Studies on the Migration of Sulphur and Chlorine in the Pyrolysis Products of Floor and Furniture Joinery
Abstract
:1. Introduction
2. Material Characteristics and Methods
2.1. Materials
- Waste floor panels made of HDF fibreboard;
- Waste furniture made of MDF fibreboard;
- Waste furniture made of chipboard;
- Waste floor planks made of natural wood;
- Energy willow chips;
- Waste floor panels made of vinyl board.
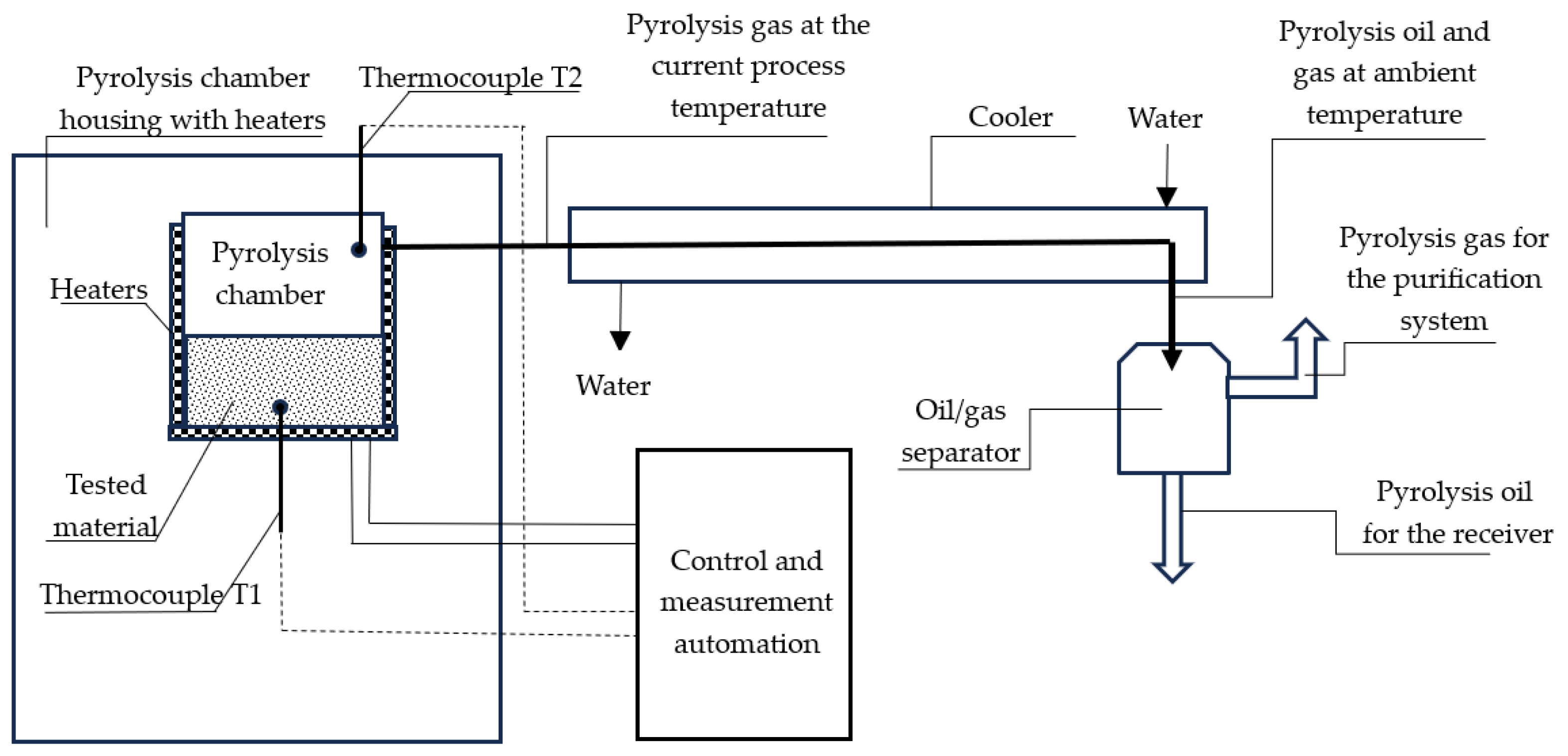
2.2. The Test Stand for Carrying out the Pyrolysis Process
- Gdi—amount of substance fed to the pyrolysis chamber, kg;
- Gwi—amount of substance removed from the pyrolysis chamber, kg;
- mSW—mass of waste sample, kg;
- mP—mass of pyrolysis products, kg;
- mCh—mass of char, kg;
- mPO—mass of pyrolysis oil, kg;
- mPG—mass of pyrolysis gas, kg.
