Prediction of Gas Hydrate Formation in the Wellbore
Abstract
:1. Introduction
2. Theories and Models
2.1. Temperature Distribution Models
2.2. Pressure Distribution Models
2.3. Gas Hydrate Formation Models
3. Results and Discussion
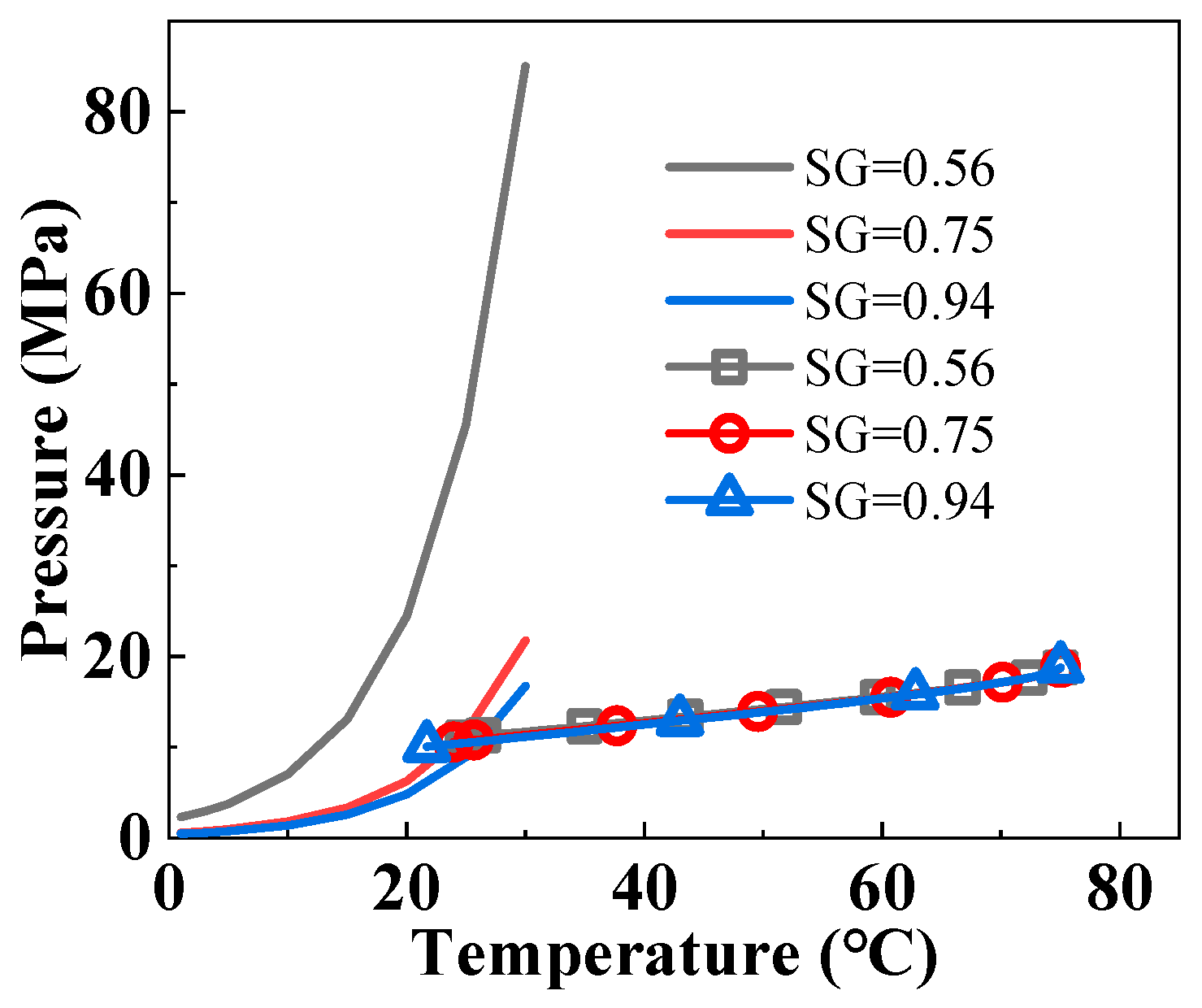
3.1. Prediction of Hydrate Formation Region
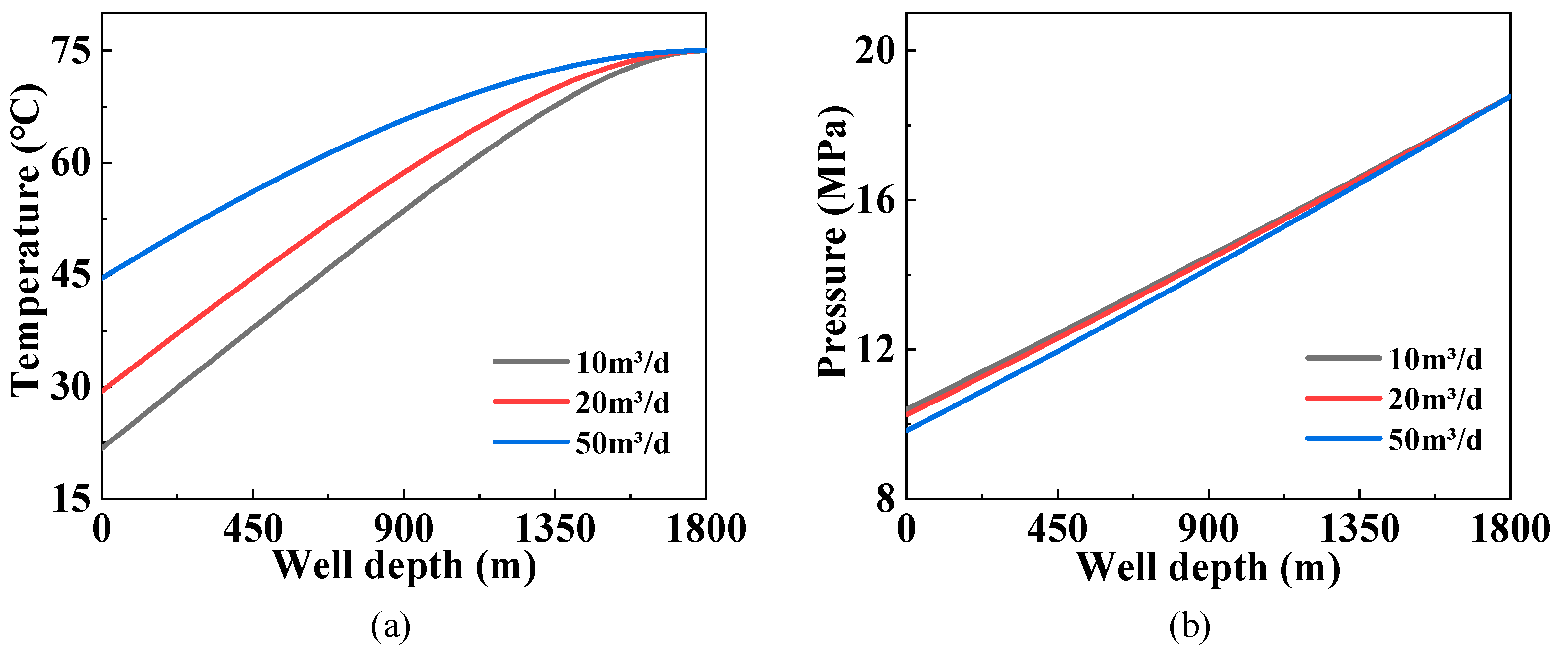
3.2. Influence of the Gas Production Rate
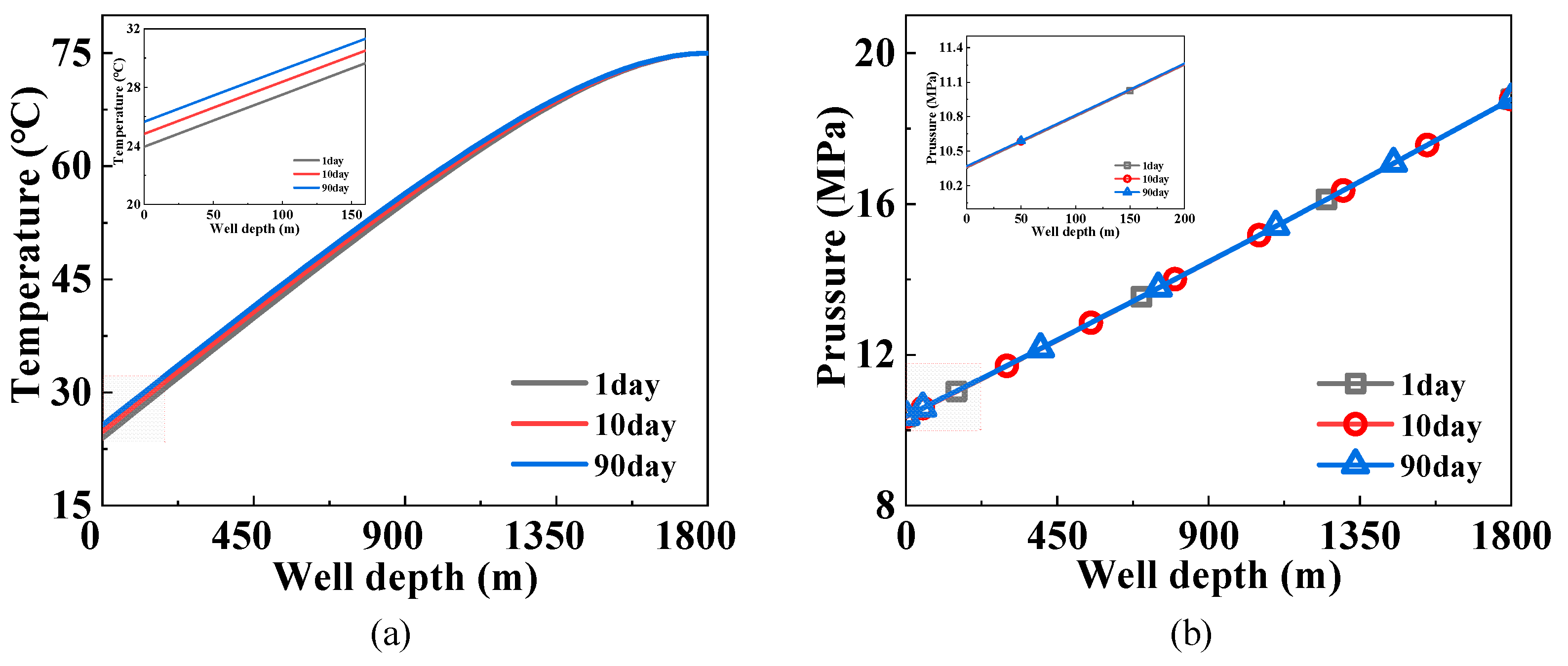
3.3. Influence of the Producing Time
3.4. Influence of the Water Production Rate
3.5. Influence of Natural Gas Components on Hydrate Formation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Klymenko, V.; Ovetskyi, S.; Martynenko, V.; Vytyaz, O.; Uhrynovskyi, A. An alternative method of methane production from deposits of subaquatic gas hydrates. Min. Miner. Depos. 2022, 16, 11–17. [Google Scholar] [CrossRef]
- Bazaluk, O.; Sai, K.; Lozynskyi, V.; Petlovanyi, M.; Saik, P. Research into Dissociation Zones of Gas Hydrate Deposits with a Heterogeneous Structure in the Black Sea. Energies 2021, 14, 1345. [Google Scholar] [CrossRef]
- Pedchenko, M.; Pedchenko, L.; Nesterenko, T.; Dyczko, A. Technological Solutions for the Realization of NGH-Technology for Gas Transportation and Storage in Gas Hydrate Form. Solid State Phenom. 2018, 277, 123–136. [Google Scholar] [CrossRef]
- Botrel, T. Hydrates Prevention and Removal in Ultra-Deepwater Drilling Systems. In Proceedings of the Offshore Technology Conference, OTC, Houston, TX, USA, 30 April–3 May 2001; p. OTC-12962-MS. [Google Scholar]
- Sahari Moghaddam, F.; Hamid, A.; Abdi, M.; James, L. Consideration of Various Parameters and Scenarios in the Simulation of Hydrate Formation. In Proceedings of the SPE Canadian Energy Technology Conference, SPE, Calgary, AB, Canada, 16–17 March 2022; p. SPE-208881-MS. [Google Scholar]
