Research on Downhole Gas Separation Method Based on a PDMS Separation Membrane
Abstract
1. Introduction
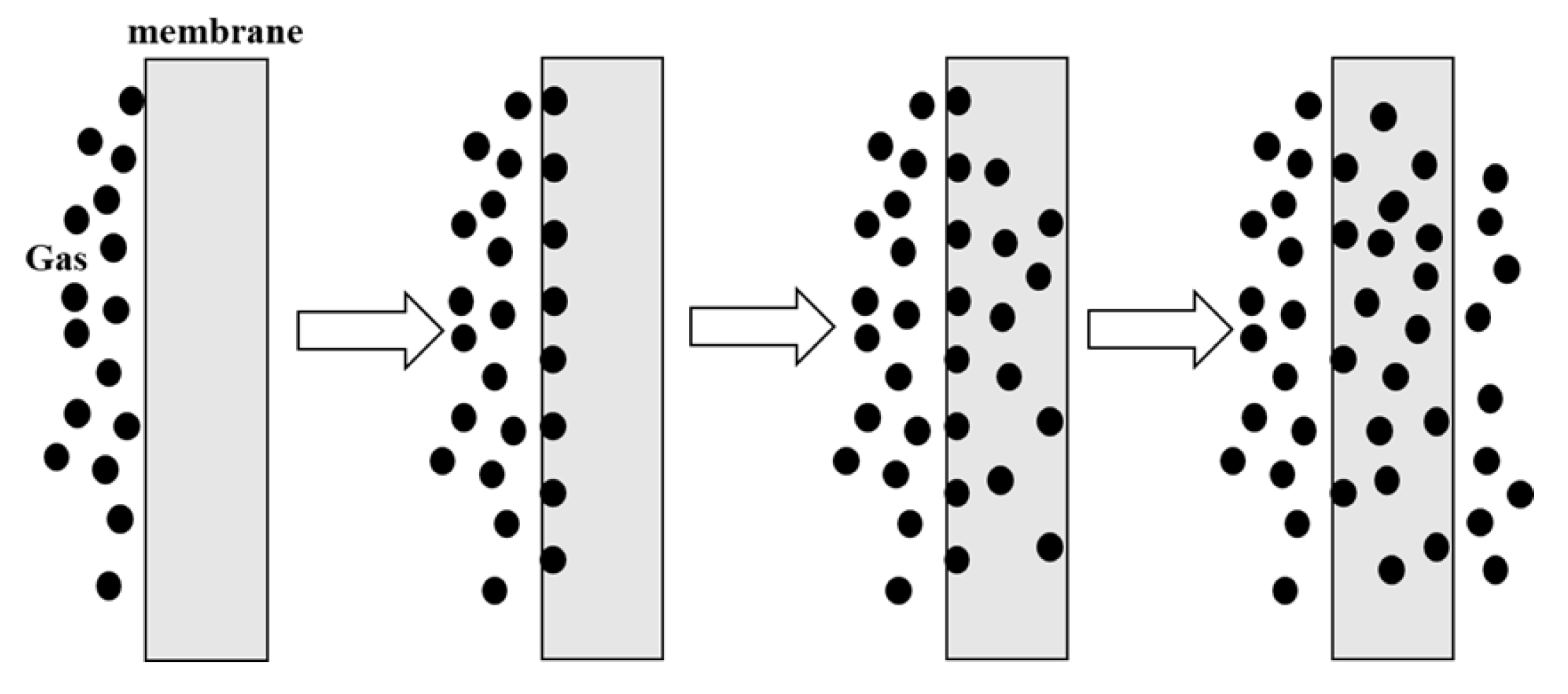
2. Gas Separation Membrane Mechanism Analysis
2.1. Basic Principle of Gas Membrane Separation
2.2. Polymer Gas Separation Membrane Performance Requirements
- (1)
- High permeability. High permeability is a fundamental requirement for a separation membrane as it needs to selectively allow the mixture being separated to pass through. The higher the permeability of the separation membrane the better, as it can increase the processing capacity and reduce the operational cost of the separation process [22,23,24].
- (2)
- Good mechanical strength. Due to the phenomena of vibration, shock, and corrosion in the drilling process, it requires the separation membrane to be invariable and non-ruptured in the long-term operation process, and the replacement cycle should be as long as possible. At the same time, it has a good film-forming ability and is easy to process [25,26].
- (3)
2.3. Comparison and Selection of Membrane Materials for Downhole Gas Separation
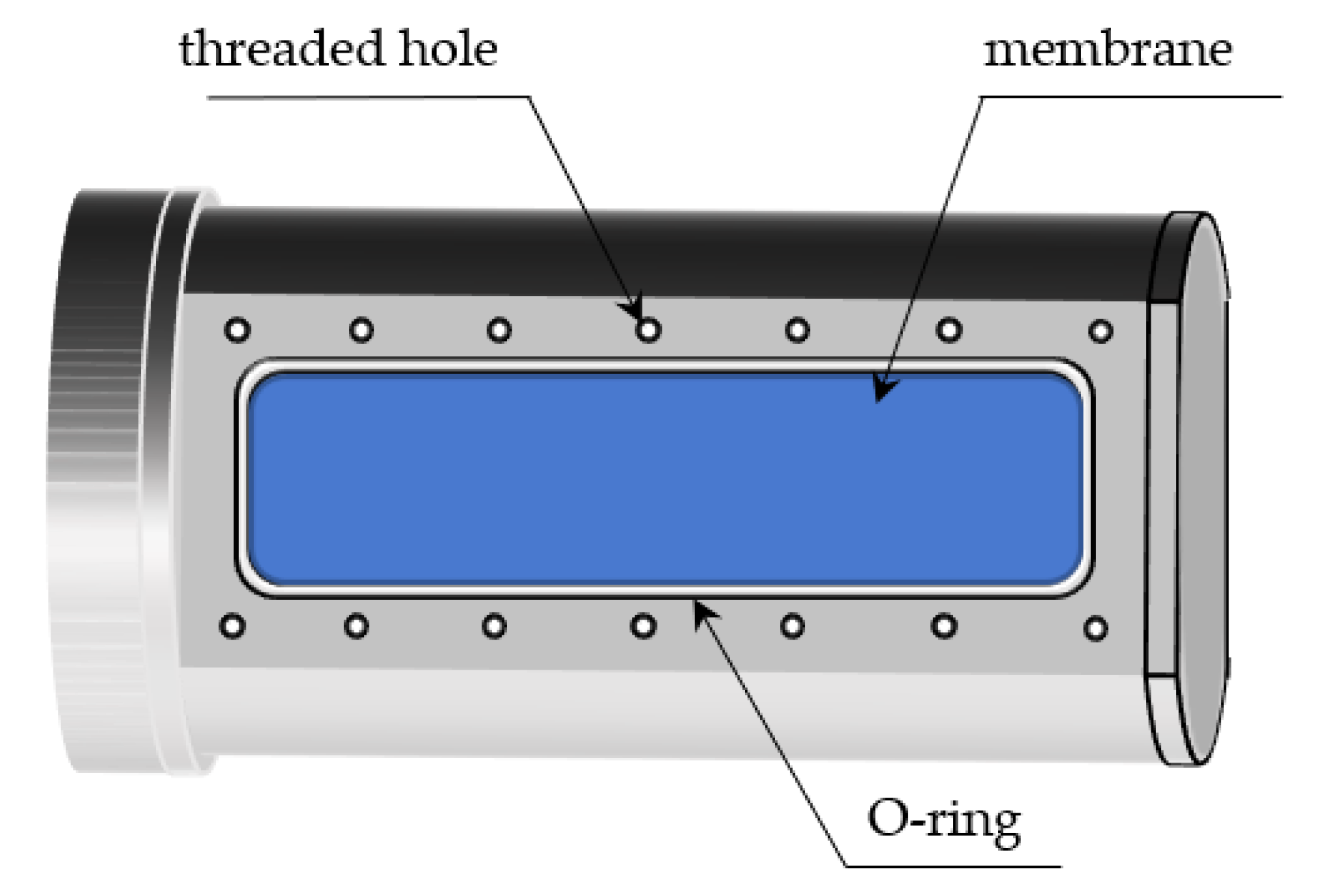
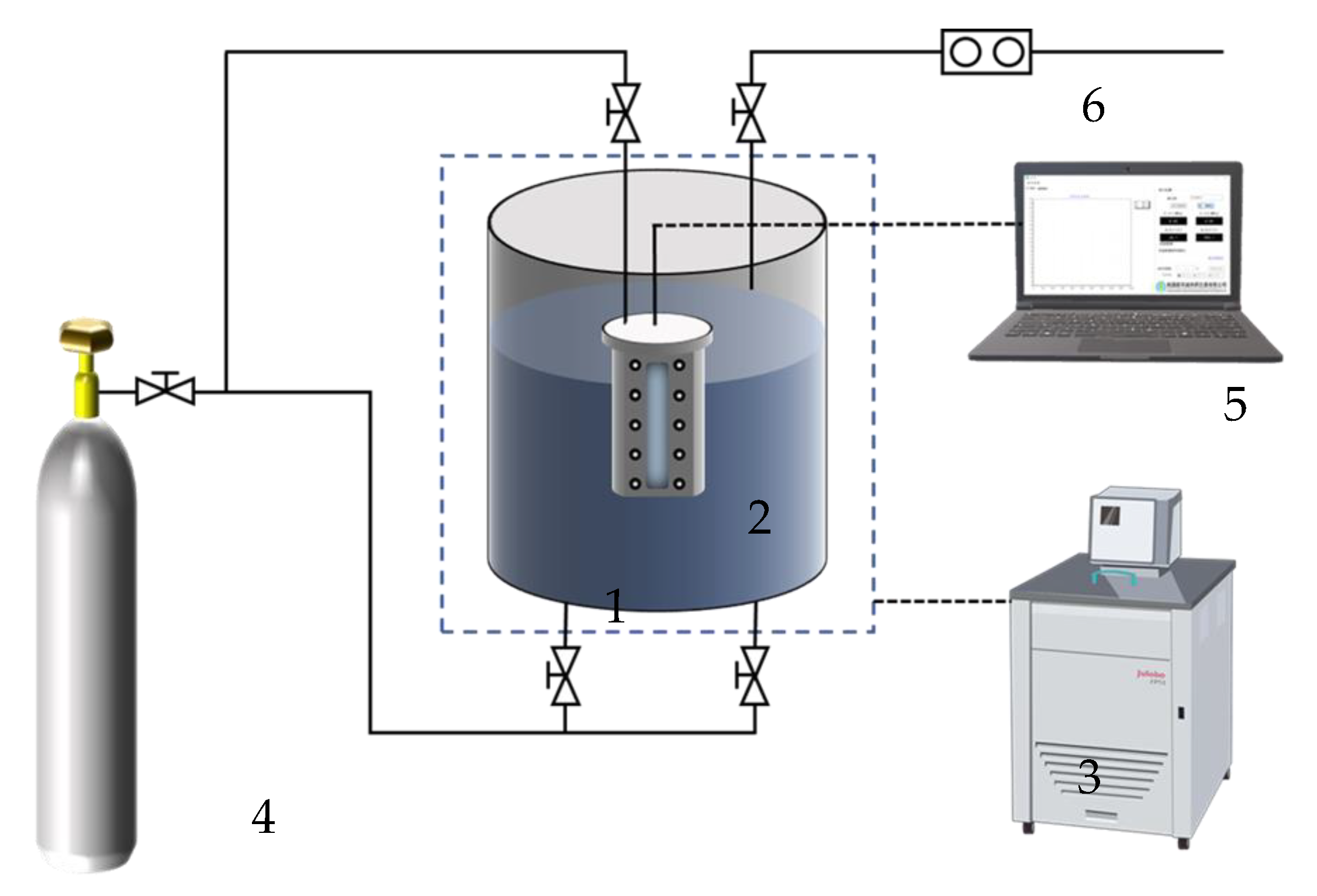
3. PDMS Separation Membrane Gas Separation Experiments
4. Gas Separation Membrane Mechanism Analysis
4.1. Separation Membrane Strength Performance Analysis
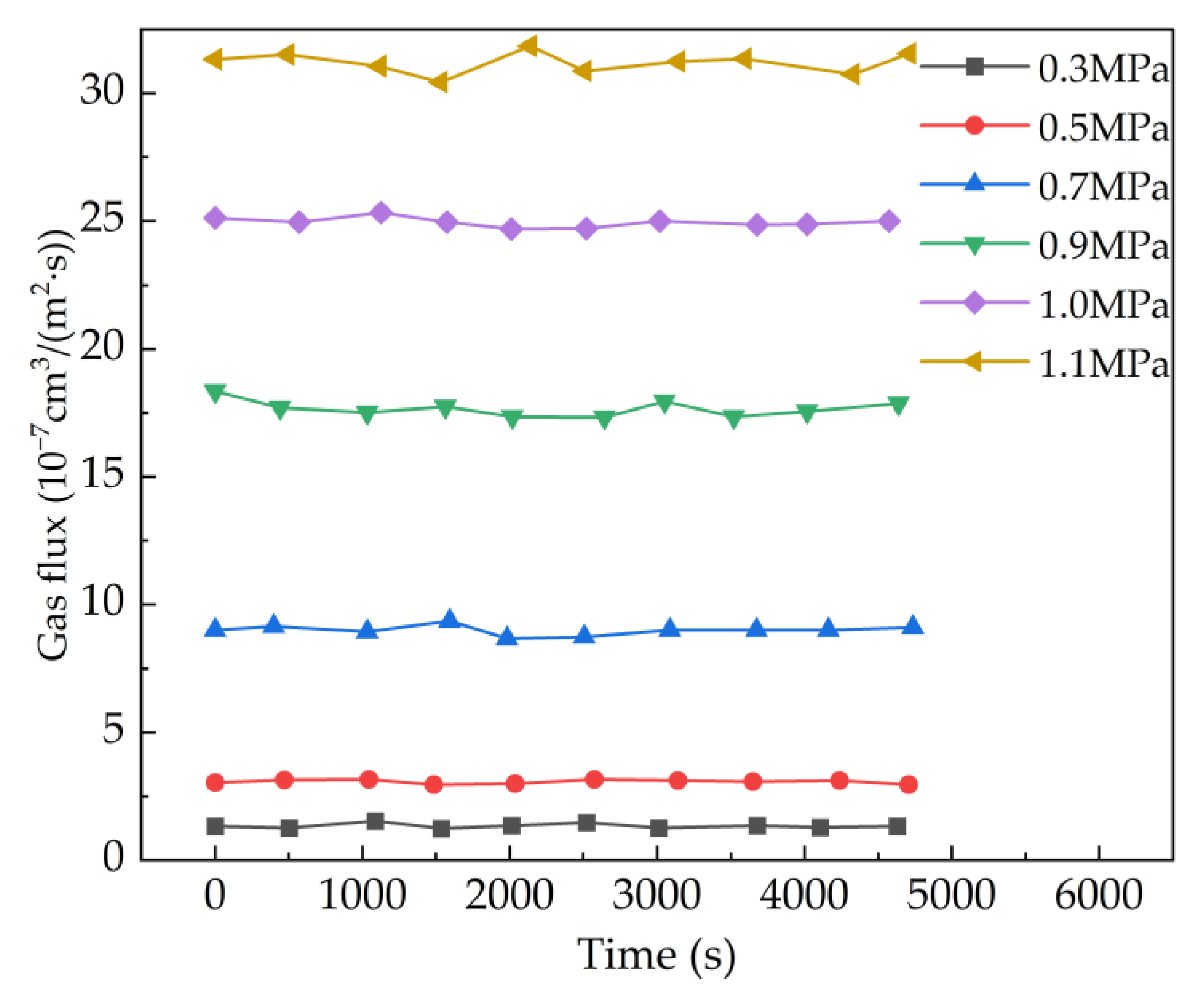
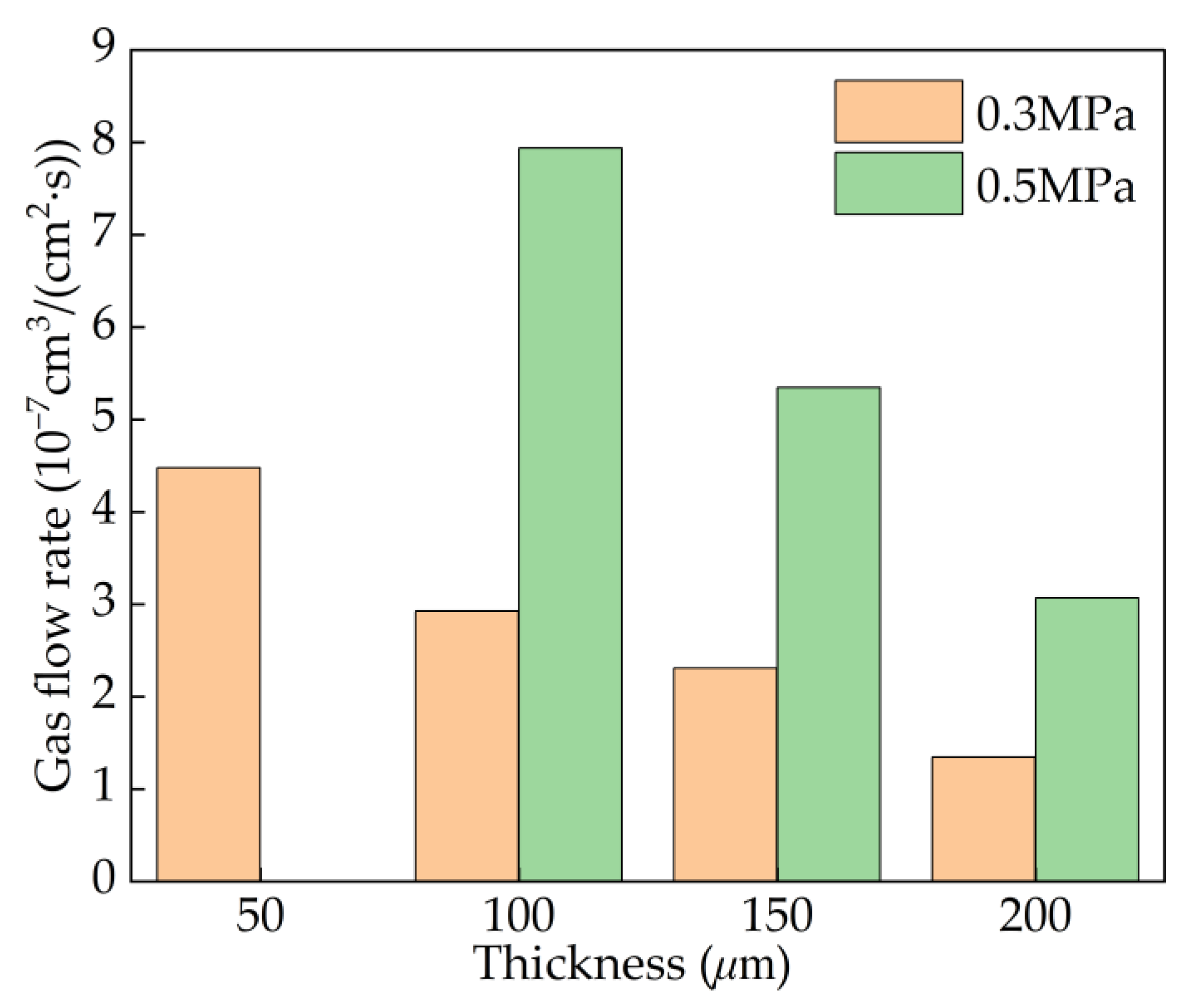