- The mass of the gas was determined from Equation (4):
- mS_SW; mCl_SW—mass of sulphur/chlorine in the waste sample, g;
- mS_Ch; mCl_Ch—mass of sulphur/chlorine content in the char, g;
- mS_PO; mCl_PO—mass of sulphur/chlorine content in pyrolysis oil, g;
- mS_PG; mCl_PG—mass of sulphur/chlorine content in the pyrolysis gas, g.
2.3. Sulphur and Chlorine Analysis
- Va—the amount of NaOH used to titrate the waste sample, (cm3);
- Vb—the amount of NaOH used for the titration of the blank, (cm3);
- 0.0008—mass of sulphur corresponding to 1 cm3 of 0.05 n sodium hydroxide solution, (g/cm3);
- m—mass of the waste sample, (g).
- Va—the amount of AgNO3 used to titrate the waste sample, (cm3);
- Vb—the amount of AgNO3 used for the titration of the blank, (cm3);
- c—concentration of silver nitrate(V) solution (0.05), (mol/dm3);
- m—mass of the waste sample, (g).
3. Results and Discussion
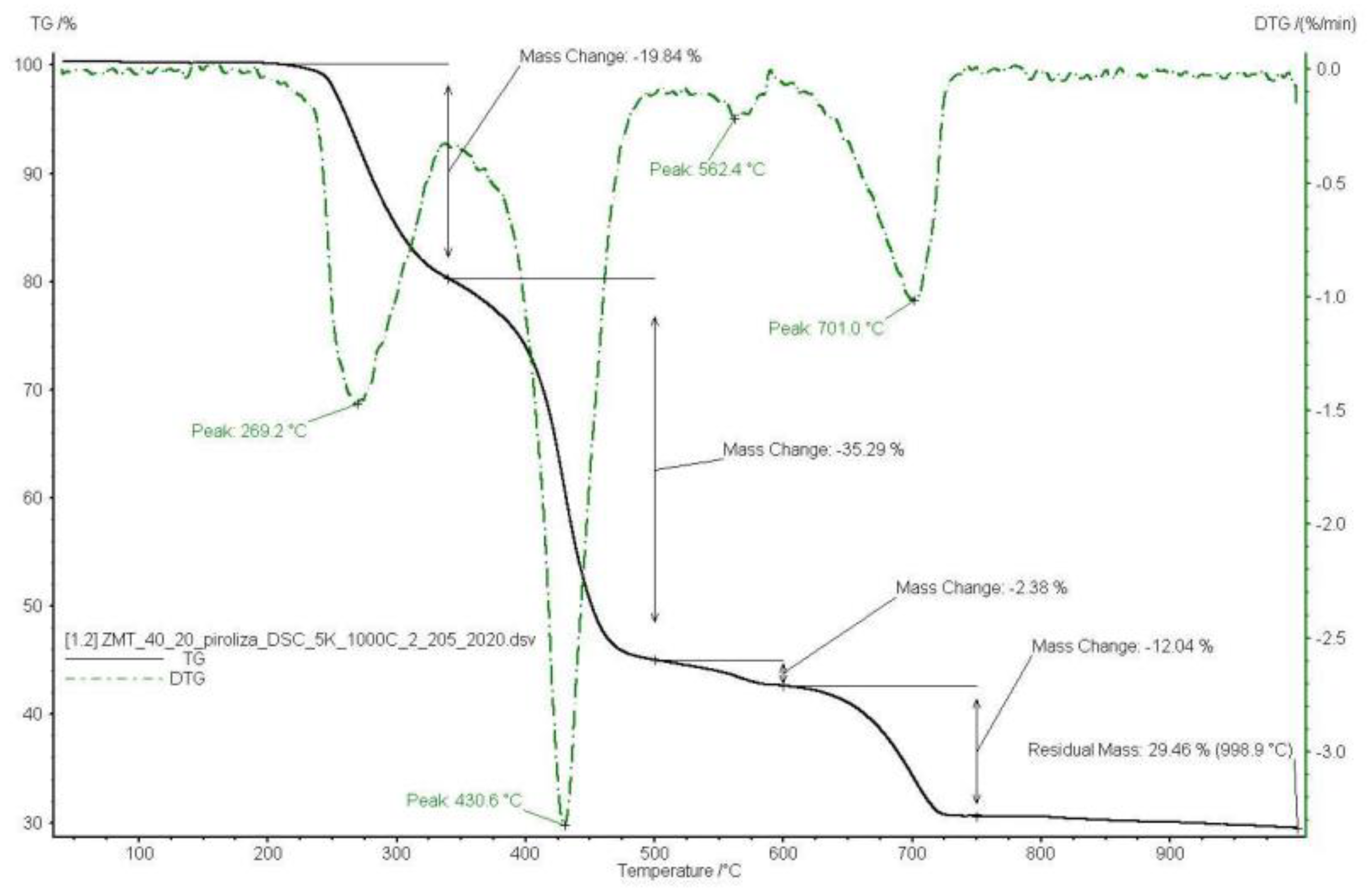
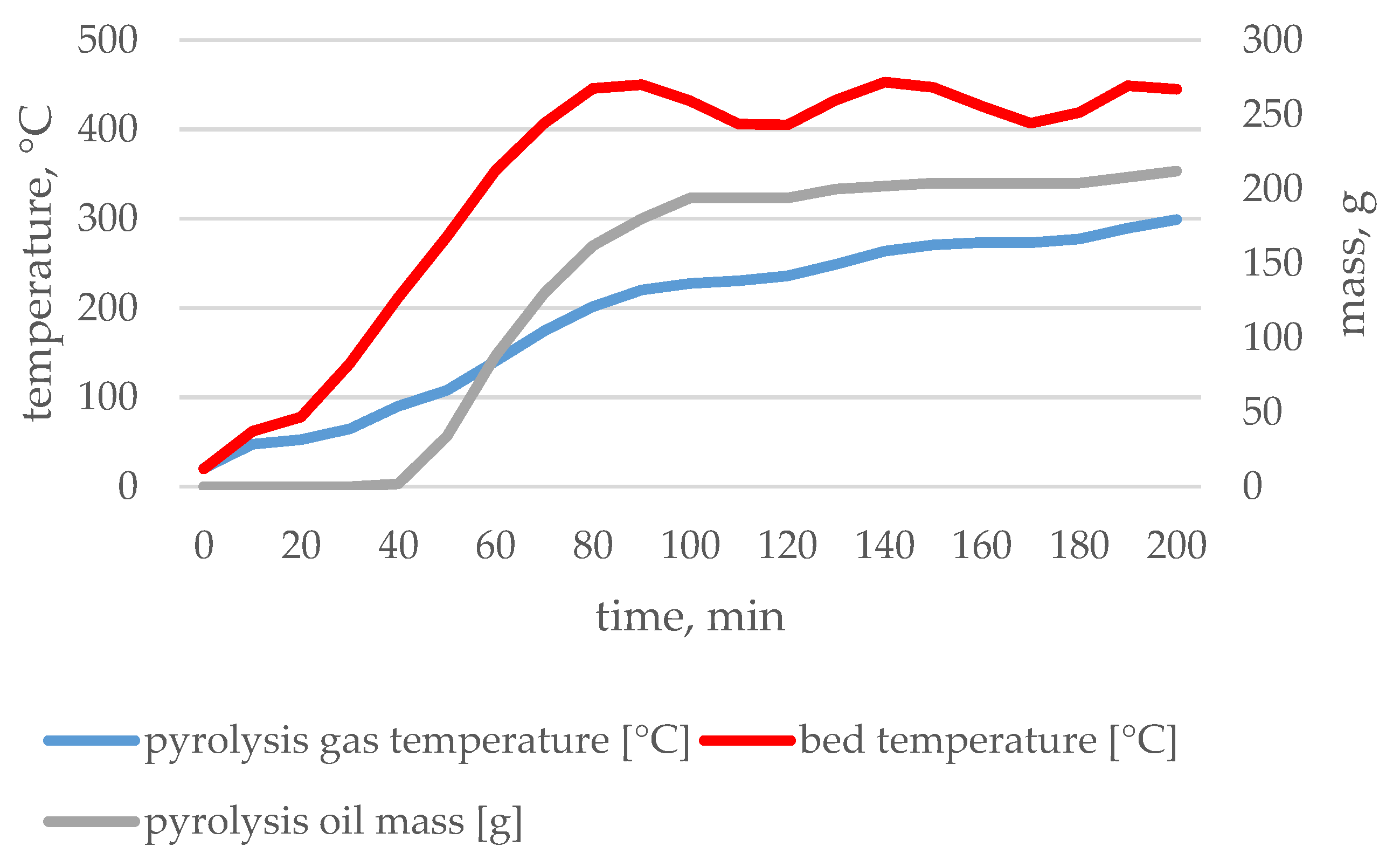
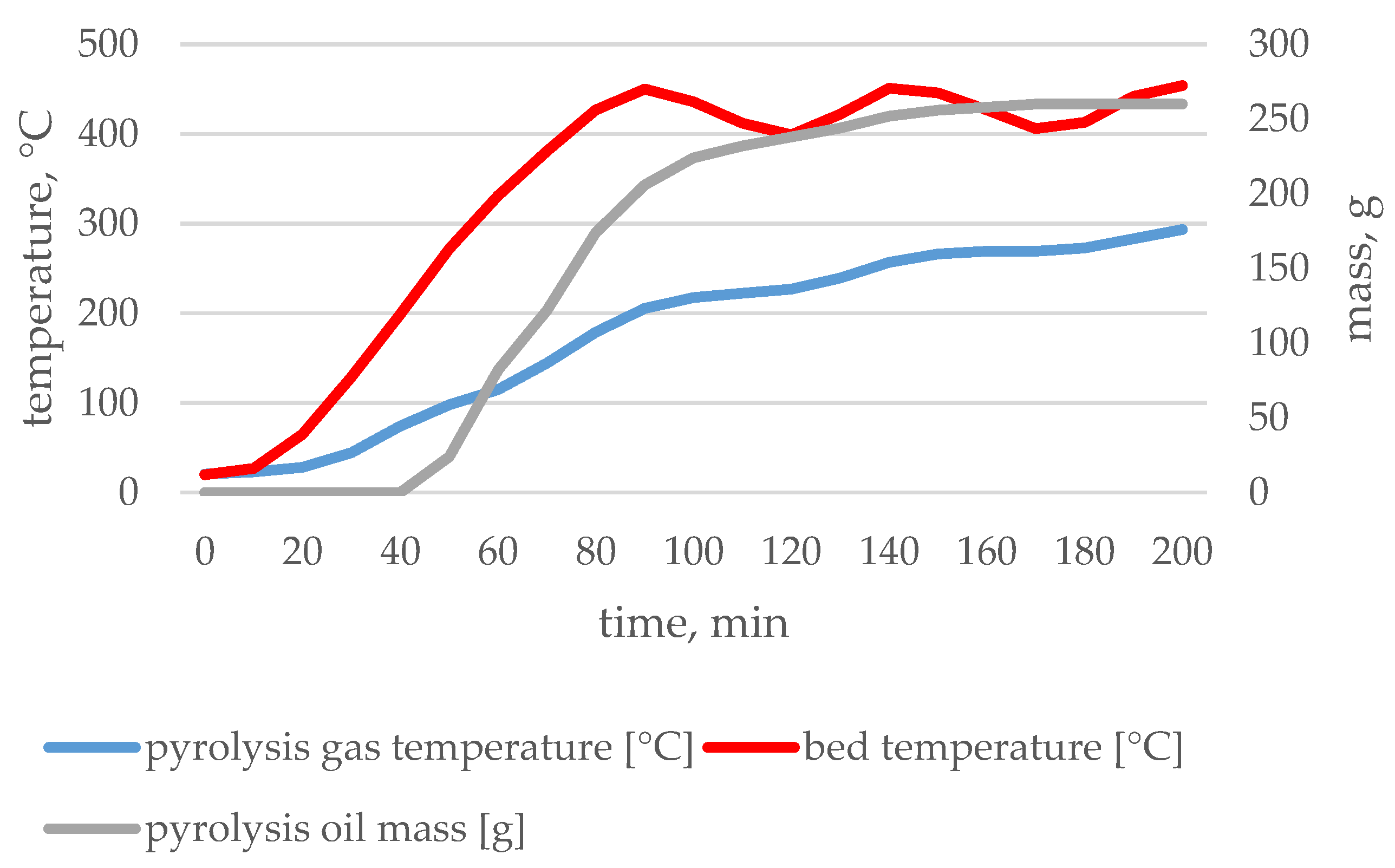
3.1. Pyrolysis Process Parameters
3.2. Migration of Acidic Elements (S and Cl)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roffael, E.; Schneider, T.; Dix, B.; Buchholz, T. Zur Hydrophobierung von mitteldichten Faserplatten (MDF) mit Paraffinen. Teil 1: Einfluss der chemischen Zusammensetzung des Paraffins und des Emulgatortyps auf die Hydrophobierung von MDF. Holz. Als. Roh.-Und. Werkst. 2005, 63, 192–203. [Google Scholar] [CrossRef]
- Wan, H.; Wang, X.M.; Bary, A.; Shen, J. Recycling wood composite panels: Characterizing recycled materials. BioResources 2014, 9, 7554–7565. [Google Scholar] [CrossRef]
- Nicewicz, D. Płyty Pilśniowe MDF (Fibreboard MDF); SGGW: Warszawa, Poland, 2006. [Google Scholar]
