Abstract
The objective of this work was to determine the potential of chicken manure as a substrate for biogas production after pretreatment. The effects of removing excess nitrogen from chicken manure by water extraction in a temperature range from 20 °C to 60 °C to increase methane production were investigated. The dynamics of the process and efficiency of biogas production were also analyzed. As a result of manure fermentation after pretreatment, 16 to 45% more biogas and 18 to 39% more methane were obtained compared to manure without pretreatment. The effect of extraction was to increase the ratio of carbon to nitrogen by 2–2.7 times, which contributed to increasing biogas efficiency. The proposed method seems to be a promising enhancing of biogas and methane production in comparison with raw chicken manure. Biomass in the form of chicken manure is a promising substrate for biogas production, due to the constantly growing poultry meat production as well as environmental aspects such as reducing gas emissions from manure into the atmosphere.
1. Introduction
World production of poultry meat between 2000 and 2017 almost doubled. Chicken represents the most commonly consumed poultry bird worldwide. As a result of the continuous development of poultry breeding and intensified poultry meat consumption, the amount of generated waste by the poultry industry is rapidly increasing. It is worth noting that in parallel with the increasing production of eggs and poultry meat, the amount of animal manure also increases [1,2,3].
Poland is the leader in poultry farming in Europe. The United Kingdom, France, Germany, Spain and Italy are also among the leading producers. It was estimated that in 2017 the total amount of poultry production was 192.1 million, of which the amount of chickens was estimated at 176.7 million [4,5]. The growing world poultry industry produces around 20,708 million tons of chicken manure annually, of which Asia produces 11,514 million tons and Europe contributes almost 2039 million tons, around one tenth of the global total. A total of 30–40% of this waste is being utilized as a feedstock for anaerobic digestion processes [6]. According to the literature, a poultry bird during 47 days of growth produces about 1 kg of poultry litter [7], while 1000 chicken broilers produce 65 kg of manure per day [8].
The growing demand for more expensive mineral fertilizers as well as the slow depletion of deposits are among the factors stimulating the development of modern technologies and economic opportunities for chicken manure (CM) utilization and nutrients recovery. Methane fermentation is considered to be the best technique for CM management due to the possibility of waste reduction and energy recovery [9,10]. However, despite the high biogas potential due to easily degradable organic matter, the nitrogen present in CM results in significant amounts of ionic and free ammonia being formed during feedstock protein hydrolysis, which in turn results in the inhibition of the process [11]. This effect can be reduced by the addition of supplements (e.g., magnetite, zeolite) or essential stimulants to improve microbial metabolism [7,12]. On the other hand, the addition of heavy metals may limit the subsequent use of digestate [13].
Other biological utilization processes—e.g., composting—allow the weight, volume and water content of CM to be reduced. Organic matter is safely stabilized, which reduces the risk of nutrient pollution. Compost is also pathogen free [2]. It is worth noting that high-quality compost is produced mostly from a mixture of CM and C-rich feedstock materials [14]. Nevertheless, during the composting process, nitrogen is lost through ammonia and N2O volatilization, denitrification and leaching. It is estimated that 50–88% of total nitrogen from CM is lost during composting [15,16,17]. Intensive poultry production not only increases the amount of biowaste in the form of manure, slaughter waste or dead birds but also increases the emission of ammonia, methane and carbon dioxide, which are greenhouse gases [16].
Alternative CM management methods include combustion, pyrolysis or gasification. Thermal methods allow for the reduction of negative aspects related to the utilization of manure in biological processes, however pyrolysis or gasification can be expensive, energy consuming and require complicated equipment. Whereas, CM combustion must be preceded by energy-intensive drying and the process itself may contribute to NOx emissions; therefore, effective methods of managing and recovering biogenic substances from manure are sought [18,19,20].
The pursuit of energy security and care for environmental aspects, in the form of greenhouse gas emissions, translates into growing interest in the development of renewable bioenergy, which is an alternative energy source to fossil fuels [21]. Biomass has a great potential as a source of renewable energy via biofuels production, electricity or heat [22]. Its use could significantly reduce the amount of greenhouse gas emissions, contributing to the mitigation of environmental crises such as climate change and global warming [23]. Biogas is a biofuel, and is also a promising way to meet the global energy demand and provide many environmental benefits [24].
Intensively reared chickens consume large amounts of protein and other nitrogen-containing substances. Utilization of dietary nitrogen is incomplete, therefore 50–80% of N is excreted. Therefore, the feces are rich in nitrogen, as well as P and K. Nitrogen in the feces is mainly excreted in the form of uric acid and undigested protein, which account for approximately 70% and 30% of excreted nitrogen, respectively. Excreted uric acid is rapidly converted to ammonia (FAN) by aerobic and anaerobic processes [25,26,27]. These forms of nitrogen can lead to inhibition of the methane fermentation process. Anaerobic processes also lead to the formation of the NH4+, which, next to ammonia (FAN), is the main form of inorganic nitrogen in the solution. Their sum is measured as total ammonia nitrogen (TAN) [28,29,30], however the FAN form is more inhibitory. FAN accumulation can cause instability of the fermentation process, which results in a decrease in the production of biogas and methane. It can even completely stop the fermentation. The ratio of concentrations of these two forms of nitrogen depends on the temperature and pH value [31]. Due to the high nitrogen content, the carbon to nitrogen ratio is only 5–10. This is less than manure from other livestock, food waste and activated sludge [11].
Depending on the source, the inhibitory concentration of TAN ranges from 1.5 to 7 g/L [31,32,33] and thus there is only low activity of methanogens. Such a large span is related to the origin of the substrate or the inoculum used, as well as environmental conditions and also from process temperature and pH [11]. It is recommended that the concentration of TAN should be below 3 g/L as this is a nontoxic range. Once the inoculum has been acclimated to high nitrogen concentrations, it is possible to successfully ferment at TAN concentrations up to 5 g/L [25]. In the case of fermentation with a high content of solids, a decrease in the activity of methanogens was found with increasing concentration of ammonium nitrogen. An increase from 170 mg/L to 3720 mg/L resulted in a 10% decrease in methanogen activity, while a 50% decrease was observed in the range of 4090–5550 mg/L [33,34]. Moreover, the TAN increase from 5800 mg/L to 6800 mg/L resulted in a 30% reduction in methane production [35]. It was found that the tolerance of thermophilic reactor on TAN was 8000 mg/L with free ammonia 2000 mg/L, whereas for mesophilic reactor it was to 16,000 mg/L with 1500 mg/L of FAN [36].
