomass tar over (W/Ni)-zeolites. J. Ind. Eng. Chem. 2021, 101, 293–306. [Google Scholar] [CrossRef]
- Wang, W.; Fan, L.W.; Zhou, P. Evolution of global fossil fuel trade dependencies. Energy 2022, 238, 121924. [Google Scholar] [CrossRef]
- Guo, F.; Dong, Y.; Fan, P.; Lv, Z.; Yang, S.; Dong, L. Catalytic decomposition of biomass tar compound by calcined coal gangue: A kinetic study. Int. J. Hydrogen Energy 2016, 41, 13380–13389. [Google Scholar] [CrossRef]
- Mei, D.; Wang, Y.; Liu, S.; Alliati, M.; Yang, H.; Tu, X. Plasma reforming of biomass gasification tars using mixed naphthalene and toluene as model compounds. Energy Convers. Manag. 2019, 195, 409–419. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Song, J.; Ahmad, S.; Liang, J.; Sun, Y. Plasma-enhanced steam reforming of different model tar compounds over Ni-based fusion catalysts. J. Hazard Mater. 2019, 377, 24–33. [Google Scholar] [CrossRef]
- Sulan, W.; Quanguo, Z.; Jihong, L. Chemical composition of biomass tar and its distillations. Acta Energ. Sol. Sin. 2006, 27, 651. [Google Scholar]
- Ellison, C.R.; Boldor, D. Mild upgrading of biomass pyrolysis vapors via ex-situ catalytic pyrolysis over an iron-montmorillonite catalyst. Fuel 2021, 291, 120226. [Google Scholar] [CrossRef]
- Gao, N.; Han, Y.; Quan, C.; Wu, C. Promoting hydrogen-rich syngas production from catalytic reforming of biomass pyrolysis oil on nanosized nickel-ceramic catalysts. Appl. Therm. Eng. 2017, 125, 297–305. [Google Scholar] [CrossRef]
- Li, B.; Yang, H.; Wei, L.; Shao, J.; Wang, X.; Chen, H. Hydrogen production from agricultural biomass wastes gasification in a fluidized bed with calcium oxide enhancing. Int. J. Hydrogen Energy 2017, 42, 4832–4839. [Google Scholar] [CrossRef]
- Xue, A.J.; Pan, J.H.; Tian, M.C. Experimental Study on Catalytic Pyrolysis of Biomass Pellet. In Applied Mechanics and Materials; Trans Tech Publications: Zurich, Switzerland, 2013; pp. 320–323. [Google Scholar]
- Li, B.; Magoua Mbeugang, C.F.; Huang, Y.; Liu, D.; Wang, Q.; Zhang, S. A review of CaO based catalysts for tar removal during biomass gasification. Energy 2022, 244, 123172. [Google Scholar] [CrossRef]
- Xu, A.; Zhou, W.; Zhang, X.; Zhao, B.; Chen, L.; Sun, L.; Ding, W.; Yang, S.; Guan, H.; Bai, B. Gas production by catalytic pyrolysis of herb residues using Ni/CaO catalysts. J. Anal. Appl. Pyrolysis 2018, 130, 216–223. [Google Scholar] [CrossRef]
- Zhao, B.; Song, G.; Zhou, W.; Chen, L.; Sun, L.; Yang, S.; Guan, H.; Zhu, D.; Chen, G.; Ding, W.; et al. Catalytic Pyrolysis of Herb Residues for the Preparation of Hydrogen-Rich Gas. Energy Fuels 2019, 34, 1131–1136. [Google Scholar] [CrossRef]
- Yue, W.; Ma, X.; Yu, Z.; Liu, H.; Li, M.; Lu, X. Ni-CaO bifunctional catalyst for biomass catalytic pyrolysis to produce hydrogen-rich gas. J. Anal. Appl. Pyrolysis 2023, 169, 105872. [Google Scholar] [CrossRef]
- Dang, C.; Li, Z.; Long, J.; Yang, W.; Cai, W. Sorption-enhanced glycerol steam reforming over hierarchical hollow Ni-CaO-Ca12Al14O33 bi-functional catalyst derived from hydrotalcite-like compounds. Fuel 2022, 324, 124468. [Google Scholar] [CrossRef]
