A Scientometric Review of CO2 Electroreduction Research from 2005 to 2022
Abstract
:1. Introduction
2. Methods
2.1. Data Collection and Processing
- i.
- Types of literature and publishing languages;
- ii.
- Annual trend of published literature during 2005–2022;
- iii.
- Subject categories and journals;
- iv.
- Author information;
- v.
- Geographical distribution;
- vi.
- Cited frequency;
- vii.
- Research hotspots and future trends.
2.2. Data Analysis
3. Results and Discussion
3.1. Types of Literature and Publishing Languages
3.2. Annual Trend of Published Literature during 2005–2022
3.3. Subject Categories and Journals
3.4. Author Information
3.5. Geographical Distribution
3.6. Cited Frequency
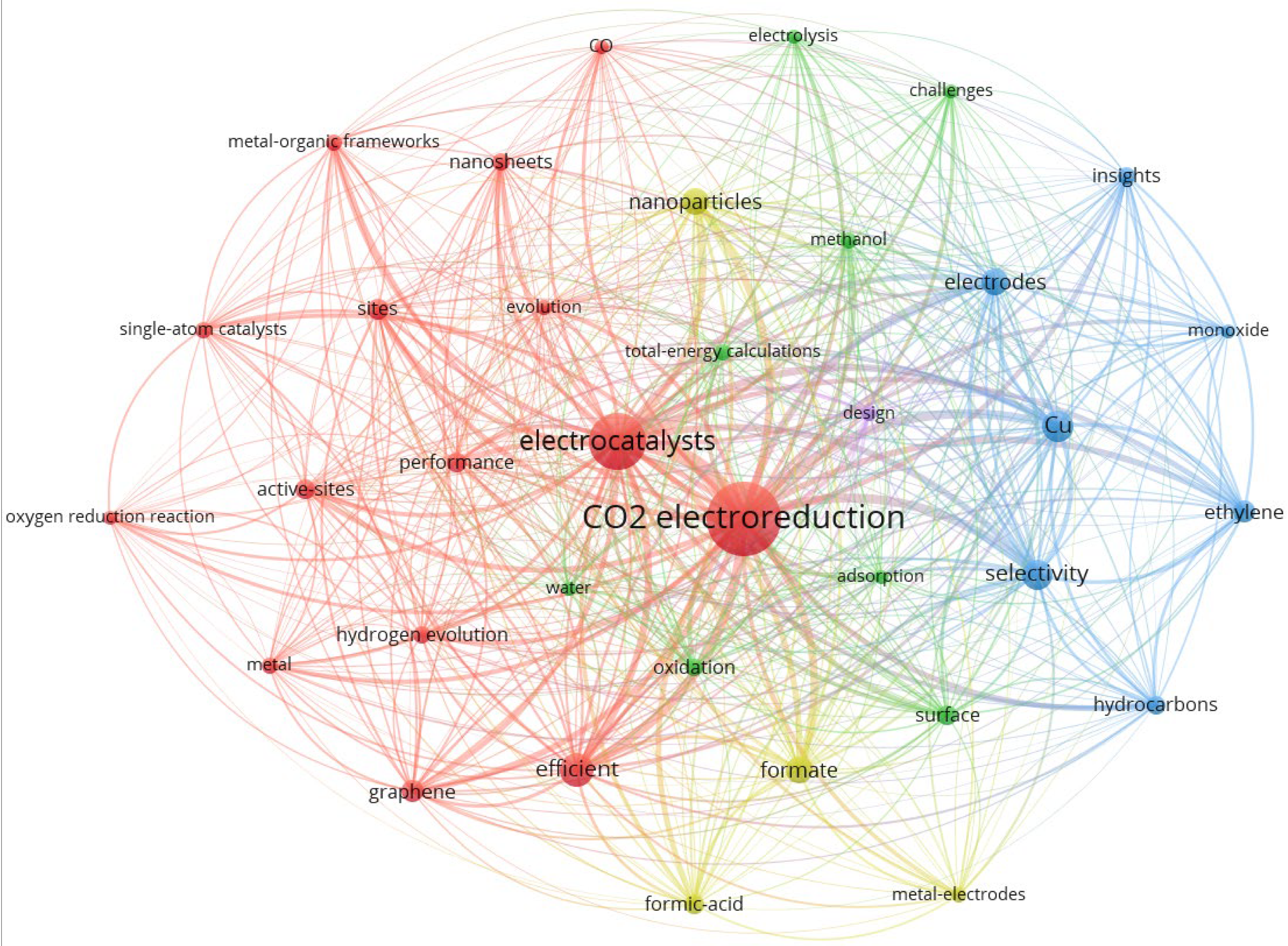
3.7. Research Hotspots and Future Trends
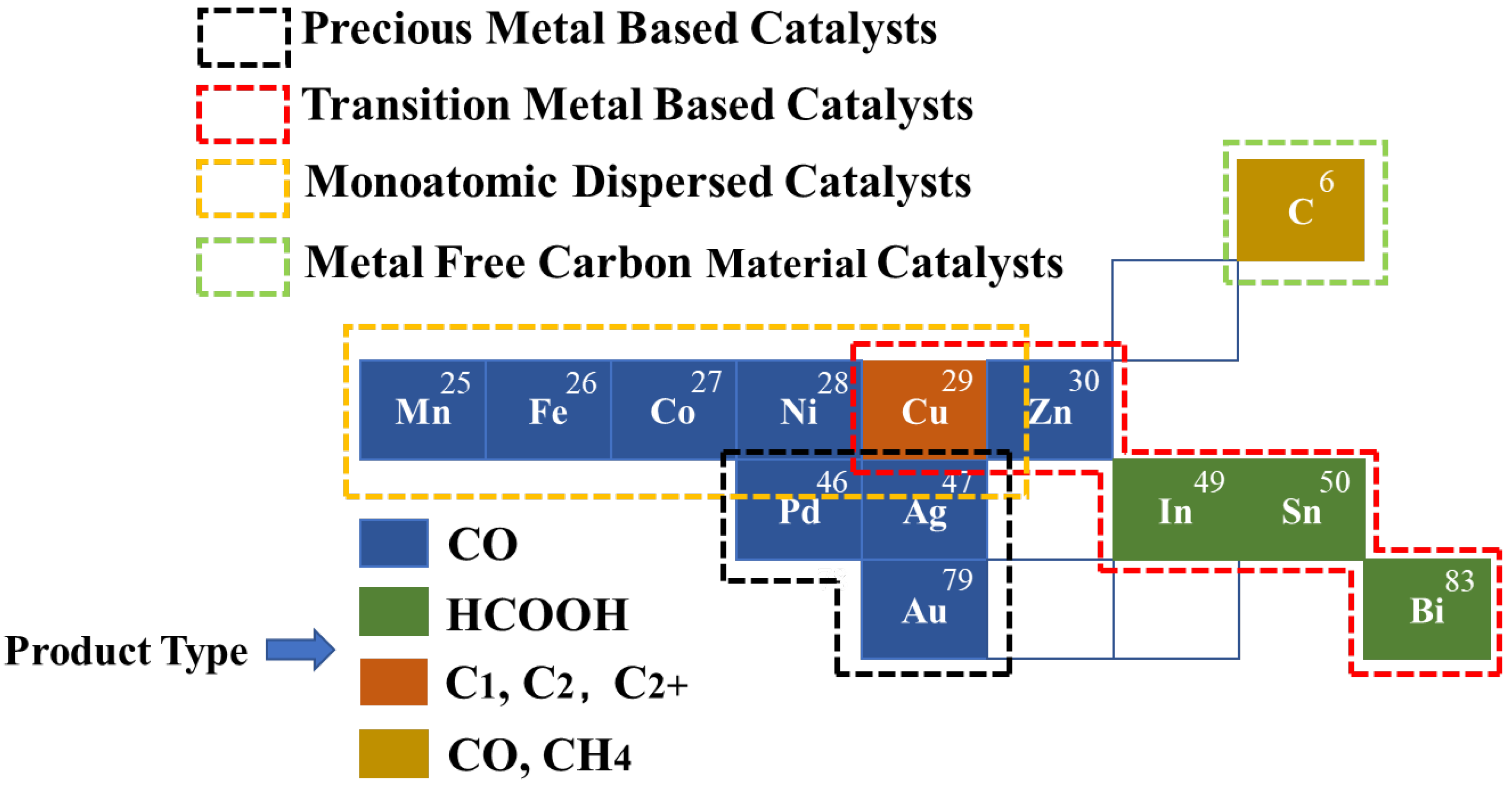
3.7.1. Cluster 1 (Red): Classification of Catalysts and Product Types
- (1)
- Metal catalyst
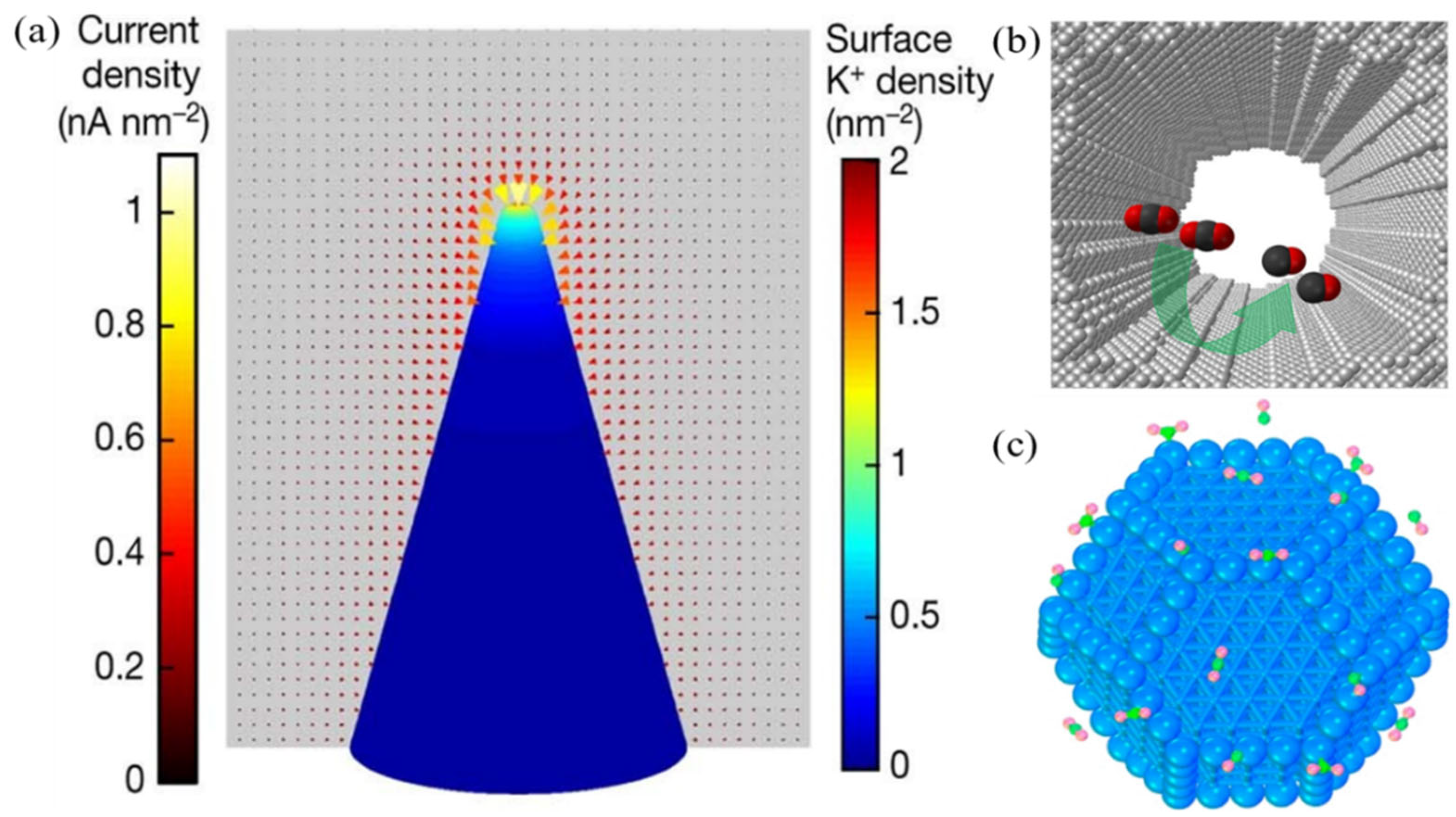
- (2)
- Non-metallic catalysts
3.7.2. Cluster 2 (Blue): Copper-Based Catalysts and Methods for Improving Product Selectivity
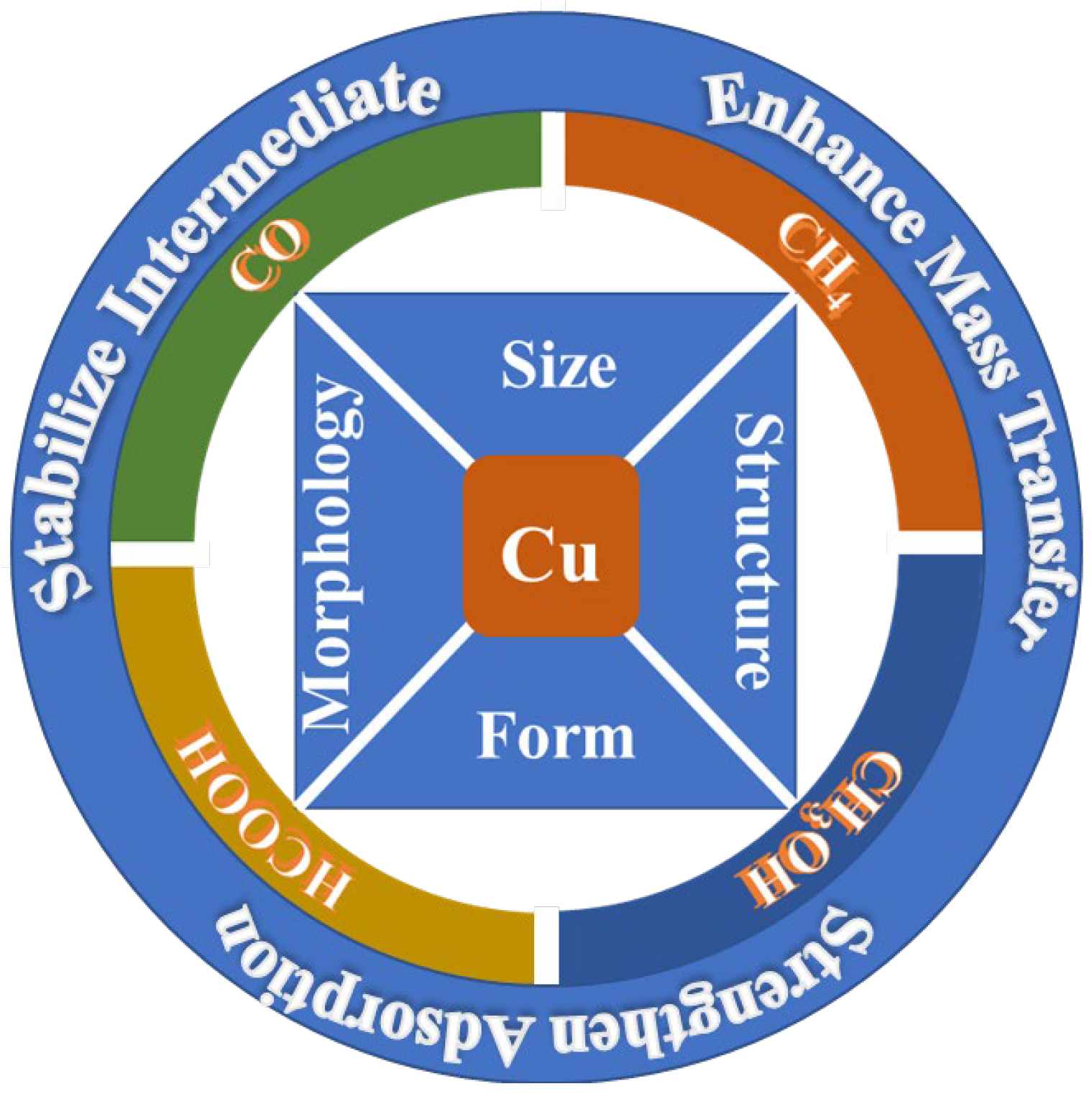
3.7.3. Cluster 3 (Green) and Cluster 4 (Yellow): Research Status of Preparation of C1 Product with a Copper-Based Catalyst
- (a).