- Ramey, H.J. Wellbore Heat Transmission. J. Pet. Technol. 1962, 14, 427–435. [Google Scholar] [CrossRef]
- Hagoort, J. Ramey’s Wellbore Heat Transmission Revisited. SPE J. 2004, 9, 465–474. [Google Scholar] [CrossRef]
- Galvao, M.S.; Carvalho, M.S.; Barreto, A.B. A Coupled Transient Wellbore/Reservoir-Temperature Analytical Model. SPE J. 2019, 24, 2335–2361. [Google Scholar] [CrossRef]
- Willhite, G.P. Over-all Heat Transfer Coefficients in Steam And Hot Water Injection Wells. J. Pet. Technol. 1967, 19, 607–615. [Google Scholar] [CrossRef]
- You, J.; Rahnema, H.; McMillan, M.D. Numerical modeling of unsteady-state wellbore heat transmission. J. Nat. Gas Sci. Eng. 2016, 34, 1062–1076. [Google Scholar] [CrossRef]
- Gray, H.E. Vertical Flow Correlation in Gas Wells. User’s Manual for API 14B Surface Controlled Subsurface Safety Valve Sizing Computer Program, 2nd ed.; American Petroleum Institute: Washington, DC, USA, 1978. [Google Scholar]
- Coddington, P.; Macian, R. A study of the performance of void fraction correlations used in the context of drift-flux two-phase flow models. Nucl. Eng. Des. 2002, 215, 199–216. [Google Scholar] [CrossRef]
- Inoue, A.; Kurosu, T.; Yagi, M.; Morooka, S.; Hoshide, A.; Ishizuka, T.; Yoshimura, K. In-bundle void measurement of a BWR fuel assembly by an x-ray CT scanner: Assessment of BWR design void correlation and development of new void correlation. In Proceedings of the 2nd ASME-JSME International Conference on Nuclear Engineering, Chiba, Japan, 1 January 1993. [Google Scholar]
- Morooka, S.; Ishizuka, T.; Iizuka, M.; Yoshimura, K. Experimental study on void fraction in a simulated BWR fuel assembly (evaluation of cross-sectional averaged void fraction). Nucl. Eng. Des. 1989, 114, 91–98. [Google Scholar] [CrossRef]
