Accurate Remaining Available Energy Estimation of LiFePO4 Battery in Dynamic Frequency Regulation for EVs with Thermal-Electric-Hysteresis Model
Abstract
:1. Introduction
1.1. Background
1.2. Literature Review
1.2.1. Lithium-Ion Battery Model
1.2.2. State-of-Charge and Remaining Available Energy Estimation Methods
1.2.3. Literature Review Summary
1.3. Contribution of This Research
2. Thermal-Electric-Hysteresis Coupling Model
2.1. Battery Experiment
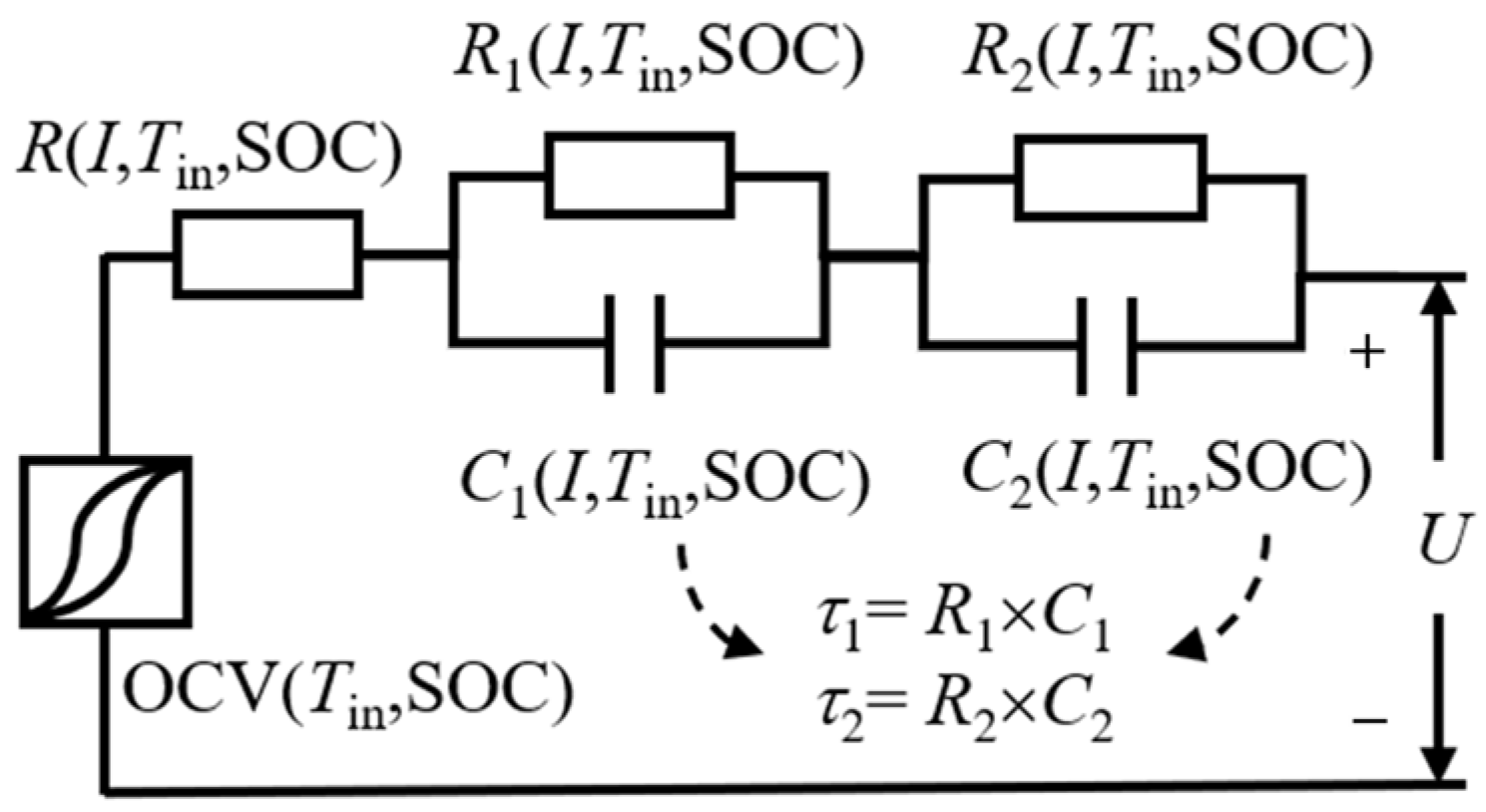
2.2. Second-Order RC Equivalent Circuit Model with Hysteresis Voltage Reconstruction