The problem of ammonia accumulation during manure fermentation can be solved by appropriate dilution of the substrate, so that it contains from 0.5% to 3% of dry matter content, however, due to the need to use significant amounts of water, this method is not economically attractive [25,37,38,39]. Another method, and the most popular, to avoid ammonia accumulation is co-digestion with lignocellulosic biomass, which is rich in carbon; this makes it possible to maintain an appropriate carbon to nitrogen ratio in the fermentation mass. Substrates like grass and different straws [6,10,40,41,42,43], maize silage [11] or agricultural or municipal wastes (organic fraction) [42,44,45,46] are used in co-digestion. The addition of 3% straw increased methane production by 18% compared to mono-fermentation of chicken manure [43]. Zhang et al. concluded that the HRT reduction relaxed the inhibition of ammonia nitrogen and improved methane fermentation performance at constant high OLR. However, a significant shortening of the process resulted in the leaching of microorganisms and thus the process failure [39].
Inorganic nitrogen and phosphorus can be removed from wastewaters of agro- and livestock production, by struvite generation which can be used as fertilizer [47,48,49,50,51]. Nutrients can also be taken up by plants [52]. Liquid rich in nutrients like N and P can be used for algae cultivation [53]. Precipitation of struvite in combination with anaerobic digestion is an advantage in the context of environment protection and renewable energy [54,55].
The aim of the study was to propose and investigate a simple pre-treatment method dedicated to chicken manure. In the present study, the removal of nutrients from chicken manure by water extraction depending on the temperature was investigated. The authors also analyzed the influence of pre-treatment of chicken manure on biogas and methane efficiency and compared it with chicken manure without pre-treatment. The dynamics of the process were also compared.
2. Materials and Methods
2.1. Substrates and Seeding Sludge
The seeding sludge for methane fermentation studies came from a mesophilic agricultural biogas plant, where mainly maize silage is fermented. To remove larger unfermented particles, the inoculum was sieved and then kept at 4 °C for two weeks to minimize its biogas production. The substrate for the pre-treatment and fermentation tests was chicken manure (CM), with straw as a bedding material. After being transported to the laboratory, the manure was ground down and stored at −18 °C until the tests. The day before the start of the pretreatment studies, the CM was stored at ambient temperature.
2.2. Pretreatment of Chicken Manure
The pretreatment consisted of extraction (by shaking) of chicken manure with water in the amount of 5 g fresh weight of manure per 100 mL of deionized water. The extraction was carried out for 10 min in a temperature range from 20 °C to 60 °C. After substrate extraction, in order to separate the liquid phase (LF) from the solid phase (SF), the samples were centrifuged for 10 min at 5000 rpm (MPW-223es, MPW Med. Instruments, Warsaw, Poland). The solid fractions of manure after centrifugation (SF-CM) were used in batch fermentation studies.
2.3. Physical and Chemical Analyses
In order to select the appropriate amount of fresh mass (FM) of inoculum and the substrate, parameters such as total solids (TS) [%FM] and volatile solids (VS) [%TS] were determined according to standard methods [56]. These parameters are also necessary for the calculation of the efficiency of biogas and methane production for the tested substrate in terms of m3/Mg FM, TS and VS. In addition, pH value (IJ44A, Ionode Pty. Ltd. Tennyson, Australia), oxygen-reduction potential (ORP) (ERPt-111, Elmetron, Zabrze, Poland) and conductivity (ECF-1, Elmetron, Zabrze, Poland) for liquid fractions of pretreated chicken manure were determined using a multifunction device (CX-705, Elmetron, Zabrze, Poland) with suitable electrodes. After the fermentation process, digestate samples were taken from each fermenter in order to determine TS, VS and pH value. Each parameter was measured three times in order to minimize experimental errors.
Liquid samples after chicken manure extraction were subjected to further chemical analyses. Ammonia, phosphate and chemical oxygen demand were determined with use of the cuvette test system (Hach Lange, Wrocław, Poland) and a DR-3900 spectrophotometer (Hach Lange, Wrocław, Poland). Samples were filtered prior to analysis using a test through a nylon syringe filter with a 0.45 µm membrane.
In order to analyze carbon to nitrogen ratio in solid phase after pretreatment, an Elemental Analyzer Flash 2000 (Thermo Scientific, Waltham, MA, USA) was used. The samples were dried and homogenized prior to the determination of C and N (weight %). Determination of C:N ratio consisted of the catalytic combustion of the sample in the appropriate amount of oxygen. The resultant gases are determined on the GC column with thermal conductivity detector and helium as a carrier gas.
2.4. Batch Fermentation
Fermentation studies were carried out in accordance with the modified German norm DIN 38 414 S8 and standardized guidelines for biogas issued by the Association of German Engineers in Dresden VDI 4630. The most important guidelines are appropriate reactor size (in range from 0.5 L to 2 L), content of volatile solids in the seeding sludge greater than 50%, the fermentation batch should contain from 1.5% to 2% by weight of VS from the seeding sludge and to prevent inhibition in the fermentation batch, and that the VS ratio of substrate to seeding sludge should not exceed 0.5. According to the guidelines, the criterion for completing the process is daily biogas production less than 1% of total production.
Methane fermentation was carried out in reactors with a capacity of 2 L, under mesophilic conditions at a temperature of 38 ± 2 °C. After placing the appropriate amounts of substrate and seeding sludge in the reactor, they were purged with nitrogen to create anaerobic conditions. Then the reactors were placed in a thermostatic water bath with a circulation pump, which simulated biogas plant conditions.
The bioreactor system works on the principle of connected vessels. Each biogas reactor is connected to a cylinder filled with barrier liquid in which the produced biogas is stored. The biogas that forms pushes the liquid out of the cylinder into the collection vessel. The liquid forms a barrier against the biogas and prevents its dissolution and diffusion. Each cylinder has a valve enabling gas sample collection.
