Advances in Selective Hydrogenation of 5-Hydroxymethylfurfural over Heterogeneous Metal Catalysts
Abstract
:1. Introduction
| Chemical | Molecular Formula Molecular | Weigh t/(g·mol−1) | Boiling Point/K | Melting Point/K | Density /(g·cm−3) |
|---|---|---|---|---|---|
| 5-hydroxym ethylfurfural (HMF) | C6H6O3 | 126.11 | 387−389 | 301−307 | 1.24 |
2. Direct Hydrogenation
2.1. Precious Metals
2.2. Non-Precious Metals
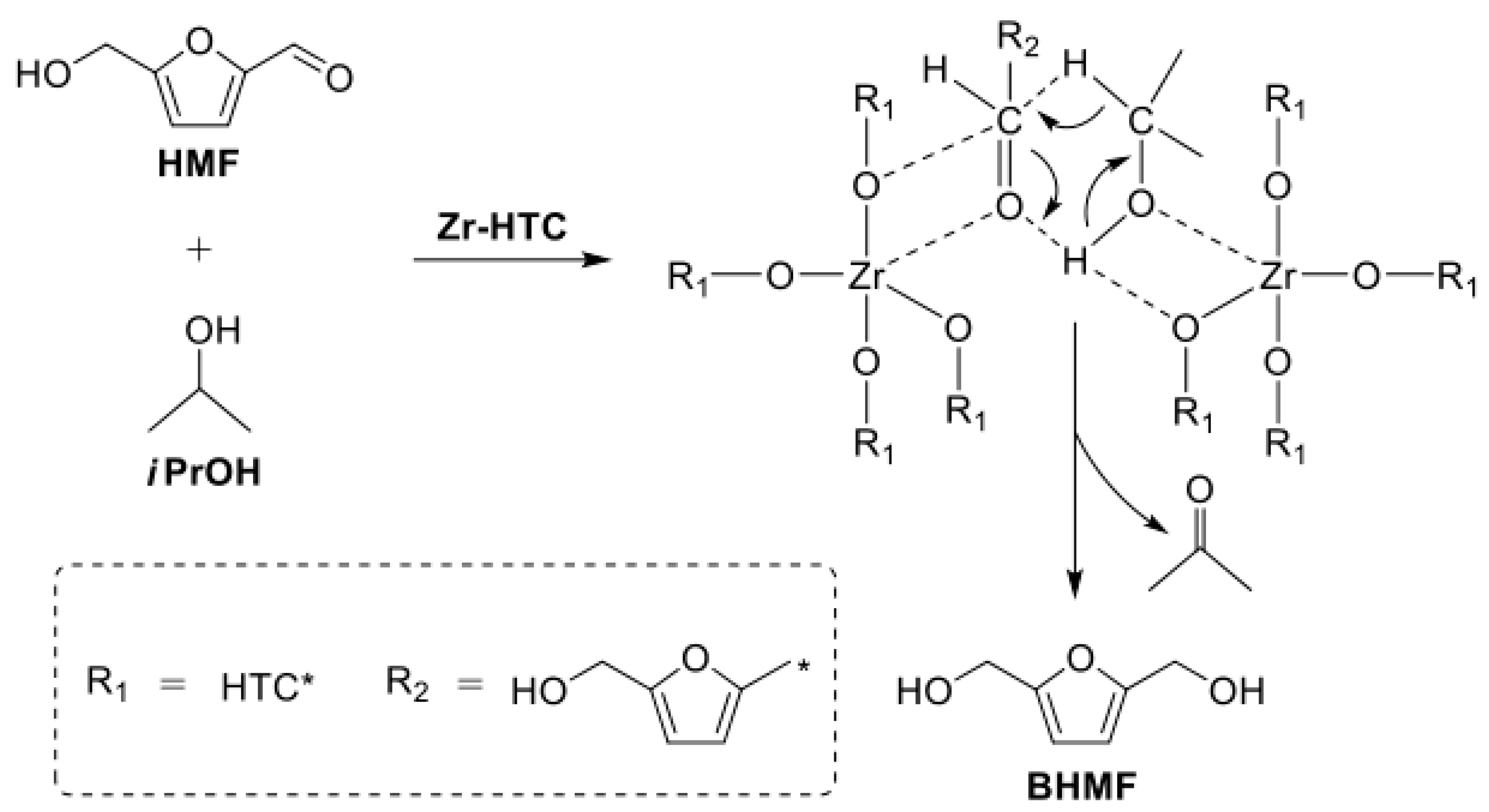
3. Catalytic Transfer Hydrogenation (CTH)
3.1. Hydrogen Donor as Alcohol
3.2. Hydrogen Donor as Acid
3.3. The Hydrogen Donor Is Other
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Wojcieszak, R.; Dumeignil, F.; Marceau, E.; Royer, S. How Catalysts and Experimental Conditions Determine the Selective Hydroconversion of Furfural and 5-Hydroxymethylfurfural. Chem. Rev. 2018, 118, 11023–11117. [Google Scholar] [CrossRef]
- Fang, W.; Riisager, A. Recent advances in heterogeneous catalytic transfer hydrogenation/hydrogenolysis for valorization of biomass-derived furanic compounds. Green Chem. 2021, 23, 670–688. [Google Scholar] [CrossRef]
- Turkin, A.; Eyley, S.; Preegel, G.; Thielemans, W.; Makshina, E.; Sels, B.F. How Trace Impurities Can Strongly Affect the Hydroconversion of Biobased 5-Hydroxymethylfurfural? ACS Catal. 2021, 11, 9204–9209. [Google Scholar] [CrossRef]
- Messori, A.; Fasolini, A.; Mazzoni, R. Advances in Catalytic Routes for the Homogeneous Green Conversion of the Bio-Based Platform 5-Hydroxymethylfurfural. ChemSusChem 2022, 15, e202200228. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Wang, X.; Liang, X.; Li, J.; Li, Q. Catalytic hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to biofuel 2, 5-dimethylfuran. Appl. Catal. A Gen. 2019, 576, 85–95. [Google Scholar] [CrossRef]
- Weng, R.; Lu, X.; Ji, N.; Fukuoka, A.; Shrotri, A.; Li, X.; Zhang, R.; Zhang, M.; Xiong, J.; Yu, Z. Taming the butterfly effect: Modulating catalyst nanostructures for better selectivity control of the catalytic hydrogenation of biomass-derived furan platform chemicals. Catal. Sci. Technol. 2021, 11, 7785–7806. [Google Scholar] [CrossRef]
- Park, D.; Lee, S.; Kim, J.; Ryu, G.Y.; Suh, Y. 5-(Chloromethyl)Furfural as a Potential Source for Continuous Hydrogenation of 5-(Hydroxymethyl)Furfural to 2,5-Bis(Hydroxymethyl)Furan. ChemPlusChem 2022, 87, e202200272. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, J.; Lei, J.; Zhao, W.; Chen, H.; Yang, Y.; Xu, Q.; Liu, X. Recent Advances in the Catalytic Hydroconversion of 5-Hydroxymethylfurfural to Valuable Diols. Front. Chem. 2022, 10, 925603. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhu, Y.; Fang, Z.; Kozinski, J.A.; Butler, I.S.; Xu, L.; Song, H.; Wei, X. Catalytic conversion of 5-hydroxymethylfurfural to some value-added derivatives. Green Chem. 2018, 20, 3657–3682. [Google Scholar] [CrossRef]