4.2. Effect of Pressure
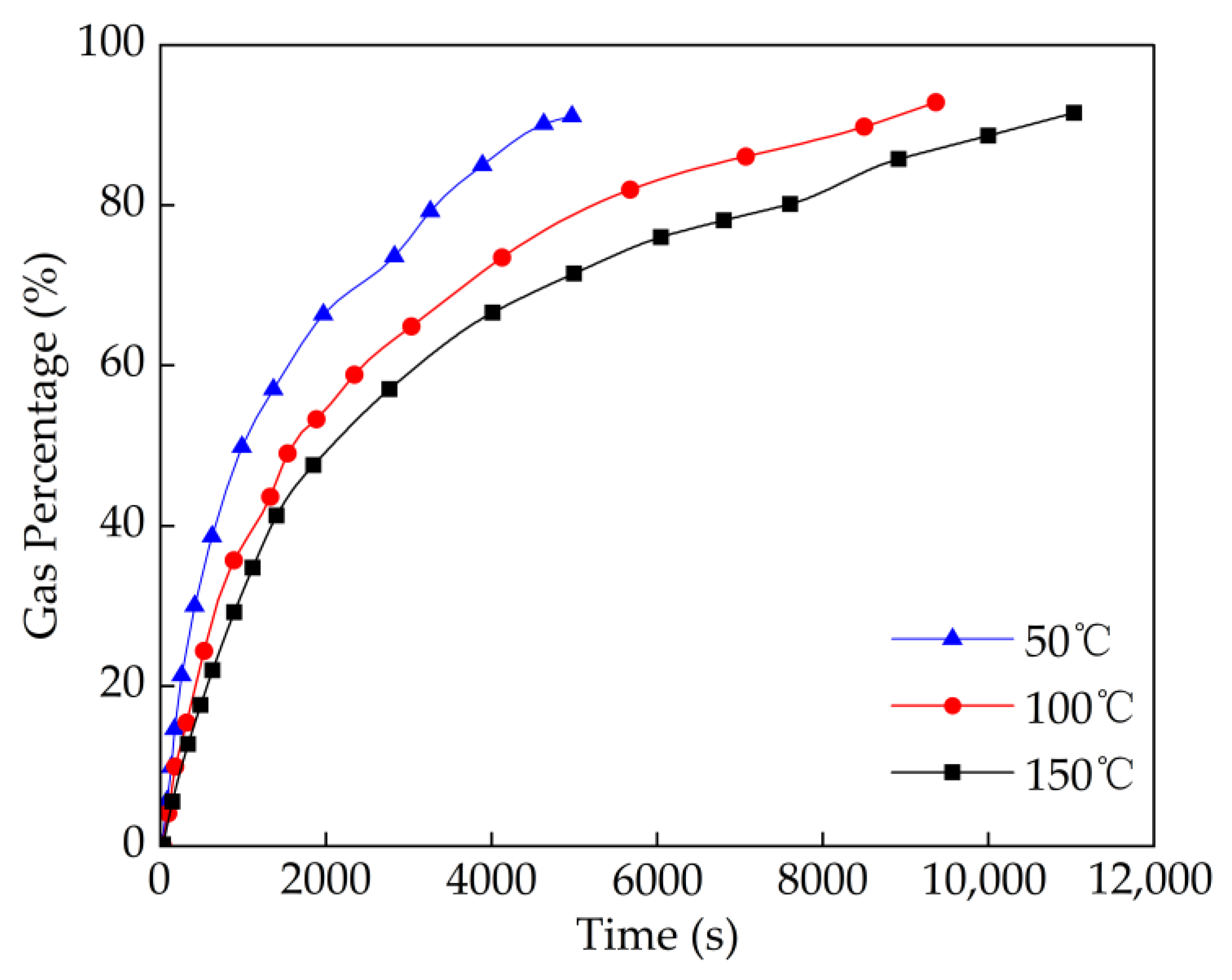
4.3. Effect of Temperature
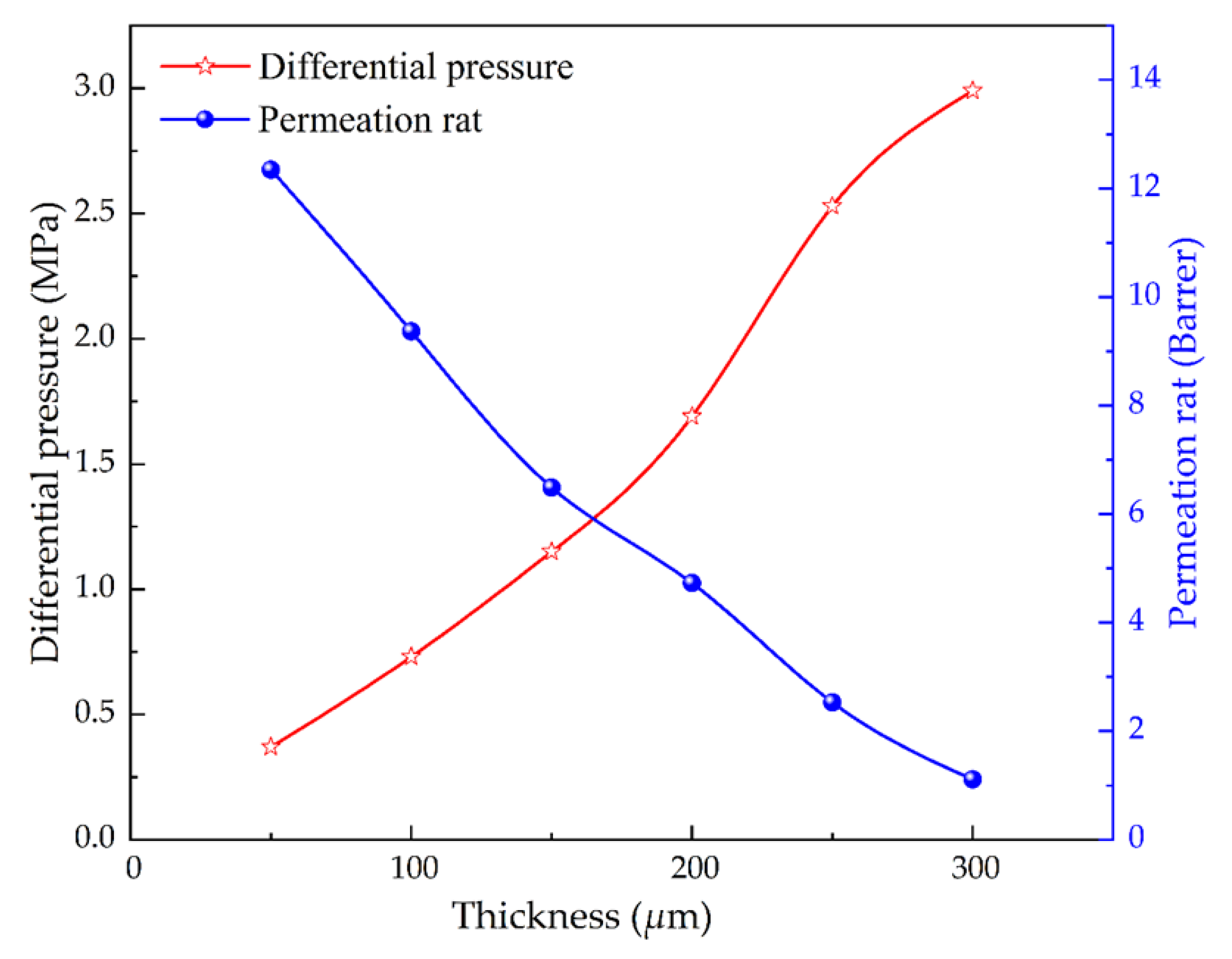
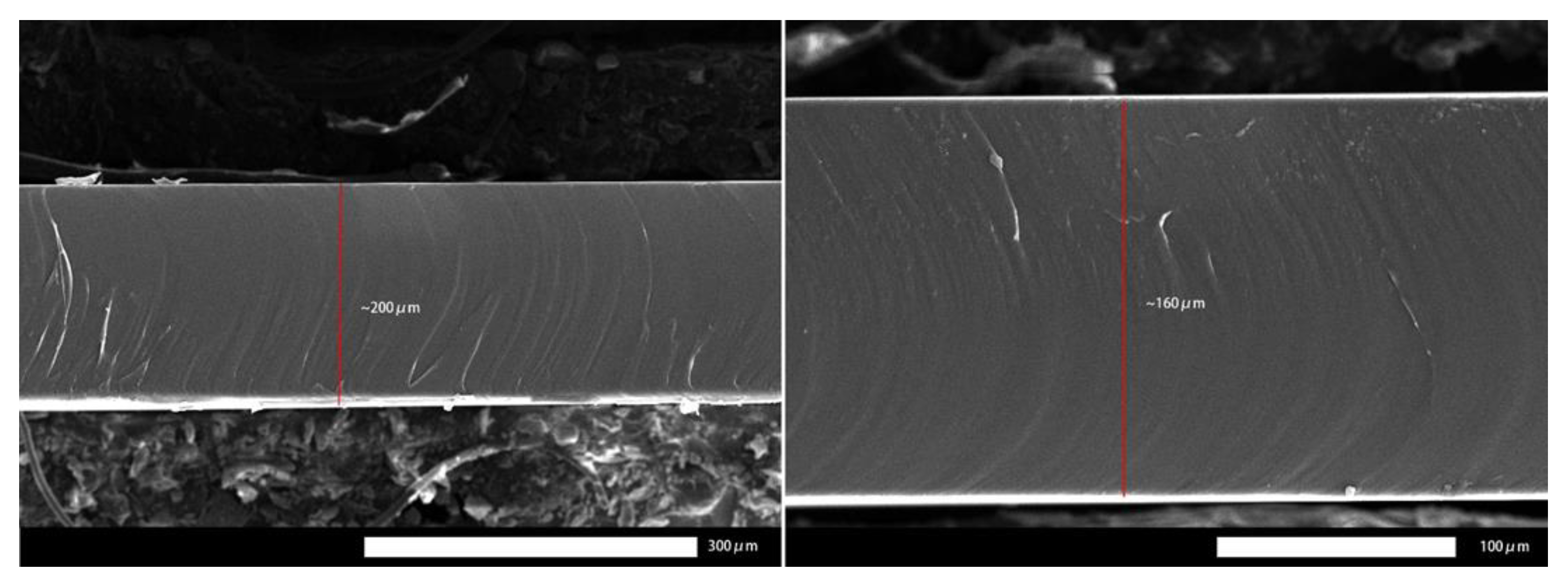
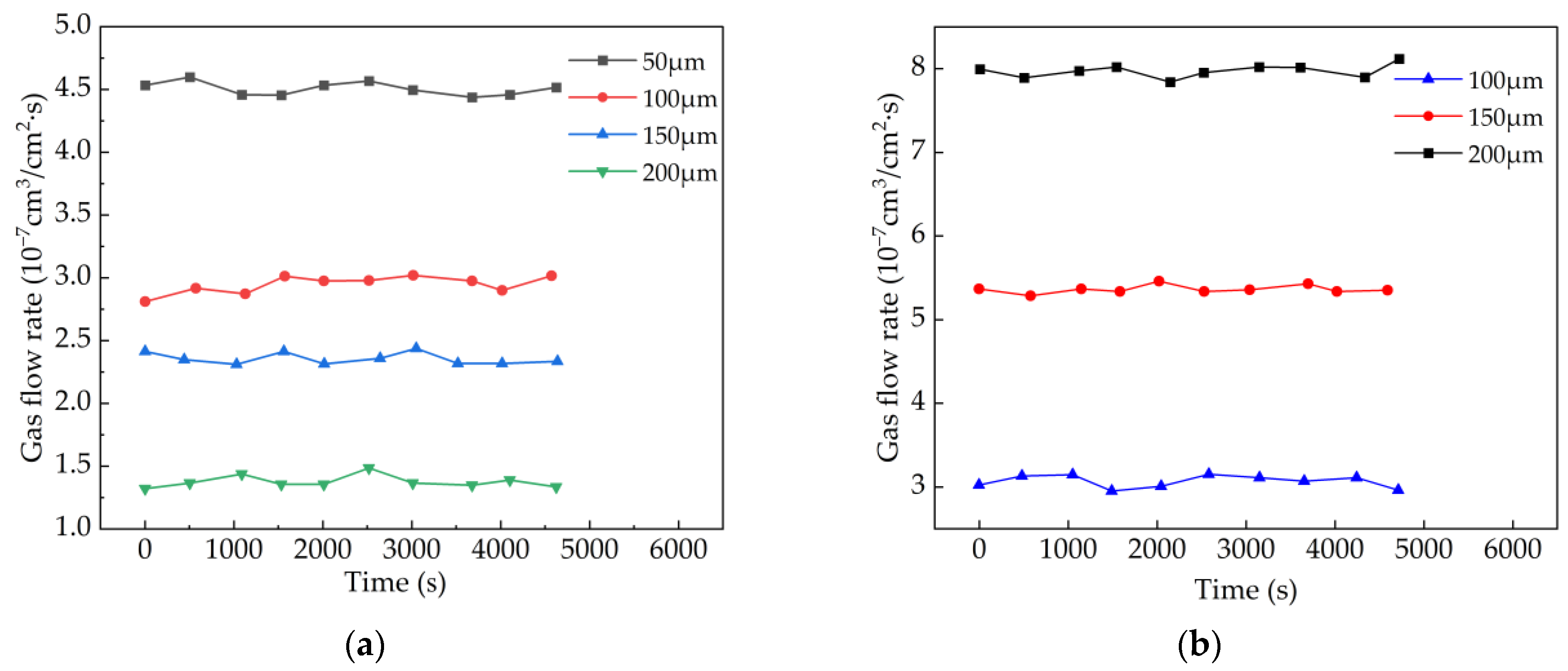
4.4. Effect of Thickness
4.5. Physical Properties of PDMS Separation Membranes at High Temperature and Pressure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Wu, Y.; Xie, R.; Jiao, J. Development and prospect of the key technologies for the drilling of deep wells in deep water. Oil Drill. Prod. Technol. 2021, 43, 139–145. [Google Scholar]
- Ilyushin, Y.V.; Fetisov, V. Experience of virtual commissioning of a process control system for the production of high-paraffin oil. Sci. Rep. 2022, 12, 18415. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Ma, T. Research status of early monitoring technology for deepwater drilling overflow. Acta Petrolei Sin. 2014, 35, 602–612. [Google Scholar]
- Martirosyan, A.V.; Kukharova, T.V.; Fedorov, M.S. Research of the Hydrogeological Objects’ Connection Peculiarities. In Proceedings of the 2021 IV International Conference on Control in Technical Systems (CTS), Saint Petersburg, Russia, 21–23 September 2021; IEEE: New York, NY, USA, 2021; pp. 34–38. [Google Scholar]
- Li, Y.; Xia, W.; Luo, F.; Liang, S.; Chen, Z.; Wang, P.; Ren, H. Principle and Experimental Research of Ultrasonic Gas Kick Detecting While Drilling Tool. Chian Pet. Mach. 2020, 48, 16–23. [Google Scholar]