On the basis of the VDI (Association of German Engineers) guidelines, batches contained 10.14 g VS/L of CM, 9.55 g VS/L of SFCW (solid fraction after cold water extraction) and 9.46 g VS/L of SFHW (solid fraction after hot water extraction).
Each experiment was performed in triplicate, including the control sample, which is the seeding sludge, without substrate addition.
2.5. Biogas Measurements
Measurements of the produced biogas were performed daily with an accuracy of 0.01 dm3. The qualitative and quantitative analysis was analyzed when its volume in the cylinder was at least 0.45 dm3, using a portable biogas analyzer (GA5000, Geotech, Manchester, UK).
The analyzer had ATEX II 2G Ex ib IIA T1 Gb (Ta = −10 °C to +50 °C), IECEx and CSA quality certificates and UKAS ISO 17025 calibration certificate.
The device enables the determination of the main components of biogas—methane and carbon dioxide— as well as oxygen, hydrogen sulfide and hydrogen. The quantification range is 0–100%, 0–100%, 0–25%, 0–5000 ppm and 0–1000 ppm, respectively. The device was calibrated at least twice a week.
When the volume of biogas was insufficient to use the analyzer (below 0.45 dm3), especially at the end of the process, a gas chromatograph (GC) was used for qualitative and quantitative determination, equipped with a thermal conductivity detector (TCD). Argon with a flow rate of 0.6 mL/h was used as a carrier gas. A ShinCarbon ST (Shimadzu)® with characteristics of 2 m/2 mm ID (inner diameter) 1/8″ OD (outer diameter), Silico was used.
Cumulative biogas and methane production were normalized to standard conditions (dry gas, 0 °C and 1013.25 hPa) [57,58] according to Equation (1):
where VSTP is volume (standard temperature and pressure), PSTP is the standard pressure (hPa), Pgas is the pressure for the measured gas, Tgas is temperature (K), TSTP is normal temperature—0 °C and Vgas is volume of measured gas.
2.6. Conversion Efficiency
At the end of the experiment VS [%] conversion was calculated for all mixtures in this study using Equation (2):
where VSb and VSe are the amounts of VS in the reactor at the beginning and at the end of the batch test, respectively. This parameter allows the assessment of the degree of conversion of volatile solids into biogas [40].
3. Results and Discussion
3.1. Sample Characterization
The results of physicochemical parameters of liquid samples after manure extraction and centrifugation depending on temperature are presented in Table 1 and in Figure 1.

Table 1.
Characterization of liquid phase after chicken manure extraction depending on the temperature.
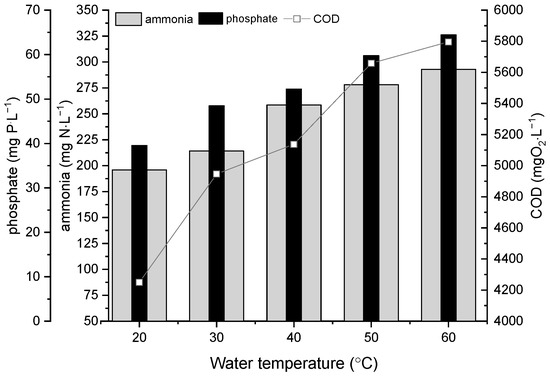
Figure 1.
Chemical characterization of liquid phase of chicken manure depending on extraction temperature.
With increasing temperature of water extraction, increasing amount of ammonium in LP-CM (liquid-phase chicken manure) was observed, from 196 mg NH4-N/L to 295 mg NH4-N/L, which is connected with increasing compounds solubility depending on temperature. For extraction at 50 °C and 60 °C, 42% and 50% more ammonium was extracted, respectively, compared to 20 °C. The increase of extraction temperature from 20 °C to 60 °C led to the growth of the phosphate content in the liquid phase from 32% to 63% (Figure 1).
The increasing extraction temperature also resulted in an increase of COD (Chemical Oxygen Demand) level in liquids from 16% to 36% compared to the extraction at 20 °C.
In the case of conductivity and ORP increasing and decreasing a trend was observed, respectively. For the mentioned parameters, an inverse relationship was observed. Increasing conductivity with temperature of tested liquids were connected with increasing amount of ammonia and phosphate in liquids (Table 1).
On the basis of the results obtained, for further experiments solid fractions of manure after extraction at 20 °C (CW) and 50 °C (HW) were used, the reference test was chicken manure (CM) without pretreatment in the form of extraction.
The environmental characterization data of inoculum, CM and SF are provided in Table 2.

Table 2.
The physicochemical characterization of substrates used in methane fermentation experiments.
The TS of raw chicken manure in our study is comparable with data from the literature [11,59]. The lower content of dry matter (approx. 25%) may be caused by excluding bedding material during breeding [60,61,62]. The reduction of TS in SF results from the extraction process. The hydration of solid phases after pretreatment process increased about three times. The content of VS was on a similar level. As a result of the extraction process, a decrease in pH was observed. A significant change was observed in the C:N ratio. For chicken manure used in this research, the determined carbon to nitrogen ratio was 10:1 [63,64,65]. As confirmed by others, as a result of the proposed pre-treatment, this ratio increased to 20 and 27 for manure treated with the extraction liquid at 20 °C and 50 °C, respectively.
The literature review shows that the optimal carbon to nitrogen ratio in the substrate is in the range of 20–30. If the carbon content is too low in relation to the nitrogen content, the efficiency of the anaerobic process may be limited. The optimal content of these elements affects the methane content in biogas, while a too low nitrogen content limits the activity of microorganisms involved in fermentation. This is because microorganisms need the right amount of N in ammonia form to grow, its limited amount makes carbon inaccessible for the production of methane [24,38,40,41,66].
3.2. Dynamics of the Biogas Production and Hydraulic Retention Time
Dynamic of methane fermentation of tested substrates is shown in Figure 2. A significant difference in dynamics can be observed depending on the substrate.