- Hu, L.; He, A.; Liu, X.; Xia, J.; Xu, J.; Zhou, S.; Xu, J. Biocatalytic Transformation of 5-Hydroxymethylfurfural into High-Value Derivatives: Recent Advances and Future Aspects. ACS Sustain. Chem. Eng. 2018, 6, 15915–15935. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, X.; Zeng, Z.; Lei, J.; Huang, Z.; Xu, Q.; Liu, X.; Yang, Y. Cu-Co nanoparticles supported on nitrogen-doped carbon: An efficient catalyst for hydrogenation of 5-hydroxymethylfurfural into 2,5-bis(hydroxymethyl)furan. Mol. Catal. 2022, 524, 112304. [Google Scholar] [CrossRef]
- Ahishakiye, R.; Wang, F.; Zhang, X.; Sun, M.; Zhai, Y.; Liu, Y.; Wu, Y.; Li, M.; Li, M.; Zhang, Q. Novel noble metal-free and recyclable Co-CoOx-FeNiCo/γ-Al2O3 catalyst for selective hydrogenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran or 2,5-Bis(hydroxymethyl)furan. Chem. Eng. J. 2022, 450, 138187. [Google Scholar] [CrossRef]
- Rao, K.T.V.; Hu, Y.; Yuan, Z.; Zhang, Y.; Xu, C.C. Green synthesis of heterogeneous copper-alumina catalyst for selective hydrogenation of pure and biomass-derived 5-hydroxymethylfurfural to 2,5-bis(hydroxymethyl)furan. Appl. Catal. A Gen. 2021, 609, 117892. [Google Scholar] [CrossRef]
- Hsiao, Y.W.; Zong, X.; Zhou, J.; Zheng, W.; Vlachos, D.G. Selective hydrodeoxygenation of 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF) over carbon supported copper catalysts using isopropyl alcohol as a hydrogen donor. Appl. Catal. B Environ. 2022, 317, 121790. [Google Scholar] [CrossRef]
- Tan, J.J.; Cui, J.; Zhu, Y.; Cui, X.; Shi, Y.; Yan, W.; Zhao, Y. Complete Aqueous Hydrogenation of 5-Hydroxymethylfurfural at Room Temperature over Bimetallic RuPd/Graphene Catalyst. ACS Sustain. Chem. Eng. 2019, 7, 10670–10678. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, C.; Liu, D.; Shang, Y.; Yin, X.; Zhang, P.; Mamba, B.B.; Kuvarega, A.T.; Gui, J. Hydrothermal carbon-supported Ni catalysts for selective hydrogenation of 5-hydroxymethylfurfural toward tunable products. J. Mater. Sci. 2020, 55, 14179–14196. [Google Scholar] [CrossRef]