- CO
- (b).
- CH4
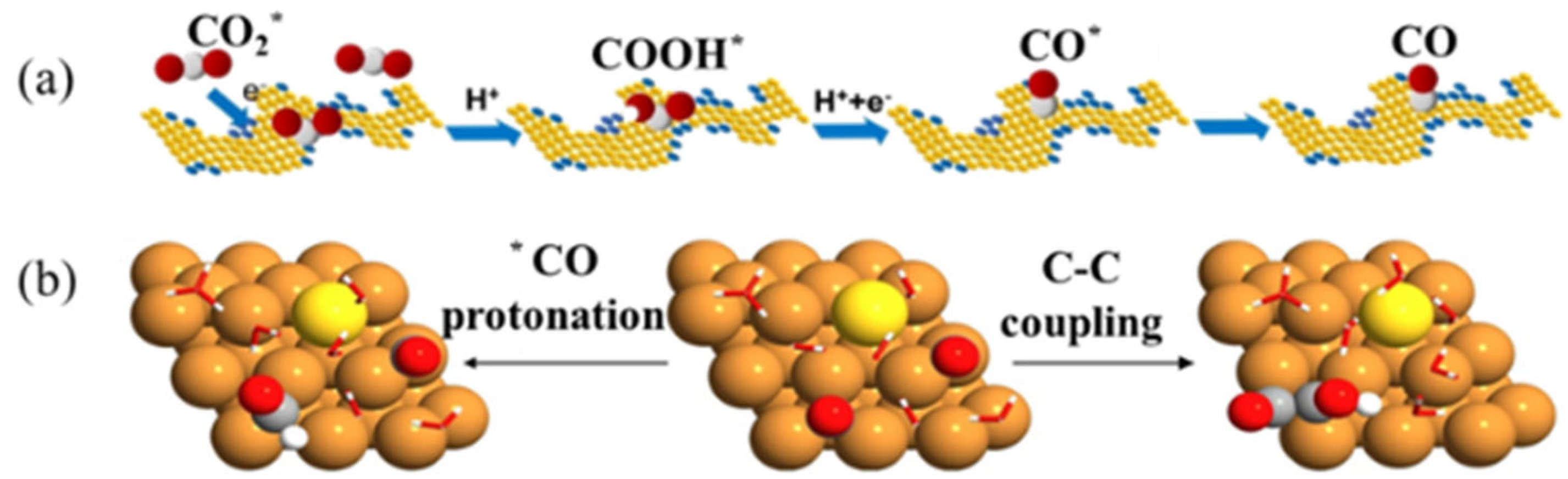
- (c).
- Methanol
- (d).
- Formic acid and formate
3.7.4. Cluster 5 (Purple): Challenges and Future Trend
- (1)
- CO2 is a nonpolar linear molecule with very stable chemical properties, which is difficult to activate and transform. In addition, the CO2 reduction process is also subject to relatively high thermodynamic and kinetic barriers [118]. In eCO2RR, the process of CO2 molecules adsorbing electrons into CO2 is the first one, which requires a lot of energy. These characteristics of eCO2RR lead to high overpotential, low selectivity, and competitive hydrogen evolution reaction, resulting in the low energy conversion efficiency of eCO2RR. At the same time, the products of eCO2RR are diverse, the selectivity of a single product is low, and the separation is difficult. Therefore, it is still necessary to continue to develop catalysts with high activity, high selectivity, and high stability (especially for a single product), especially copper-based catalysts for C1 production.
- (2)
- At present, although there have been many studies on eCO2RR, the mechanism of this reaction process is still not clear enough. Most of the studies are still in a relatively simple stage, and researchers need to continue to dig deeply.
- (3)
- In addition, the low solubility of CO2 in aqueous solution limits the current density of eCO2RR. For this problem, in addition to improving the catalyst, it can also be improved by optimizing the structure of the electrochemical reactor. In addition to the most commonly used H-type reactor, the membrane electrode assembly (MEA) reactor is also the focus of future research. The CO2 gas in the reactor is not in direct contact with the electrolyte, which can not only enhance mass transfer and reduce ohmic resistance, but it can also effectively avoid hydrogen evolution.
- (4)
- At present, the research on eCO2RR is still in the stage of laboratory research, and there is still a long way to go from commercial application. Therefore, it is of great practical significance to develop a CO2 electrochemical reactor with industrial current density considering the amplification of the CO2 electroreduction device [119,120].
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, V.K.; Scinocca, J.F.; Boer, G.J.; Christian, J.R.; Denman, K.L.; Flato, G.M.; Kharin, V.V.; Lee, W.G.; Merryfield, W.J. Carbon emission limits required to satisfy future representative concentration pathways of greenhouse gases. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Shakun, J.D.; Clark, P.U.; He, F.; Marcott, S.A.; Mix, A.C.; Liu, Z.; Otto-Bliesner, B.; Schmittner, A.; Bard, E. Global warming preceded by increasing carbon dioxide concentrations during the last deglaciation. Nature 2012, 484, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.R.; Hawkins, E.; Jones, P. CO2, the greenhouse effect and global warming: From the pioneering work of Arrhenius and Callendar to today’s Earth System Models. Endeavour 2016, 40, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eren, E.O.; Ozkar, S. Recent advances in heterogeneous catalysts for the effective electroreduction of carbon dioxide to carbon monoxide. J. Power Sources 2021, 506, 230215. [Google Scholar] [CrossRef]
- Yuan, X.L.; Sheng, X.R.; Chen, L.P.; Tang, Y.Z.; Li, Y.; Jia, Y.S.; Qu, D.F.; Wang, Q.S.; Ma, Q.; Zuo, J. Carbon footprint and embodied carbon transfer at the provincial level of the Yellow River Basin. Sci. Total Environ. 2022, 803, 149993. [Google Scholar] [CrossRef]
- De Luna, P.; Hahn, C.; Higgins, D.; Jaffer, S.A.; Jaramillo, T.F.; Sargent, E.H. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 2019, 364, eaav3506. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.S.; Xia, B.Y.; Li, Y.; Yan, Y.; Yang, Y.; Sun, Q.; Chan, S.H.; Fisher, A.; Wang, X. Amino acid modified copper electrodes for the enhanced selective electroreduction of carbon dioxide towards hydrocarbons. Energy Environ. Sci. 2016, 9, 1687–1695. [Google Scholar] [CrossRef]
- Zhang, T.; Zhong, H.; Qiu, Y.; Li, X.; Zhang, H. Zn electrode with a layer of nanoparticles for selective electroreduction of CO2 to formate in aqueous solutions. J. Mater. Chem. A 2016, 4, 16670–16676. [Google Scholar] [CrossRef]
- Hong, D.; Tsukakoshi, Y.; Kotani, H.; Ishizuka, T.; Kojima, T. Visible-Light-Driven Photocatalytic CO2 Reduction by a Ni(II) Complex Bearing a Bioinspired Tetradentate Ligand for Selective CO Production. J. Am. Chem. Soc. 2017, 139, 6538–6541. [Google Scholar] [CrossRef]
- Homayoni, H.; Chanmanee, W.; De Tacconi, N.R.; Dennis, B.H.; Rajeshwar, K. Continuous Flow Photoelectrochemical Reactor for Solar Conversion of Carbon Dioxide to Alcohols. J. Electrochem. Soc. 2015, 162, E115–E122. [Google Scholar] [CrossRef]
- Li, Q.; Rao, X.; Sheng, J.; Xu, J.; Yi, J.; Liu, Y.; Zhang, J. Energy storage through CO2 electroreduction: A brief review of advanced Sn-based electrocatalysts and electrodes. J. CO2 Util. 2018, 27, 48–59. [Google Scholar] [CrossRef]
- Nam, D.H.; Kuk, S.K.; Choe, H.; Lee, S.; Ko, J.W.; Son, E.J.; Choi, E.-G.; Kim, Y.H.; Park, C.B. Enzymatic photosynthesis of formate from carbon dioxide coupled with highly efficient photoelectrochemical regeneration of nicotinamide cofactors. Green Chem. 2016, 18, 5989–5993. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, Y.; Jiang, Z.; Wang, X.; Wang, X.; Zhang, S.; Han, P.; Yang, C. Enzymatic conversion of carbon dioxide. Chem. Soc. Rev. 2015, 44, 5981–6000. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef]
- Zheng, T.; Jiang, K.; Wang, H. Recent Advances in Electrochemical CO2-to-CO Conversion on Heterogeneous Catalysts. Adv. Mater. 2018, 30, e1802066. [Google Scholar] [CrossRef]
- Handoko, A.D.; Wei, F.; Jenndy; Yeo, B.S.; Seh, Z.W. Understanding heterogeneous electrocatalytic carbon dioxide reduction through operando techniques. Nat. Catal. 2018, 1, 922–934. [Google Scholar] [CrossRef]