- Jaworski, T.; Kajda-Szcześniak, M. Research on the Kinetics of Pyrolysis of Wood-Based Panels in Terms of Waste Management. Energies 2019, 12, 3705. [Google Scholar] [CrossRef]
- Mu, J.; Lai, Z. Pyrolysis Characteristics of Wood-Based Panels and Its Products; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.B.; Xue, L.; Chu, D.M.; Mu, J. Influence of a urea–formaldehyde resin adhesive on pyrolysis characteristics and volatiles emission of poplar particleboard. RSC Adv. 2016, 6, 12850–12861. [Google Scholar] [CrossRef]
- Gaze, B.; Wojtko, P.; Knutel, B.; Kobel, P.; Bobrowicz, K.; Bukowski, P.; Chojnacki, J.; Kielar, J. Influence of Catalytic Additive Application on the Wood-Based Waste Combustion Process. Energies 2023, 16, 2055. [Google Scholar] [CrossRef]
- Jaworski, T.; Kajda-Szcześniak, M. Study on the Similarity of the Parameters of Biomass and Solid Waste Fuel Combustion for the Needs of Thermal Power Engineering. Sustainability 2020, 12, 7894. [Google Scholar] [CrossRef]
- Pikoń, K.; Kajda-Szcześniak, M.; Bogacka, M. Environmental effect of co-combustion of coal with wood waste in low-power boilers. Przem. Chem. 2015, 94, 1548–1550. [Google Scholar] [CrossRef]
- Plis, A.; Kotyczka-Morańska, M.; Kopczyński, M.; Łabojko, G. Furniture wood waste as a potential renewable energy source. J. Therm. Anal. Calorim. 2016, 125, 1357–1371. [Google Scholar] [CrossRef]
- Kajda-Szcześniak, M.; Czop, M. Comparison of Pyrolysis and Combustion Processes of Vinyl Floor Panels Using Thermogravimetric Analysis (TG-FTIR) in Terms of the Circular Economy. Energies 2022, 15, 1516. [Google Scholar] [CrossRef]
- Ustawa o Odpadach (Dz. U. 2013 Poz. 23 z Póz. zm). Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20130000021 (accessed on 27 July 2023).