2.2.1. Hysteresis Voltage Reconstruction
2.2.2. Second-Order RC Equivalent Circuit Model
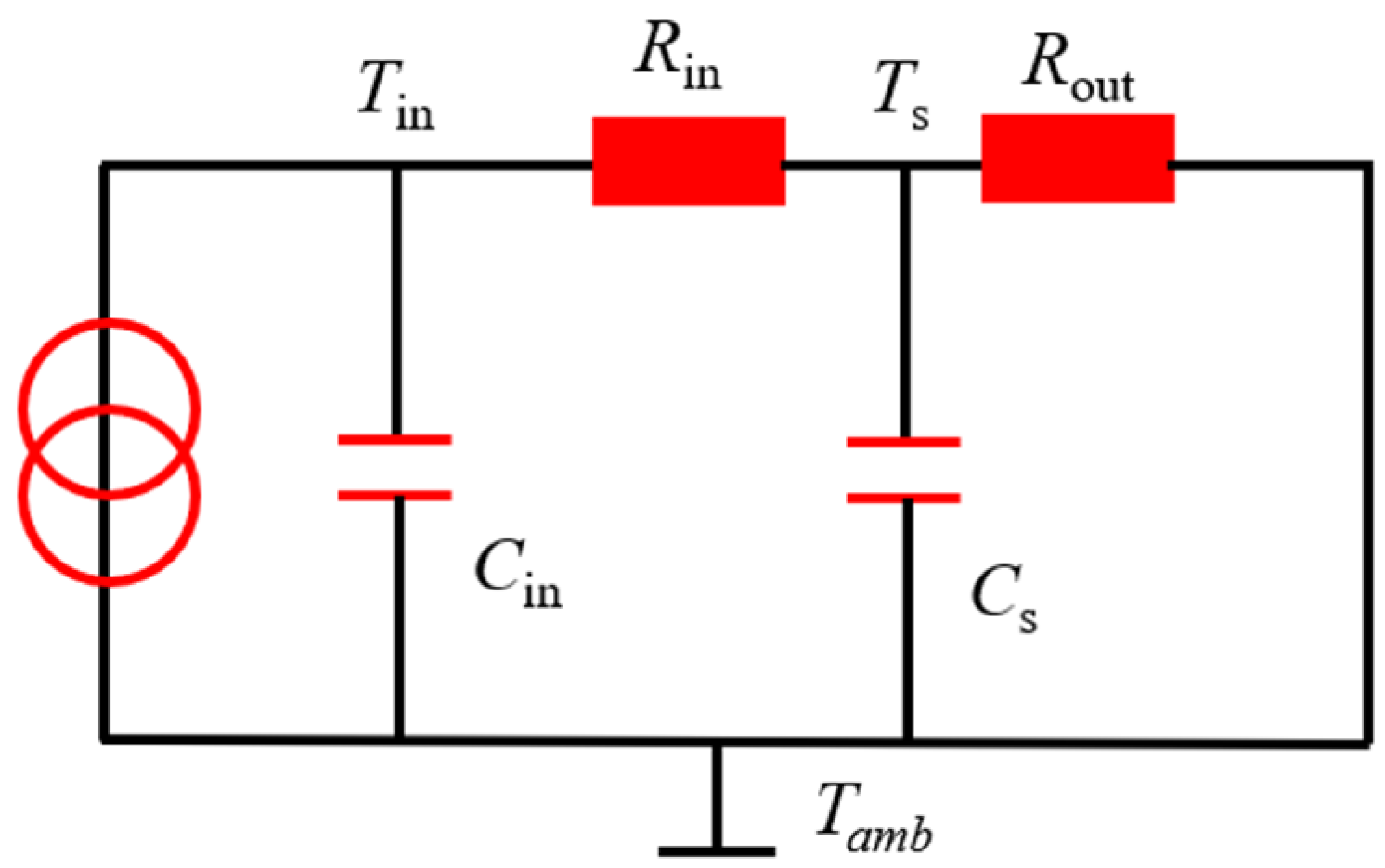
2.3. Estimation of Battery Core Temperature
2.3.1. Two-State Thermal Model
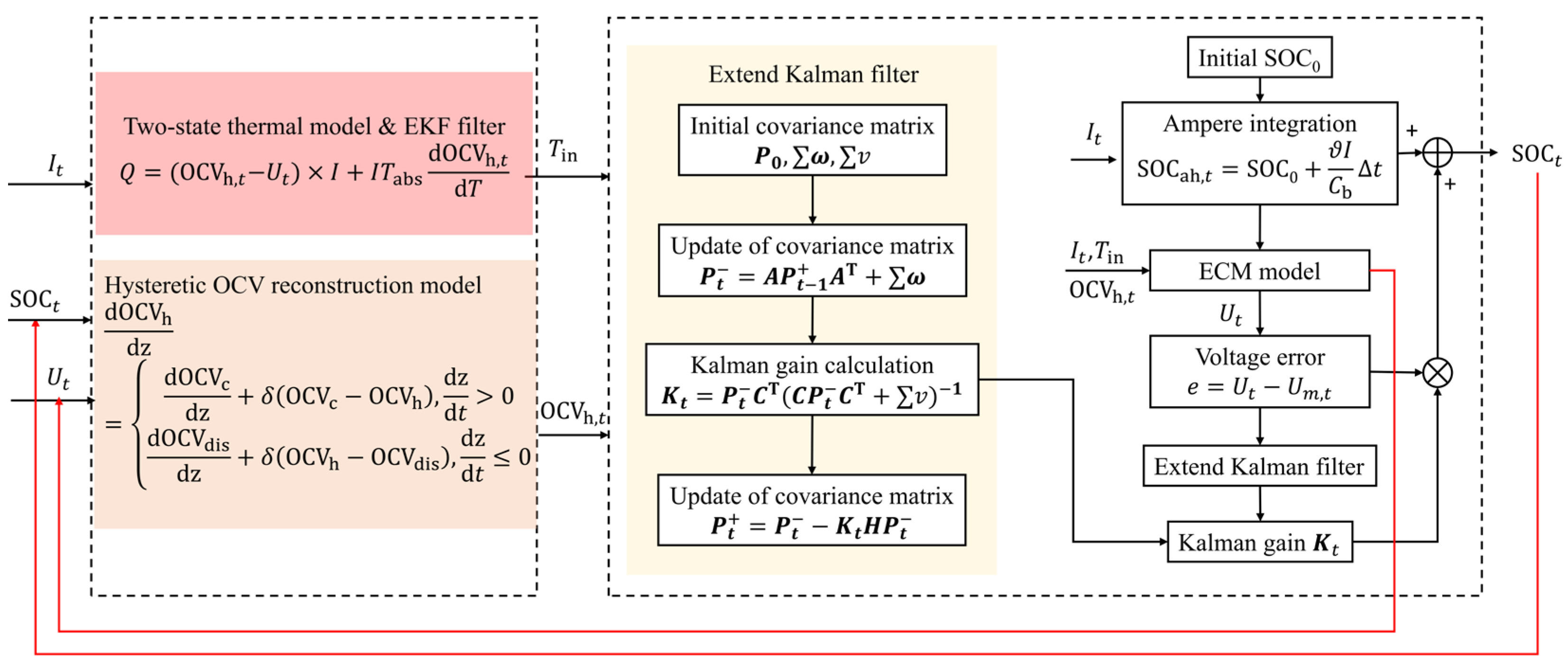
2.3.2. Battery Core Temperature Estimation Based on the Extended Kalman Filter (EKF) Algorithm
2.4. Terminal Voltage Simulation under Dynamic Frequency Regulation Working Conditions Using the Thermal-Electrochemical Coupling Model