- Hasan, A.R.; Kabir, C.S.; Sayarpour, M. Simplified two-phase flow modeling in wellbores. J. Pet. Sci. Eng. 2010, 72, 42–49. [Google Scholar] [CrossRef]
- Duru, O.O.; Horne, R.N. Modeling Reservoir Temperature Transients and Reservoir-Parameter Estimation Constrained to the Model. SPE Reserv. Eval. Eng. 2010, 13, 873–883. [Google Scholar] [CrossRef] [Green Version]
- Duru, O.O.; Horne, R.N. Simultaneous Interpretation of Pressure, Temperature, and Flow-Rate Data Using Bayesian Inversion Methods. SPE Reserv. Eval. Eng. 2011, 14, 225–238. [Google Scholar] [CrossRef]
- Hasan, A.R.; Kabir, C.S.; Wang, X. Wellbore Two-Phase Flow and Heat Transfer During Transient Testing. SPE J. 1998, 3, 174–180. [Google Scholar] [CrossRef]
- Ghiasi, M.M. Initial estimation of hydrate formation temperature of sweet natural gases based on new empirical correlation. J. Nat. Gas Chem. 2012, 21, 508–512. [Google Scholar] [CrossRef]
- Woldesemayat, M.A.; Ghajar, A.J. Comparison of void fraction correlations for different flow patterns in horizontal and upward inclined pipes. Int. J. Multiph. Flow 2007, 33, 347–370. [Google Scholar] [CrossRef]
- Lockhart, R.W. Proposed Correlation of Data for Isothermal Two-Phase, Two-Component Flow in Pipes. Chem. Eng. Prog. 1949, 45, 39–48. [Google Scholar]
- Petalas, N.; Aziz, K. A Mechanistic Model for Multiphase Flow in Pipes. J. Can. Pet. Technol. 2000, 39, PETSOC-00-06-04. [Google Scholar] [CrossRef]
- Motiee, M. Estimate possibility of hydrates. Hydrocarb. Process. 1991, 70, 98–99. [Google Scholar]
- ali Ghayyem, M.; Izadmehr, M.; Tavakoli, R. Developing a simple and accurate correlation for initial estimation of hydrate formation temperature of sweet natural gases using an eclectic approach. J. Nat. Gas Sci. Eng. 2014, 21, 184–192. [Google Scholar] [CrossRef]