- Jiang, K.; Siahrostami, S.; Zheng, T.; Hu, Y.; Hwang, S.; Stavitski, E.; Peng, Y.; Dynes, J.; Gangisetty, M.; Su, D.; et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ. Sci. 2018, 11, 893–903. [Google Scholar] [CrossRef]
- Liu, A.; Gao, M.; Ren, X.; Meng, F.; Yang, Y.; Gao, L.; Yang, Q.; Ma, T. Current progress in electrocatalytic carbon dioxide reduction to fuels on heterogeneous catalysts. J. Mater. Chem. A 2020, 8, 3541–3562. [Google Scholar] [CrossRef]
- Siahrostami, S.; Jiang, K.; Karamad, M.; Chan, K.; Wang, H.; Nørskov, J. Theoretical Investigations into Defected Graphene for Electrochemical Reduction of CO2. ACS Sustain. Chem. Eng. 2017, 5, 11080–11085. [Google Scholar] [CrossRef]
- Zheng, T.; Jiang, K.; Ta, N.; Hu, Y.; Zeng, J.; Liu, J.; Wang, H. Large-Scale and Highly Selective CO2 Electrocatalytic Reduction on Nickel Single-Atom Catalyst. Joule 2019, 3, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Han, P.; Wang, Z.; Kuang, M.; Wang, Y.; Liu, J.; Hu, L.; Qian, L.; Zheng, G. 2D Assembly of Confined Space toward Enhanced CO2 Electroreduction. Adv. Energy Mater. 2018, 8, 1801230. [Google Scholar] [CrossRef]
- Wu, J.; Sharifi, T.; Gao, Y.; Zhang, T.; Ajayan, P.M. Emerging Carbon-Based Heterogeneous Catalysts for Electrochemical Reduction of Carbon Dioxide into Value-Added Chemicals. Adv. Mater. 2019, 31, e1804257. [Google Scholar] [CrossRef] [PubMed]
- Mun, Y.; Kim, K.; Kim, S.; Lee, S.; Lee, S.; Kim, S.; Choi, W.; Kim, S.-K.; Han, J.W.; Lee, J. A novel strategy to develop non-noble metal catalyst for CO2 electroreduction: Hybridization of metal-organic polymer. Appl. Catal. B Environ. 2018, 236, 154–161. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Z.; Bao, A.; Tang, X.; Huang, X.; Yi, H.; Zhao, S.; Sun, T.; Wang, J.; Gao, F. Recent advances on electrocatalytic CO2 reduction to resources: Target products, reaction pathways and typical catalysts. Chem. Eng. J. 2023, 453, 139663. [Google Scholar] [CrossRef]
- Peterson, A.A.; Abild-Pedersen, F.; Studt, F.; Rossmeisl, J.; Nørskov, J.K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 2010, 3, 1311–1315. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Gao, S.; Lin, Y.; Jiao, X.; Sun, Y.; Luo, Q.; Zhang, W.; Xie, Y. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 2016, 529, 68. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Mul, G.; Baltrusaitis, J.; Larrazabal, G.O.; Perez-Ramirez, J. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy Environ. Sci. 2013, 6, 3112–3135. [Google Scholar] [CrossRef] [Green Version]
- Costentin, C.; Robert, M.; Saveant, J.M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef] [PubMed]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef] [Green Version]
- Dinh, C.T.; Burdyny, T.; Kibria, M.G.; Seifitokaldani, A.; Gabardo, C.M.; de Arquer, F.P.G.; Kiani, A.; Edwards, J.P.; De Luna, P.; Bushuyev, O.S.; et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 2018, 360, 783–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.W.; Ciston, J.; Kanan, M.W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 2014, 508, 504. [Google Scholar] [CrossRef]
- Chen, C.; Kotyk, J.F.K.; Sheehan, S.W. Progress toward Commercial Application of Electrochemical Carbon Dioxide Reduction. Chem 2018, 4, 2571–2586. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Zhao, X.; Wu, M.; Li, Q.; Wang, Y.; Yao, J. Metal-Free Fluorine-Doped Carbon Electrocatalyst for CO2 Reduction Outcompeting Hydrogen Evolution. Angew. Chem. Int. Ed. 2018, 57, 9640–9644. [Google Scholar] [CrossRef]
- Zhao, S.; Jin, R.X.; Jin, R.C. Opportunities and Challenges in CO2 Reduction by Gold- and Silver-Based Electrocatalysts: From Bulk Metals to Nanoparticles and Atomically Precise Nanoclusters. ACS Energy Lett. 2018, 3, 452–462. [Google Scholar] [CrossRef]
- Liu, M.; Pang, Y.; Zhang, B.; De Luna, P.; Voznyy, O.; Xu, J.; Zheng, X.; Dinh, C.T.; Fan, F.; Cao, C.; et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 2016, 537, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-C.; Betancourt, L.E.; Senanayake, S.D.; Hu, E.; Zhang, Y.; Xu, W.; Polyansky, D.E. Modification of CO2 Reduction Activity of Nanostructured Silver Electrocatalysts by Surface Halide Anions. ACS Appl. Energy Mater. 2018, 2, 102–109. [Google Scholar] [CrossRef]
- Luan, C.; Shao, Y.; Lu, Q.; Gao, S.; Huang, K.; Wu, H.; Yao, K. High-Performance Carbon Dioxide Electrocatalytic Reduction by Easily Fabricated Large-Scale Silver Nanowire Arrays. ACS Appl. Mater. Interfaces 2018, 10, 17950–17956. [Google Scholar] [CrossRef]
- Peng, X.; Karakalos, S.G.; Mustain, W.E. Preferentially Oriented Ag Nanocrystals with Extremely High Activity and Faradaic Efficiency for CO2 Electrochemical Reduction to CO. ACS Appl. Mater. Interfaces 2018, 10, 1734–1742. [Google Scholar] [CrossRef]
- Lu, Q.; Rosen, J.; Zhou, Y.; Hutchings, G.; Kimmel, Y.C.; Chen, J.G.; Jiao, F. A selective and efficient electrocatalyst for carbon dioxide reduction. Nat. Commun. 2014, 5, 3242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.; Zhou, H.; Wang, J.; Miao, S.; Yang, F.; Wang, G.; Wang, J.; Bao, X. Size-Dependent Electrocatalytic Reduction of CO2 over Pd Nanoparticles. J. Am. Chem. Soc. 2015, 137, 4288–4291. [Google Scholar] [CrossRef] [PubMed]
- Wakerley, D.; Lamaison, S.; Ozanam, F.; Menguy, N.; Mercier, D.; Marcus, P.; Fontecave, M.; Mougel, V. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface. Nat. Mater. 2019, 18, 1222–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Cheng, C.Q.; Zou, C.Q.; Zheng, X.L.; Mao, J.; Liu, H.; Li, Z.; Dong, C.K.; Du, X.W. Electroreduction of Carbon Dioxide in Metallic Nanopores through a Pincer Mechanism. Angew. Chem. Int. Ed. 2020, 59, 19297–19303. [Google Scholar] [CrossRef]