- Panigrahy, S.; Mishra, R.; Panda, P.; Kempasiddaiah, M.; Barman, S. Carbon-Supported Ag Nanoparticle Aerogel for Electrocatalytic Hydrogenation of 5-(Hydroxymethyl)furfural to 2,5-Hexanedione Under Acidic Conditions. ACS Appl. Nano Mater. 2022, 5, 8314–8323. [Google Scholar] [CrossRef]
- Kim, J.; Bathula, H.B.; Yun, S.; Jo, Y.; Lee, S.; Baik, J.H.; Suh, Y.-W. Hydrogenation of 5-hydroxymethylfurfural into 2,5-bis(hydroxymethyl)furan over mesoporous Cu–Al2O3 catalyst: From batch to continuous processing. J. Ind. Eng. Chem. 2021, 102, 186–194. [Google Scholar] [CrossRef]
- Xia, J.; Gao, D.; Han, F.; Li, Y.; Waterhouse, G.I.N. Efficient and Selective Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran Over a Non-noble CoNCx/NiFeO Catalyst. Catal. Lett. 2022, 152, 3400–3413. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Fu, J.; Miao, C.; Jia, S.; Yan, P.; Huang, J. Highly selective hydrogenation of 5-hydroxymethylfurfural to 2,5-bis(hydroxymethyl)furan over metal-oxide supported Pt catalysts: The role of basic sites. Appl. Catal. A Gen. 2022, 643, 118762. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Sun, K.; Gao, G.; Fan, M.; Li, C.; Ming, C.; Zhang, L.; Zhang, S.; Hu, X. Cu-Based Nanoparticles as Catalysts for Selective Hydrogenation of Biomass-Derived 5-Hydroxymethylfurfural to 1,2-Hexanediol. ACS Appl. Nano Mater. 2022, 5, 5882–5894. [Google Scholar] [CrossRef]
- Gilcher, E.B.; Chang, H.; Rebarchik, M.; Huber, G.W.; Dumesic, J.A. Effects of Water Addition to Isopropanol for Hydrogenation of Compounds Derived from 5-Hydroxymethyl Furfural over Pd, Ru, and Cu Catalysts. ACS Catal. 2022, 12, 10186–10198. [Google Scholar] [CrossRef]
- Ledesma, B.; Juárez, J.; Mazarío, J.; Domine, M.; Beltramone, A. Bimetallic platinum/iridium modified mesoporous catalysts applied in the hydrogenation of HMF. Catal. Today 2021, 360, 147–156. [Google Scholar] [CrossRef]
- Silva, W.R.; Matsubara, E.Y.; Rosolen, J.M.; Donate, P.M.; Gunnella, R. Pd catalysts supported on different hydrophilic or hydrophobic carbonaceous substrate for furfural and 5-(hydroxymethyl)-furfural hydrogenation in water. Mol. Catal. 2021, 504, 111496. [Google Scholar] [CrossRef]
- Turkin, A.A.; Makshina, E.V.; Sels, B.F. Catalytic Hydroconversion of 5-HMF to Value-Added Chemicals: Insights into the Role of Catalyst Properties and Feedstock Purity. ChemSusChem 2022, 15, e202200412. [Google Scholar] [CrossRef]
- Wang, L.; Zuo, J.; Zhang, Q.; Peng, F.; Chen, S.; Liu, Z. Catalytic Transfer Hydrogenation of Biomass-Derived 5-Hydroxymethylfurfural into 2,5-Dihydroxymethylfuran over Co/UiO-66-NH2. Catal. Lett. 2021, 152, 361–371. [Google Scholar] [CrossRef]
