Recent Progresses on Vanadium Sulfide Cathodes for Aqueous Zinc-Ion Batteries
Abstract
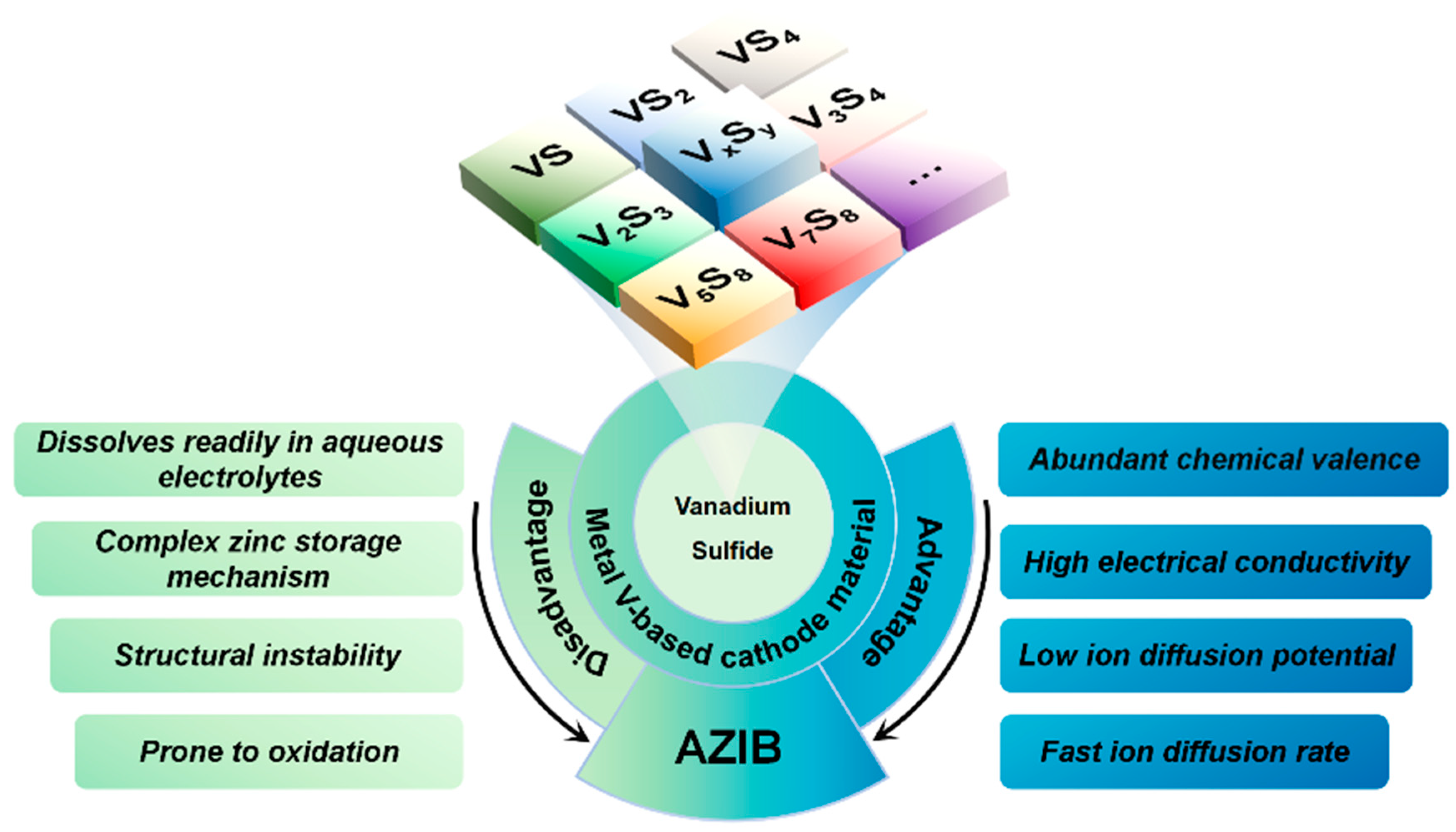
:1. Introduction
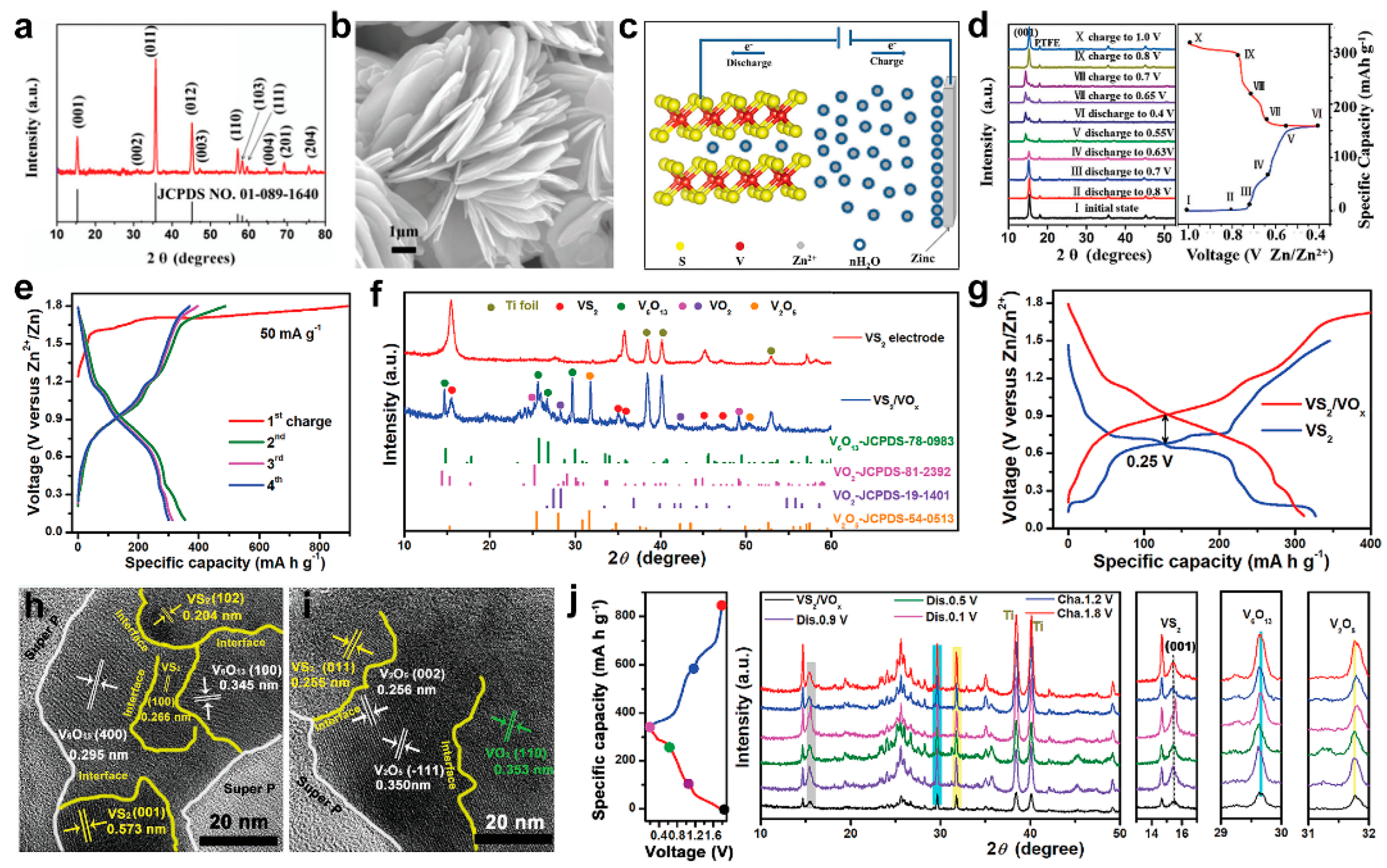
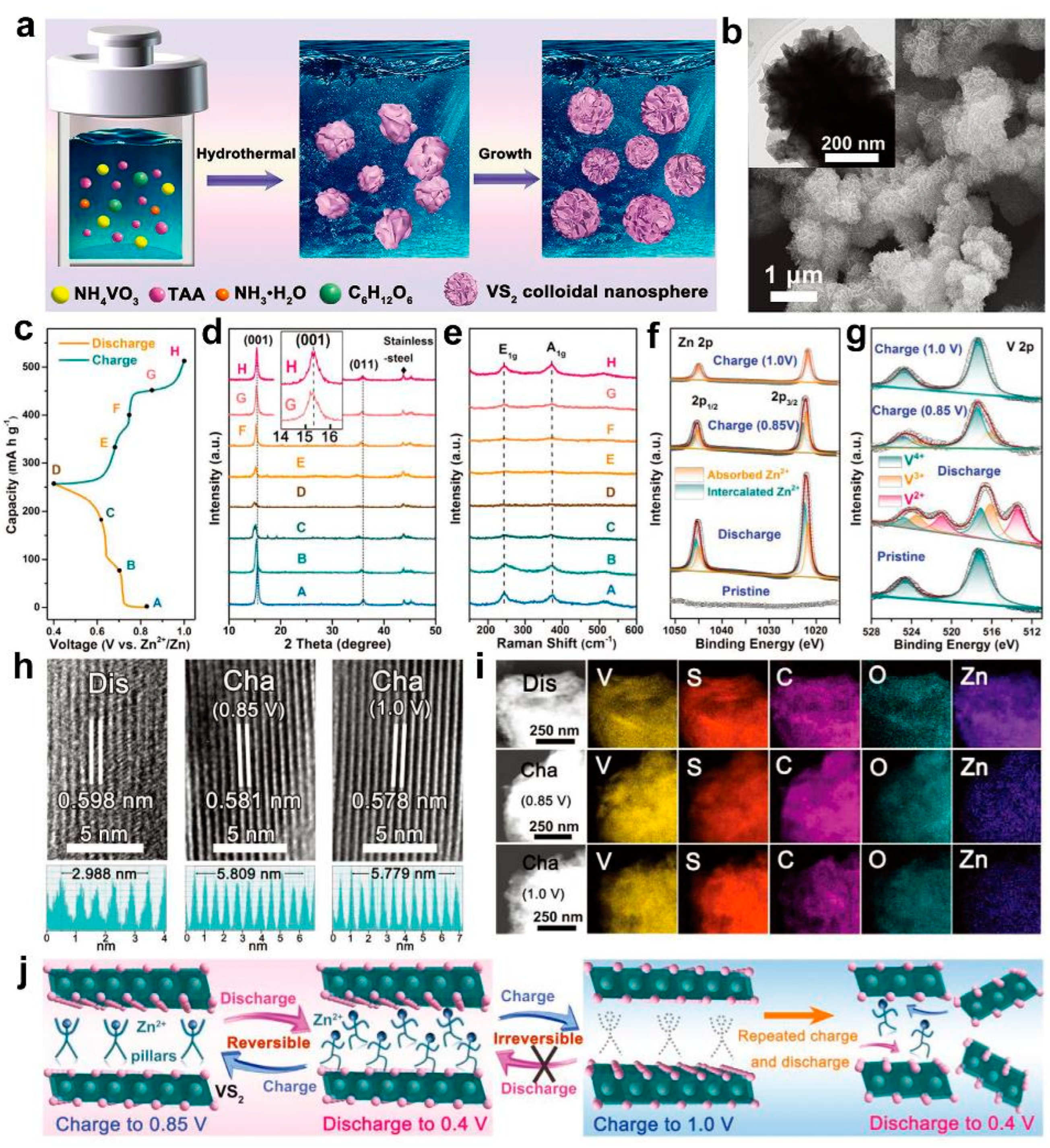
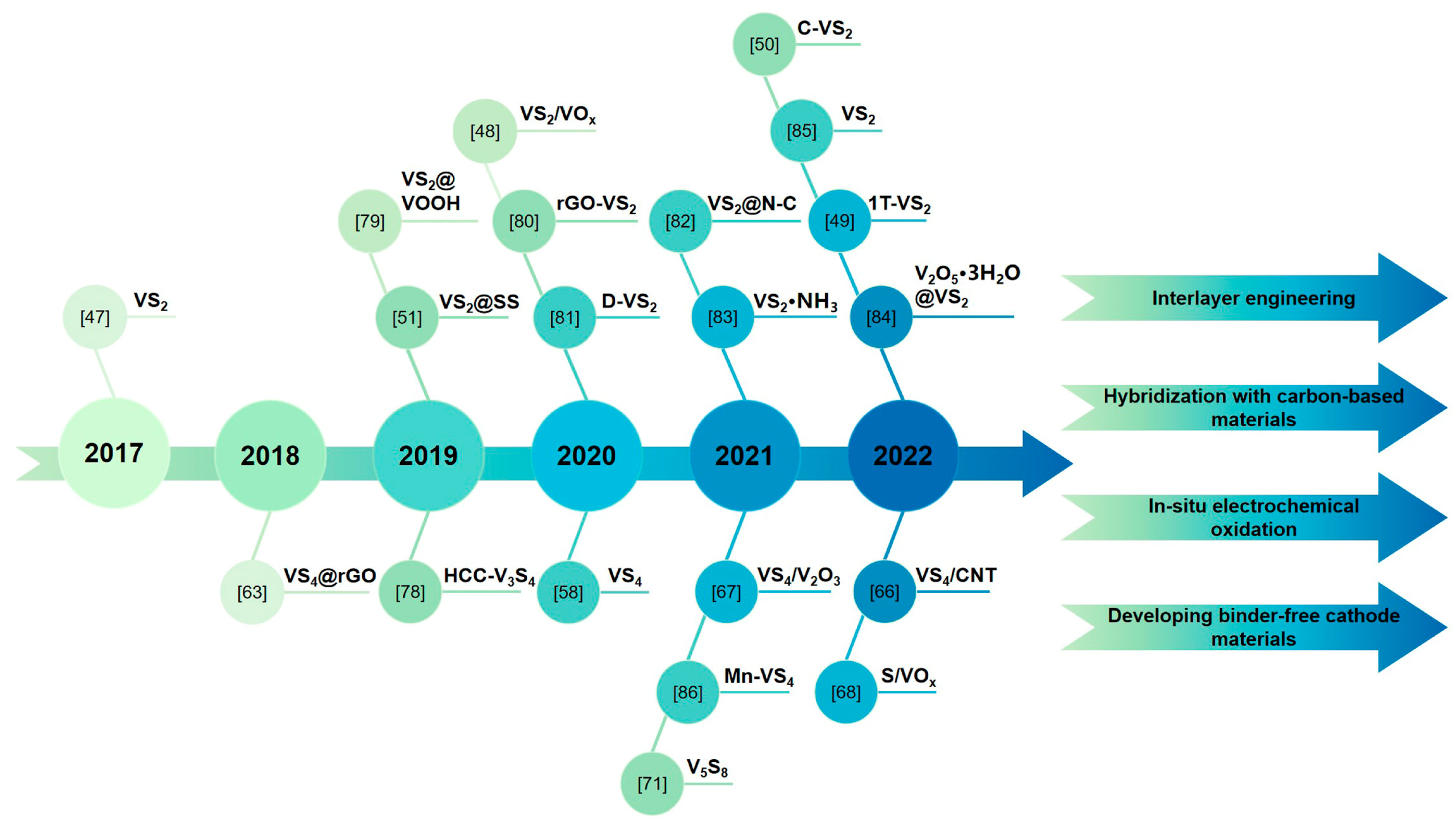
2. VS2 and Its Composites in AZIBs
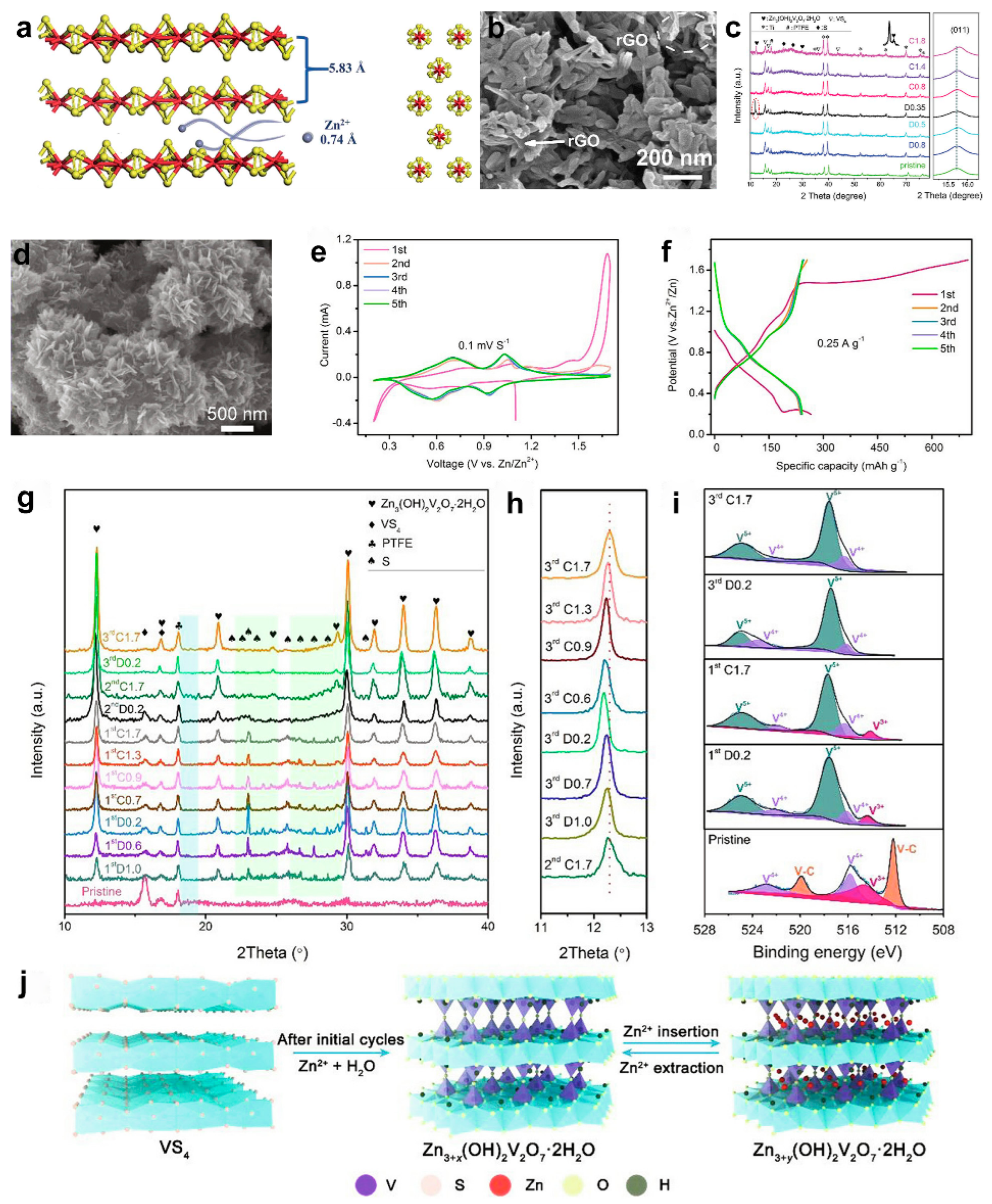
3. VS4 and Its Composites in AZIBs
4. Other Vanadium Sulfides and Their Composites in AZIBs
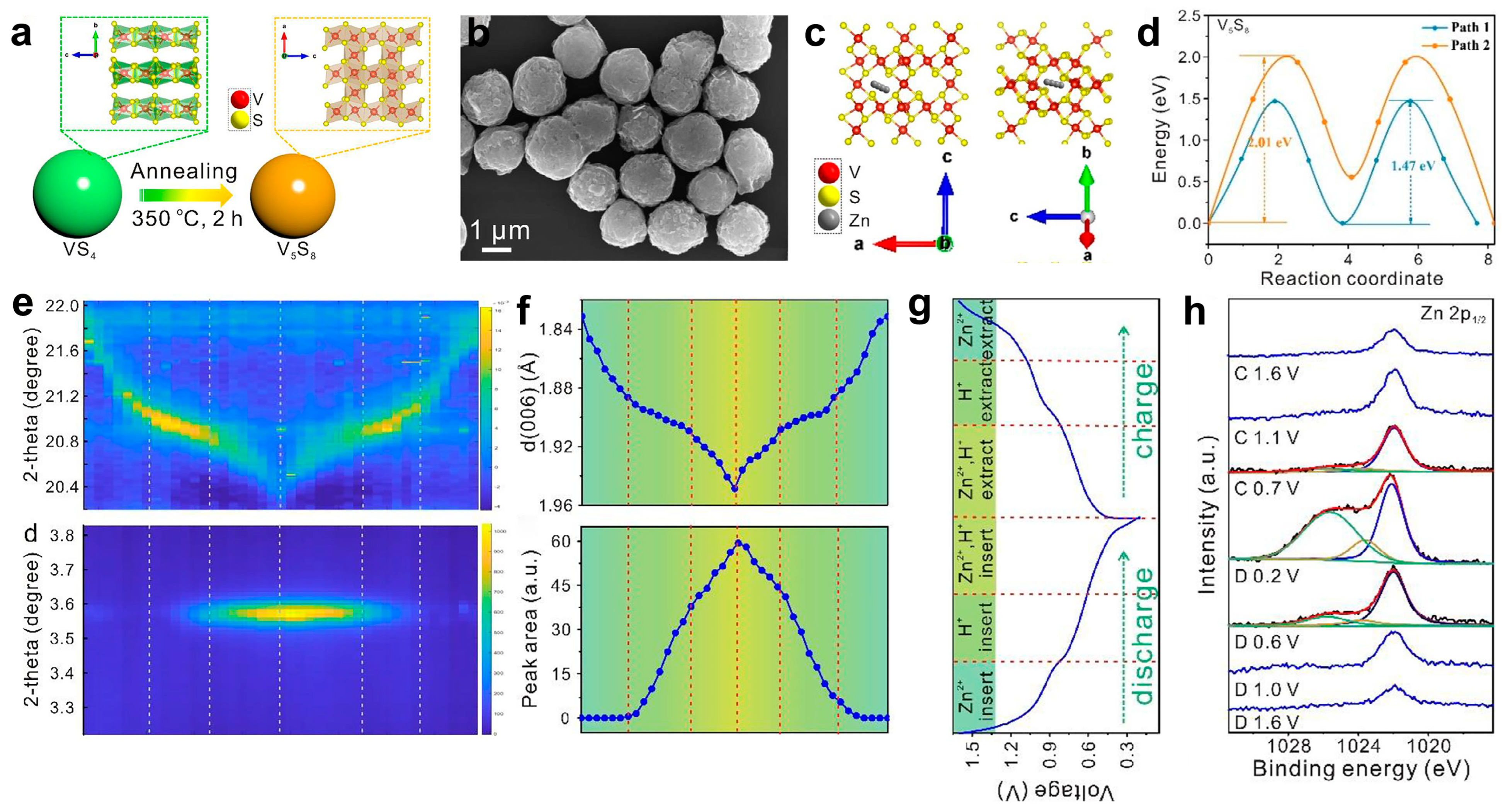
4.1. V5S8
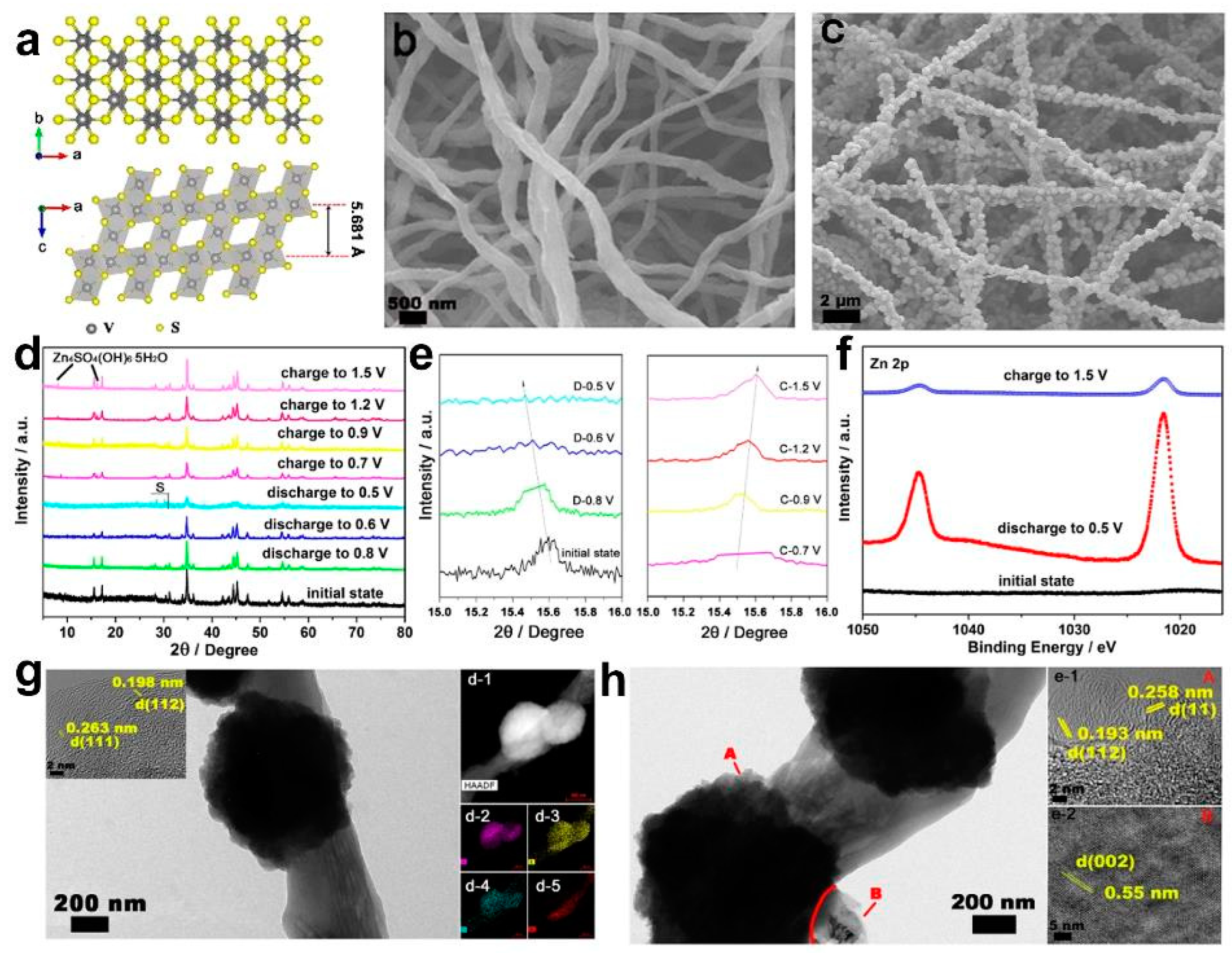
4.2. V3S4
5. Summary and Outlook
Funding
Data Availability Statement
Conflicts of Interest
References
- Ao, H.; Zhao, Y.; Zhou, J.; Cai, W.; Zhang, X.; Zhu, Y.; Qian, Y. Rechargeable aqueous hybrid ion batteries: Developments and prospects. J. Mater. Chem. A 2019, 7, 18708–18734. [Google Scholar] [CrossRef]
- Ling, J.; Kunwar, R.; Li, L.; Peng, S.; Misnon, I.I.; Ab Rahim, M.H.; Yang, C.-C.; Jose, R. Self-rechargeable energizers for sustainability. eScience 2022, 2, 347–364. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, R.; He, C.; Xia, C.; Guo, W.; Xu, Z.-L.; Xia, B. Advanced polymer-based electrolytes in zinc–air batteries. eScience 2022, 2, 453–466. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Xi, B.; Chen, W.; Jia, Y.; Feng, J.; Xiong, S. Advances and Perspectives of Cathode Storage Chemistry in Aqueous Zinc-Ion Batteries. ACS Nano 2021, 15, 9244–9272. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, H.; Wang, X.; Gu, Z.-Y.; Xu, C.; Li, H.; Zhang, G.; He, Y.; Wu, X.-L. Tetrafunctional template-assisted strategy to preciously construct co-doped Sb@C nanofiber with longitudinal tunnels for ultralong-life and high-rate sodium storage. Energy Stor. Mater. 2022, 48, 90–100. [Google Scholar] [CrossRef]
- Yang, Y.; Bremner, S.; Menictas, C.; Kay, M. Battery energy storage system size determination in renewable energy systems: A review. Renew. Sust. Energy Rev. 2018, 91, 109–125. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, G.; Rutt, A.; Shi, T.; Kim, H.; Wang, J.; Koettgen, J.; Sun, Y.; Ouyang, B.; Chen, T.; et al. Promises and Challenges of Next-Generation "Beyond Li-ion" Batteries for Electric Vehicles and Grid Decarbonization. Chem. Rev. 2021, 121, 1623–1669. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; He, Y.; Sun, H.; Xu, C.; Li, H.; Wang, X.; Zhang, G.; Sun, Z.; Wei, Q.; et al. An integrated strategy based on Schiff base reactions to construct unique two-dimensional nanostructures for intrinsic pseudocapacitive sodium/lithium storage. Chem. Eng. J. 2022, 429, 132339. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, X.; Yang, Z.; Xia, M.; Xu, C.; Liu, Y.; Yu, H.; Zhang, L.; Shu, J. Insight into the electrolyte strategies for aqueous zinc ion batteries. Coordin. Chem. Rev. 2022, 452, 7517–7556. [Google Scholar] [CrossRef]