- Wang, X.; Huang, L.; Li, X.; Bi, S.; Li, H.; Zhang, J.; Sun, X. Wellbore multiphase flow behaviors of gas kick in deep water horizontal drilling. Front. Phys. 2022, 10, 1059. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, B.; Gao, Y.; Li, H.; Wu, C. Gas kick during carbonate reservoirs drilling and its risk assessment. Petrol. Explor. Dev. 2017, 44, 462–469. [Google Scholar] [CrossRef]
- Sidhikku Kandath Valappil, R.; Ghasem, N.; Al-Marzouqi, M. Current and future trends in polymer membrane-based gas separation technology: A comprehensive review. J. Ind. Eng. Chem. 2021, 98, 103–129. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef]
- Li, S.; Xie, G. Experimental Study of Drilling Fluid Oil and Gas Separator Based on Semi-permeable Membrane. Sci. Technol. Rev. 2012, 30, 19–22. [Google Scholar]
- Dalane, K.; Dai, Z.; Mogseth, G.; Hillestad, M.; Deng, L. Potential applications of membrane separation for subsea natural gas processing: A review. J. Nat. Gas Sci. Eng. 2017, 39, 101–117. [Google Scholar] [CrossRef]
- Yang, M. Research on Hydrocarbon Quantitative Separation and Detection Method of Drilling Fluids. Ph.D. Thesis, China University of Geosciences, Beijing, China, 2015. [Google Scholar]
- Wang, B.; Wen, X.; Chen, C. Application of membrane separation technology in petrochemical industry. Chem. Ind. Eng. Prog. 2002, 21, 880–884. [Google Scholar]