- Pomeroy, B.; Grilc, M.; Gyergyek, S.; Likozar, B. Catalyst structure-based hydroxymethylfurfural (HMF) hydrogenation mechanisms, activity and selectivity over Ni. Chem. Eng. J. 2021, 412, 127553. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.N.; Yuan, H.R.; Wang, S.R.; Yong, C.H.E.N. Advances on the catalytic hydrogenation of bio-mass-derived furfural and 5-hydroxymethylfurfural. J. Fuel Chem. Technol. 2021, 49, 1752–1766. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhang, J.; Chen, H.; Wang, T.; Lu, Q. Catalytic Transfer Hydrogenation of 5-Hydroxymethylfurfural with Primary Alcohols over Skeletal CuZnAl Catalysts. ChemSusChem 2022, 15, e202200237. [Google Scholar] [CrossRef]
- Qu, D.; He, S.; Chen, L.; Ye, Y.; Ge, Q.; Cong, H.; Jiang, N.; Ha, Y. Paired electrocatalysis in 5-hydroxymethylfurfural valorization. Front. Chem. 2022, 10, 1055865. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Z.; Hu, C. Selective Hydrogenation of the Carbonyls in Furfural and 5-Hydroxymethylfurfural Catalyzed by PtNi Alloy Supported on SBA-15 in Aqueous Solution Under Mild Conditions. Front. Chem. 2021, 9, 759512. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ding, H.; Wang, S.; Deng, Y.; Feng, H.; Liang, Y.; Chen, L.; Zhang, X. Crystal Phase Sensitivity of 5-Hydroxymethylfurfural Hydrodeoxygenation over a Pt1Co Single-Atom Alloy Catalyst. J. Phys. Chem. C 2022, 126, 20807–20815. [Google Scholar] [CrossRef]
- Gan, T.; Liu, Y.; He, Q.; Zhang, H.; He, X.; Ji, H. Facile Synthesis of Kilogram-Scale Co-Alloyed Pt Single-Atom Catalysts via Ball Milling for Hydrodeoxygenation of 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2020, 8, 8692–8699. [Google Scholar] [CrossRef]
- Vikanova, K.V.; Chernova, M.S.; Redina, E.A.; Kapustin, G.I.; Tkachenko, O.P.; Kustov, L.M. Heterogeneous additive-free highly selective synthesis of 2,5-bis(hydroxymethyl)furan over catalysts with ultra-low Pt content. J. Chem. Technol. Biotechnol. 2021, 96, 2421–2425. [Google Scholar] [CrossRef]
- Kumar, A.; Ananthakrishnan, R. Strategic Design of a WO3/PdOx-Carbon Shell Composite Photocatalyst: Visible Light-Mediated Selective Hydrogenation of 5-Hydroxymethylfurfural. ACS Appl. Energy Mater. 2022, 5, 2706–2719. [Google Scholar] [CrossRef]
- Nishimura, S.; Shimura, T.; Ebitani, K. Transfer hydrogenation of furaldehydes with sodium phosphinate as a hydrogen source using Pd-supported alumina catalyst. J. Taiwan Inst. Chem. Eng. 2017, 79, 97–102. [Google Scholar] [CrossRef]