- Wang, M.; Meng, Y.; Li, K.; Ahmad, T.; Chen, N.; Xu, Y.; Sun, J.; Chuai, M.; Zheng, X.; Yuan, Y.; et al. Toward dendrite-free and anti-corrosion Zn anodes by regulating a bismuth-based energizer. eScience 2022, 2, 509–517. [Google Scholar] [CrossRef]
- Ruan, P.; Liang, S.; Lu, B.; Fan, H.J.; Zhou, J. Design Strategies for High-Energy-Density Aqueous Zinc Batteries. Angew. Chem. Int. Ed. Engl. 2022, 61, e202200598. [Google Scholar] [CrossRef]
- Wang, H.; Ye, W.; Yang, Y.; Zhong, Y.; Hu, Y. Zn-ion hybrid supercapacitors: Achievements, challenges and future perspectives. Nano Energy 2021, 85, 105942. [Google Scholar] [CrossRef]
- Zhang, F.; Liao, T.; Liu, C.; Peng, H.; Luo, W.; Yang, H.; Yan, C.; Sun, Z. Biomineralization-inspired dendrite-free Zn-electrode for long-term stable aqueous Zn-ion battery. Nano Energy 2022, 103, 107830. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent Advances in Zn-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, A.; Sun, J. Promise and challenge of vanadium-based cathodes for aqueous zinc-ion batteries. J. Energy Chem. 2021, 54, 655–667. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, M.; Qin, L.; Chen, M.; Chen, Z.; Guo, S.; Wang, L.; Fang, G.; Liang, S. Simultaneous regulation of cations and anions in an electrolyte for high-capacity, high-stability aqueous zinc–vanadium batteries. eScience 2022, 2, 209–218. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Jin, T.; Meng, J.; Jiao, L.; Zhu, M.; Chen, J. Electrospun Thin-Walled CuCo2O4@C Nanotubes as Bifunctional Oxygen Electrocatalysts for Rechargeable Zn-Air Batteries. Nano Lett. 2017, 17, 7989–7994. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Fu, H. Recent advances in rechargeable Zn-based batteries. J. Power Sources 2021, 493, 229677. [Google Scholar] [CrossRef]
- Hua, F.; Fan, M. Research progress on Mn based oxide as cathode materia for ZIBs. New Chem. Mater. 2021, 49, 1006–3536. [Google Scholar]
- Zhang, B.; Chen, J.; Sun, W.; Shao, Y.; Zhang, L.; Zhao, K. Challenges and Perspectives for Doping Strategy for Manganese-Based Zinc-ion Battery Cathode. Energies 2022, 15, 4698. [Google Scholar] [CrossRef]
- Chuai, M.; Yang, J.; Wang, M.; Yuan, Y.; Liu, Z.; Xu, Y.; Yin, Y.; Sun, J.; Zheng, X.; Chen, N.; et al. High-performance Zn battery with transition metal ions co-regulated electrolytic MnO2. eScience 2021, 1, 178–185. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, J. Recent advances in cathode materials of rechargeable aqueous zinc-ion batteries. Mater. Today Adv. 2020, 7, 100078. [Google Scholar] [CrossRef]
- Li, S.; Wei, X.; Chen, H.; Lai, G.; Wang, X.; Zhang, S.; Wu, S.; Tang, W.; Lin, Z. A mixed-valent vanadium oxide cathode with ultrahigh rate capability for aqueous zinc-ion batteries. J. Mater. Chem. A 2021, 9, 22392–22398. [Google Scholar] [CrossRef]
- Tolstopyatova, E.G.; Kamenskii, M.A.; Kondratiev, V.V. Vanadium Oxide–Conducting Polymers Composite Cathodes for Aqueous Zinc-Ion Batteries: Interfacial Design and Enhancement of Electrochemical Performance. Energies 2022, 15, 8966. [Google Scholar] [CrossRef]