- Landrat, M. Możliwości zagospodarowania odpadów florystycznej gąbki polifenolowej. Przem. Chem. 2017, 96, 1704–1706. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Kulczycka, J.; Pędziwiatr, E. Gospodarka o Obiegu Zamkniętym w Polityce i Badaniach Naukowych; IGSMiE PAN: Kraków, Poland, 2019. [Google Scholar]
- Sauvé, S.; Bernard, S.; Sloan, P. Environmental sciences, sustainable development and circular economy: Alternative concepts for trans-disciplinary research. Environ. Dev. 2016, 17, 48–56. [Google Scholar] [CrossRef]
- Sakthipriya, N. Plastic waste management: A road map to achieve circular economy and recent innovations in pyrolysis. Sci. Total Environ. 2022, 809, 151160. [Google Scholar] [CrossRef]
- Poskart, A.; Skrzyniarz, M.; Sajdak, M.; Zajemska, M.; Skibiński, A. Management of Lignocellulosic Waste towards Energy Recovery by Pyrolysis in the Framework of Circular Economy Strategy. Energies 2021, 14, 5864. [Google Scholar] [CrossRef]
- Sherwood, J. The significance of biomass in a circular economy. Bioresour. Technol. 2020, 300, 122755. [Google Scholar] [CrossRef] [PubMed]
- Andooz, A.; Eqbalpour, M.; Kowsari, E.; Ramakrishna, S.; Cheshmeh, Z.A. A comprehensive review on pyrolysis from the circular economy point of view and its environmental and social effects. J. Clean. Prod. 2023, 388, 136021. [Google Scholar] [CrossRef]
- Stelmach, S. Piroliza Odpadów Jako Element Gospodarki o Obiegu Zamkniętym (Pyrolysis of Waste as a Component of Circular Economy); Wyd. Politechnika Śląska: Gliwice, Poland, 2019. [Google Scholar]
- Basu, P. Biomass Gasification and Pyrolysis Practical Design and Theory, 2nd ed.; Elsevier: Oxford, UK, 2010. [Google Scholar]