- Liu, L.; Hong, D.; Wang, N.; Guo, X. High purity H2 production from sorption enhanced bio-ethanol reforming via sol-gel-derived Ni–CaO–Al2O3 bi-functional materials. Int. J. Hydrogen Energy 2020, 45, 34449–34460. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, W.; Jia, X.; Chen, B.; An, S.; Xie, X.; Huang, L. Ca–Al layered double hydroxides-derived Ni-based catalysts for hydrogen production via auto-thermal reforming of acetic acid. Int. J. Hydrogen Energy 2019, 44, 20007–20016. [Google Scholar] [CrossRef]
- Donphai, W.; Piriyawate, N.; Witoon, T.; Jantaratana, P.; Varabuntoonvit, V.; Chareonpanich, M. Effect of magnetic field on CO2 conversion over Cu-ZnO/ZrO2 catalyst in hydrogenation reaction. J. CO2 Util. 2016, 16, 204–211. [Google Scholar] [CrossRef]
- Kiatphuengporn, S.; Jantaratana, P.; Limtrakul, J.; Chareonpanich, M. Magnetic field-enhanced catalytic CO2 hydrogenation and selective conversion to light hydrocarbons over Fe/MCM-41 catalysts. Chem. Eng. J. 2016, 306, 866–875. [Google Scholar] [CrossRef]
- Zhao, B.; Yang, H.; Zhang, H.; Zhong, C.; Wang, J.; Zhu, D.; Guan, H.; Sun, L.; Yang, S.; Chen, L.; et al. Study on hydrogen-rich gas production by biomass catalytic pyrolysis assisted with magnetic field. J. Anal. Appl. Pyrolysis 2021, 157, 105227. [Google Scholar] [CrossRef]
- Guo, F.; Dong, Y.; Fan, P.; Lv, Z.; Yang, S.; Dong, L. Detailed kinetic study of phenol decomposition under isothermal conditions to understand tar catalytic cracking process. J. Anal. Appl. Pyrolysis 2016, 118, 155–163. [Google Scholar] [CrossRef]
- Gai, C.; Dong, Y.; Lv, Z.; Zhang, Z.; Liang, J.; Liu, Y. Pyrolysis behavior and kinetic study of phenol as tar model compound in micro fluidized bed reactor. Int. J. Hydrogen Energy 2015, 40, 7956–7964. [Google Scholar] [CrossRef]
- Gai, C.; Zhang, Y.; Chen, W.-T.; Zhang, P.; Dong, Y. Thermogravimetric and kinetic analysis of thermal decomposition characteristics of low-lipid microalgae. Bioresour. Technol. 2013, 150, 139–148. [Google Scholar] [CrossRef] [PubMed]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Shokrollahi Yancheshmeh, M.; Radfarnia, H.R.; Iliuta, M.C. High temperature CO2 sorbents and their application for hydrogen production by sorption enhanced steam reforming process. Chem. Eng. J. 2016, 283, 420–444. [Google Scholar] [CrossRef]
- Xu, P.; Zhou, Z.; Zhao, C.; Cheng, Z. Ni/CaO-Al2O3 bifunctional catalysts for sorption-enhanced steam methane reforming. AIChE J. 2014, 60, 3547–3556. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Sun, L.; Xie, X.; Zhao, B.; Si, H.; Zhang, X.; Hua, D. Novel Ni–Al nanosheet catalyst with homogeneously embedded nickel nanoparticles for hydrogen-rich syngas production from biomass pyrolysis. Int. J. Hydrogen Energy 2021, 46, 1762–1776. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, X.; Xu, A.; Ding, W.; Sun, L.; Chen, L.; Guan, H.; Yang, S.; Zhou, W. A study of the in-situ CO2 removal pyrolysis of Chinese herb residue for syngas production. Sci Total Environ. 2018, 626, 703–709. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, J.; Zhu, D.; Song, G.; Yang, H.; Chen, L.; Sun, L.; Yang, S.; Guan, H.; Xie, X. Adsorption Characteristics of Gas Molecules (H2O, CO2, CO, CH4, and H2) on CaO-Based Catalysts during Biomass Thermal Conversion with In Situ CO2 Capture. Catalysts 2019, 9, 757. [Google Scholar] [CrossRef]