3. SOC and RAE Estimation under Dynamic Frequency Regulation Working Condition
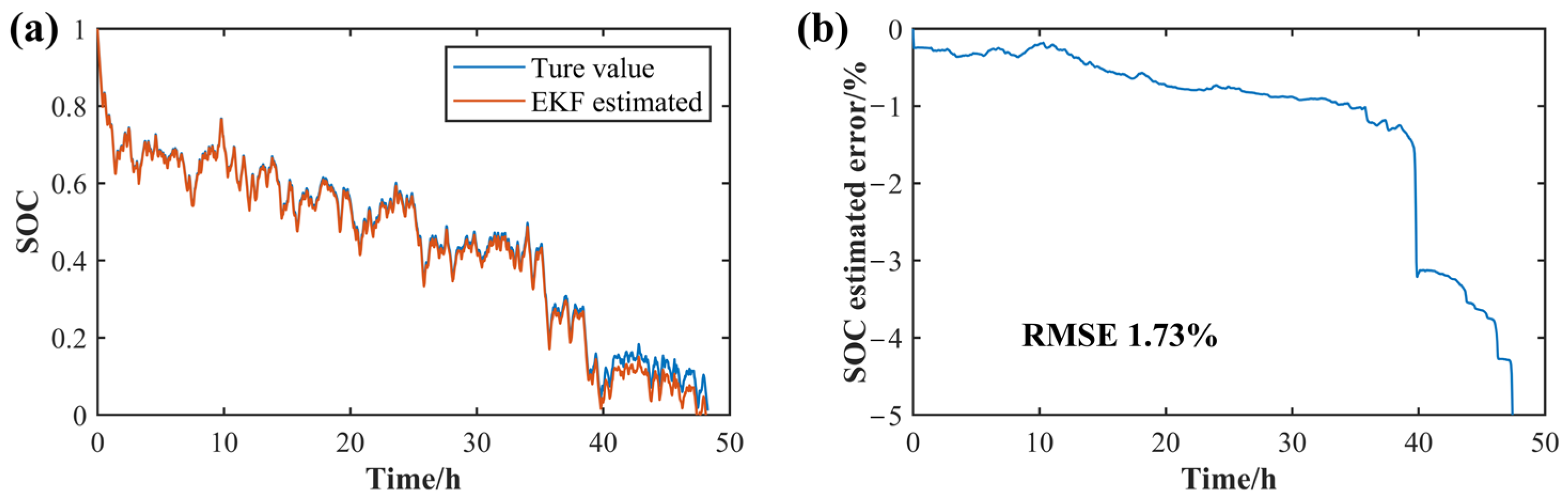
3.1. SOC Estimation Based on the EKF Algorithm
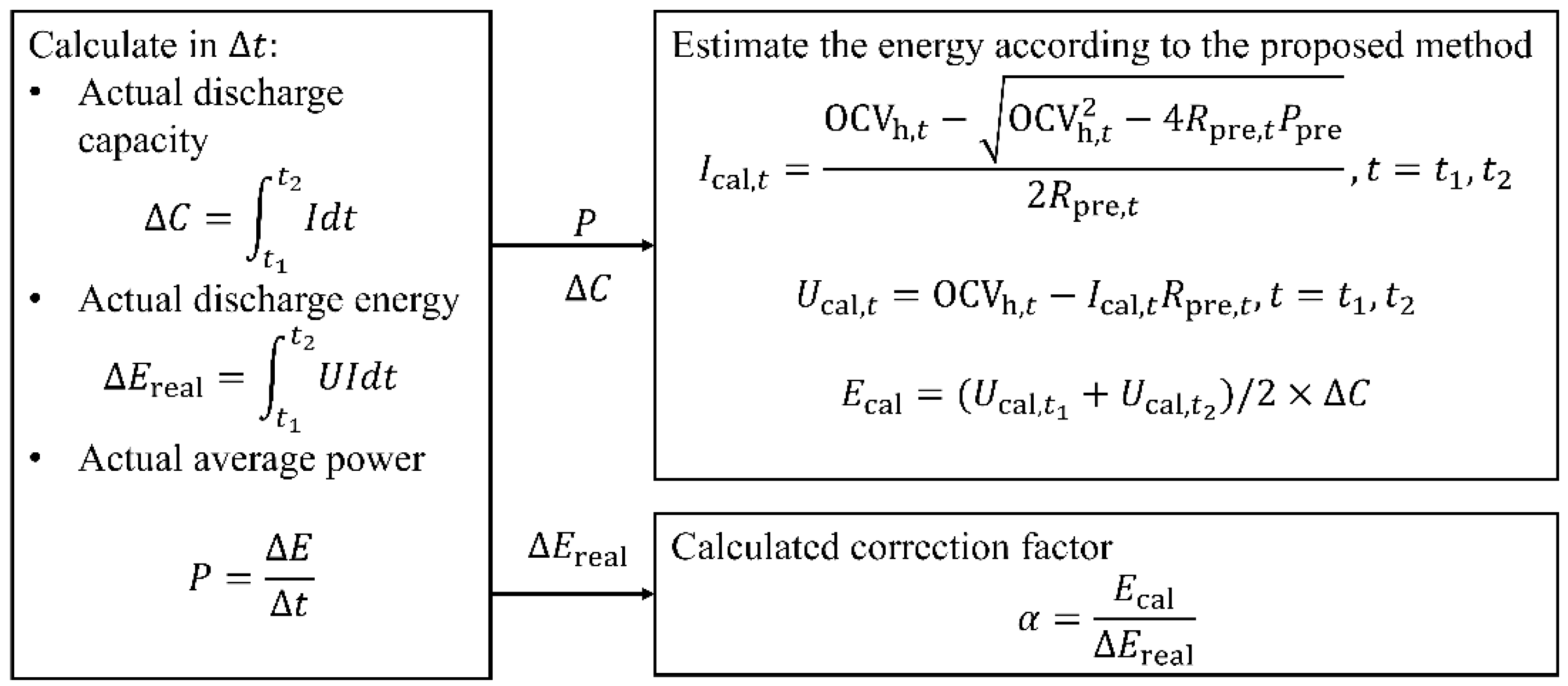
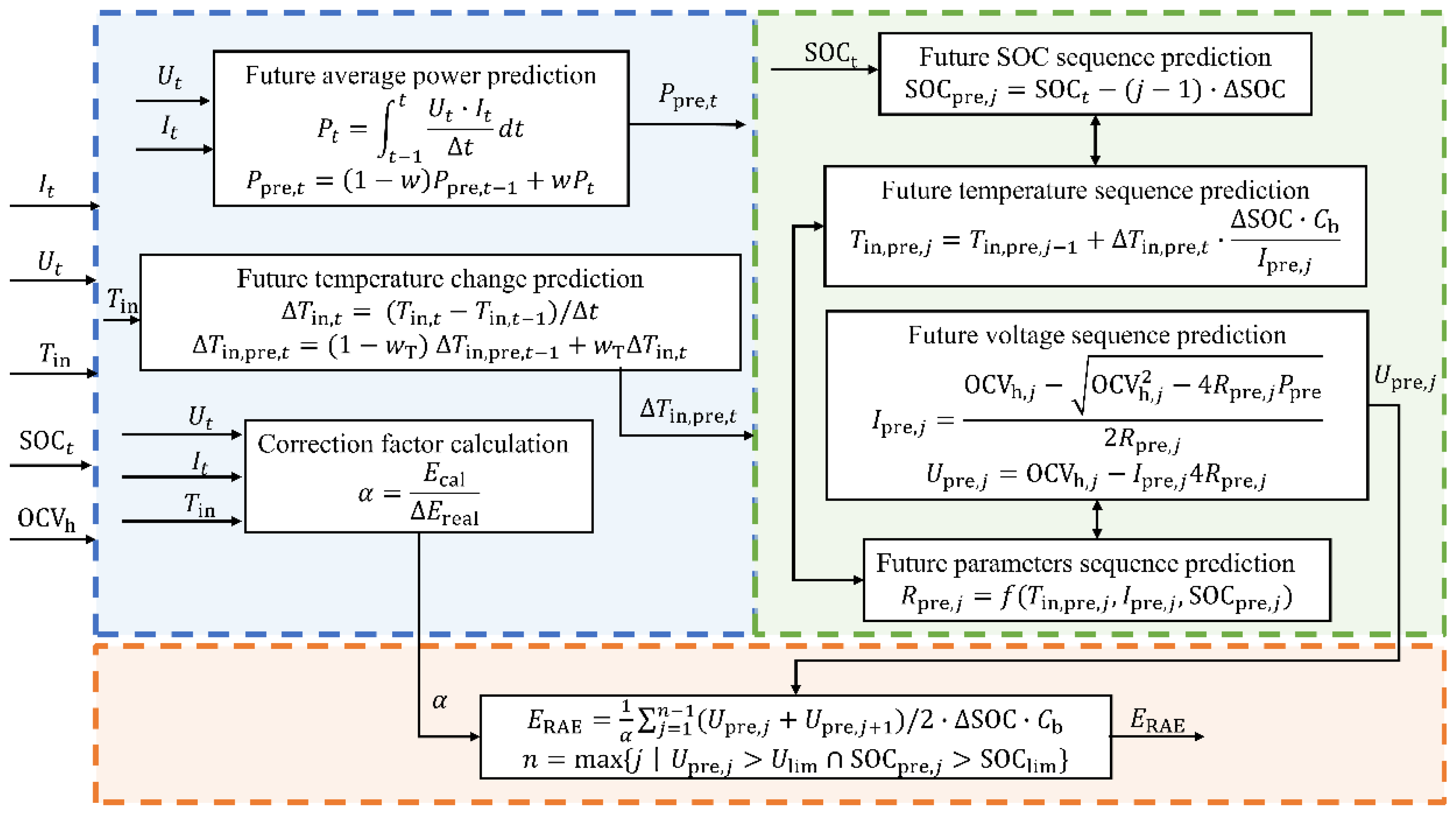
3.2. RAE Estimation Algorithm Based on Future Voltage Sequence Prediction
3.2.1. Future Operational Condition Prediction Based on Historical Information
3.2.2. Model-Based Future Voltage Prediction
3.2.3. Determination of Discharge Cutoff Point and Estimation of RAE
- (1)
- The use of the ohmic resistance value from the SRCM.