- He, P.; Chen, Q.; Yan, M.; Xu, X.; Zhou, L.; Mai, L.; Nan, C.-W. Building better zinc-ion batteries: A materials perspective. Energy Chem. 2019, 1, 100022. [Google Scholar] [CrossRef]
- Liang, Y.; Dong, H.; Aurbach, D.; Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 2020, 5, 646–656. [Google Scholar] [CrossRef]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific Challenges for the Implementation of Zn-Ion Batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Xie, R.; Li, J.; Tian, Z.; Chai, G.; Zhang, Y.; Lai, F.; He, G.; Liu, C.; et al. Structural engineering of cathodes for improved Zn-ion batteries. J. Energy Chem. 2021, 58, 147–155. [Google Scholar] [CrossRef]
- Du, M.; Miao, Z.; Li, H.; Sang, Y.; Liu, H.; Wang, S. Strategies of structural and defect engineering for high-performance rechargeable aqueous zinc-ion batteries. J. Mater. Chem. A 2021, 9, 19245–19281. [Google Scholar] [CrossRef]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Yi, T.-F.; Qiu, L.; Qu, J.-P.; Liu, H.; Zhang, J.-H.; Zhu, Y.-R. Towards high-performance cathodes: Design and energy storage mechanism of vanadium oxides-based materials for aqueous Zn-ion batteries. Coord. Chem. Rev. 2021, 446, 214124. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.-G.; You, Z.; Wei, C.; Kang, F.; Wei, B. Rechargeable aqueous zinc-ion batteries: Mechanism, design strategies and future perspectives. Mater. Today 2021, 42, 73–98. [Google Scholar] [CrossRef]
- Xiaoru, Y.; Yufang, C.; Peitao, X.; Chunman, Z. Review on Oxygen-Free Vanadium-Based Cathodes for Aqueous Zinc-Ion Batteries. J. Electrochem. 2022, 28, 2219004. [Google Scholar]
- Xu, X.; Xiong, F.; Meng, J.; Wang, X.; Niu, C.; An, Q.; Mai, L. Vanadium-Based Nanomaterials: A Promising Family for Emerging Metal-Ion Batteries. Adv. Funct. 2020, 30, 1904398. [Google Scholar] [CrossRef]
- Li, T.; Li, H.; Yuan, J.; Xia, Y.; Liu, Y.; Sun, A. Recent Advance and Modification Strategies of Transition Metal Dichalcogenides (TMDs) in Aqueous Zinc Ion Batteries. Materials 2022, 15, 2654. [Google Scholar] [CrossRef]
- Liu, B. Transition Metal Dichalcogenides for High−Performance Aqueous Zinc Ion Batteries. Batteries 2022, 8, 62. [Google Scholar] [CrossRef]
- Ding, J.; Gao, H.; Ji, D.; Zhao, K.; Wang, S.; Cheng, F. Vanadium-based cathodes for aqueous zinc-ion batteries: From crystal structures, diffusion channels to storage mechanisms. J. Mater. Chem. A 2021, 9, 5258–5275. [Google Scholar] [CrossRef]
- Yun, Q.; Lu, Q.; Zhang, X.; Tan, C.; Zhang, H. Three-Dimensional Architectures Constructed from Transition-Metal Dichalcogenide Nanomaterials for Electrochemical Energy Storage and Conversion. Angew. Chem. Int. Ed. 2018, 57, 626–646. [Google Scholar] [CrossRef]