- Wilcox, W.I.; Carson, D.B.; Katz, D.L. Natural Gas Hydrates. Ind. Eng. Chem. 1941, 33, 662–665. [Google Scholar] [CrossRef]
- Van der Waals, J.H.; Platteeuw, J.C. Clathrate Solutions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 1–57. [Google Scholar] [CrossRef]
- Ballard, A.L.; Sloan, E.D. The next generation of hydrate prediction: Part III. Gibbs energy minimization formalism. Fluid Phase Equilib. 2004, 218, 15–31. [Google Scholar] [CrossRef]
- Ballard, A.L.; Sloan, E.D., Jr. The next generation of hydrate prediction: I. Hydrate standard states and incorporation of spectroscopy. Fluid Phase Equilib. 2002, 194–197, 371–383. [Google Scholar] [CrossRef]
- Jager, M.D.; Ballard, A.L.; Sloan, E.D. The next generation of hydrate prediction: II. Dedicated aqueous phase fugacity model for hydrate prediction. Fluid Phase Equilib. 2003, 211, 85–107. [Google Scholar] [CrossRef]
- Cheng, W.-L.; Huang, Y.-H.; Lu, D.-T.; Yin, H.-R. A novel analytical transient heat-conduction time function for heat transfer in steam injection wells considering the wellbore heat capacity. Energy 2011, 36, 4080–4088. [Google Scholar] [CrossRef]
- Chen, N.H. An Explicit Equation for Friction Factor in Pipe. Ind. Eng. Chem. Fundam. 1979, 18, 296–297. [Google Scholar] [CrossRef]
- Hammerschmidt, E.G. Formation of Gas Hydrates in Natural Gas Transmission Lines. Ind. Eng. Chem. 1934, 26, 851–855. [Google Scholar] [CrossRef]
- Carson, D.B.; Katz, D.L. Natural Gas Hydrates. Trans. AIME 1942, 146, 150–158. [Google Scholar] [CrossRef]
- Cao, F.; Wang, T.; Cao, L.; Wang, B. Formation Conditions of Forecasting Natural Gas Hydrate with Numerical Calculation Method. Guangzhou Chem. Ind. 2013, 5, 82–83. [Google Scholar]