- (2)
- The Rint model is relatively simple, and there may be errors when calculating future voltage based on predicted future power values.
- (3)
- Even if the voltage prediction is accurate, there is an inherent approximation error in approximating the area of the light orange shaded region in Figure 13 using .
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Abbreviations | ||
| Item | Description | |
| FR | Frequency regulation | |
| LiFePO4 | Lithium iron phosphate | |
| EVs | Electric vehicles | |
| SOC | State of charge | |
| RAE | Remaining available energy | |
| OCV | Open-circuit voltage | |
| NNMs | Neural network models | |
| P2D | Pseudo-two-dimensions | |
| SP | Single particle | |
| SP2D | Simplified pseudo-two-dimensions | |
| ROM | Reduced-order model | |
| FUDS | Federal urban driving schedule | |
| DST | Dynamic stress test | |
| 1D | One-dimensional | |
| 2D | Two-dimensional | |
| 3D | Three-dimensional | |
| EKF | Extended Kalman filter | |
| KF | Kalman filter | |
| PF | Particle filter | |
| SOE | State of energy | |
| HVRM | Hysteresis voltage reconstruction model | |
| HPPC | Hybrid Pulse Power Characterization | |
| PSO | Particle Swarm Optimization | |
| MAP | Multi-Dimensional Parameter | |
| SRCM | Second-order RC equivalent circuit model | |
| CAN | Controller area network | |
| PJM | Pennsylvania-New Jersey-Maryland | |
| CC | Constant current | |
| CCCV | Constant current and constant voltage | |
| RMSE | Root mean square error | |
| Symbols | ||
| Item | Description | Unit |
| Hysteretic open circuit voltage. | V | |
| Main hysteresis charging voltage. | V | |
| Main hysteresis discharging voltage. | V | |
| Correction factor. | / | |
| z | State of charge. | / |
| The voltage across the i-th parallel RC branch, i = 1, 2. | V | |
| I | Current (positive for charging). | A |
| Ohmic resistance. | Ω | |
| Polarization resistance of the i-th parallel RC branch, i = 1, 2. | Ω | |
| Polarization capacitance of the i-th parallel RC branch, i = 1, 2. | Ω | |
| Time constant of the i-th parallel RC branch, i = 1, 2. | s | |
| Simulated voltage at time t. | V | |
| N | The number of voltage sampling data. | / |
| Measured voltage. | V | |
| Heat generation in the battery core. | W | |
| Absolute temperature. | °C | |
| Temperature change. | °C | |
| Internal temperature change. | °C | |
| The data sampling time (1 s in this study). | s | |
| The specific heat capacity of the battery core. | J/(kg·K) | |
| The internal thermal resistance. | K/W | |
| The thermal resistances in the width direction. | K/W | |
| The thermal resistances in the height direction. | K/W | |
| The thermal resistances in the length direction. | K/W | |
| The thermal conductivity coefficients in the width direction. | W/(m·K) | |
| The thermal conductivity coefficients in the height direction. | W/(m·K) | |
| The thermal conductivity coefficients in the length direction. | W/(m·K) | |
| The length of the battery. | m | |
| The width of the battery. | m | |
| The height of the battery. | m | |
| The specific heat capacity of the casing. | J/(kg·K) | |
| Surface temperature of the battery. | °C | |
| Ambient temperature. | °C | |
| External thermal resistance. | K/W | |
| W | Convective heat transfer coefficient. | W/(m2·K) |
| The state vector of the state-space equation. | / | |
| The output vector of the state-space equation. | / | |
| The input vector of the state-space equation. | / | |
| Process noise. | / | |
| Sampling noise. | / | |
| Parameter transition matrices. | / | |
| Initial covariance matrix. | / | |
| The prior estimate of the state variable. | / | |
| The posterior estimate of the state variable. | / | |
| Kalman gain matrix | / | |
| The measured surface temperature. | °C | |
| SOC value calculated through the ampere-hour integration method. | / | |
| Initial SOC value. | / | |
| Coulomb efficiency. | / | |
| The battery’s rated capacity. | Ah | |