- Brumboiu, A.O.; Hawker, D.P.; Norquay, D.A.; Wolcott, D.K. Application of Semipermeable Membrane Technology in the Measurement of Hydrocarbon Gases in Drilling Fluids. In Proceedings of the SPE/AAPG Western Regional Meeting, Long Beach, CA, USA, 19–22 June 2000; p. SPE-62525-MS. [Google Scholar]
- Jiao, F. Preparation and Application on Membrane of Oil & Gas Separation from Drilling Fluid. Master’s Thesis, Xiamen University, Xiamen, China, 2014. [Google Scholar]
- Liang, B.; He, X.; Hou, J.; Li, L.; Tang, Z. Membrane Separation in Organic Liquid: Technologies, Achievements, and Opportunities. Adv. Mater. 2018, 31, 1806090. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, R.; Geng, X.; Yang, Y.; Yang, H. Study of Gas Detection in Drilling Fluid Using Membrane Separation. J. Chenggde Pet. Coll. 2015, 17, 10–13+19. [Google Scholar]
- Saleh, T.A.; Gupta, V.K. Applications of nanomaterial-polymer membranes for oil and gas separation. Nanomater. Polym. Membr 2016, 10, 251–265. [Google Scholar]
- Wang, S.F.; Li, X.Q.; Wu, H.; Tian, Z.Z.; Xin, Q.P.; He, G.W.; Peng, D.D.; Chen, S.L.; Yin, Y.; Jiang, Z.Y.; et al. Advances in high permeability polymer-based membrane materials for CO2 separations. Energ. Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Wang, L.; Corriou, J.P.; Castel, C.; Favre, E. Transport of Gases in Glassy Polymers under Transient Conditions: Limit-Behavior Investigations of Dual-Mode Sorption Theory. Ind. Eng. Chem. Res. 2013, 52, 1089–1101. [Google Scholar] [CrossRef]