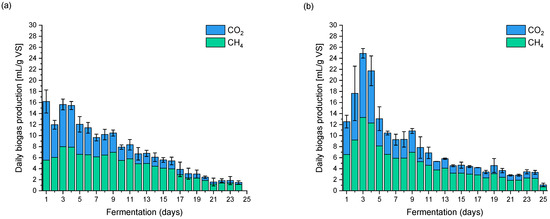
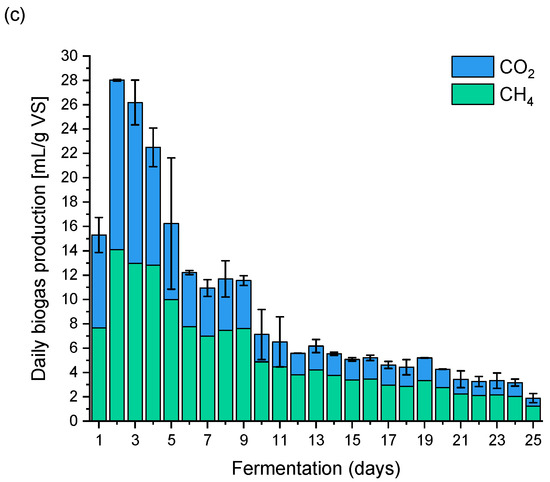
Figure 2.
Biogas dynamics production (a) CM without pretreatment; (b) SFCW; (c) SFHW.
During the fermentation of CM without water extraction treatment, several successive peaks can be observed of a similar intensity, indicating the complex structure of the fermented substrate. Based on the dynamics of the process, it can be concluded that the various stages of fermentation occurred in parallel. The situation was different on the first day, where a greater share of carbon dioxide in the produced gas was observed, which may indicate the ongoing hydrolysis of the substrate. At the end of the first week and at the turn of the first and second week of fermentation (between 4 and 8 days), a slight decrease in methane production was observed, which may indicate inhibition of the fermentation process. Fermentation of chicken manure lasted 24 days.
The dynamics of manure samples’ fermentation after pre-treatment is significant compared to untreated manure; this is particularly evident in the first week of the process (Figure 2b,c). The dynamics of methane fermentation are comparable to those obtained by Li et al. [60] in their research; the authors used a substrate that had comparable hydration.
Regardless of the extraction temperature used, the dynamics of the fermentation processes were similar. During the SFCW fermentation, clear peaks of biogas production were observed, with the most intense peaks being on the third day of the process, and several smaller peaks in the second and third week of fermentation (9th, 13th and 19th days). As previously mentioned, the dynamics of the fermentation after the treatment of Figure 2c) was similar because clear biogas production peaks were also observed in the first week of the process on (2nd, 3rd and 4th days) and less intense in the second week and at the end of the experiment (8th, 9th and 19th day).
In the case of fermentation of all samples, slight process breakdowns can be observed, manifested by a decrease in methane concentration in the biogas, falling between the fourth and eighth fermentation day.
As can be seen in Figure 3, the total methane fermentation time of the samples was comparable. The use of pre-treatment resulted in an extension of the process by one day, which is within the margin of error.
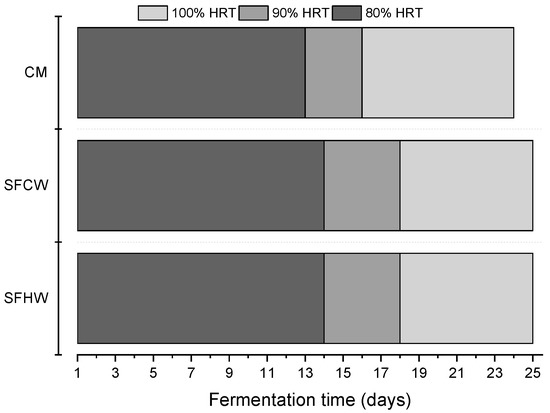
Figure 3.
Time required to reach 80%, 90% and 100% HRT.
It should be noted that fermentation tests are on a laboratory scale. The criterion for the completion of the process conducted in accordance with the guidelines is fermentation until the daily biogas production is less than 1% of the total biogas production. Thus, the sample is almost completely fermented. However, on a technological scale, in a continuous process, where the substrate is added continuously; the substrate is not completely digested. On a technological scale, the use of such a long HRT (hydraulic retention time) (lab scale) is economically unprofitable due to the low production of biogas in the last phase of fermentation [67]. It should also be noted that larger working volumes of the bioreactor involve the need to increase the energy to mix the suspension contained in it.
Figure 3 also shows the time in which the anaerobic decomposition of the substrate occurs in 80 and 90%. The 80% HRT for the substrates after pretreatment was one day longer (total 14 days) compared to untreated manure. The time necessary to reach 90% HRT for manure was 16 days, while for manure after treatment it was 18 days, in terms of the length of the entire process, it was 66.6% and 72%, respectively.
3.3. Biogas Efficiency and VS Reduction
Methane concentration in biogas for all tested substrates was comparable. Obtained biogas contained 60.29%, 60.38% and 57.28% methane for CM, SFCW and SFHW, respectively. For fresh chicken manure 77.44 m3 of methane and 128.43 m3 of biogas per Mg FM (fresh matter) was recorded. Li et al. [60] obtained an average methane content of almost 65%.
The cumulative methane production in terms of fresh mass were 29.56 m3 and 32.46 m3 (about 62% and 58% lower), while cumulative biogas was 48.95 m3 and 56.67 m3 (about 62% and 56% lower), respectively, for SFCW and SFHW. The observed decrease in methane and biogas productivity is caused by a hydration of samples, for pretreated samples the water content was about 48% higher than for manure without pretreatment.
Figure 4 shows cumulative biogas and methane efficiency per ton (Mg) of organic dry matter. The largest biogas production was observed for SFHW (527.8 m3); for SFCW it was 420.6 m3 and for CM about 45% less than for SFHW (362.5 m3). An increase in biogas yield was observed of 16% and 45% for SFCW and SFHW, respectively. Obtained results are consistent with those presented by Böjti et al., where differences in gas production before and after water extraction from CM were recorded according to VDI methodology [68]. Lisboa and Lansing [62] and Li et al. [60] obtained similar amount of gases for chicken manure on a VS basis.
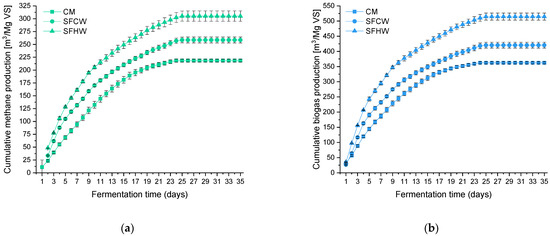
Figure 4.