- Lee, W.S.V.; Xiong, T.; Wang, X.; Xue, J. Unraveling MoS2 and Transition Metal Dichalcogenides as Functional Zinc-Ion Battery Cathode: A Perspective. Small Methods 2021, 5, e2000815. [Google Scholar] [CrossRef] [PubMed]
- Hoang Huy, V.P.; Ahn, Y.N.; Hur, J. Recent Advances in Transition Metal Dichalcogenide Cathode Materials for Aqueous Rechargeable Multivalent Metal-Ion Batteries. Nanomaterials 2021, 11, 1517. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Q.; Li, H.; Sun, J.; Li, H.; He, Y.; Liu, Z. Iron-chalcogenide-based electrode materials for electrochemical energy storage. J. Mater. Chem. A 2022, 10, 7517–7556. [Google Scholar] [CrossRef]
- Wu, Y.; Song, T.-Y.; Chen, L.-N. A review on recent developments of vanadium-based cathode for rechargeable zinc-ion batteries. Tungsten 2021, 3, 289–304. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Liu, J.-H.; Gao, Y.; Cao, X.; Zhan, C.; Wang, Y.; Wang, S.; Chou, S.-L.; Dou, S.-X.; et al. Vanadium-based cathodes for aqueous zinc-ion batteries: Mechanism, design strategies and challenges. Energy Stor. Mater. 2022, 50, 21–46. [Google Scholar] [CrossRef]
- Wan, F.; Niu, Z. Design Strategies for Vanadium-based Aqueous Zinc-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 16358–16367. [Google Scholar] [CrossRef]
- He, P.; Yan, M.; Zhang, G.; Sun, R.; Chen, L.; An, Q.; Mai, L. Layered VS2 Nanosheet-Based Aqueous Zn Ion Battery Cathode. Adv. Energy Mater. 2017, 7, 1601920. [Google Scholar] [CrossRef]
- Yu, D.; Wei, Z.; Zhang, X.; Zeng, Y.; Wang, C.; Chen, G.; Shen, Z.X.; Du, F. Boosting Zn2+ and NH4+ Storage in Aqueous Media via In-Situ Electrochemical Induced VS2/VOx Heterostructures. Adv. Funct. Mater. 2020, 31, 2008743. [Google Scholar] [CrossRef]
- Tan, Y.; Li, S.W.; Zhao, X.D.; Wang, Y.; Shen, Q.Y.; Qu, X.H.; Liu, Y.C.; Jiao, L.F. Unexpected Role of the Interlayer "Dead Zn2+" in Strengthening the Nanostructures of VS2 Cathodes for High-Performance Aqueous Zn-Ion Storage. Adv. Energy Mater. 2022, 12, 2104001. [Google Scholar] [CrossRef]
- Jiao, T.; Yang, Q.; Wu, S.; Wang, Z.; Chen, D.; Shen, D.; Liu, B.; Cheng, J.; Li, H.; Ma, L.; et al. Binder-free hierarchical VS2 electrodes for high-performance aqueous Zn ion batteries towards commercial level mass loading. J. Mater. Chem. A 2019, 7, 16330–16338. [Google Scholar] [CrossRef]
- Feng, M.; Wang, W.; Hu, Z.; Fan, C.; Zhao, X.; Wang, P.; Li, H.; Yang, L.; Wang, X.; Liu, Z. Engineering chemical-bonded Ti3C2 MXene@carbon composite films with 3D transportation channels for promoting lithium-ion storage in hybrid capacitors. Sci. China Mater. 2022, 1248. [Google Scholar] [CrossRef]
- Wang, W.; Feng, M.; Hu, E.; Hu, Z.; Fan, C.; Li, H.; Wang, P.; Wang, X.; Liu, Z. Interlayer and intralayer co-modified flexible V2CTX MXene@SWCNT films for high-power Li-ion capacitors. J. Energy Chem. 2022, 22, 2095–4956. [Google Scholar] [CrossRef]