- Chen, T.; Ma, F.; Wang, L.; Han, D.; Zhang, G. Application of Polymers Membrane in Dissolved Gas Analysis: A Review. Trans. China Electrotech. Soc. 2022, 37, 750–766. [Google Scholar]
- Qiu, W.L.; Zhang, K.; Li, F.S.; Zhang, K.; Koros, W.J. Gas Separation Performance of Carbon Molecular Sieve Membranes Based on 6FDA-mPDA/DABA(3:2) Polyimide. ChemSusChem 2014, 7, 1186–1194. [Google Scholar] [CrossRef]
- Yu, Y.W.; Liu, S.Y.; Wang, Y.; Zhang, H.B.; Li, X.B.; Jiang, Z.H.; Liu, B.J. Asymmetric membranes prepared with trifluoromethylphenylated poly(ether ether ketone) for gas separation. High Perform Polym. 2015, 27, 10–18. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Chu, L.Y.; Wu, X.M.; Wang, Z.; Jiang, X.B.; Ju, X.J.; Ruan, X.H.; He, G.H. Membrane-based separation technologies: From polymeric materials to novel process: An outlook from China. Rev. Chem. Eng. 2020, 36, 67–105. [Google Scholar] [CrossRef]
- Feng, S.; Wang, Y.; Zhang, G.; Zhao, Y.; Wang, S.; Gao, R.; Xiao, E. Estimation of optimal drilling efficiency and rock strength by using controllable drilling parameters in rotary non-percussive drilling. J. Petrol. Sci. Eng. 2020, 193, 107376. [Google Scholar] [CrossRef]
- Gelfgat, M.; Sledkov, V. Superdeep Scientific Drilling‐Lessons Learned for the Development of Modern Drilling Technologies. Acta Geol. Sin. 2019, 93, 186–187. [Google Scholar] [CrossRef]