- Ścierski, W.; Landrat, M.; Pikoń, K.; Bogacka, M. Analysis of chlorine migration in low temperature pyrolysis process. Przem. Chem. 2019, 98, 1448–1450. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Johan, R.B. Pyrolysis: A Sustainable Way to Generate Energy from Waste; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Ścierski, W. Migration of Sulfur and Nitrogen in the Pyrolysis Products of Waste and Contaminated Plastics. Appl. Sci. 2021, 11, 4374. [Google Scholar] [CrossRef]
- Gaoa, N.; Li, A.; Quana, C.; Dub, L.; Duan, Y. TG–FTIR and Py–GC/MS analysis on pyrolysis and combustion of pine sawdust. J. Anal. Appl. Pyrolysis 2013, 100, 26–32. [Google Scholar] [CrossRef]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. ChemInform 2004, 45, 651–671. [Google Scholar]
- Wu, J.; Wang, L.; Ma, H.; Zhou, J. Investigation of element migration characteristics and product properties during biomass pyrolysis: A case study of pine cones rich in nitrogen. RSC Adv. 2021, 11, 34795–34805. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, Y.; Ryu, C.; Park, Y.-K. Slow pyrolysis of rice straw: Analysis of products properties, carbon and energy yields. Bioresour. Technol. 2014, 155, 63–70. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An Overview of Biomass Pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Liu, Z.; Han, G. Production of solid fuel biochar from waste biomass by low temperature pyrolysis. Fuel 2015, 158, 159–165. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Wang, Z.; Wei, R.; Wang, X.; He, J.; Wang, J. Pyrolysis and Combustion of Polyvinyl Chloride (PVC) Sheath for New and Aged Cables via Thermogravimetric Analysis-Fourier Transform Infrared (TG-FTIR) and Calorimeter. Materials 2018, 11, 1997. [Google Scholar] [CrossRef]