- Wang, X.; Wang, R.; Zhao, Z.; Bi, S.; Niu, Z. Controllable spatial engineering of flexible all-in-one graphene-based supercapacitors with various architectures. Energy Stor. Mater. 2019, 23, 269–276. [Google Scholar] [CrossRef]
- Sun, H.; Yang, L.; Hu, E.; Feng, M.; Fan, C.; Wang, W.; Li, H.; Wang, X.; Liu, Z. Rose-like VS2 Nanosheets Chemically Anchored on Carbon Nanotubes for Flexible Zinc-Ion Batteries with Enhanced Properties. ACS Appl. Mater. Interfaces 2022, 14, 40247–40256. [Google Scholar] [CrossRef]
- Yao, K.; Wu, M.; Chen, D.; Liu, C.; Xu, C.; Yang, D.; Yao, H.; Liu, L.; Zheng, Y.; Rui, X. Vanadium Tetrasulfide for Next-Generation Rechargeable Batteries: Advances and Challenges. Chem. Rec. 2022, 22, e202200117. [Google Scholar] [CrossRef]
- Allmann, R.; Baumann, I.; Kutoglu, A.; Rösch, H.; Hellner, E. Die Kristallstruktur des Patronits V(S2)2. Naturwissenschaften 1964, 51, 263–264. [Google Scholar] [CrossRef]
- Hillebrand, W.F. The Vanadium Sulphide, Patronite, and its Mineral Associates from Minasragra, Peru. J. Am. Chem. Soc. 1907, 29, 1019–1029. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Xiao, Q.; Zhang, B.; Yan, Z.; Liu, X.; Chen, S.; Ren, Z.; Yu, Y. VS4 with a chain crystal structure used as an intercalation cathode for aqueous Zn-ion batteries. J. Mater. Chem. A 2020, 8, 10761–10766. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Wang, C.; Yi, X.; Chen, R.; Ma, L.; Hu, Y.; Zhu, G.; Chen, T.; Tie, Z.; et al. Highly Branched VS4 Nanodendrites with 1D Atomic-Chain Structure as a Promising Cathode Material for Long-Cycling Magnesium Batteries. Adv. Mater. 2018, 30, e1802563. [Google Scholar] [CrossRef]
- Shen, C.; Li, X.; Li, N.; Xie, K.; Wang, J.G.; Liu, X.; Wei, B. Graphene-Boosted, High-Performance Aqueous Zn-Ion Battery. ACS Appl. Mater. Interfaces 2018, 10, 25446–25453. [Google Scholar] [CrossRef]
- Ruan, P.; Xu, X.; Feng, J.; Yu, L.; Gao, X.; Shi, W.; Wu, F.; Liu, W.; Zang, X.; Ma, F.; et al. Boosting zinc storage performance via conductive materials. Mater. Res. Bull. 2021, 133, 111077. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, L.; Hou, Y. MXenes: Synthesis strategies and lithium-sulfur battery applications. eScience 2022, 2, 164–182. [Google Scholar] [CrossRef]
- Qin, H.; Yang, Z.; Chen, L.; Chen, X.; Wang, L. A high-rate aqueous rechargeable zinc ion battery based on the VS4@rGO nanocomposite. J. Mater. Chem. A 2018, 6, 23757–23765. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Niu, Z. Carbon-based materials for lithium-ion capacitors. Mater. Chem. Front. 2019, 3, 1265–1279. [Google Scholar] [CrossRef]
- Xu, C.; Wei, Q.; Li, M.; You, J.; Song, W.; Wang, X.; Zhang, G.; Li, H.; He, Y.; Liu, Z. Ultrafast and simple integration engineering of graphene-based flexible films with extensive tunability and simple trial in lithium-ion batteries. J. Alloys Compd. 2022, 922, 166282. [Google Scholar] [CrossRef]