- Xie, H.; Qi, T.; Lyu, Y.-J.; Zhang, J.-F.; Si, Z.-B.; Liu, L.-J.; Zhu, L.-F.; Yang, H.-Q.; Hu, C.-W. Molecular mechanism comparison of decarbonylation with deoxygenation and hydrogenation of 5-hydroxymethylfurfural catalyzed by palladium acetate. Phys. Chem. Chem. Phys. 2019, 21, 3795–3804. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Gilcher, E.B.; Huber, G.W.; Dumesic, J.A. Synthesis of performance-advantaged polyurethanes and polyesters from biomass-derived monomers by aldol-condensation of 5-hydroxymethyl furfural and hydrogenation. Green Chem. 2021, 23, 4355–4364. [Google Scholar] [CrossRef]
- Mishra, D.K.; Lee, H.J.; Truong, C.C.; Kim, J.; Suh, Y.-W.; Baek, J.; Kim, Y.J. Ru/MnCo2O4 as a catalyst for tunable synthesis of 2,5-bis(hydroxymethyl)furan or 2,5-bis(hydroxymethyl)tetrahydrofuran from hydrogenation of 5-hydroxymethylfurfural. Mol. Catal. 2020, 484, 110722. [Google Scholar] [CrossRef]
- Ji, K.; Xu, M.; Xu, S.; Wang, Y.; Ge, R.; Hu, X.; Sun, X.; Duan, H. Electrocatalytic Hydrogenation of 5-Hydroxymethylfurfural Promoted by a Ru1Cu Single-Atom Alloy Catalyst. Angew. Chem. Int. Ed. 2022, 61, e202209849. [Google Scholar] [CrossRef]
- Feng, L.; Li, X.; Lin, Y.; Liang, Y.; Chen, Y.; Zhou, W. Catalytic hydrogenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran over Ru based catalyst: Effects of process parameters on conversion and products selectivity. Renew. Energy 2020, 160, 261–268. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Lu, Y.; Cao, Q.-E.; Fang, W. Efficient hydrogenation of 5-hydroxymethylfurfural using a synergistically bimetallic Ru–Ir/C catalyst. Chem. Commun. 2021, 57, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Gilcher, E.B.; Chang, H.; Huber, G.W.; Dumesic, J.A. Controlled hydrogenation of a biomass-derived platform chemical formed by aldol-condensation of 5-hydroxymethyl furfural (HMF) and acetone over Ru, Pd, and Cu catalysts. Green Chem. 2022, 24, 2146–2159. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, T.; Peng, M.; Zhang, Q.; Liu, J.; Zhang, J.; Fu, Y.; Li, W. Highly selective electrocatalytic hydrogenation of 5-hydroxymethylfurfural to 2,5-dihydroxymethylfuran over AgCu nanoalloys. Int. J. Hydrogen Energy 2022, 47, 28904–28914. [Google Scholar] [CrossRef]