- Reske, R.; Mistry, H.; Behafarid, F.; Cuenya, B.R.; Strasser, P. Particle Size Effects in the Catalytic Electroreduction of CO2 on Cu Nanoparticles. J. Am. Chem. Soc. 2014, 136, 6978–6986. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhao, S.; Johannessen, B.; Veder, J.; Saunders, M.; Rowles, M.R.; Cheng, M.; Liu, C.; Chisholm, M.F.; De Marco, R.; et al. Atomically Dispersed Transition Metals on Carbon Nanotubes with Ultrahigh Loading for Selective Electrochemical Carbon Dioxide Reduction. Adv. Mater. 2018, 30, e1706287. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Li, G.; Lin, Q.; Hu, Q.; Zhang, Q.; Liu, J.; He, C. Scalable Production of Efficient Single-Atom Copper Decorated Carbon Membranes for CO2 Electroreduction to Methanol. J. Am. Chem. Soc. 2019, 141, 12717–12723. [Google Scholar] [CrossRef]
- Ju, W.; Bagger, A.; Hao, G.-P.; Varela, A.S.; Sinev, I.; Bon, V.; Cuenya, B.R.; Kaskel, S.; Rossmeisl, J.; Strasser, P. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat. Commun. 2017, 8, 944. [Google Scholar] [CrossRef] [Green Version]
- Xin, Z.; Liu, J.; Wang, X.; Shen, K.; Yuan, Z.; Chen, Y.; Lan, Y.-Q. Implanting Polypyrrole in Metal-Porphyrin MOFs: Enhanced Electrocatalytic Performance for CO2RR. ACS Appl. Mater. Interfaces 2021, 13, 54959–54966. [Google Scholar] [CrossRef]
- Cheng, Y.; Veder, J.-P.; Thomsen, L.; Zhao, S.; Saunders, M.; Demichelis, R.; Liu, C.; De Marco, R.; Jiang, S.P. Electrochemically substituted metal phthalocyanines, e-MPc (M = Co, Ni), as highly active and selective catalysts for CO2reduction. J. Mater. Chem. A 2017, 6, 1370–1375. [Google Scholar] [CrossRef]
- Xin, Z.; Wang, Y.-R.; Chen, Y.; Li, W.-L.; Dong, L.-Z.; Lan, Y.-Q. Metallocene implanted metalloporphyrin organic framework for highly selective CO2 electroreduction. Nano Energy 2019, 67, 104233. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhang, Y.; Cheng, K.; Quan, X.; Fan, X.; Su, Y.; Chen, S.; Zhao, H.; Zhang, Y.; Yu, H.; et al. Selective Electrochemical Reduction of Carbon Dioxide to Ethanol on a Boron- and Nitrogen-Co-doped Nanodiamond. Angew. Chem. Int. Ed. 2017, 56, 15607–15611. [Google Scholar] [CrossRef]
- Wang, H.; Jia, J.; Song, P.; Wang, Q.; Li, D.; Min, S.; Qian, C.; Wang, L.; Li, Y.F.; Ma, C.; et al. Efficient Electrocatalytic Reduction of CO2by Nitrogen-Doped Nanoporous Carbon/Carbon Nanotube Membranes: A Step Towards the Electrochemical CO2Refinery. Angew. Chem. Int. Ed. 2017, 56, 7847–7852. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.P.; Wu, J.; Yadav, R.M.; Liu, M.; Wright, C.J.; Tiwary, C.S.; Yakobson, B.I.; Lou, J.; Ajayan, P.M.; Zhou, X.-D. Nitrogen-Doped Carbon Nanotube Arrays for High-Efficiency Electrochemical Reduction of CO2: On the Understanding of Defects, Defect Density, and Selectivity. Angew. Chem. Int. Ed. 2015, 54, 13701–13705. [Google Scholar] [CrossRef]
- Grosse, P.; Gao, D.; Scholten, F.; Sinev, I.; Mistry, H.; Cuenya, B.R. Dynamic Changes in the Structure, Chemical State and Catalytic Selectivity of Cu Nanocubes during CO2Electroreduction: Size and Support Effects. Angew. Chem. Int. Ed. 2018, 57, 6192–6197. [Google Scholar] [CrossRef] [Green Version]
- Nellaiappan, S.; Katiyar, N.K.; Kumar, R.; Parui, A.; Malviya, K.D.; Pradeep, K.G.; Singh, A.K.; Sharma, S.; Tiwary, C.S.; Biswas, K. High-Entropy Alloys as Catalysts for the CO2 and CO Reduction Reactions: Experimental Realization. ACS Catal. 2020, 10, 3658–3663. [Google Scholar] [CrossRef]
- Luo, W.; Nie, X.; Janik, M.J.; Asthagiri, A. Facet Dependence of CO2 Reduction Paths on Cu Electrodes. ACS Catal. 2016, 6, 219–229. [Google Scholar] [CrossRef]
- Jiang, K.; Sandberg, R.B.; Akey, A.J.; Liu, X.; Bell, D.C.; Nørskov, J.K.; Chan, K.; Wang, H. Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. Nat. Catal. 2018, 1, 111–119. [Google Scholar] [CrossRef]
- De Gregorio, G.L.; Burdyny, T.; Loiudice, A.; Iyengar, P.; Smith, W.A.; Buonsanti, R. Facet-Dependent Selectivity of Cu Catalysts in Electrochemical CO2 Reduction at Commercially Viable Current Densities. ACS Catal. 2020, 10, 4854–4862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karapinar, D.; Huan, N.T.; Sahraie, N.R.; Li, J.; Wakerley, D.; Touati, N.; Zanna, S.; Taverna, D.; Tizei, L.H.G.; Zitolo, A.; et al. Electroreduction of CO 2 on Single-Site Copper-Nitrogen-Doped Carbon Material: Selective Formation of Ethanol and Reversible Restructuration of the Metal Sites. Angew. Chem. Int. Ed. 2019, 58, 15098–15103. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; de Arquer, F.P.G.; Dinh, C.-T.; Ozden, A.; Li, Y.C.; Nam, D.-H.; Li, J.; Liu, Y.-S.; Wicks, J.; et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 2020, 5, 478–486. [Google Scholar] [CrossRef]
- Zhao, K.; Nie, X.; Wang, H.; Chen, S.; Quan, X.; Yu, H.; Choi, W.; Zhang, G.; Kim, B.; Chen, J.G. Selective electroreduction of CO2 to acetone by single copper atoms anchored on N-doped porous carbon. Nat. Commun. 2020, 11, 2455. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.-H.; Bushuyev, O.S.; Li, J.; De Luna, P.; Seifitokaldani, A.; Dinh, C.-T.; de Arquer, F.P.G.; Wang, Y.; Liang, Z.; Proppe, A.H.; et al. Metal–Organic Frameworks Mediate Cu Coordination for Selective CO2Electroreduction. J. Am. Chem. Soc. 2018, 140, 11378–11386. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.X.; Hou, S.Z.; Zhang, X.D.; Xu, M.; Yang, H.F.; Cao, P.S.; Gu, Z. Cathodized copper porphyrin metal-organic framework nanosheets for selective formate and acetate production from CO2 electroreduction. Chem. Sci. 2019, 10, 2199–2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Mensi, M.; Oveisi, E.; Mantella, V.; Buonsanti, R. Structural Sensitivities in Bimetallic Catalysts for Electrochemical CO2 Reduction Revealed by Ag–Cu Nanodimers. J. Am. Chem. Soc. 2019, 141, 2490–2499. [Google Scholar] [CrossRef]
- Fang, X.; Men, Y.; Wu, F.; Zhao, Q.; Singh, R.; Xiao, P.; Du, T.; Webley, P.A. Improved methanol yield and selectivity from CO2 hydrogenation using a novel Cu-ZnO-ZrO2 catalyst supported on Mg-Al layered double hydroxide (LDH). J. CO2 Util. 2019, 29, 57–64. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, A.; Jiang, X.; Liu, M.; Sun, Y.; Song, C.; Guo, X. Selective CO2 Hydrogenation to Hydrocarbons on Cu-Promoted Fe-Based Catalysts: Dependence on Cu-Fe Interaction. ACS Sustain. Chem. Eng. 2018, 6, 10182–10190. [Google Scholar] [CrossRef]