- Mercadelli, E.; Gondolini, A.; Ardit, M.; Cruciani, G.; Melandri, C.; Escolástico, S.; Serra, J.M.; Sanson, A. Chemical and mechanical stability of BCZY-GDC membranes for hydrogen separation. Sep. Purif. Technol. 2022, 289, 120795. [Google Scholar] [CrossRef]
- Swain, S.S.; Unnikrishnan, L.; Mohanty, S.; Nayak, S.K. Insight into mechanical, thermal, and chemical stability of polysulfone-based membranes for the separation of O2/N2. Korean J. Chem. Eng. 2022, 39, 785–797. [Google Scholar] [CrossRef]
- Ghosh, A.; Sen, S.K.; Banerjee, S.; Voit, B. Solubility improvements in aromatic polyimides by macromolecular engineering. Rsc. Adv. 2012, 2, 5900–5926. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, H.S.; Kwon, S.K. Synthesis and characterization of highly soluble and oxygen permeable new polyimides based on twisted biphenyl dianhydride and spirobifluorene diamine. Macromolecules 2005, 38, 7950–7956. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Wang, C.; Wang, T. Preparation of PDMS composite membranes with flexible supports and their performance in C3 hydrocarbon gases/N2 separation. J. Chem. Eng. Chin. Univ. 2016, 30, 1036–1042. [Google Scholar]
- Lu, G.; Cui, H.; Weng, X.; Sun, Y.; Chang, Z. Investigation and optimization of PDMS/PES membrane on morphology and performance for methane-water separation. Microporous Mesoporous Mater. 2022, 341, 112078. [Google Scholar] [CrossRef]