- Chadderdon, X.H.; Chadderdon, D.J.; Pfennig, T.; Shanks, B.H.; Li, W. Paired electrocatalytic hydrogenation and oxidation of 5-(hydroxymethyl)furfural for efficient production of biomass-derived monomers. Green Chem. 2019, 21, 6210–6219. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Lin, Z.; Xie, J.; Chen, J.G. Understanding the Role of M/Pt(111) (M = Fe, Co, Ni, Cu) Bimetallic Surfaces for Selective Hydrodeoxygenation of Furfural. ACS Catal. 2017, 7, 5758–5765. [Google Scholar] [CrossRef]
- Kong, F.; Wang, M. Preparation of Sulfur-Modulated Nickel/Carbon Composites from Lignosulfonate for the Electrocatalytic Oxidation of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid. ACS Appl. Energy Mater. 2021, 4, 1182–1188. [Google Scholar] [CrossRef]
- Jurado-Vázquez, T.; Arévalo, A.; García, J.J. Furfural and 5-(hydroxymethyl)furfural valorization using homogeneous Ni(0) and Ni(II) catalysts by transfer hydrogenation. J. Organomet. Chem. 2022, 957, 122162. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Huang, B.; Zhang, L.; Ma, X.; Zhang, S.; Zhu, Z.; Lü, H.; Yang, K. Hydrogenation and hydrogenolysis of 5-hydroxymethylfurfural to 2,5-dimethylfuran via synergistic catalysis of Ni2In and acid-base sites. Appl. Surf. Sci. 2022, 604, 154579. [Google Scholar] [CrossRef]
- Xia, Z.; Niu, L.; An, Y.; Bian, G.; Li, T.; Bai, G. Ni–Al/CoOx-catalyzed hydrodeoxygenation of 5-hydroxymethylfurfural into 2,5-dimethylfuran at low temperatures without external hydrogen. Green Chem. 2021, 23, 7763–7772. [Google Scholar] [CrossRef]
- Ma, N.; Song, Y.; Han, F.; Waterhouse, G.I.N.; Li, Y.; Ai, S. Multifunctional NiCoTi Catalyst Derived from Layered Double Hydroxides for Selective Hydrogenation of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran. Catal. Lett. 2020, 151, 517–525. [Google Scholar] [CrossRef]
- Morales, M.V.; Conesa, J.M.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Tunable selectivity of Ni catalysts in the hydrogenation reaction of 5-hydroxymethylfurfural in aqueous media: Role of the carbon supports. Carbon 2021, 182, 265–275. [Google Scholar] [CrossRef]
- Yuan, X.; Lee, K.; Bender, M.T.; Schmidt, J.R.; Choi, K. Mechanistic Differences between Electrochemical Hydrogenation and Hydrogenolysis of 5-Hydroxymethylfurfural and Their pH Dependence. ChemSusChem 2022, 15, e202200952. [Google Scholar] [CrossRef]
- Wang, T.; Li, F.; Xue, W.; Wang, Y. Highly efficient catalytic transfer hydrogenation of 5-hydroxymethylfurfural to 2,5-dimethylfuran over CuxZnAl catalysts. Asia-Pac. J. Chem. Eng. 2021, 17, e2736. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, S.; Wang, C.; Liu, M.; Zhang, F.; Hu, X.; Chen, H.; Gou, X.; Chen, K.; Zhu, Y.; et al. CuZnCoOx multifunctional catalyst for in situ hydrogenation of 5-hydroxymethylfurfural with ethanol as hydrogen carrier. J. Catal. 2019, 373, 314–321. [Google Scholar] [CrossRef]