- Gao, S.; Ju, P.; Liu, Z.; Zhai, L.; Liu, W.; Zhang, X.; Zhou, Y.; Dong, C.; Jiang, F.; Sun, J. Electrochemically induced phase transition in a nanoflower vanadium tetrasulfide cathode for high-performance zinc-ion batteries. J. Energy Chem. 2022, 69, 356–362. [Google Scholar] [CrossRef]
- Ding, J.; Gao, H.; Liu, W.; Wang, S.; Wu, S.; Fang, S.; Cheng, F. Operando constructing vanadium tetrasulfide-based heterostructures enabled by extrinsic adsorbed oxygen for enhanced zinc ion storage. J. Mater. Chem. A 2021, 9, 11433–11441. [Google Scholar] [CrossRef]
- Deng, L.; Yu, D.; Sun, G.; Zhang, X.; Wang, C.; Tian, R.; Du, F. In-situ electrochemical synthesis of a high-performance S/VOx composite for aqueous zinc ion batteries. J. Phys. D Appl. Phys. 2022, 55, 274001. [Google Scholar] [CrossRef]
- Wang, X.; Wang, F.; Wang, L.; Li, M.; Wang, Y.; Chen, B.; Zhu, Y.; Fu, L.; Zha, L.; Zhang, L.; et al. An Aqueous Rechargeable Zn//Co3O4 Battery with High Energy Density and Good Cycling Behavior. Adv. Mater. 2016, 28, 4904–4911. [Google Scholar] [CrossRef]
- Li, Y.; Fu, J.; Zhong, C.; Wu, T.; Chen, Z.; Hu, W.; Amine, K.; Lu, J. Recent Advances in Flexible Zinc-Based Rechargeable Batteries. Adv. Energy Mater. 2018, 9, 1802605. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Sun, Y.; Yin, Y.; Zhang, J.; Ren, X.; Zhao, Y.; Liang, Z.; Huai, P.; Song, F.; Jiang, Z.; et al. Metallic V5S8 microparticles with tunnel-like structure for high-rate and stable zinc-ion energy storage. Energy Stor. Mater. 2021, 42, 786–793. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, S.; Zhang, X.; Wei, J.; Liu, Y.; Hou, L.; Yuan, C. Few-layered V2C MXene derived 3D V3S4 nanocrystal functionalized carbon flakes boosting polysulfide adsorption and catalytic conversion towards Li–S batteries. J. Mater. Chem. A 2022, 10, 18679–18689. [Google Scholar] [CrossRef]
- Yao, G.; Niu, P.; Li, Z.; Xu, Y.; Wei, L.; Niu, H.; Yang, Y.; Zheng, F.; Chen, Q. Construction of flexible V3S4@CNF films as long-term stable anodes for sodium-ion batteries. Chem. Eng. J. 2021, 423, 130229. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, W.; Feng, Y.; Ma, J. Necklace-like carbon nanofibers encapsulating V3S4 microspheres for ultrafast and stable potassium-ion storage. J. Mater. Chem. A 2020, 8, 2618–2626. [Google Scholar] [CrossRef]
- Zhong, Y.; Chai, S.; Huang, X.; Zhou, S.; He, Q.; Lu, R.; Su, Q.; Pan, A. Hierarchical 1D/2D V3S4@N, S-Codoped rGO Hybrids as High-Performance Anode Materials for Fast and Stable Lithium-Ion Storage. ACS Appl. Energy Mater. 2022, 5, 4722–4732. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Ma, L.; Li, H.; Xu, X.; Liu, X.; Lu, T.; Pan, L. Insights into the storage mechanism of 3D nanoflower-like V3S4 anode in sodium-ion batteries. Chem. Eng. J. 2022, 427, 130936. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Y.; Meng, Y.; Xiao, M.; Hu, J.; Zhao, G.; Liu, S.; Zhu, F. Composite V3S4@rGO nanowires as a high-performance anode material for lithium-/sodium-ion batteries. Ionics 2021, 27, 5067–5077. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Zhang, Q.; Zhou, J.; Cai, Z.; Pan, A. Fabrication of an Inexpensive Hydrophilic Bridge on a Carbon Substrate and Loading Vanadium Sulfides for Flexible Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 36676–36684. [Google Scholar] [CrossRef]