- Lin, W.; Chen, H.; Li, Z.; Sasaki, K.; Yao, S.; Zhang, Z.; Li, J.; Fu, J. A Cu2O-derived Polymeric Carbon Nitride Heterostructured Catalyst for the Electrochemical Reduction of Carbon Dioxide to Ethylene. Chemsuschem 2021, 14, 3190–3197. [Google Scholar] [CrossRef]
- Ma, X.; Tian, J.; Wang, M.; Jin, X.; Shen, M.; Zhang, L. Metal-organic framework derived carbon supported Cu-In nanoparticles for highly selective CO2 electroreduction to CO. Catal. Sci. Technol. 2021, 11, 6096–6102. [Google Scholar] [CrossRef]
- Zhu, H.L.; Huang, J.R.; Zhang, X.W.; Wang, C.; Huang, N.Y.; Liao, P.Q.; Chen, X.M. Highly Efficient Electroconversion of CO2 into CH4 by a Metal-Organic Framework with Trigonal Pyramidal Cu(I)N3 Active Sites. ACS Catal. 2021, 11, 11786–11792. [Google Scholar] [CrossRef]
- Chen, C.; Pang, Y.; Zhang, F.; Zhong, J.; Zhang, B.; Cheng, Z. Sharp Cu@Sn nanocones on Cu foam for highly selective and efficient electrochemical reduction of CO2 to formate. J. Mater. Chem. A 2018, 6, 19621–19630. [Google Scholar] [CrossRef]
- Ye, K.; Cao, A.; Shao, J.; Wang, G.; Si, R.; Ta, N.; Xiao, J.; Wang, G. Synergy effects on Sn-Cu alloy catalyst for efficient CO2 electroreduction to formate with high mass activity. Sci. Bull. 2020, 65, 711–719. [Google Scholar] [CrossRef]
- Mellmann, D.; Sponholz, P.; Junge, H.; Beller, M. Formic acid as a hydrogen storage material—Development of homogeneous catalysts for selective hydrogen release. Chem. Soc. Rev. 2016, 45, 3954–3988. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xie, Y.; Wang, X.; Gao, C.; Chen, Z.; Wu, J.; Meng, H.; Song, Z.; Du, S.; Ren, Z. Copper-triggered delocalization of bismuth p-orbital favours high-throughput CO2 electroreduction. Appl. Catal. B Environ. 2022, 301, 120781. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.Q.; Ni, B.; Wang, L.; Peng, H.J. A perspective on the electrocatalytic conversion of carbon dioxide to methanol with metallomacrocyclic catalysts. J. Energy Chem. 2022, 64, 263–275. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, C.; Hu, R.; Du, Z.; Gu, J.; Cui, Y.; Chen, X.; Xu, W.; Cheng, Z.; Li, S.; et al. Selective Etching Quaternary MAX Phase toward Single Atom Copper Immobilized MXene (Ti3C2Clx) for Efficient CO2 Electroreduction to Methanol. ACS Nano 2021, 15, 4927–4936. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Shi, Q.R.; Fu, S.F.; Song, J.H.; Xia, H.B.; Du, D.; Lin, Y.H. Efficient Synthesis of MCu (M = Pd, Pt, and Au) Aerogels with Accelerated Gelation Kinetics and their High Electrocatalytic Activity. Adv. Mater. 2016, 28, 8779–8783. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, C.; Zhu, Y.; He, D.; Huang, W. Tunable Syngas Formation from Electrochemical CO2 Reduction on Copper Nanowire Arrays. ACS Appl. Energy Mater. 2020, 3, 9841–9847. [Google Scholar] [CrossRef]
- Fan, Z.X.; Chen, Y.; Zhu, Y.H.; Wang, J.; Li, B.; Zong, Y.; Han, Y.; Zhang, H. Epitaxial growth of unusual 4H hexagonal Ir, Rh, Os, Ru and Cu nanostructures on 4H Au nanoribbons. Chem. Sci. 2017, 8, 795–799. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Mahyoub, S.A.; Zhong, J.; Chen, C.; Zhang, F.; Cheng, Z. Ultrathin and dense Ag nanosheets synthesis under suppressed face (111) growth and surface diffusion. J. Power Sources 2021, 488, 229484. [Google Scholar] [CrossRef]
- Ming, M.; Zhang, Y.; He, C.; Zhao, L.; Niu, S.; Fan, G.Y.; Hu, J.S. Room-Temperature Sustainable Synthesis of Selected Platinum Group Metal (PGM = Ir, Rh, and Ru) Nanocatalysts Well-Dispersed on Porous Carbon for Efficient Hydrogen Evolution and Oxidation. Small 2019, 15, e1903057. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Z.; Suematsu, K.; Li, P.; Yu, Z.; Zhang, W.; Hu, J.; Shimanoe, K. Rapid and Stable Detection of Carbon Monoxide in Changing Humidity Atmospheres Using Clustered In2O3/CuO Nanospheres. ACS Sens. 2020, 5, 1040–1049. [Google Scholar] [CrossRef]
- Lu, L.; Sun, X.; Ma, J.; Yang, D.; Wu, H.; Zhang, B.; Zhang, J.; Han, B. Highly Efficient Electroreduction of CO2 to Methanol on Palladium-Copper Bimetallic Aerogels. Angew. Chem. Int. Ed. 2018, 57, 14149–14153. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Yang, S.; Chu, M.G.; Zhu, Q.; Zhuang, Y.; Ren, C.; Chen, Z.Y.; Lu, L.; Qin, P.Y. Amorphous Copper-modified Gold Interface Promotes Selective CO2 Electroreduction to CO. Chemcatchem 2022, 14, e202200109. [Google Scholar] [CrossRef]
- Zhi, X.; Jiao, Y.; Zheng, Y.; Vasileff, A.; Qiao, S.Z. Selectivity roadmap for electrochemical CO2 reduction on copper-based alloy catalysts. Nano Energy 2020, 71, 104601. [Google Scholar] [CrossRef]
- Kim, D.; Resasco, J.; Yu, Y.; Asiri, A.M.; Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nat. Commun. 2014, 5, 4948. [Google Scholar] [CrossRef] [Green Version]
- Mahyoub, S.A.; Qaraah, F.A.; Yan, S.; Hezam, A.; Chen, C.; Zhong, J.; Cheng, Z. 3D Cu/In nanocones by morphological and interface engineering design in achieving a high current density for electroreduction of CO2 to syngas under elevated pressure. J. CO2 Util. 2022, 61, 102033. [Google Scholar] [CrossRef]
- Cheng, T.; Xiao, H.; Goddard, W.A., III. Free-Energy Barriers and Reaction Mechanisms for the Electrochemical Reduction of CO on the Cu(100) Surface, Including Multiple Layers of Explicit Solvent at pH 0. J. Phys. Chem. Lett. 2015, 6, 4767–4773. [Google Scholar] [CrossRef]
