Quantitative Evaluation of Underground Coal Gasification Based on a CO2 Gasification Agent
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Sample Information
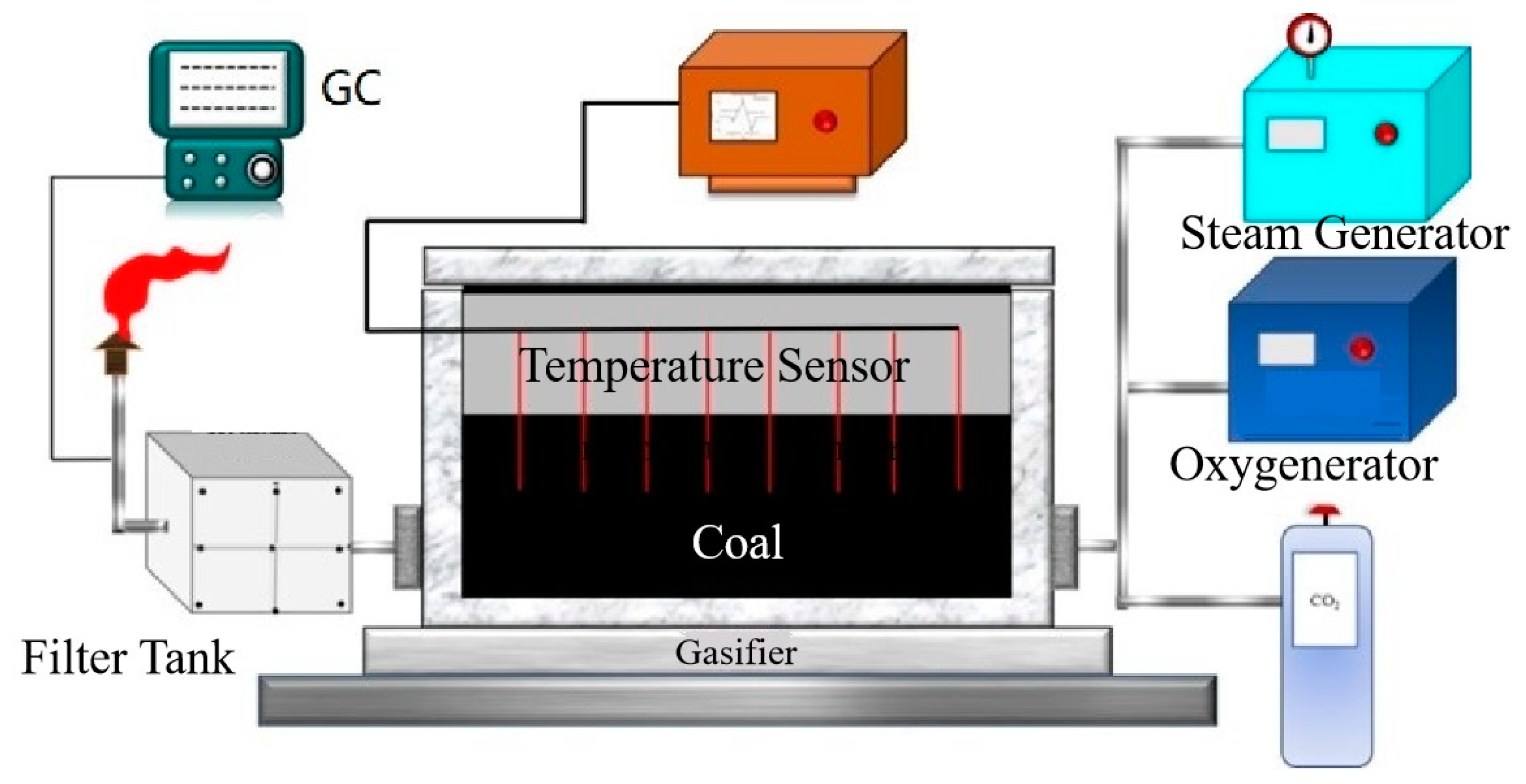
2.2. Experimental Setup
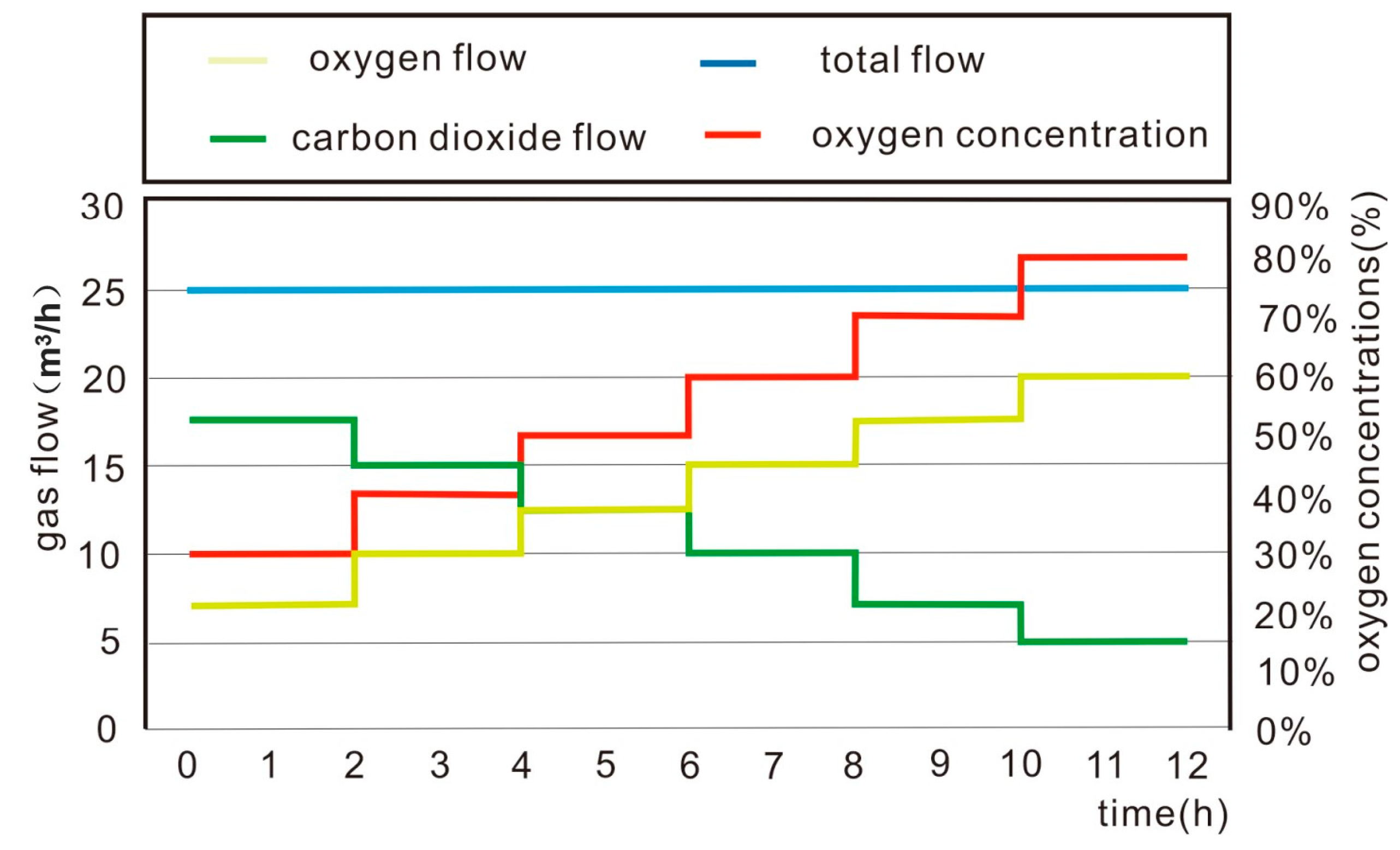
2.3. Experimental Design
2.4. Experimental Procedure
3. Experimental Results
4. Discussion