| Remaining available energy. | Wh | |
| The future terminal voltage sequence. j denotes the index of the sequence point. | V | |
| n | The discharge cutoff point. | / |
| Average power during the time interval from time t − 1 to t. | W | |
| The predicted value of future average power. | W | |
| Weight factor. | / | |
| The average internal temperature change during the time interval from time t − 1 to t. | °C | |
| Predicted value of future internal temperature change. | °C | |
| Weight factor of temperature. | / | |
| The future SOC sequence. | ||
| The internal temperature at time t. | °C | |
| The predicted future internal temperature change. | °C | |
| Predicted future current value. | A | |
| The predicted output power. | Wh | |
| The predicted current value. | A | |
| The predicted value of future internal resistance parameters. | Ω | |
| The predicted future voltage sequence. | V | |
| The discharge cutoff voltage. | V | |
| The discharge cutoff SOC. | / | |
| Correction coefficient. | / | |
| The estimation error of the remaining available energy | / | |
| The estimated remaining available energy | Wh | |
| The true remaining available energy obtained through reverse power integration after discharging | Wh | |
References
- Li, Y.; Wei, Y.; Zhu, F.; Du, J.; Zhao, Z.; Ouyang, M. The path enabling storage of renewable energy toward carbon neutralization in China. Etransportation 2023, 16, 100226. [Google Scholar] [CrossRef]
- Dixon, J.; Bukhsh, W.; Bell, K.; Brand, C. Vehicle to grid: Driver plug-in patterns, their impact on the cost and carbon of charging, and implications for system flexibility. Etransportation 2022, 13, 100180. [Google Scholar] [CrossRef]
- Hu, G.; Huang, P.; Bai, Z.; Wang, Q.; Qi, K. Comprehensively analysis the failure evolution and safety evaluation of automotive lithium ion battery. Etransportation 2021, 10, 100140. [Google Scholar] [CrossRef]
- Wang, X.; Wei, X.; Zhu, J.; Dai, H.; Zheng, Y.; Xu, X.; Chen, Q. A review of modeling, acquisition, and application of lithium-ion battery impedance for onboard battery management. Etransportation 2021, 7, 100093. [Google Scholar] [CrossRef]
- Xu, C.; Li, L.; Xu, Y.; Han, X.; Zheng, Y. A vehicle-cloud collaborative method for multi-type fault diagnosis of lithium-ion batteries. Etransportation 2022, 12, 100172. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Jiang, J.; Zhang, L.; Zhang, W.; Wang, L.Y. Deduction of the transformation regulation on voltage curve for lithium-ion batteries and its application in parameters estimation. Etransportation 2022, 12, 100164. [Google Scholar] [CrossRef]
- Doyle, M.; Fuller, T.F.; Newman, J. Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J. Electrochem. Soc. 1993, 140, 1526. [Google Scholar] [CrossRef]
- Han, X.; Ouyang, M.; Lu, L.; Li, J. Simplification of physics-based electrochemical model for lithium ion battery on electric vehicle. Part I: Diffusion simplification and single particle model. J. Power Sources 2015, 278, 802–813. [Google Scholar] [CrossRef]
- Han, X.; Ouyang, M.; Lu, L.; Li, J. Simplification of physics-based electrochemical model for lithium ion battery on electric vehicle. Part II: Pseudo-two-dimensional model simplification and state of charge estimation. J. Power Sources 2015, 278, 814–825. [Google Scholar] [CrossRef]
- Rodríguez, A.; Plett, G.L.; Trimboli, M.S. Improved transfer functions modeling linearized lithium-ion battery-cell internal electrochemical variables. J. Energy Storage 2018, 20, 560–575. [Google Scholar] [CrossRef]
- Hu, X.; Feng, F.; Liu, K.; Zhang, L.; Xie, J.; Liu, B. State estimation for advanced battery management: Key challenges and future trends. Renew. Sustain. Energy Rev. 2019, 114, 109334. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.; Sun, Z.; Wang, L.; Xu, R.; Li, M.; Chen, Z. A comprehensive review of battery modeling and state estimation approaches for advanced battery management systems. Renew. Sustain. Energy Rev. 2020, 131, 110015. [Google Scholar] [CrossRef]