Cumulative methane (a) and biogas (b) production for tested substrates during methane fermentation calculated for m3 of gas per one ton of organic dry matter.
The extraction process allowed for an increase in methane yield (Figure 4a) by 18% and 39% for SFCW and SFHW, respectively, per m3 per ton VS of substrate, compared to manure without pretreatment. Increasing the extraction temperature from 20 to 50 °C resulted in an increase in methane production by 18% and 25%, respectively, for biogas. A significant increase in the volume of biogas (Figure 4b) produced is related to the improvement of the carbon and nitrogen ratio. For the manure, the C:N ratio was only 10, which is consistent with data from the literature [11,43,60], while for the treated samples it was higher and ranged from 20 to 27. Values in the range of 20 to 30 are considered favorable for a stable fermentation process [65,66,69]. It was proven that by increasing the C to N ratio in the reactor, gas production is improved mostly by minimizing ammonia inhibition [33].
After the fermentation processes were completed, the VS reduction efficiency was found to be at a comparable level, ranging from 26.4 to 28.5% with an average of 27.3%. Bujoczek et al. [37] observed that the final reductions in VS were lowest for high TS samples, which causes lower biogas production rates. Li et al. [60] obtained 61% biodegradability after 30 days of digestion, while obtaining about 47% for their previous studies. They indicated that this may be due to the utilization of a different sludge and feedstock source.
The methane concentration in the obtained biogas was comparable for all tested substrates. However, the methane and biogas productivity was significantly lower for pretreated samples due to increased water content. The highest gas production was observed for the substrate SFHW, followed by SFCW and CM. The extraction process resulted in increased methane yield and improved carbon-to-nitrogen ratio, leading to higher biogas volume. Increasing the extraction temperature also increased methane production. Overall, the fermentation processes showed comparable levels of volatile solids reduction efficiency. These findings highlight the importance of pretreatment methods and substrate characteristics in optimizing methane production in biogas systems.
The physicochemical characterization of liquid samples extracted from chicken manure revealed that higher extraction temperatures increased the solubility of compounds, resulting in higher ammonium and phosphate content. Conductivity increased with temperature, indicating greater ammonia and phosphate presence, while the ORP decreased. The solid fractions obtained after extraction showed reduced total solids and a higher carbon-to-nitrogen ratio compared to untreated manure. During fermentation, untreated manure exhibited complex dynamics, while pretreated samples displayed clear biogas production peaks, with slight process breakdowns. Methane concentrations in biogas were comparable for all substrates, and cumulative methane and biogas production was lower for pretreated samples due to increased water content. However, methane yield per unit of volatile solids increased with extraction, reaching up to 38% improvement. The results suggest that extraction temperature and pretreatment influenced the efficiency of the anaerobic fermentation process, impacting biogas production and methane yield.
This comprehensive study aims to explore the potential of a simple pre-treatment method for chicken manure to remove nutrients and improve biogas and methane efficiency. By optimizing the management of chicken manure, it is possible to reduce waste, recover valuable nutrients, and harness renewable bioenergy, contributing to environmental sustainability and energy security.
The advantage of the proposed method is that it is simple. In addition, no additional chemical reagents are used, which makes it environmentally friendly. The solid residue after extraction with a more favorable C:N ratio, used in the biogas plant, is an additional input in the form of heat and electricity, while the post-extraction liquid can be used for phosphorus recovery or for irrigation.
4. Conclusions
Chicken manure has a relatively high biogas production potential, however due to the high nitrogen content the process is inhibited due to the toxic effects on methanogen bacteria population [38].
The production of biogas from manure after applying pre-treatment in the extraction form seems to be an interesting alternative that allows an increase in its yield. Extraction with water allowed an increase in the carbon to nitrogen ratio in the range of 2–2.7 times, which resulted in a 16–45% increase in biogas and 16–38% methane production.
Process water savings are mainly related to the possible return of the extraction water back to the extraction process. There is also a possibility of recovering the extracted substances from the post-process solution and then recycling the “recovered solvent” to the process.
The global poultry industry has witnessed significant growth, leading to a surge in waste generation. Chicken manure, a major waste product, poses environmental challenges due to its high nitrogen content. To address this issue, researchers proposed a pre-treatment method involving water extraction at various temperatures. The study aimed to investigate the removal of nutrients from chicken manure and assess its impact on biogas and methane efficiency. The experiment involved extracting manure with water at temperatures ranging from 20 °C to 60 °C, separating the liquid and solid phases, and conducting batch fermentation tests. The results indicated that increasing the extraction temperature resulted in higher amounts of ammonium, phosphate and chemical oxygen demand in the liquid phase. Moreover, the pre-treatment process altered the carbon-to-nitrogen ratio, potentially optimizing the anaerobic digestion process. The dynamics of bio-gas production showed distinct patterns depending on the substrate, with treated manure exhibiting more consistent fermentation. Overall, the study provided insights into a simple and effective pre-treatment method for chicken manure, emphasizing its potential for waste management and energy recovery in the poultry industry.
The simple extraction method for chicken manure as a pretreatment technique offers several advantages. It is cost-effective, easy to implement, making it accessible and environmentally friendly. This method preserves the valuable organic nutrients in the substrate, improves methane yield, and is compatible with existing biogas infrastructure. Overall, the simple extraction method is a practical and efficient way to enhance biogas production from chicken manure while minimizing costs and environmental impact.