- Okamoto, Y.; Chiang, H.; Fang, M.; Galizia, M.; Merkel, T.; Yavari, M.; Nguyen, H.; Lin, H. Perfluorodioxolane Polymers for Gas Separation Membrane Applications. Membranes 2020, 10, 394. [Google Scholar] [CrossRef]
- Ariati, R.; Sales, F.; Souza, A.; Lima, R.A.; Ribeiro, J. Polydimethylsiloxane Composites Characterization and Its Applications: A Review. Polymers 2021, 13, 4258. [Google Scholar] [CrossRef]
- Xiao, D.M.; Liu, H.L. Permeability study of high polymer membranes for gas-oil separation. In Proceedings of the 2000 IEEE Power Engineering Society Winter Meeting—Vols 1–4, Conference Proceedings, 2000 Winter Meeting of the IEEE Power Engineering-Society, Singapore, 23–27 January 2000; pp. 2229–2232. [Google Scholar]
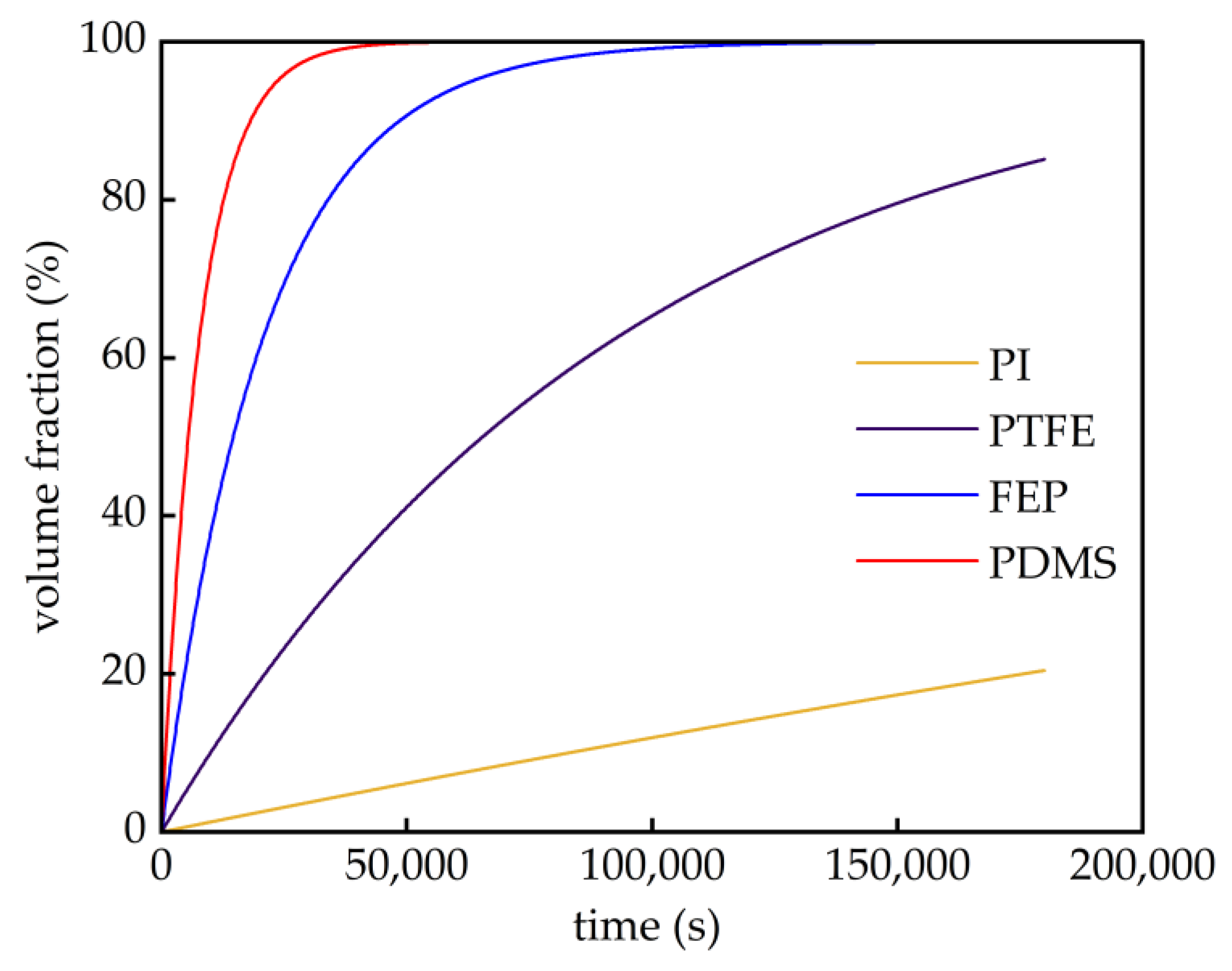


| Membrane | Advantages | Disadvantages |
|---|---|---|
| PI | Good chemical resistance. Good thermal stability. | Low gas permeation. Not suitable for multicomponent gas detection. |
| PTFE | Good chemical resistance. Good thermal stability. Excellent anti-adhesion performance. | Excellent anti-adhesion performance. Susceptible to damage. |
| FEP | Easy to process. Good chemical resistance. Good thermal stability. | High viscosity. Poor wear resistance. |
| PDMS | Good gas permeability. Good chemical resistance. Easy to process. | Low mechanical strength. Sensitive to organic solvents. |
| Separation Membrane | CH4 | C2H4 | C2H6 |
|---|---|---|---|
| PI | 0.015 | -- | -- |