- Hu, X.; Li, S.; Peng, H. A comparative study of equivalent circuit models for Li-ion batteries. J. Power Sources 2012, 198, 359–367. [Google Scholar] [CrossRef]
- Srinivasan, V.; Newman, J. Existence of path-dependence in the LiFePO4 electrode. Electrochem. Solid-State Lett. 2006, 9, A110. [Google Scholar] [CrossRef]
- Dreyer, W.; Jamnik, J.; Guhlke, C.; Huth, R.; Moškon, J.; Gaberšček, M. The thermodynamic origin of hysteresis in insertion batteries. Nat. Mater. 2010, 9, 448–453. [Google Scholar] [CrossRef]
- Huria, T.; Ludovici, G.; Lutzemberger, G. State of charge estimation of high power lithium iron phosphate cells. J. Power Sources 2014, 249, 92–102. [Google Scholar] [CrossRef]
- Mao, S.; Han, M.; Han, X.; Lu, L.; Feng, X.; Su, A.; Wang, D.; Chen, Z.; Lu, Y.; Ouyang, M. An Electrical–Thermal Coupling Model with Artificial Intelligence for State of Charge and Residual Available Energy Co-Estimation of LiFePO4 Battery System under Various Temperatures. Batteries 2022, 8, 140. [Google Scholar]
- Wang, Z.; Du, C. A comprehensive review on thermal management systems for power lithium-ion batteries. Renew. Sustain. Energy Rev. 2021, 139, 110685. [Google Scholar] [CrossRef]
- Wang, T.; Tseng, K.-J.; Yin, S.; Hu, X. Development of a one-dimensional thermal-electrochemical model of lithium ion battery. In Proceedings of the IECON 2013-39th Annual Conference of the IEEE Industrial Electronics Society, Vienna, Austria, 10–13 November 2013; pp. 6709–6714. [Google Scholar]
- Xu, M.; Zhang, Z.; Wang, X.; Jia, L.; Yang, L. Two-dimensional electrochemical–thermal coupled modeling of cylindrical LiFePO4 batteries. J. Power Sources 2014, 256, 233–243. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Bahiraei, F.; Nazri, G.-A.; Saif, M. Electrochemical–thermal model of pouch-type lithium-ion batteries. Electrochim. Acta 2017, 247, 569–587. [Google Scholar] [CrossRef]
- Lin, X.; Perez, H.E.; Mohan, S.; Siegel, J.B.; Stefanopoulou, A.G.; Ding, Y.; Castanier, M.P. A lumped-parameter electro-thermal model for cylindrical batteries. J. Power Sources 2014, 257, 1–11. [Google Scholar] [CrossRef]
- Dai, H.; Zhu, L.; Zhu, J.; Wei, X.; Sun, Z. Adaptive Kalman filtering based internal temperature estimation with an equivalent electrical network thermal model for hard-cased batteries. J. Power Sources 2015, 293, 351–365. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, J.; Wang, Z.; Jiang, Y. Study of SOC Estimation by the Ampere-Hour Integral Method with Capacity Correction Based on LSTM. Batteries 2022, 8, 170. [Google Scholar] [CrossRef]
- He, H.; Zhang, X.; Xiong, R.; Xu, Y.; Guo, H. Online model-based estimation of state-of-charge and open-circuit voltage of lithium-ion batteries in electric vehicles. Energy 2012, 39, 310–318. [Google Scholar] [CrossRef]
- Ning, B.; Xu, J.; Cao, B.; Wang, B.; Xu, G. A sliding mode observer SOC estimation method based on parameter adaptive battery model. Energy Procedia 2016, 88, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Plett, G.L. Extended Kalman filtering for battery management systems of LiPB-based HEV battery packs: Part 3. State and parameter estimation. J. Power Sources 2004, 134, 277–292. [Google Scholar] [CrossRef]
- Sassi, H.B.; Errahimi, F.; Es-Sbai, N.; Alaoui, C. Comparative study of ANN/KF for on-board SOC estimation for vehicular applications. J. Energy Storage 2019, 25, 100822. [Google Scholar] [CrossRef]
- Xia, B.; Sun, Z.; Zhang, R.; Cui, D.; Lao, Z.; Wang, W.; Sun, W.; Lai, Y.; Wang, M. A comparative study of three improved algorithms based on particle filter algorithms in soc estimation of lithium ion batteries. Energies 2017, 10, 1149. [Google Scholar] [CrossRef]