- Esteves, L.M.; Brijaldo, M.H.; Oliveira, E.G.; Martinez, J.J.; Rojas, H.; Caytuero, A.; Passos, F.B. Effect of support on selective 5-hydroxymethylfurfural hydrogenation towards 2,5-dimethylfuran over copper catalysts. Fuel 2020, 270, 117524. [Google Scholar] [CrossRef]
- Xia, J.; Gao, D.; Han, F.; Lv, R.; Waterhouse, G.I.N.; Li, Y. Hydrogenolysis of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran Over a Modified CoAl-Hydrotalcite Catalyst. Front. Chem. 2022, 10, 907649. [Google Scholar] [CrossRef]
- Xiang, S.; Dong, L.; Wang, Z.-Q.; Han, X.; Daemen, L.L.; Li, J.; Cheng, Y.; Guo, Y.; Liu, X.; Hu, Y.; et al. A unique Co@CoO catalyst for hydrogenolysis of biomass-derived 5-hydroxymethylfurfural to 2,5-dimethylfuran. Nat. Commun. 2022, 13, 3657. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Q.; Ma, Q.; Guo, Z.; Qin, F.; Ismagilov, Z.R.; Shen, W. Constructing Co@N-doped graphene shell catalyst via Mott-Schottky effect for selective hydrogenation of 5-hydroxylmethylfurfural. Appl. Catal. B Environ. 2020, 263, 118339. [Google Scholar] [CrossRef]
- Chen, N.; Zhu, Z.; Su, T.; Liao, W.; Deng, C.; Ren, W.; Zhao, Y.; Lü, H. Catalytic hydrogenolysis of hydroxymethylfurfural to highly selective 2,5-dimethylfuran over FeCoNi/h-BN catalyst. Chem. Eng. J. 2020, 381, 122755. [Google Scholar] [CrossRef]
- Zhou, S.; Dai, F.; Chen, Y.; Dang, C.; Zhang, C.; Liu, D.; Qi, H. Sustainable hydrothermal self-assembly of hafnium–lignosulfonate nanohybrids for highly efficient reductive upgrading of 5-hydroxymethylfurfural. Green Chem. 2019, 21, 1421–1431. [Google Scholar] [CrossRef]
- He, A.; Hu, L.; Zhang, Y.; Jiang, Y.; Wang, X.; Xu, J.; Wu, Z. High-Efficiency Catalytic Transfer Hydrogenation of Biomass-Based 5-Hydroxymethylfurfural to 2,5-Bis(hydroxymethyl)furan over a Zirconium–Carbon Coordination Catalyst. ACS Sustain. Chem. Eng. 2021, 9, 15557–15570. [Google Scholar] [CrossRef]
- Cortez-Elizalde, J.; Córdova-Pérez, G.E.; Silahua-Pavón, A.A.; Pérez-Vidal, H.; Cervantes-Uribe, A.; Cordero-García, A.; Arévalo-Pérez, J.C.; Becerril-Altamirano, N.L.; Castillo-Gallegos, N.C.; Lunagómez-Rocha, M.A.; et al. 2,5-Dimethylfuran Production by Catalytic Hydrogenation of 5-Hydroxymethylfurfural Using Ni Supported on Al2O3-TiO2-ZrO2 Prepared by Sol-Gel Method: The Effect of Hydrogen Donors. Molecules 2022, 27, 4187. [Google Scholar] [CrossRef] [PubMed]