Author Contributions
Conceptualization, I.K.; methodology, I.K.; software, I.K.; validation, I.K. and L.Ś.; formal analysis, I.K.; investigation, I.K.; resources, I.K.; data curation, I.K.; writing—original draft preparation, I.K.; writing—review and editing, I.K., L.Ś. and A.C.; visualization, I.K; supervision, A.C.; project administration, A.C. and funding acquisition, I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the European Regional Development Fund within the Interreg South Baltic Programme 2014–2020, under project no. WASTEMAN STHB.02.02.00-0131/17 and Provincial Fund for Environmental Protection and Water Management in Gdansk under project no. WFOŚ/D/748/20/2018.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jurgutis, L.; Slepetiene, A.; Volungevicius, J.; Amaleviciute-Volunge, K. Biogas Production from Chicken Manure at Different Organic Loading Rates in a Mesophilic Full Scale Anaerobic Digestion Plant. Biomass Bioenergy 2020, 141, 105693. [Google Scholar] [CrossRef]
- Ksheem, A.M.; Bennett, J.M.L.; Antille, D.L.; Raine, S.R. Towards a Method for Optimized Extraction of Soluble Nutrients from Fresh and Composted Chicken Manures. Waste Manag. 2015, 45, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Stiborova, H.; Kronusova, O.; Kastanek, P.; Brazdova, L.; Lovecka, P.; Jiru, M.; Belkova, B.; Poustka, J.; Stranska, M.; Hajslova, J.; et al. Waste Products from the Poultry Industry: A Source of High-Value Dietary Supplements. J. Chem. Technol. Biotechnol. 2020, 95, 985–992. [Google Scholar] [CrossRef]
- Cieślak, A.; Dach, J.; Horbańczuk, J.; Józefiak, D.; Pszczoła, M.; Strabel, T.; Szumacher-Strabel, M.; Tomasik, C. Stock Take of Regional Activities and Needs in Relation to Livestock Research—Poland. In Proceedings of the Workshop on the Global Research Alliance on Agricultural Greenhouse Gases, Ministry of Agronomy, Warsaw, Poland, 8–9 March 2014. [Google Scholar]
- Dróżdż, D.; Wystalska, K.; Malińska, K.; Grosser, A.; Grobelak, A.; Kacprzak, M. Management of Poultry Manure in Poland – Current State and Future Perspectives. J. Environ. Manag. 2020, 264, 110327. [Google Scholar] [CrossRef]
- Bayrakdar, A.; Molaey, R.; Sürmeli, R.Ö.; Sahinkaya, E.; Çalli, B. Biogas Production from Chicken Manure: Co-Digestion with Spent Poppy Straw. Int. Biodeterior. Biodegrad. 2017, 119, 205–210. [Google Scholar] [CrossRef]
- Ma, Q.; Paudel, K.P.; Bhandari, D.; Theegala, C.; Cisneros, M. Implications of Poultry Litter Usage for Electricity Production. Waste Manag. 2019, 95, 493–503. [Google Scholar] [CrossRef]
- Augustyńska-Prejsnar, A.; Ormian, M.; Sokołowicz, Z.; Topoczewska, J.; Lechowska, J. Environmental Impacts of Pig and Poultry Farms. Proc. ECOpole 2018, 12, 117–129. [Google Scholar] [CrossRef]
- Niu, Q.; Qiao, W.; Qiang, H.; Hojo, T.; Li, Y.Y. Mesophilic Methane Fermentation of Chicken Manure at a Wide Range of Ammonia Concentration: Stability, Inhibition and Recovery. Bioresour. Technol. 2013, 137, 358–367. [Google Scholar] [CrossRef]
- Li, C.; Strömberg, S.; Liu, G.; Achu, I.; Liu, J. Assessment of Regional Biomass as Co-Substrate in the Anaerobic Digestion of Chicken Manure: Impact of Co-Digestion with Chicken Processing Waste, Seagrass and Miscanthus. Biochem. Eng. J. 2017, 118, 1–10. [Google Scholar] [CrossRef]
- Sun, C.; Cao, W.; Banks, C.J.; Heaven, S.; Liu, R. Biogas Production from Undiluted Chicken Manure and Maize Silage: A Study of Ammonia Inhibition in High Solids Anaerobic Digestion. Bioresour. Technol. 2016, 218, 1215–1223. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, T.; Wan, H.; Chen, Y.; Wang, X.; Yang, G.; Ren, G. Anaerobic Co-Digestion of Animal Manure and Wheat Straw for Optimized Biogas Production by the Addition of Magnetite and Zeolite. Energy Convers. Manag. 2015, 97, 132–139. [Google Scholar] [CrossRef]
- Li, K.; Liu, R.; Yu, Q.; Ma, R. Removal of Nitrogen from Chicken Manure Anaerobic Digestion for Enhanced Biomethanization. Fuel 2018, 232, 395–404. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Reubens, B.; Willekens, K.; De Neve, S. Composting for Increasing the Fertilizer Value of Chicken Manure: Effects of Feedstock on P Availability. Waste Biomass Valorization 2014, 5, 491–503. [Google Scholar] [CrossRef]
- Kelleher, B.P.; Leahy, J.J.; Henihan, A.M.; O’Dwyer, T.F.; Sutton, D.; Leahy, M.J. Advances in Poultry Litter Disposal Technology—A Review. Bioresour. Technol. 2002, 83, 27–36. [Google Scholar] [CrossRef]
- Myszograj, S.; Puchalska, E. Waste from Rearing and Slaughter of Poultry—Treat to the Environment or Feedstock for Energy. Environ. Med. 2012, 15, 106–115. [Google Scholar]
- Agyarko-Mintah, E.; Cowie, A.; Van Zwieten, L.; Singh, B.P.; Smillie, R.; Harden, S.; Fornasier, F. Biochar Lowers Ammonia Emission and Improves Nitrogen Retention in Poultry Litter Composting. Waste Manag. 2017, 61, 129–137. [Google Scholar] [CrossRef]
- Turzyński, T.; Kluska, J.; Kardaś, D. Study on Chicken Manure Combustion and Heat Production in Terms of Thermal Self-Sufficiency of a Poultry Farm. Renew. Energy 2022, 191, 84–91. [Google Scholar] [CrossRef]
- Manogaran, M.D.; Shamsuddin, R.; Mohd Yusoff, M.H.; Lay, M.; Siyal, A.A. A Review on Treatment Processes of Chicken Manure. Clean. Circ. Bioecon. 2022, 2, 100013. [Google Scholar] [CrossRef]