- Lin, C.; Mu, H.; Xiong, R.; Shen, W. A novel multi-model probability battery state of charge estimation approach for electric vehicles using H-infinity algorithm. Appl. Energ. 2016, 166, 76–83. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, P.; Cheng, Z. A novel deep neural network model for estimating the state of charge of lithium-ion battery. J. Energy Storage 2022, 54, 105308. [Google Scholar] [CrossRef]
- El Fallah, S.; Kharbach, J.; Hammouch, Z.; Rezzouk, A.; Jamil, M.O. State of charge estimation of an electric vehicle’s battery using Deep Neural Networks: Simulation and experimental results. J. Energy Storage 2023, 62, 106904. [Google Scholar] [CrossRef]
- Hong, J.; Wang, Z.; Chen, W.; Wang, L.-Y.; Qu, C. Online joint-prediction of multi-forward-step battery SOC using LSTM neural networks and multiple linear regression for real-world electric vehicles. J. Energy Storage 2020, 30, 101459. [Google Scholar] [CrossRef]
- Cui, Z.; Kang, L.; Li, L.; Wang, L.; Wang, K. A hybrid neural network model with improved input for state of charge estimation of lithium-ion battery at low temperatures. Renew. Energ 2022, 198, 1328–1340. [Google Scholar] [CrossRef]
- Fasahat, M.; Manthouri, M. State of charge estimation of lithium-ion batteries using hybrid autoencoder and Long Short Term Memory neural networks. J. Power Sources 2020, 469, 228375. [Google Scholar] [CrossRef]
- He, H.; Zhang, Y.; Xiong, R.; Wang, C. A novel Gaussian model based battery state estimation approach: State-of-Energy. Appl. Energ. 2015, 151, 41–48. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, C.; Li, S.; Zhou, W. State monitor for lithium-ion power battery pack. In Proceedings of the 2010 International Conference on Measuring Technology and Mechatronics Automation, Changsha, China, 13–14 March 2010; pp. 481–484. [Google Scholar]
- Liu, G.; Ouyang, M.; Lu, L.; Li, J.; Hua, J. A highly accurate predictive-adaptive method for lithium-ion battery remaining discharge energy prediction in electric vehicle applications. Appl. Energ. 2015, 149, 297–314. [Google Scholar] [CrossRef]
- Esfahani, M.M.; Mohammed, O. Real-time distribution of en-route Electric Vehicles for optimal operation of unbalanced hybrid AC/DC microgrids. Etransportation 2019, 1, 100007. [Google Scholar] [CrossRef]

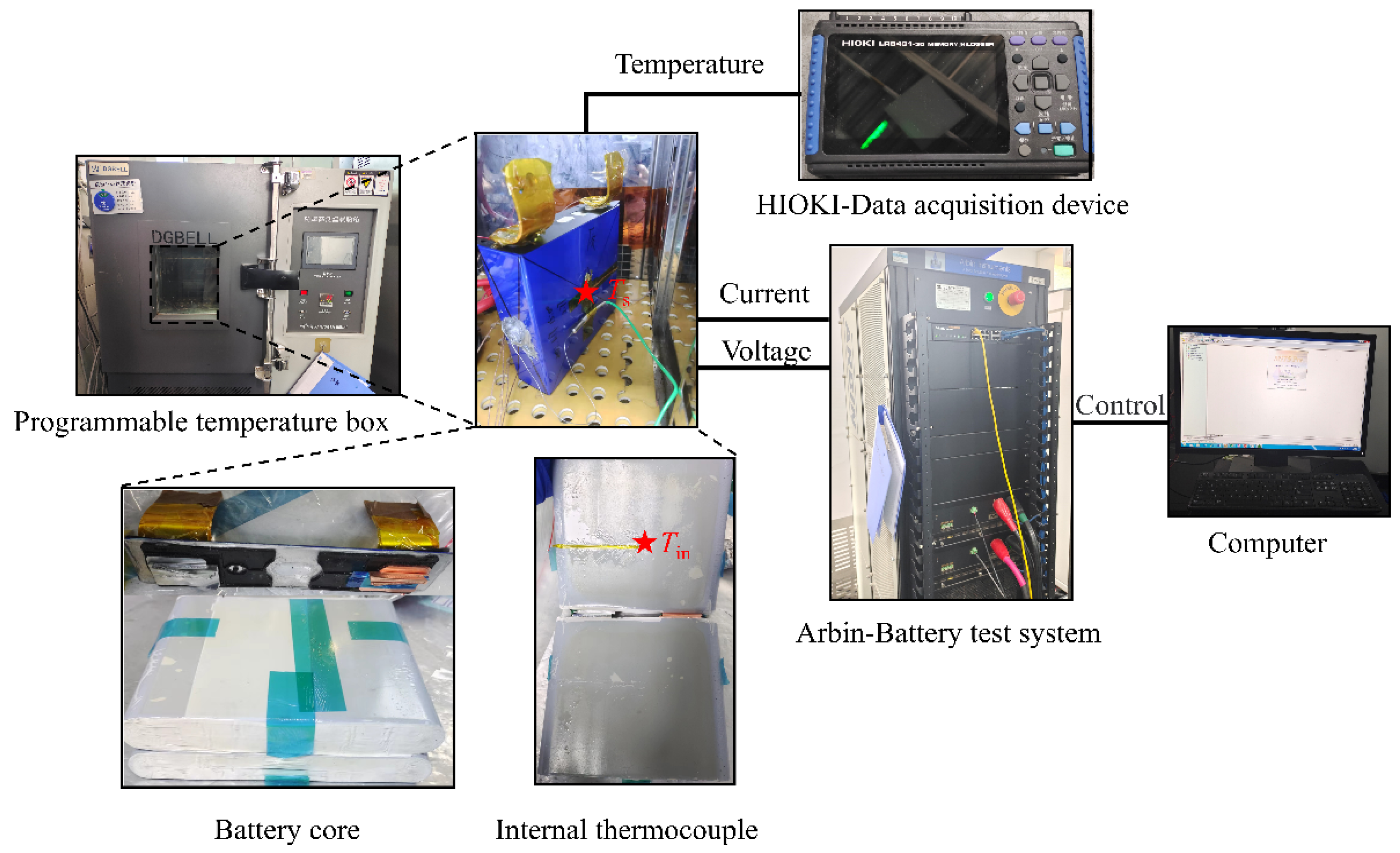






| Item | Specification |
|---|---|
| Cathode material | LiFePO4 |
| Anode material | Graphite |
| Nominal capacity | 120 Ah |
| Nominal voltage | 3.2 V |
| Rated discharging power | 190 W |
| Rated discharging energy | 380 Wh |
| Operating voltage | 2.5~3.65 V |
| Length × width × height | 174 mm × 48 mm × 170.5 mm |
| Weight | 2.86 kg |
| Item | Main Experiment Steps |
|---|---|
| 1. Battery capacity experiment | ① Discharge at a constant current (CC) of 1/3C at 25 °C until the cut-off voltage of 2.5 V. ② Charge at a constant current and constant voltage (CCCV) of 1/3C at 25 °C, with a cut-off current of 6 A (1/20C). ③ Allow the battery to rest for two hours at 25 °C. ④ Discharge at a CC of 1/3C at 25 °C until the cut-off voltage of 2.5 V. ⑤ Allow the battery to rest for two hours at 25 °C. ⑥ Repeat steps ② to ⑤ three times and take the average of the discharge capacities obtained from the three cycles. Experimental result: 127.64 Ah |
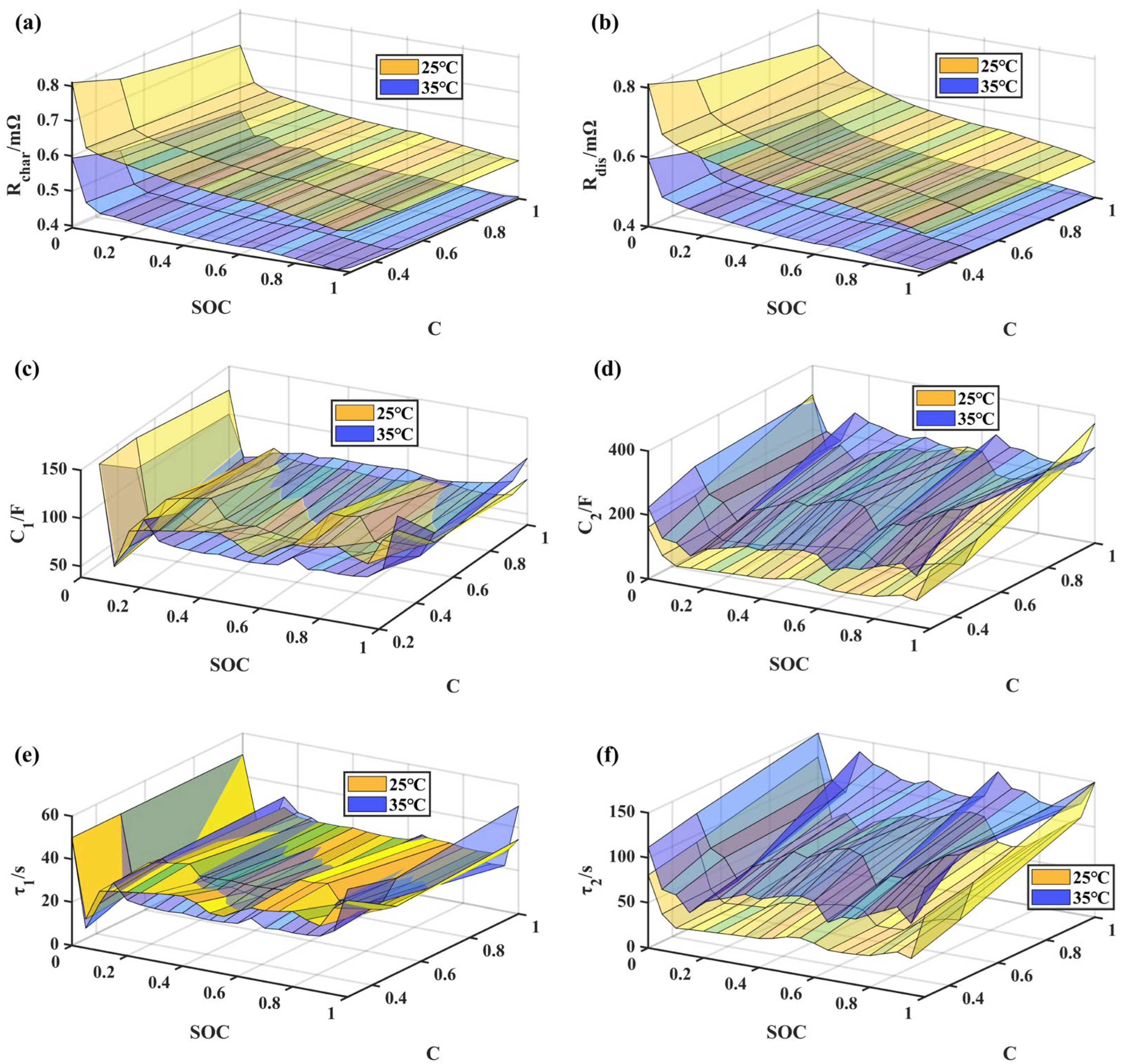
| 2. HPPC experiment | ① Charge at a CCCV of 1/3C at 25 °C, with a cut-off current of 6 A (1/20C). ② Perform SOC adjustment by discharging at 1/3C current and adjusting SOC by 0.05 at each step at temperatures of 25 °C and 35 °C. The SOC adjustment path: 1 → 0.95 → 0.9… 0.1 → 0.05 → 0. ③ Perform current pulse excitation at each SOC experimental point at temperatures of 25 °C and 35 °C:
Experimental results are shown in Figure 3a,b. |
| 3.1. Main hysteresis experiment | ① Charge at a CCCV of 1/3C at 25 °C, with a cut-off current of 6 A (1/20C). ② Adjust SOC by discharging at a current of 1/3C at temperatures of 25 °C and 35 °C, with each adjustment being 0.05 SOC. The SOC adjustment path is as follows: 1 → 0.95 → 0.9 →… → 0.1 → 0.05 → 0 → 0.05 → 0.1 → … → 0.95 → 1 ③ At temperatures of 25 °C and 35 °C, allow the battery to rest for 3 h after reaching each SOC node and record the OCV value. Experimental results are shown in Figure 3c. |