- Gattrell, M.; Gupta, N.; Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Wang, X.; Ou, P.; Wicks, J.; Xie, Y.; Wang, Y.; Li, J.; Tam, J.; Ren, D.; Howe, J.Y.; Wang, Z.; et al. Gold-in-copper at low *CO coverage enables efficient electromethanation of CO2. Nat. Commun. 2021, 12, 3387. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jin, H.; Xia, B.Y.; Davey, K.; Zheng, Y.; Qiao, S.Z. Molecular Cleavage of Metal-Organic Frameworks and Application to Energy Storage and Conversion. Adv. Mater. 2021, 33, 2104341. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Zhu, H.; Song, K.; Wei, N.; Liu, L.; Zhang, D.; Yin, J.; Dong, X.; Yao, K.; Wang, N.; et al. Investigating the Origin of Enhanced C2+ Selectivity in Oxide-/Hydroxide-Derived Copper Electrodes during CO2 Electroreduction. J. Am. Chem. Soc. 2020, 142, 4213–4222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Tan, H.Y.; Zhu, Y.; Chu, H.; Chen, H.M. Linking the Dynamic Chemical State of Catalysts with the Product Profile of Electrocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2021, 60, 17254–17267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Shan, J.Q.; Chen, L.; Xia, B.Y.; Ling, T.; Duan, J.J.; Jiao, Y.; Zheng, Y.; Qiao, S.Z. Stabilizing Cu2+ Ions by Solid Solutions to Promote CO2 Electroreduction to Methane. J. Am. Chem. Soc. 2022, 144, 2079–2084. [Google Scholar] [CrossRef]
- Gao, F.Y.; Hu, S.J.; Zhang, X.L.; Zheng, Y.R.; Wang, H.J.; Niu, Z.Z.; Yang, P.P.; Bao, R.C.; Ma, T.; Dang, Z.; et al. High-Curvature Transition-Metal Chalcogenide Nanostructures with a Pronounced Proximity Effect Enable Fast and Selective CO2 Electroreduction. Angew. Chem. Int. Ed. 2020, 59, 8706–8712. [Google Scholar] [CrossRef]
- Yang, P.P.; Zhang, X.L.; Gao, F.Y.; Zheng, Y.R.; Niu, Z.Z.; Yu, X.; Liu, R.; Wu, Z.Z.; Qin, S.; Chi, L.P.; et al. Protecting Copper Oxidation State via Intermediate Confinement for Selective CO2 Electroreduction to C2+ Fuels. J. Am. Chem. Soc. 2020, 142, 6400–6408. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, Q.; Kang, X.; Liu, H.; Qian, Q.; Zhang, Z.; Han, B. Molybdenum-Bismuth Bimetallic Chalcogenide Nanosheets for Highly Efficient Electrocatalytic Reduction of Carbon Dioxide to Methanol. Angew. Chem. Int. Ed. 2016, 55, 6771–6775. [Google Scholar] [CrossRef]
- Yang, H.P.; Yue, Y.N.; Qin, S.; Wang, H.; Lu, J.X. Selective electrochemical reduction of CO2 to different alcohol products by an organically doped alloy catalyst. Green Chem. 2016, 18, 3216–3220. [Google Scholar] [CrossRef]
- Li, Y.; Cui, F.; Ross, M.B.; Kim, D.; Sun, Y.; Yang, P. Structure-Sensitive CO2 Electroreduction to Hydrocarbons on Ultrathin 5-fold Twinned Copper Nanowires. Nano Lett. 2017, 17, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Chang, C.-C.; Chiu, S.-Y.; Pai, H.-T.; Liao, T.-Y.; Hsu, C.-S.; Chiang, W.-H.; Tsai, M.-K.; Chen, H.M. Operando time-resolved X-ray absorption spectroscopy reveals the chemical nature enabling highly selective CO2 reduction. Nat. Commun. 2020, 11, 3525. [Google Scholar] [CrossRef]
- Tan, X.; Yu, C.; Zhao, C.; Huang, H.; Yao, X.; Han, X.; Guo, W.; Cui, S.; Huang, H.; Qiu, J. Restructuring of Cu2O to Cu2O@Cu-Metal-Organic Frameworks for Selective Electrochemical Reduction of CO2. ACS Appl. Mater. Interfaces 2019, 11, 9904–9910. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Deng, Y.; Handoko, A.D.; Chen, C.S.; Malkhandi, S.; Yeo, B.S. Selective Electrochemical Reduction of Carbon Dioxide to Ethylene and Ethanol on Copper(I) Oxide Catalysts. ACS Catal. 2015, 5, 2814–2821. [Google Scholar] [CrossRef]
- Qiu, Y.L.; Zhong, H.X.; Zhang, T.T.; Xu, W.B.; Su, P.P.; Li, X.F.; Zhang, H.M. Selective Electrochemical Reduction of Carbon Dioxide Using Cu Based Metal Organic Framework for CO2 Capture. ACS Appl. Mater. Interfaces 2018, 10, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, L.; Yang, J.; Zhang, Y.; Wan, Z.; Zhou, L. Pd-Ru-Bi nanoalloys modified three-dimensional reduced graphene oxide/MOF-199 composites as a highly efficient electrocatalyst for ethylene glycol electrooxidation. Appl. Surf. Sci. 2019, 492, 617–625. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, L.; Jiang, D.; Xia, W.; Xu, X.; Yamauchi, Y.; Ge, J.; Tang, J. Nanoengineering Metal-Organic Framework-Based Materials for Use in Electrochemical CO2 Reduction Reactions. Small 2021, 17, 202006590. [Google Scholar] [CrossRef]
- Diercks, C.S.; Liu, Y.; Cordova, K.E.; Yaghi, O.M. The role of reticular chemistry in the design of CO2 reduction catalysts. Nat. Mater. 2018, 17, 301–307. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, J.; Yang, X.; Xu, Y.; Sun, W.F.; Zhou, J.H. MOF-derived Cu@Cu2O heterogeneous electrocatalyst with moderate intermediates adsorption for highly selective reduction of CO2 to methanol. Chem. Eng. J. 2022, 431, 134171. [Google Scholar] [CrossRef]
- Gu, J.; Héroguel, F.; Luterbacher, J.; Hu, X. Densely Packed, Ultra Small SnO Nanoparticles for Enhanced Activity and Selectivity in Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2018, 57, 2943–2947. [Google Scholar] [CrossRef]
- Zhang, A.; Liang, Y.; Li, H.; Wang, S.; Chang, Q.; Peng, K.; Geng, Z.; Zeng, J. Electronic Tuning of SnS2 Nanosheets by Hydrogen Incorporation for Efficient CO2 Electroreduction. Nano Lett. 2021, 21, 7789–7795. [Google Scholar] [CrossRef]
- Hou, X.; Cai, Y.; Zhang, D.; Li, L.; Zhang, X.; Zhu, Z.; Peng, L.; Liu, Y.; Qiao, J. 3D core-shell porous-structured Cu@Sn hybrid electrodes with unprecedented selective CO2-into-formate electroreduction achieving 100%. J. Mater. Chem. A 2019, 7, 3197–3205. [Google Scholar] [CrossRef]
- Lu, W.; Zhou, J.; Kong, F.; Fang, H.; Wang, W. Porous tin-based film deposited on copper foil for electrochemical reduction of carbon dioxide to formate. Int. J. Hydrogen Energy 2016, 41, 1585–1591. [Google Scholar] [CrossRef]
- Jeon, H.S.; Timoshenko, J.; Rettenmaier, C.; Herzog, A.; Yoon, A.; Chee, S.W.; Oener, S.; Hejral, U.; Haase, F.T.; Cuenya, B.R. Selectivity Control of Cu Nanocrystals in a Gas-Fed Flow Cell through CO2 Pulsed Electroreduction. J. Am. Chem. Soc. 2021, 143, 7578–7587. [Google Scholar] [CrossRef]
- Chen, D.; Yao, Q.; Cui, P.; Liu, H.; Xie, J.; Yang, J. Tailoring the Selectivity of Bimetallic Copper-Palladium Nanoalloys for Electrocatalytic Reduction of CO2 to CO. ACS Appl. Energy Mater. 2018, 1, 883–890. [Google Scholar] [CrossRef]