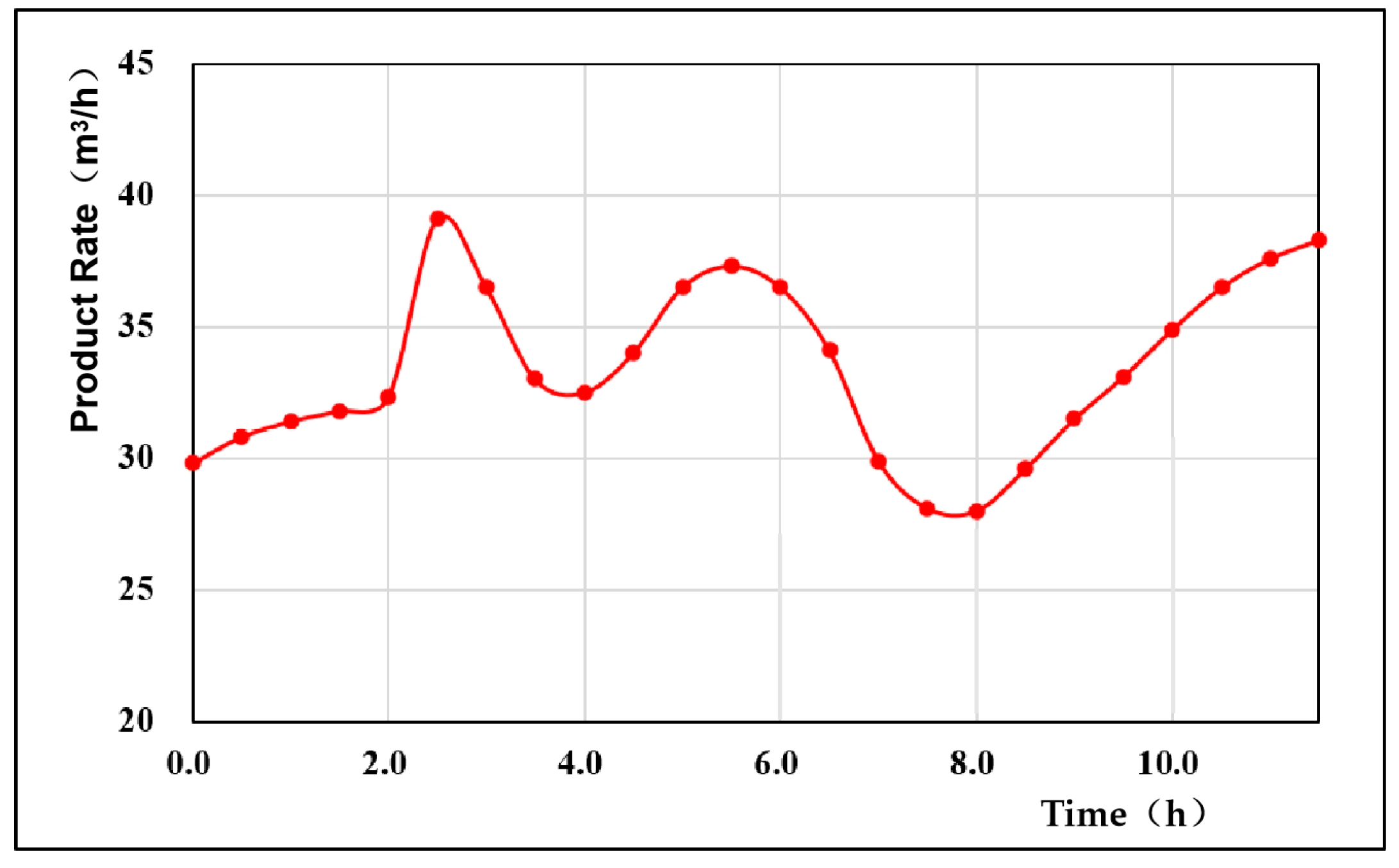
4.1. Generation Rate of the Product Gas
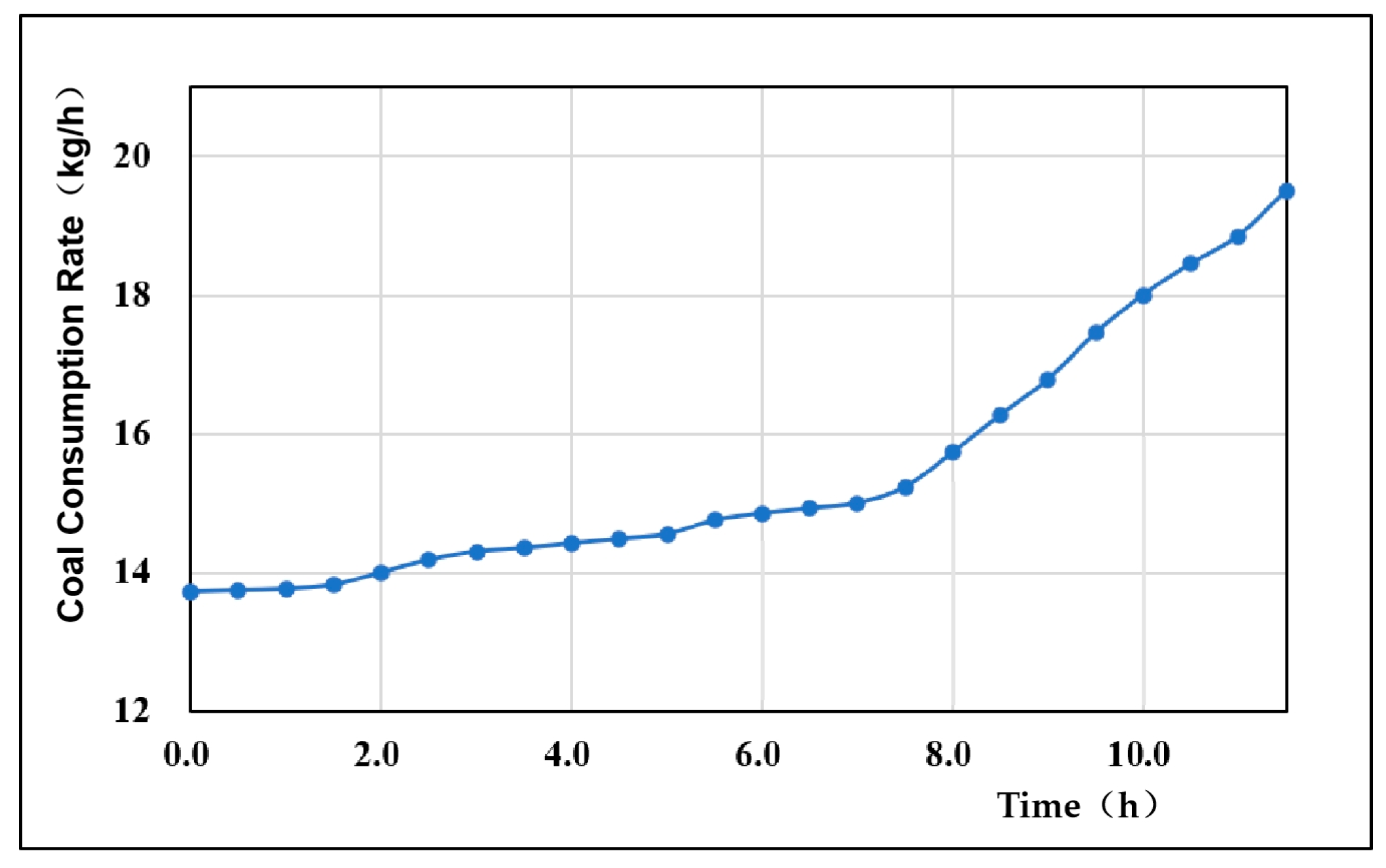
4.2. Coal Consumption Rate
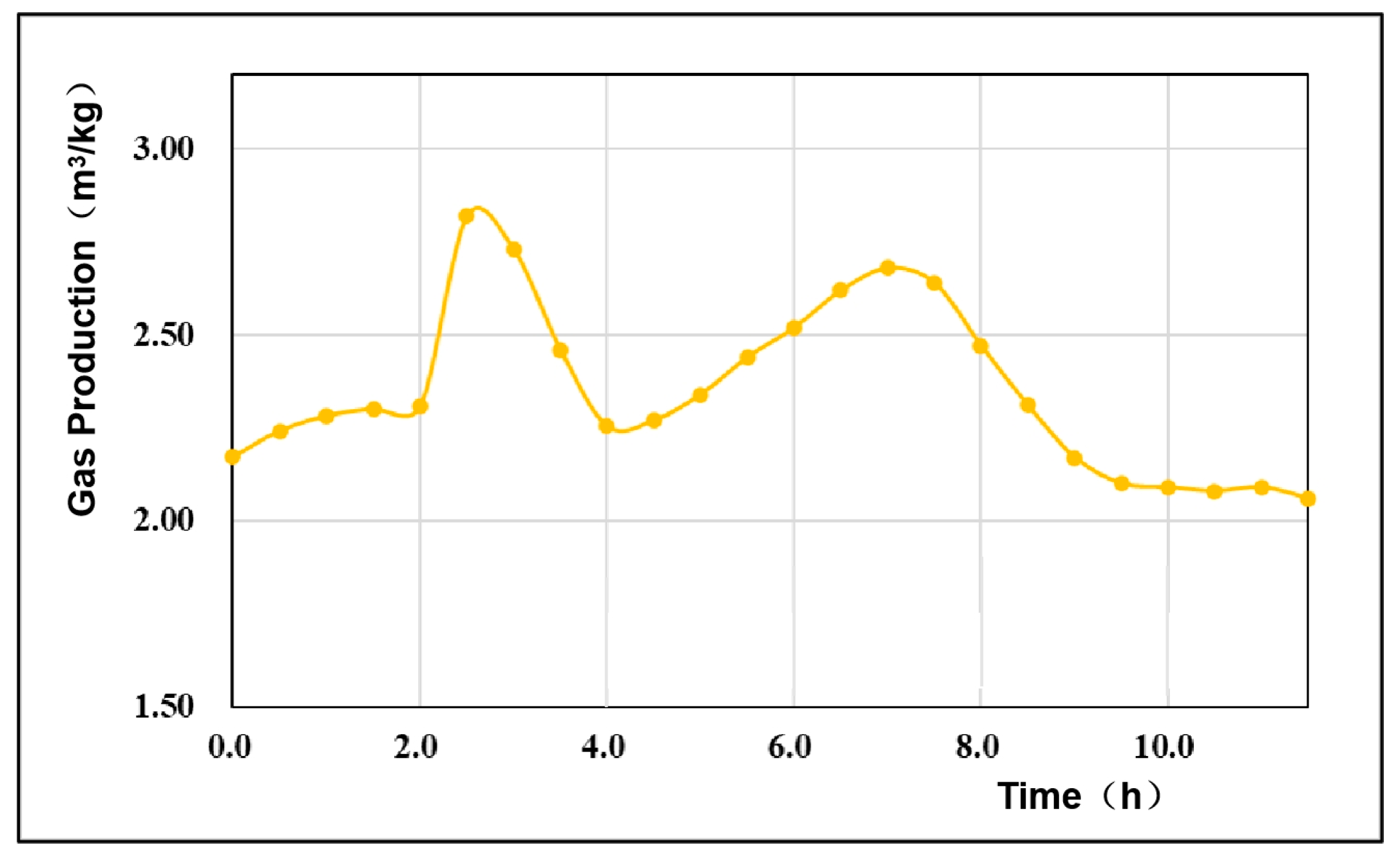
4.3. Gas Production Per Unit Mass of Coal
4.4. Energy Recovery Rate
5. Conclusions
- (1)
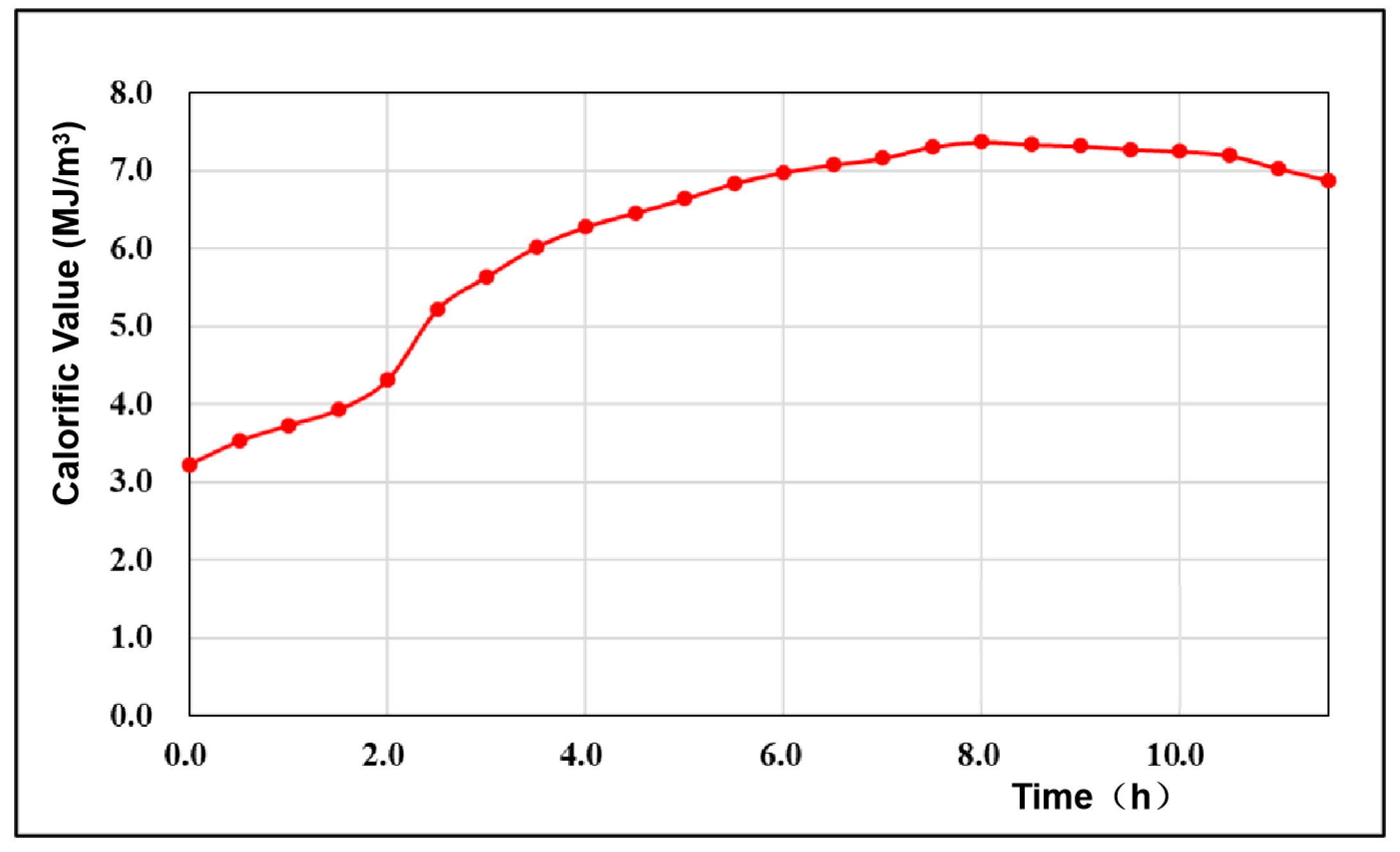
- In the oxygen-enriched carbon dioxide gasification experiment, with increasing oxygen content, the effective product and calorific value of the gasification product increased. The best gas production effect was obtained when the oxygen concentration was 50–60%. The content of active components in the generated gas was the highest under gasification conditions, with a hydrogen content of 25.1%, CO content of 29.6%, and calorific value of 7.3 MJ/m3, indicating that the redox reaction in the gasification reaction was close to equilibrium.
- (2)
- The gas production rate and the gas production per unit mass of coal fluctuated, mainly due to the change in the dominance of the oxidation reaction and the reduction reaction during the gasification process. When the oxidation reaction was dominant, the gas production was larger, and when the reduction reaction was dominant, the gas production decreased. The overall coal consumption rate in the experiment fluctuated minimally, the coal consumption rate increased with time, and the average coal consumption rate was 15.46 kg/h. The gas production rate of coal in this experiment showed a cyclic process of gas production increasing first and then decreasing, and then increasing again, with an average value of 2.35 m3/kg. According to the theoretical calculation of the gasification energy recovery evaluation system, the overall energy recovery rate was 56.34%. The energy utilization rate was high.