| 3.2. Minor hysteresis experiment-I | ① Charge at a CCCV of 1/3C at 25 °C, with a cut-off current of 6 A (1/20C). ② Adjust SOC by discharging at a current of 1/3C at temperatures of 25 °C and 35 °C, with each adjustment being 0.05 SOC. The SOC adjustment path is as follows: 1 → 0.95 → … → 0.15 → 0.2 → 0.25 → … → 0.75 → 0.8 → 0.75 → … → 0.15 → 0.2 → 0.25 → … → 0.95 → 1 ③ At temperatures of 25 °C and 35 °C, allow the battery to rest for 3 h after reaching each SOC node. Experimental results are shown in Figure 3d. |
| 3.3. Minor hysteresis experiment-II | ① Charge at a CCCV of 1/3C at 25 °C, with a cut-off current of 6 A (1/20C). ② Adjust SOC by discharging at a current of 1/3C at temperatures of 25 °C and 35 °C, with each adjustment being 0.05 SOC. The SOC adjustment path is as follows: 1 → 0.95 → … → 0.35 → 0.4 → 0.45 → … → 0.55 → 0.6 → 0.55 → … → 0.35 → 0.4 → 0.45 → … → 0.95 → 1 ③ At temperatures of 25 °C and 35 °C, allow the battery to rest for 3 h after reaching each SOC node. Experimental results are shown in Figure 3e. |
| 4. Battery entropy heat experiment. | ① Charge at a CCCV of 1/3C at 25 °C, with a cut-off current of 6 A (1/20C). ② Discharge at a current of 1/3C at 25 °C to adjust SOC, with each adjustment being 0.1. The SOC adjustment path is as follows: 1 → 0.9 → … → 0.1 → 0 ③ At each SOC node, adjust the temperature of the thermal chamber. The temperature adjustment path is as follows: 25 °C → 35 °C → 25 °C → 15 °C → 5 °C → −5 °C Rest for 5 h at 5 °C and −5 °C, and rest for 3 h at other temperature points. Calculate the derivative of OCV to temperature. Experimental results are shown in Figure 3f. |
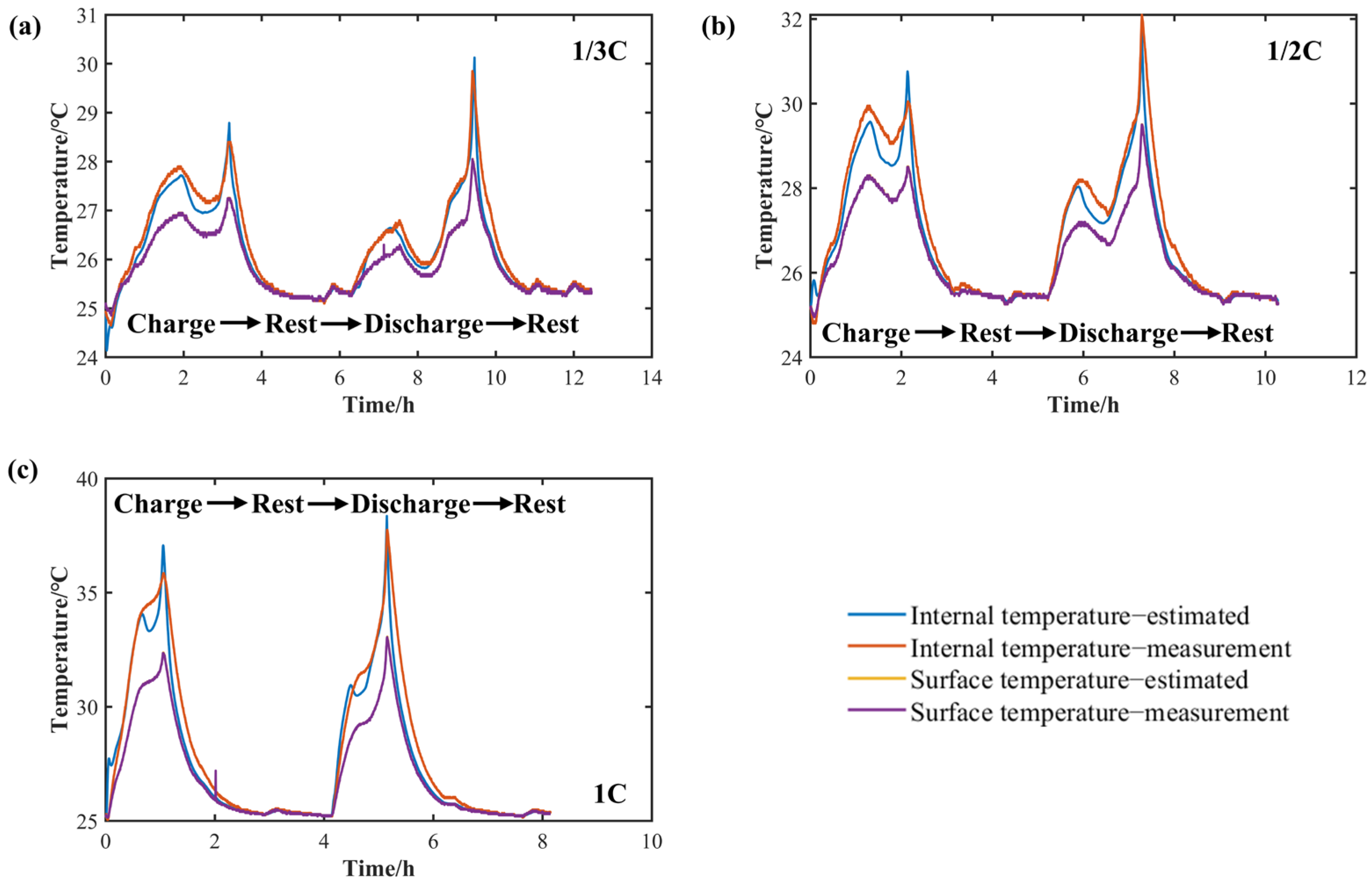
| 5. Multi-rate CC core temperature acquisition experiment. | ① Charge at a CC of 1/3C, 1/2C, and 1C at 25 °C until the upper cut-off voltage of 3.65 V. ② Discharge at a CC of 1/3C, 1/2C, and 1C at 25 °C until the lower cut-off voltage of 2.5 V. Experimental results are shown in the Section 2.3.2. |
| 6. FR working condition experiment | ① Charge at a CCCV of 1/3C at 25 °C, with a cut-off current of 6 A (1/20C). ② Simulate operating conditions using the processed FR instructions at 25 °C. The experimental results are shown in the Section 2.4. |
| Item | Symbol | Specification |
|---|---|---|
| Heat conductivity coefficient (W/(m·K)) | 7.5 | |
| 5 | ||
| 1.5 | ||
| Convective heat transfer coefficient (W/(m2·K)) | 5 | |
| Specific heat capacity (J/(kg·K)) | 880 | |
| 880 |
| Item | Mathematical Expression |
|---|---|
| Initialize state variables and covariance matrix | |
| Prior estimation | |
| Update covariance matrix | |
| Calculate Kalman gain | |
| Posterior estimation | |
| Update covariance matrix |
| Item | 1/3C | 1/2C | 1C |
|---|---|---|---|
| Internal temperature simulation RMSE (°C) | 0.22 | 0.39 | 0.75 |
| Surface temperature simulation RMSE (°C) | 0.007 | 0.008 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Lu, L.; Li, Y.; Wang, H.; Ouyang, M. Accurate Remaining Available Energy Estimation of LiFePO4 Battery in Dynamic Frequency Regulation for EVs with Thermal-Electric-Hysteresis Model. Energies 2023, 16, 5239. https://doi.org/10.3390/en16135239
Zhang Z, Lu L, Li Y, Wang H, Ouyang M. Accurate Remaining Available Energy Estimation of LiFePO4 Battery in Dynamic Frequency Regulation for EVs with Thermal-Electric-Hysteresis Model. Energies. 2023; 16(13):5239. https://doi.org/10.3390/en16135239
Chicago/Turabian StyleZhang, Zhihang, Languang Lu, Yalun Li, Hewu Wang, and Minggao Ouyang. 2023. "Accurate Remaining Available Energy Estimation of LiFePO4 Battery in Dynamic Frequency Regulation for EVs with Thermal-Electric-Hysteresis Model" Energies 16, no. 13: 5239. https://doi.org/10.3390/en16135239