- Lin, P.; Peng, J.; Hou, B.; Fu, Y. Effects of magnetic field on catalytic activity of CO monoxide oxidation and O2 adsorption over Ln0.7Sr0.3MnO3. J. Phys. Chem. B 1993, 97, 1471–1473. [Google Scholar] [CrossRef]
- Pan, L.; Ai, M.; Huang, C.; Yin, L.; Liu, X.; Zhang, R.; Wang, S.; Jiang, Z.; Zhang, X.; Zou, J.J.; et al. Manipulating spin polarization of titanium dioxide for efficient photocatalysis. Nat. Commun. 2020, 11, 418. [Google Scholar] [CrossRef]
- Mammadova, T.; Abbasov, M.; Movsumov, N.; Latifova, T.; Hasanova, A.; Kocharli, Z.; Khalafova, İ.; Abbasov, V. Production of diesel fractions by catalytic cracking of vacuum gas oil and its mixture with cottonseed oil under the influence of a magnetic field. Egypt. J. Pet. 2018, 27, 1029–1033. [Google Scholar] [CrossRef]
- Matas Güell, B.; Babich, I.V.; Lefferts, L.; Seshan, K. Steam reforming of phenol over Ni-based catalysts—A comparative study. Appl. Catal. B Environ. 2011, 106, 280–286. [Google Scholar] [CrossRef]
- Rodgers, C.T. Magnetic field effects in chemical systems. Pure Appl. Chem. 2009, 81, 19–43. [Google Scholar] [CrossRef]


| Catalyst | Ms (emu/g) | Mr (emu/g) | Hc (Oe) |
|---|---|---|---|
| Unreduced | 0 | 0 | 0 |
| Reduced | 4.62 | 0.71 | 10117.28 |
| Catalyst | SBET (m2/g) | Pore Volume (cm3/g) | Pore Size (nm) |
|---|---|---|---|
| 10%Ni/CaO-Ca12Al14O33 | 4.18 | 0.049 | 23.4 |
| Ni | Ca | Al | Na | |
|---|---|---|---|---|
| Content (wt.%) | 10.2 | 59.5 | 29.47 | 0.83 |
| Phenol (g) | Gas (g) | Solid (g) | Carbon (g) | |
|---|---|---|---|---|
| 1 | 2 | 0.71 | 0.14 | 1.06 |
| 2 | 2 | 0.68 | 0.17 | 1.08 |
| 3 | 2 | 0.66 | 0.15 | 1.11 |
| Magnetic Field Strength (mT) | H2 (mL/g) | CH4 (mL/g) | CO (mL/g) | CO2 (mL/g) |
|---|---|---|---|---|
| 0 | 357.55 | 13.30 | 114.30 | 7.55 |
| 20 | 367.35 | 12.70 | 117.90 | 7.30 |
| 40 | 395.40 | 12 | 128.55 | 6.80 |
| 60 | 419.95 | 13.35 | 134.77 | 7.60 |
| 80 | 458.88 | 16.7 | 145.60 | 8.15 |
| Magnetic Field Intensity (mT) | Temperature (°C) | Ea (kJ/mol) | A (s−1) | R2 |
|---|---|---|---|---|
| 0 | 550 | 45.54 | 3.23 | 0.9828 |
| 600 | ||||
| 650 | ||||
| 700 | ||||
| 80 | 550 | 23.78 | 7.36 | 0.9737 |
| 600 | ||||
| 650 | ||||
| 700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhao, B.; Guan, H.; Liu, S.; Zhu, D.; Song, A.; Li, H.; Sun, L. Hydrogen Production from Catalytic Pyrolysis of Phenol as Tar Model Compound in Magnetic Field. Energies 2023, 16, 4140. https://doi.org/10.3390/en16104140
Li Y, Zhao B, Guan H, Liu S, Zhu D, Song A, Li H, Sun L. Hydrogen Production from Catalytic Pyrolysis of Phenol as Tar Model Compound in Magnetic Field. Energies. 2023; 16(10):4140. https://doi.org/10.3390/en16104140
Chicago/Turabian StyleLi, Yalong, Baofeng Zhao, Haibin Guan, Suxiang Liu, Di Zhu, Angang Song, Huan Li, and Laizhi Sun. 2023. "Hydrogen Production from Catalytic Pyrolysis of Phenol as Tar Model Compound in Magnetic Field" Energies 16, no. 10: 4140. https://doi.org/10.3390/en16104140
APA StyleLi, Y., Zhao, B., Guan, H., Liu, S., Zhu, D., Song, A., Li, H., & Sun, L. (2023). Hydrogen Production from Catalytic Pyrolysis of Phenol as Tar Model Compound in Magnetic Field. Energies, 16(10), 4140. https://doi.org/10.3390/en16104140