- (3)
- Various quantitative parameters, namely, the product generation rate, coal consumption rate, gas production per unit mass of coal, and energy recovery rate, have good practical significance for evaluating the gasification efficiency of UCG. The energy recovery evaluation method of the gasification process can be used to better evaluate the reaction process of UCG.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, L.; Zhang, N.; Kan, J.; Wang, Y. Concept, model and prediction of green coal resources in China. J. China Univ. Min. Technol. 2018, 47, 1–8. [Google Scholar]
- Zhang, J.; Li, H. Commercialization path of medium deep underground coal gasification in China. Nat. Gas Ind. 2020, 40, 156–165. [Google Scholar]
- Zou, C.; Chen, Y.; Kong, L.; Sun, F.; Chen, S.; Dong, Z. Underground coal gasification and its strategic significance to the development of natural gas industry in China. Pet. Explor. Dev. 2019, 46, 195–204. [Google Scholar] [CrossRef]
- Khan, M.M.; Mmbaga, J.P.; Shirazi, A.S.; Trivedi, J.; Liu, Q.; Gupta, R. Modelling Underground Coal Gasification-A Review. Energies 2015, 8, 12603–12668. [Google Scholar] [CrossRef]
- Liu, S.; Chang, Z.; Liu, J. Key technologies and prospect for in-situ gasification mining of deep coal resources. J. Min. Sci. Technol. 2021, 6, 261–270. [Google Scholar]
- Feng, L.; Dong, M.; Wang, B.; Qin, B. Gas production performance of underground coal gasification with continuously moving injection: Effect of direction and speed. Fuel 2023, 347, 128425. [Google Scholar] [CrossRef]
- Kapusta, K.; Wiatowski, M.; Thomas, H.R.; Zagorščak, R.; Sadasivam, S.; Masum, S.; Kempka, T.; Otto, C.; Basa, W.; Szyja, M.; et al. Experimental simulations of methane-oriented underground coal gasification using hydrogen—The effect of coal rank and gasification pressure on the hydrogasification process. Int. J. Hydrogen Energy 2023, 48, 921–932. [Google Scholar] [CrossRef]
- Perkins, G. Underground coal gasification-Part I: Field demonstrations and process performance. Prog. Energy Combust. Sci. 2018, 67, 158–187. [Google Scholar] [CrossRef]
- Vyas, D.U.; Singh, R.P. Worldwide Developments in UCG and Indian Initiative. Procedia Earth Planet. Sci. 2015, 11, 29–37. [Google Scholar] [CrossRef]
- Derbin, Y.; Walker, J.; Wanatowski, D.; Marshall, A. Soviet experience of underground coal gasification focusing on surface subsidence. J. Zhejiang Univ.-Sci. A 2015, 16, 12. [Google Scholar] [CrossRef]
- Perkins, G. Underground coal gasification—Part II: Fundamental phenomena and modeling. Prog. Energy Combust. Sci. 2018, 67, 234–274. [Google Scholar] [CrossRef]
- Stańczyk, K.; Howaniec, N.; Smoliński, A.; Świądrowski, J.; Kapusta, K.; Wiatowski, M.; Grabowski, J.; Rogut, J. Gasification of lignite and hard coal with air and oxygen enriched air in a pilot scale ex situ reactor for underground gasification. Fuel 2011, 90, 1953–1962. [Google Scholar] [CrossRef]
- Pang, X.; Pan, X.; Liu, H.; Yang, L.; Chen, F. Experimental study on underground gasification model of lean coal. Coal Sci. Technol. 2011, 39, 120–124. [Google Scholar]
- Liang, J.; Xi, J.; Sun, J.; Liang, X.; Lou, Y. Model test of oxygen-enriched underground gasification of thin coal seam in Ezhuang. J. China Coal Soc. 2007, 10, 1031–1035. [Google Scholar]
- Huang, W.; Wang, Z.; Duan, T.; Duan, T.; Xin, L. Study on underground gasification characteristics of Huating coal air, oxygen-rich and pure oxygen. Clean Coal Technol. 2011, 17, 71–74. [Google Scholar]