- Cavalaglio, G.; Coccia, V.; Cotana, F.; Gelosia, M.; Nicolini, A.; Petrozzi, A. Energy from Poultry Waste: An Aspen Plus-Based Approach to the Thermo-Chemical Processes. Waste Manag. 2018, 73, 496–503. [Google Scholar] [CrossRef]
- Zentou, H.; Zainal Abidin, Z.; Yunus, R.; Awang Biak, D.R.; Abdullah Issa, M.; Yahaya Pudza, M. A New Model of Alcoholic Fermentation under a Byproduct Inhibitory Effect. ACS Omega 2021, 6, 4137–4146. [Google Scholar] [CrossRef]
- Lauri, P.; Havlík, P.; Kindermann, G.; Forsell, N.; Böttcher, H.; Obersteiner, M. Woody Biomass Energy Potential in 2050. Energy Policy 2014, 66, 19–31. [Google Scholar] [CrossRef]
- Tursi, A. A Review on Biomass: Importance, Chemistry, Classification, and Conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on Research Achievements of Biogas from Anaerobic Digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Fuchs, W.; Wang, X.; Gabauer, W.; Ortner, M.; Li, Z. Tackling Ammonia Inhibition for Efficient Biogas Production from Chicken Manure: Status and Technical Trends in Europe and China. Renew. Sustain. Energy Rev. 2018, 97, 186–199. [Google Scholar] [CrossRef]
- Kirchmann, H.; Witter, E. Ammonia Volatilization during Aerobic and Anaerobic Manure Decomposition. Plant Soil 1989, 115, 35–41. [Google Scholar] [CrossRef]
- Sakar, S.; Yetilmezsoy, K.; Kocak, E. Anaerobic Digestion Technology in Poultry and Livestock Waste Treatment - A Literature Review. Waste Manag. Res. 2009, 27, 3–18. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A Critical Review on Inhibition of Anaerobic Digestion Process by Excess Ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Yenigün, O.; Demirel, B. Ammonia Inhibition in Anaerobic Digestion: A Review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Nahm, K.H. Factors Influencing Nitrogen Mineralization during Poultry Litter Composting and Calculations for Available Nitrogen. Worlds. Poult. Sci. J. 2005, 61, 238–255. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of Anaerobic Digestion Process: A Review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Hejnfelt, A.; Angelidaki, I. Anaerobic Digestion of Slaughterhouse By-Products. Biomass Bioenergy 2009, 33, 1046–1054. [Google Scholar] [CrossRef]
- Yuan, H.; Zhu, N. Progress in Inhibition Mechanisms and Process Control of Intermediates and By-Products in Sewage Sludge Anaerobic Digestion. Renew. Sustain. Energy Rev. 2016, 58, 429–438. [Google Scholar] [CrossRef]
- Lay, J.-J.; Li, Y.-Y.; Noike, T. The Influence of PH and Ammonia Concentration on the Methane Production in High-Solids Digestion Processes. Water Environ. Res. 1998, 70, 1075–1082. [Google Scholar] [CrossRef]
- Bi, S.; Qiao, W.; Xiong, L.; Mahdy, A.; Wandera, S.M.; Yin, D.; Dong, R. Improved High Solid Anaerobic Digestion of Chicken Manure by Moderate in Situ Ammonia Stripping and Its Relation to Metabolic Pathway. Renew. Energy 2020, 146, 2380–2389. [Google Scholar] [CrossRef]
- Niu, Q.; Takemura, Y.; Kubota, K.; Li, Y.-Y. Comparing Mesophilic and Thermophilic Anaerobic Digestion of Chicken Manure: Microbial Community Dynamics and Process Resilience. Waste Manag. 2015, 43, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Bujoczek, G.; Oleszkiewicz, J.; Sparling, R.; Cenkowski, S. High Solid Anaerobic Digestion of Chicken Manure. J. Agric. Eng. Res. 2000, 76, 51–60. [Google Scholar] [CrossRef]
- Nie, H.; Jacobi, H.F.; Strach, K.; Xu, C.; Zhou, H.; Liebetrau, J. Mono-Fermentation of Chicken Manure: Ammonia Inhibition and Recirculation of the Digestate. Bioresour. Technol. 2015, 178, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lang, Q.; Pan, Z.; Jiang, Y.; Liebetrau, J.; Nelles, M.; Dong, H.; Dong, R. Performance Evaluation of a Novel Anaerobic Digestion Operation Process for Treating High-Solids Content Chicken Manure: Effect of Reduction of the Hydraulic Retention Time at a Constant Organic Loading Rate. Waste Manag. 2017, 64, 340–347. [Google Scholar] [CrossRef]
- Rahman, M.A.; Møller, H.B.; Saha, C.K.; Alam, M.M.; Wahid, R.; Feng, L. Optimal Ratio for Anaerobic Co-Digestion of Poultry Droppings and Lignocellulosic-Rich Substrates for Enhanced Biogas Production. Energy Sustain. Dev. 2017, 39, 59–66. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing Feeding Composition and Carbon-Nitrogen Ratios for Improved Methane Yield during Anaerobic Co-Digestion of Dairy, Chicken Manure and Wheat Straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef]
- Matheri, A.N.; Ndiweni, S.N.; Belaid, M.; Muzenda, E.; Hubert, R. Optimising Biogas Production from Anaerobic Co-Digestion of Chicken Manure and Organic Fraction of Municipal Solid Waste. Renew. Sustain. Energy Rev. 2017, 80, 756–764. [Google Scholar] [CrossRef]
- Feng, L.; Lin, X.; Li, X. Combined Anaerobic Digestion of Chicken Manure and Corn Straw: Study on Methanogenic Potential and Microbial Diversity. Ann. Microbiol. 2022, 72, 44. [Google Scholar] [CrossRef]
- Abouelenien, F.; Namba, Y.; Kosseva, M.R.; Nishio, N.; Nakashimada, Y. Enhancement of Methane Production from Co-Digestion of Chicken Manure with Agricultural Wastes. Bioresour. Technol. 2014, 159, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, J.; Ye, B.; Zhang, X.; Tyagi, R.D.; Gao, X. Energy Balance Assessment on Chicken Manure for Biogas Production in Rabat-Salé-Zemmour-Zaïr of Morocco. J. Environ. Manag. 2021, 299, 113656. [Google Scholar] [CrossRef] [PubMed]