- Lemmon, E.W.; Huber, M.; Mclinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties—REFPROP, Version 9.1; Standard Reference Data Program; NIST: Gaithersburg, MD, USA, 2010. [Google Scholar]

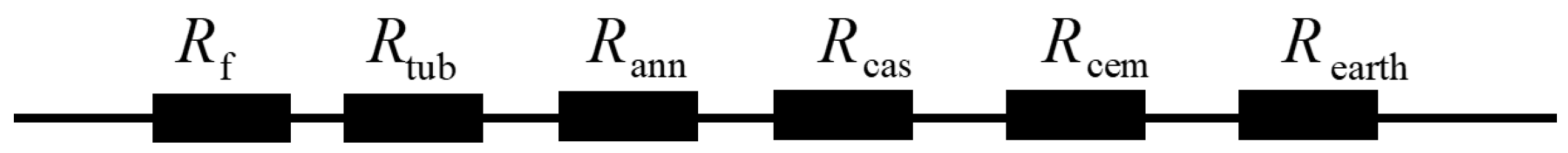


| Specific Gravity | 0.56 | 0.60 | 0.64 | 0.66 | 0.68 | 0.70 | 0.75 | 0.80 | 0.85 | 0.90 | 0.95 | 1.0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 24.25 | 17.67 | 15.47 | 14.76 | 14.34 | 14.00 | 13.32 | 12.47 | 12.18 | 11.66 | 11.17 | 10.77 |
| B1 | 77.4 | 64.2 | 48.6 | 46.9 | 45.6 | 44.4 | 42.0 | 39.9 | 37.9 | 36.2 | 34.5 | 33.1 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Well depth/m | 1800.0 | Producing time/day | 10.0 |
| The inner diameter of tubing/mm | 72.0 | Water production rate t/d | 12.69 |
| The outer diameter of tubing/mm | 114.0 | Gas production rate m³/d | 7292 |
| The inner diameter of casing/mm | 159.0 | Pressure of bottom-hole/MPa | 18.779 |
| The outer diameter of casing/mm | 177.8 | Temperature of bottom-hole/°C | 75.0 |
| The outer diameter of cement/mm | 245.0 | Mean annual ground temperature/°C | 12.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, X.; Zuo, J.; Li, J.; Tian, Y.; Zhu, C.; Gong, L. Prediction of Gas Hydrate Formation in the Wellbore. Energies 2023, 16, 5579. https://doi.org/10.3390/en16145579
Duan X, Zuo J, Li J, Tian Y, Zhu C, Gong L. Prediction of Gas Hydrate Formation in the Wellbore. Energies. 2023; 16(14):5579. https://doi.org/10.3390/en16145579
Chicago/Turabian StyleDuan, Xinyue, Jiaqiang Zuo, Jiadong Li, Yu Tian, Chuanyong Zhu, and Liang Gong. 2023. "Prediction of Gas Hydrate Formation in the Wellbore" Energies 16, no. 14: 5579. https://doi.org/10.3390/en16145579
APA StyleDuan, X., Zuo, J., Li, J., Tian, Y., Zhu, C., & Gong, L. (2023). Prediction of Gas Hydrate Formation in the Wellbore. Energies, 16(14), 5579. https://doi.org/10.3390/en16145579