- Pu, X.; Song, T.; Tang, L.; Tao, Y.; Cao, T.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Rose-like vanadium disulfide coated by hydrophilic hydroxyvanadium oxide with improved electrochemical performance as cathode material for aqueous zinc-ion batteries. J. Power Sources 2019, 437, 226917. [Google Scholar] [CrossRef]
- Chen, T.; Zhu, X.; Chen, X.; Zhang, Q.; Li, Y.; Peng, W.; Zhang, F.; Fan, X. VS2 nanosheets vertically grown on graphene as high-performance cathodes for aqueous zinc-ion batteries. J. Power Sources 2020, 477, 228652. [Google Scholar] [CrossRef]
- Yin, B.-S.; Zhang, S.-W.; Xiong, T.; Shi, W.; Ke, K.; Lee, W.S.V.; Xue, J.; Wang, Z.-B. Engineering sulphur vacancy in VS2 as high performing zinc-ion batteries with high cyclic stability. New J. Chem. 2020, 44, 15951–15957. [Google Scholar] [CrossRef]
- Liu, J.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. A VS2@N-doped carbon hybrid with strong interfacial interaction for high-performance rechargeable aqueous Zn-ion batteries. J. Mater. Chem. C 2021, 9, 6308–6315. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Z.; Ben, H.; Zhao, M.; Luo, J.; Chen, D.; Lu, Z.; Wang, L.; Liu, C. Boosting the zinc ion storage capacity and cycling stability of interlayer-expanded vanadium disulfide through in-situ electrochemical oxidation strategy. J. Colloid Interface Sci. 2022, 607, 68–75. [Google Scholar] [CrossRef]
- Gao, P.; Pan, Z.; Ru, Q.; Zhang, J.; Zheng, M.; Zhao, X.; Ling, F.C.-C.; Wei, L. Synergetic V2O5·3H2O/Metallic VS2 Nanocomposites Endow a Long Life and High Rate Capability to Aqueous Zinc-Ion Batteries. Energy Fuels 2022, 36, 3319–3327. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Chen, P.; Wang, A.; Huang, K.J.; Fang, L.; Wu, X. Interlayer-expanded VS2 nanosheet: Fast ion transport, dynamic mechanism and application in Zn2+ and Mg2+/Li+ hybrid batteries systems. J. Colloid Interface Sci. 2022, 620, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Ghosh, S.; Jang, W.; Yang, C.-M.; Murmu, N.C.; Kuila, T. A Reversible Anodizing Strategy in a Hybrid Electrolyte Zn-Ion Battery through Structural Modification of a Vanadium Sulfide Cathode. ACS Appl. Energy Mater. 2021, 4, 10656–10667. [Google Scholar] [CrossRef]

| Types of Vanadium Sulfides | Crystal Structure Models | Interplanar Crystal Spacing (Å) | Reaction Mechanisms | Ref. |
|---|---|---|---|---|
| VS2 |  | 5.76 | Interlayer mechanism | [50] |
| VS4 | 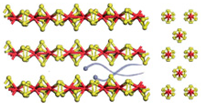 | 5.83 | Transformation/ interlayer mechanism | [58] |
| V5S8 | 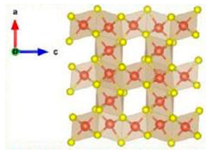 | 11.32 | Interlayer mechanism | [71] |