- Manogaran, M.D.; Hakimi, M.; Basheer Ahmad, M.H.N.; Shamsuddin, R.; Lim, J.W.; Hassan, M.A.M.; Sahrin, N.T. Effect of Temperature on Co-Anaerobic Digestion of Chicken Manure and Empty Fruit Bunch: A Kinetic Parametric Study. Sustainability 2023, 15, 5813. [Google Scholar] [CrossRef]
- Yilmazel, Y.D.; Demirer, G.N. Removal and Recovery of Nutrients as Struvite from Anaerobic Digestion Residues of Poultry Manure. Environ. Technol. 2011, 32, 783–794. [Google Scholar] [CrossRef]
- Demeestere, K.; Smet, E.; Van Langenhove, H.; Galbacs, Z. Optimalisation of Magnesium Ammonium Phosphate Precipitation and Its Applicability to the Removal of Ammonium. Environ. Technol. 2001, 22, 1419–1428. [Google Scholar] [CrossRef]
- Ryu, H.D.; Lee, S.I. Struvite Recovery from Swine Wastewater and Its Assessment as a Fertilizer. Environ. Eng. Res. 2016, 21, 29–35. [Google Scholar] [CrossRef]
- Rahman, M.M.; Salleh, M.A.M.; Rashid, U.; Ahsan, A.; Hossain, M.M.; Ra, C.S. Production of Slow Release Crystal Fertilizer from Wastewaters through Struvite Crystallization—A Review. Arab. J. Chem. 2014, 7, 139–155. [Google Scholar] [CrossRef]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: A Review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Wongkiew, S.; Koottatep, T.; Polprasert, C.; Prombutara, P.; Jinsart, W.; Khanal, S.K. Bioponic System for Nitrogen and Phosphorus Recovery from Chicken Manure: Evaluation of Manure Loading and Microbial Communities. Waste Manag. 2021, 125, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, G.; Cao, Z.; Sui, C.; Zou, J.; Wang, Z. Effects of Different Addition Ratios of Unsterilized Chicken Manure Biogas Slurry on Chlorella Cultivation. Energy Sources Part A Recover. Util. Environ. Eff. 2020. [Google Scholar] [CrossRef]
- Yetilmezsoy, K.; Sertyesilisik, B.; Kocak, E.; Sapci-Zengin, Z. Ameliorative Effect of Different Doses of MgNH4PO4 6H2O Precipitate Recovered from the Effluent of UASB Treating Poultry Manure Wastewater: Growth of Lolium Perenne. J. Food Agric. Environ. 2009, 7, 823–831. [Google Scholar]
- Luo, W.; Fang, Y.; Song, L.; Niu, Q. Production of Struvite by Magnesium Anode Constant Voltage Electrolytic Crystallisation from Anaerobically Digested Chicken Manure Slurry. Environ. Res. 2022, 214, 113991. [Google Scholar] [CrossRef]
- American Public Health Association, Inc. (APHA). Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association, Inc. (APHA): Washington, DC, USA, 1998. [Google Scholar]
- Raposo, F.; Banks, C.J.; Siegert, I.; Heaven, S.; Borja, R. Influence of Inoculum to Substrate Ratio on the Biochemical Methane Potential of Maize in Batch Tests. Process Biochem. 2006, 41, 1444–1450. [Google Scholar] [CrossRef]
- Strömberg, S.; Nistor, M.; Liu, J. Towards Eliminating Systematic Errors Caused by the Experimental Conditions in Biochemical Methane Potential (BMP) Tests. Waste Manag. 2014, 34, 1939–1948. [Google Scholar] [CrossRef]
- Žalys, B.; Venslauskas, K.; Navickas, K.; Buivydas, E.; Rubežius, M. The Influence of CO2 Injection into Manure as a Pretreatment Method for Increased Biogas Production. Sustain. 2023, 15. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Chen, C.; Liu, G.; He, Y.; Liu, X. Biogas Production from Co-Digestion of Corn Stover and Chicken Manure under Anaerobic Wet, Hemi-Solid, and Solid State Conditions. Bioresour. Technol. 2013, 149, 406–412. [Google Scholar] [CrossRef]
- Abouelenien, F.; Nakashimada, Y.; Nishio, N. Dry Mesophilic Fermentation of Chicken Manure for Production of Methane by Repeated Batch Culture. J. Biosci. Bioeng. 2009, 107, 293–295. [Google Scholar] [CrossRef]
- Lisboa, M.S.; Lansing, S. Characterizing Food Waste Substrates for Co-Digestion through Biochemical Methane Potential (BMP) Experiments. Waste Manag. 2013, 33, 2664–2669. [Google Scholar] [CrossRef]
- Kozłowski, K.; Dach, J.; Lewicki, A.; Malińska, K.; Do Carmo, I.E.P.; Czekała, W. Potential of Biogas Production from Animal Manure in Poland. Arch. Environ. Prot. 2019, 45, 99–108. [Google Scholar] [CrossRef]
- Janczak, D.; Malinska, K.; Czekała, W.; Cáceres, R.; Lewicki, A.; Dach, J. Biochar to Reduce Ammonia Emissions in Gaseous and Liquid Phase during Composting of Poultry Manure with Wheat Straw. Waste Manag. 2017, 66, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Świątek, M.; Lewicki, A.; Szymanowska, D.; Kubiak, P. The Effect of Introduction of Chicken Manure on the Biodiversity and Performance of an Anaerobic Digester. Electron. J. Biotechnol. 2019, 37, 25–33. [Google Scholar] [CrossRef]
- Resch, C.; Wörl, A.; Waltenberger, R.; Braun, R.; Kirchmayr, R. Enhancement Options for the Utilisation of Nitrogen Rich Animal By-Products in Anaerobic Digestion. Bioresour. Technol. 2011, 102, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Bohn, I.; Björnsson, L.; Mattiasson, B. The Energy Balance in Farm Scale Anaerobic Digestion of Crop Residues at 11–37 °C. Process Biochem. 2007, 42, 57–64. [Google Scholar] [CrossRef]
- Böjti, T.; Kovács, K.L.; Kakuk, B.; Wirth, R.; Rákhely, G.; Bagi, Z. Pretreatment of Poultry Manure for Efficient Biogas Production as Monosubstrate or Co-Fermentation with Maize Silage and Corn Stover. Anaerobe 2017, 46, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic Co-Digestion Process for Biogas Production: Progress, Challenges and Perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).