- Yan, Y.J.; Peng, Y.Y.; Song, Y.C.; Wang, R.Y.; Wang, H.; Bian, Z.Y. Polyethyleneimine-reinforced Sn/Cu foam dendritic self-supporting catalytic cathode for CO2 reduction to HCOOH. Chemosphere 2022, 301, 134704. [Google Scholar] [CrossRef]
- Chen, G.Q.; Ye, D.D.; Chen, R.; Li, J.; Zhu, X.; Liao, Q. Enhanced efficiency for carbon dioxide electroreduction to formate by electrodeposition Sn on Cu nanowires. J. CO2 Util. 2021, 44, 101409. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, Y.; Ma, J.; Zhang, Z.; Hu, W. Recent Advances in Atomic-Level Engineering of Nanostructured Catalysts for Electrochemical CO2 Reduction. Adv. Funct. Mater. 2020, 30, 1910534. [Google Scholar] [CrossRef]
- Liu, C.; Gong, J.; Gao, Z.; Xiao, L.; Wang, G.; Lu, J.; Zhuang, L. Regulation of the activity, selectivity, and durability of Cu-based electrocatalysts for CO2 reduction. Sci. China Chem. 2021, 64, 1660–1678. [Google Scholar] [CrossRef]
- Yin, Z.; Peng, H.; Wei, X.; Zhou, H.; Gong, J.; Huai, M.; Xiao, L.; Wang, G.; Lu, J.; Zhuang, L. An alkaline polymer electrolyte CO2 electrolyzer operated with pure water. Energy Environ. Sci. 2019, 12, 2455–2462. [Google Scholar] [CrossRef]


| Subject Categories | Publications | Percentage (%) |
|---|---|---|
| Chemistry Physical | 1938 | 42.6% |
| Chemistry Multidisciplinary | 1627 | 35.8% |
| Materials Science Multidisciplinary | 1505 | 33.1% |
| Nanoscience Nanotechnology | 844 | 18.6% |
| Energy Fuels | 690 | 15.2% |
| Engineering Chemical | 637 | 14.0% |
| Physics Applied | 544 | 12.0% |
| Electrochemistry | 496 | 10.9% |
| Physics Condensed Matter | 346 | 7.6% |
| Green Sustainable Science Technology | 229 | 5.0% |
| Journal | IF (2021) | TP | % |
|---|---|---|---|
| ACS Catalysis | 13.700 | 227 | 5.0% |
| Angewandte Chemie International Edition | 16.823 | 200 | 4.4% |
| Journal of Materials Chemistry A | 14.511 | 181 | 4.0% |
| Journal of the American Chemical Society | 16.383 | 122 | 2.7% |
| Journal of Physical Chemistry C | 4.177 | 118 | 2.6% |
| Applied Catalysis B Environmental | 24.319 | 111 | 2.4% |
| ACS Applied Materials Interfaces | 10.383 | 108 | 2.4% |
| Abstracts of Papers of the American Chemical Society | \ | 105 | 2.3% |
| Journal of CO2 Utilization | 8.321 | 97 | 2.1% |
| Electrochimica Acta | 7.336 | 93 | 2.0% |
| Authors | Affiliations | Publications | Percentage (%) | H-Index |
|---|---|---|---|---|
| Han, Buxing | Institute of Chemistry, CAS | 53 | 1.2% | 90 |
| Roldan Cuenya, Beatriz | Fritz Haber Institute of the Max Planck Society | 45 | 1.0% | 64 |
| Sargent, Edward H. | University of Toronto | 44 | 1.0% | 152 |
| Wang, Guoxiong | State Key Laboratory of Catalysis, CAS | 43 | 0.9% | 28 |
| Sinton, David | University of Toronto | 42 | 0.9% | 70 |
| Koper, Marc T. M. | Leiden University | 35 | 0.8% | 105 |
| Bao, Xin | State Key Laboratory of Catalysis, CAS | 35 | 0.8% | 68 |
| Dinh, Cao Thang | Queen′s University | 35 | 0.8% | 56 |
| Broekmann, Peter | University of Bern | 32 | 0.7% | 28 |
| Irabien, Angel | Universidad de Cantabria | 30 | 0.7% | 55 |
| Authors | Title | Affiliations | Total Citation | References |
|---|---|---|---|---|
| Peterson, A.A. et al. | “How Copper Catalyzes the Electroreduction of Carbon Dioxide into Hydrocarbon Fuels” | Technical University of Denmark | 1969 | [26] |
| Qiao, J.L. et al. | “A Review of Catalysts for the Electroreduction of Carbon Dioxide to Produce Low-Carbon Fuels” | Donghua University | 1925 | [14] |
| Kuhl, K.P. et al. | “New Insights into the Electrochemical Reduction of Carbon Dioxide on Metallic Copper Surfaces” | Stanford University | 1834 | [27] |
| Gao, S. et al. | “Partially Oxidized Atomic Cobalt Layers for Carbon Dioxide Electroreduction to Liquid Fuel” | University of Science and Technology of China | 1246 | [28] |
| Kondratenko, E.V. et al. | “Status and Perspectives of CO2 Conversion into Fuels and Chemicals by Catalytic, Photocatalytic and Electrocatalytic Processes” | Leibniz Institut fur Katalyse e.V. an der Universitat Rostock (LIKAT) | 1200 | [29] |
| Costentin, C. et al. | “Catalysis of the Electrochemical Reduction of Carbon Dioxide” | Centre National de la Recherche Scientifique (CNRS) | 1171 | [30] |
| Kortlever, R. et al. | “Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide” | Leiden University | 1154 | [31] |
| Chen, Y.J. et al. | “Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications” | Tsinghua University | 1104 | [32] |
| Dinh, C.T. et al. | “CO2 Electroreduction to Ethylene via Hydroxide-Mediated Copper Catalysis at an Abrupt Interface” | Institute of Chemical Process and Environmental Technology, Canada | 1076 | [33] |
| Li, C.W. et al. | “Electroreduction of Carbon Monoxide to Liquid Fuel on Oxide-Derived Nanocrystalline Copper” | Stanford University | 1057 | [34] |
| Cluster | Occurrences | Percentage |
|---|---|---|
| 1 (red) | 7195 | 61.5% |
| 2 (blue) | 2164 | 18.5% |
| 3 (green) | 1114 | 9.5% |
| 4 (yellow) | 1094 | 9.3% |
| 5 (purple) | 136 | 1.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yu, Z.; Zhou, J.; Li, C.; Jayanarasimhan, A.; Zhao, X.; Zhang, H. A Scientometric Review of CO2 Electroreduction Research from 2005 to 2022. Energies 2023, 16, 616. https://doi.org/10.3390/en16020616
Wang H, Yu Z, Zhou J, Li C, Jayanarasimhan A, Zhao X, Zhang H. A Scientometric Review of CO2 Electroreduction Research from 2005 to 2022. Energies. 2023; 16(2):616. https://doi.org/10.3390/en16020616
Chicago/Turabian StyleWang, Hongfei, Zhipeng Yu, Jie Zhou, Chengming Li, Ananthanarasimhan Jayanarasimhan, Xiqiang Zhao, and Hao Zhang. 2023. "A Scientometric Review of CO2 Electroreduction Research from 2005 to 2022" Energies 16, no. 2: 616. https://doi.org/10.3390/en16020616
APA StyleWang, H., Yu, Z., Zhou, J., Li, C., Jayanarasimhan, A., Zhao, X., & Zhang, H. (2023). A Scientometric Review of CO2 Electroreduction Research from 2005 to 2022. Energies, 16(2), 616. https://doi.org/10.3390/en16020616