| V3S4 | 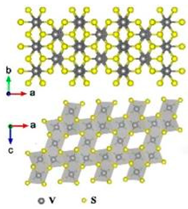 | 5.681 | Interlayer mechanism | [78] |
| Cathode Material | Electrolyte | Voltage Range (V) | Capacity (mAh·g−1@A g−1) | Cycle Stability | Ref. |
|---|---|---|---|---|---|
| VS2 | 1 M ZnSO4 | 0.4–1.0 | 190.3@0.05 | 98% after 200 cycles at 0.5 A g−1 | [47] |
| VS2@VOOH | 3 M ZnSO4 | 0.4–1.0 | 184.2@0.05 | 82% after 400 cycles at 2.5 A g−1 | [79] |
| VS2@SS | 1 M ZnSO4 | 0.4–1.0 | 98@0.05 | 80% after 1600 cycles at 1.0 A g−1 | [50] |
| rGO-VS2 | 3 M Zn (CF3SO3)2 | 0.2–1.8 | 238@0.1 | 93% after 1000 cycles at 5.0 A g−1 | [80] |
| D-VS2 | 1 M ZnSO4 | 0.2–1.7 | 262@0.1 | 94% after 100 cycles at 0.1 A g−1 | [81] |
| VS2@N-C | 3 M Zn (CF3SO3)2 | 0.2–1.8 | 203@0.05 | 97% after 600 cycles at 1.0 A g−1 | [82] |
| VS2·NH3 | 2 M Zn (CF3SO3)2 | 0.2–1.7 | 392@0.1 | 110% after 2000 cycles at 3.0 A g−1 | [83] |
| VS2/VOx | 25 M ZnCl2 | 0.1–1.8 | 301@0.05 | 75% after 3000 cycles at 1.0 A g−1 | [48] |
| V2O5·3H2O@VS2 (SVO) | 3 M ZnSO4 | 0.3–1.6 | 290@0.5 | 69.7% after 6700 cycles at 5.0 A g−1 | [84] |
| VS2 | 1 M ZnSO4 | 0.2–1.0 | 450.7@0.1 | 72% after 200 cycles at 1.0 A g−1 | [85] |
| 1T-VS2 | 2.5 M Zn (CF3SO3)2 | 0.4–0.85 | 212.9@0.1 | 86.7% after 2000 cycles at 2.0 A g−1 | [49] |
| VS2@SWCNT(C-VS2) | 3 M Zn (CF3SO3)2 | 0.3–1.5 | 205.3@0.1 | 72% after 1500 cycles at 5.0 A g−1 | [54] |
| VS4@rGO | 1 M Zn (CF3SO3)2 | 0.35–1.8 | 180@1.0 | 93.3% after 165 cycles at 1.0 A g−1 | [63] |
| VS4 | 1 M ZnSO4 | 0.2–1.6 | 310@0.1 | 85% after 500 cycles at 2.5 A g−1 | [58] |
| VS4/CNTs | 2 M Zn (CF3SO3)2 | 0.2–1.7 | 265@0.25 | 93% after 1200 cycles at 5.0 A g−1 | [66] |
| S/VOx | 30 M ZnCl2 | 0.1–1.8 | 376@0.05 | 100% after 2000 cycles at 2.0 A g−1 | [68] |
| VS4/V2O3 | 3 M Zn (CF3SO3)2 | 0.3–1.2 | 163@0.1 | - | [67] |
| Mn-VS4 | 1 M Zn (CF3SO3)2 /ACN (1:1) | 0.3–2.0 V | 547@0.1 | 97.83% after 1000 cycles at 1.0 A g−1 | [86] |
| V5S8 | 3 M ZnSO4 | 0.2–1.6 | 240@0.1 | 94% after 1000 cycles at 10.0 A g−1 | [71] |
| HCC-V3S4 | 2 M ZnSO4 | 0.5–1.5 | 148@0.5 | 95% after 200 cycles at 0.5 A g−1 | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, E.; Li, H.; Zhang, Y.; Wang, X.; Liu, Z. Recent Progresses on Vanadium Sulfide Cathodes for Aqueous Zinc-Ion Batteries. Energies 2023, 16, 917. https://doi.org/10.3390/en16020917
Hu E, Li H, Zhang Y, Wang X, Liu Z. Recent Progresses on Vanadium Sulfide Cathodes for Aqueous Zinc-Ion Batteries. Energies. 2023; 16(2):917. https://doi.org/10.3390/en16020917
Chicago/Turabian StyleHu, Enze, Huifang Li, Yizhou Zhang, Xiaojun Wang, and Zhiming Liu. 2023. "Recent Progresses on Vanadium Sulfide Cathodes for Aqueous Zinc-Ion Batteries" Energies 16, no. 2: 917. https://doi.org/10.3390/en16020917
APA StyleHu, E., Li, H., Zhang, Y., Wang, X., & Liu, Z. (2023). Recent Progresses on Vanadium Sulfide Cathodes for Aqueous Zinc-Ion Batteries. Energies, 16(2), 917. https://doi.org/10.3390/en16020917