- Bai, Y.; Zhu, W.; Zuo, Y.; Li, F. Research Progress and Application Prospect of Coal Coke Water Vapor and CO2 Cogasification Technology. Coal Chem. Ind. 2013, 41, 13–15. [Google Scholar]
- Sadasivam, S.; Zagorščak, R.; Thomas, H.; Kapusta, K.; Stańczyk, K. Experimental study of methane-oriented gasification of semi-anthracite and bituminous coals using oxygen and steam in the context of underground coal gasification (UCG): Effects of pressure, temperature, gasification reactant supply rates and coal rank. Fuel 2020, 268, 117330. [Google Scholar]
- Chen, F.; Pan, X.; Liu, H.; Yao, K. O2/CO2 underground coal gasification model test. J. China Coal Soc. 2013, 38, 495–500. [Google Scholar]
- Zhao, J.; Liu, H.; Pan, X.; Chen, F. Experimental Study on O2/CO2 underground gasification under different Oxygen-enriched Conditions. Coal Sci. Technol. 2017, 45, 214–220. [Google Scholar]
- Bhutto, A.W.; Bazmi, A.A.; Zahedi, G. Underground coal gasification: From fundamentals to applications. Prog. Energy Combust. Sci. 2013, 39, 189–214. [Google Scholar] [CrossRef]
- Wiatowski, M.; Kapusta, K.; Stanczyk, K.; Stanczyk, K. Efficiency assessment of underground gasification of ortho-and meta-lignite: High-pressure ex situ experimental simulations. Fuel 2019, 236, 221–227. [Google Scholar] [CrossRef]
- Mishra, A.; Gautam, S.; Sharma, T. Effect of operating parameters on coal gasification. Int. J. Coal Sci. Technol. 2018, 5, 113–125. [Google Scholar] [CrossRef]
- Guo, W.; Liu, H.; Chang, Z.; Cao, D.; Liu, S. Study on Effects of Thermal Resistance and Thermal Buoyancy on Oxygen Flow Patterns during Underground Coal Gasification. ACS Omega 2021, 6, 32977–32986. [Google Scholar]
- Su, F.; Jing, S.; Gao, X.; Pu, H.; Fan, W.; Yu, G.; Wu, J.; Deng, Q.; Zhang, T. Evaluation of gasification cavity growth and gas energy recovery in underground coal gasification. J. China Coal Soc. 2021, 46, 3682–3691. [Google Scholar]
- Hamanaka, A.; Su, F.-Q.; Itakura, K.-I.; Takahashi, K.; Kodama, J.-I.; Deguchi, G. Experimental study on evaluation of underground coal gasification with a horizontal hole using two different coals. Fuel 2021, 305, 121556. [Google Scholar] [CrossRef]


| Moisture (%) | Ash (%) | Volatile Component (%) | Fixed Carbon (%) | Calorific Value (MJ/kg) | Fuel Ratio | Ash Melting Point (/°C) |
|---|---|---|---|---|---|---|
| 8.19 | 4.2 | 33.06 | 63 | 25.736 | 1.09 | 1350 |
| Calorific Value | Elemental Analysis (%) | H/C | O/C | ||||
|---|---|---|---|---|---|---|---|
| MJ/kg | C | H | N | S | O | ||
| 25.736 | 79.745 | 4.42 | 1.005 | 0.185 | 10.445 | 0.891 | 0.092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Qin, Y.; Chen, Y.; Dong, Z.; Xue, J.; Chen, S.; Zhang, M.; Zhao, Y. Quantitative Evaluation of Underground Coal Gasification Based on a CO2 Gasification Agent. Energies 2023, 16, 6993. https://doi.org/10.3390/en16196993
Chen H, Qin Y, Chen Y, Dong Z, Xue J, Chen S, Zhang M, Zhao Y. Quantitative Evaluation of Underground Coal Gasification Based on a CO2 Gasification Agent. Energies. 2023; 16(19):6993. https://doi.org/10.3390/en16196993
Chicago/Turabian StyleChen, Hao, Yong Qin, Yanpeng Chen, Zhen Dong, Junjie Xue, Shanshan Chen, Mengyuan Zhang, and Yufeng Zhao. 2023. "Quantitative Evaluation of Underground Coal Gasification Based on a CO2 Gasification Agent" Energies 16, no. 19: 6993. https://doi.org/10.3390/en16196993