- Mudhulu, S.; Gong, Z.-J.; Ku, H.-C.; Lu, Y.-H.; Yu, W.-Y. Recent advances in heterogeneous catalytic hydrodeoxygenation of biomass-derived oxygenated furanics mediated by formic acid. Mater. Today Sustain. 2022, 19, 100199. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Li, S.; Chen, C.; Wu, T.; Mei, Q.; Wang, Y.; Chen, B.; Liu, H.; Han, B. Hydrogenolysis of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran under Mild Conditions without Any Additive. ACS Sustain. Chem. Eng. 2019, 7, 5711–5716. [Google Scholar] [CrossRef]
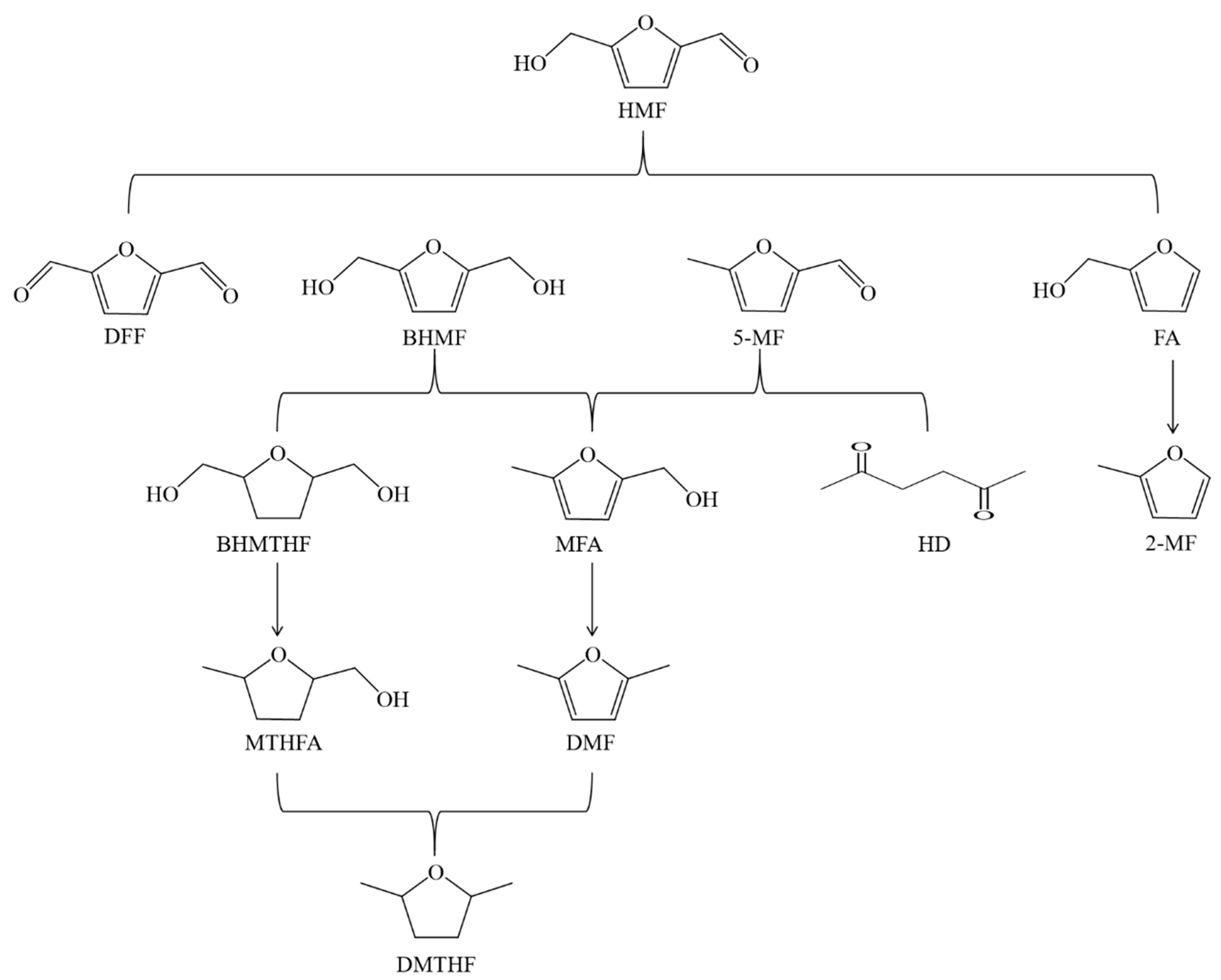

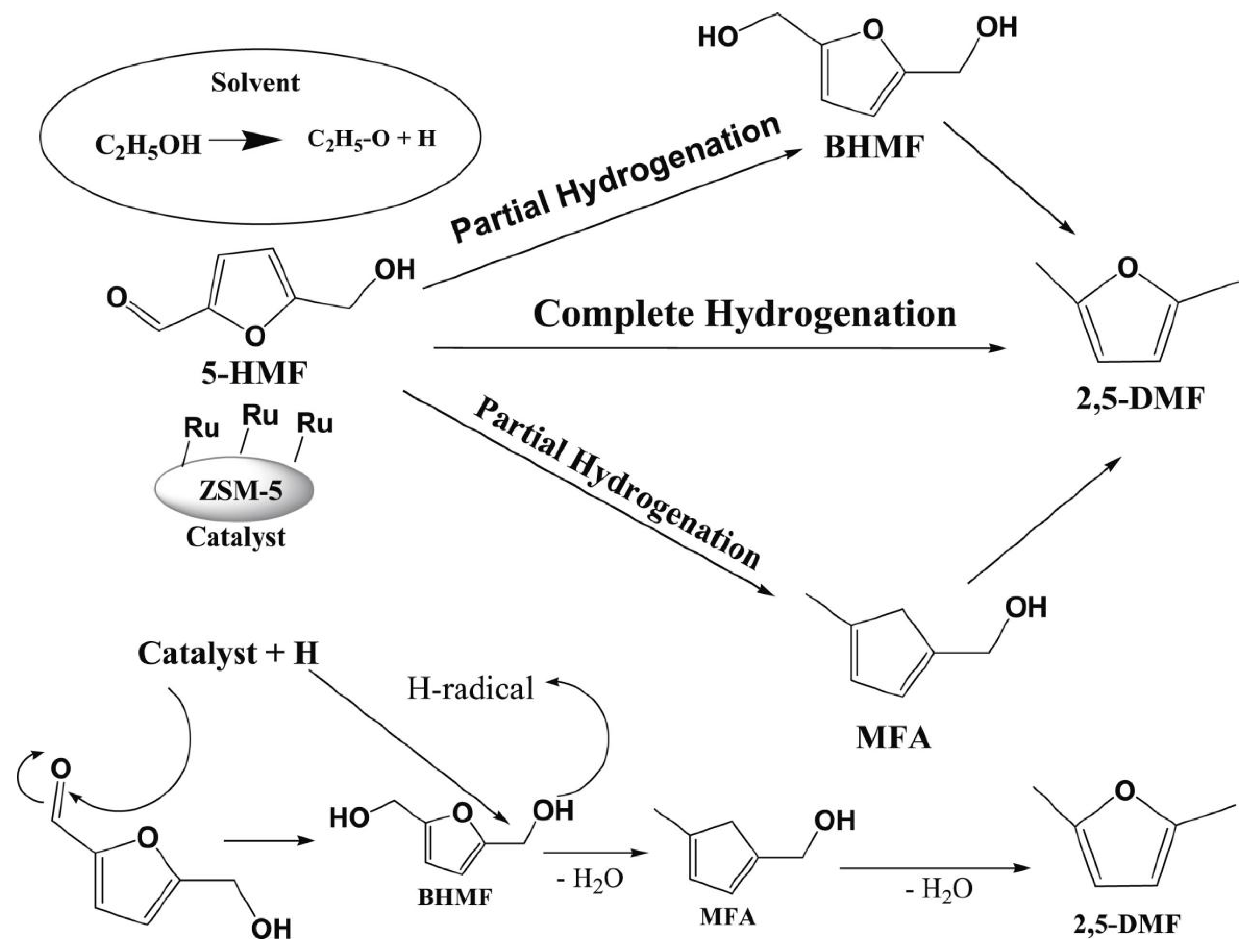
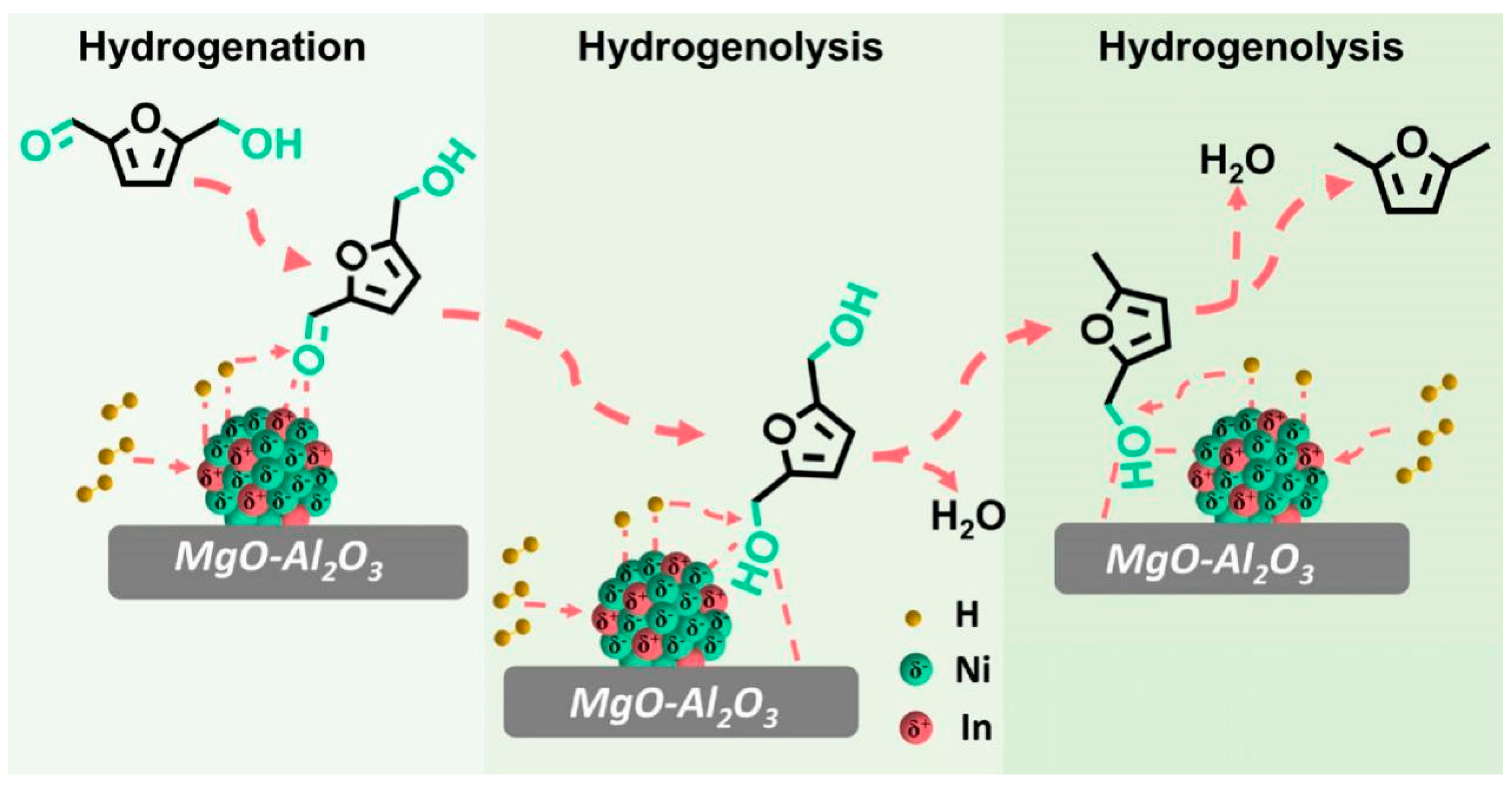
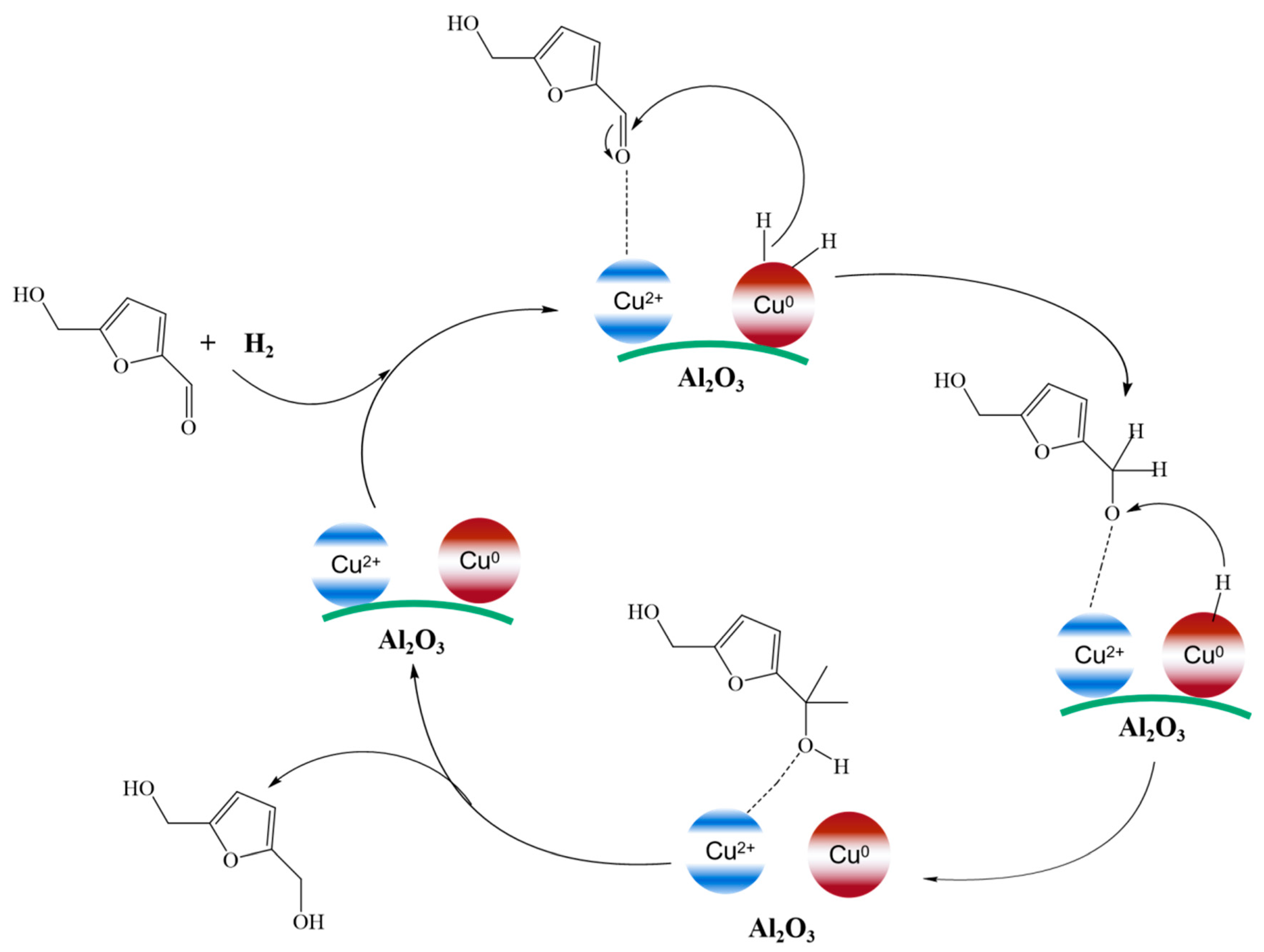

| Entry | Catalyst | Solvent | Time/h | Temp/K | Press/MPa | Conv/% | Product | Yield/% | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PtNi/SBA-15 | H2O | 2 | 303 | 1.5 | 77.0% | BHMF | 68.2% | [32] |
| 2 | Pt1/Co | THF | 2 | 453 | 1 | 100% | DMF | 92.9% | [34] |
| 3 | Ru/ZSM-5 | ethanol | 3 | 453 | 1.72 | 98% | DMF | 97% | [42] |
| 4 | Ru-Ir/C | THF | 12 | 393 | 1 | 100% | DNF | 99% | [43] |
| 5 | Ag−aerogel−CNx | H2O | 3 | 303 | 0 | 78% | HD | 77% | [18] |
| 6 | Ni/HHT | H2O | 4 | 333 | 3 | 98% | BHMF | 90% | [54] |
| 7 | 20 mol% Cu-Al2O3 | methanol | 1 | 403 | 3 | 99% | BHMF | 93% | [14] |
| 8 | meso-CuA | ethanol | 0.2 | 373 | 5 | 90% | DMF | 85% | [19] |
| 9 | CuCoNiAl-MMO | THF | 6 | 453 | 1 | 99.8 | DMF | 95.3 | [59] |
| 10 | Cu-Co/N-C | THF | 4 | 373 | 1 | 93.7 | BHMF | 92.4% | [12] |
| 11 | Co@NGs | ethanol | 4 | 473 | 2 | 100% | DMF | 94.7% | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, H.; Li, J.; Zhou, M. Advances in Selective Hydrogenation of 5-Hydroxymethylfurfural over Heterogeneous Metal Catalysts. Energies 2023, 16, 6793. https://doi.org/10.3390/en16196793
Xia H, Li J, Zhou M. Advances in Selective Hydrogenation of 5-Hydroxymethylfurfural over Heterogeneous Metal Catalysts. Energies. 2023; 16(19):6793. https://doi.org/10.3390/en16196793
Chicago/Turabian StyleXia, Haihong, Jing Li, and Minghao Zhou. 2023. "Advances in Selective Hydrogenation of 5-Hydroxymethylfurfural over Heterogeneous Metal Catalysts" Energies 16, no. 19: 6793. https://doi.org/10.3390/en16196793
APA StyleXia, H., Li, J., & Zhou, M. (2023). Advances in Selective Hydrogenation of 5-Hydroxymethylfurfural over Heterogeneous Metal Catalysts. Energies, 16(19), 6793. https://doi.org/10.3390/en16196793