| PTFE | 1.255 | 0.015 | 0.2 |
| FEP | 5.622 | 2.083 | 1.154 |
| PDMS | 15.06 | -- | -- |
| Group | Volume of Air Chamber/cm3 | Separation Film Area/cm2 | Starting Pressure/MPa | Separation Film Thickness/μm | Test Temperature/°C |
|---|---|---|---|---|---|
| 1 | 8.5 | 9 | 0.3 | 50 | 25 |
| 100 | |||||
| 150 | |||||
| 200 | |||||
| 2 | 8.5 | 9 | 0.5 | 100 | 25 |
| 150 | 25 | ||||
| 200 | 25 | ||||
| 50 | |||||
| 100 | |||||
| 150 | |||||
| 3 | 8.5 | 9 | 0.7 | 200 | 25 |
| 0.9 | |||||
| 1.0 | |||||
| 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, X.; Zhang, L.; Ma, Y.; Zhang, H.; Zhao, X.; Gao, Y. Research on Downhole Gas Separation Method Based on a PDMS Separation Membrane. Energies 2023, 16, 4255. https://doi.org/10.3390/en16104255
Pei X, Zhang L, Ma Y, Zhang H, Zhao X, Gao Y. Research on Downhole Gas Separation Method Based on a PDMS Separation Membrane. Energies. 2023; 16(10):4255. https://doi.org/10.3390/en16104255
Chicago/Turabian StylePei, Xueliang, Lei Zhang, Yongqian Ma, Hengtong Zhang, Xinxin Zhao, and Yonghai Gao. 2023. "Research on Downhole Gas Separation Method Based on a PDMS Separation Membrane" Energies 16, no. 10: 4255. https://doi.org/10.3390/en16104255
APA StylePei, X., Zhang, L., Ma, Y., Zhang, H., Zhao, X., & Gao, Y. (2023). Research on Downhole Gas Separation Method Based on a PDMS Separation Membrane. Energies, 16(10), 4255. https://doi.org/10.3390/en16104255