- Huggett, C.; Levin, B.C. Toxicity of the Pyrolysis and Combustion Products of Poly (Vinyl Chlorides): A Literature Assessment. Fire Mater. 1987, 11, 131–142. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef]
- Zhu, H.M.; Jiang, X.G.; Yan, J.H.; Chi, Y.; Cen, K.F. TG-FTIR analysis of PVC thermal degradation and HCl removal. J. Anal. Appl. Pyrolysis 2008, 82, 1–9. [Google Scholar] [CrossRef]
- Knümann, R.; Bockhorn, H. Investigation of the Kinetics of Pyrolysis of PVC by TG-MS-Analysis. Combust. Sci. Technol. 1994, 101, 285–299. [Google Scholar] [CrossRef]
- Miranda, R.; Yang, J.; Roy, C.; Vasilec, C. Vacuum pyrolysis of PVC I. Kinetic study. Polym. Degrad. Stab. 1999, 64, 127–144. [Google Scholar] [CrossRef]
- Sieradzka, M.; Kirczuk, C.; Kalemba-Rec, I.; Mlonka-Mędrala, A.; Magdziarz, A. Pyrolysis of Biomass Wastes into Carbon Materials. Energies 2022, 15, 1941. [Google Scholar] [CrossRef]
- Sajdak, M.; Muzyka, R.; Gałko, G.; Ksepko, E.; Zajemska, M.; Sobek, S.; Tercki, D. Actual Trends in the Usability of Biochar as a High-Value Product of Biomass Obtained through Pyrolysis. Energies 2023, 16, 355. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Rahman, M.M.; Won, S.; Shim, S.; Ra, C. Biochar Properties and Eco-Friendly Applications for Climate Change Mitigation, Waste Management, and Wastewater Treatment: A Review. Renew. Sustain. Energy Rev. 2017, 79, 255–273. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Routledge: London, UK, 2012; pp. 1–416. [Google Scholar]
- Soffian, M.S.; Abdul Halim, F.Z.; Aziz, F.; Rahman, M.A.; Mohamed Amin, M.A.; Awang Chee, D.N. Carbon-Based Material Derived from Biomass Waste for Wastewater Treatment. Environ. Adv. 2022, 9, 100259. [Google Scholar] [CrossRef]
- Treviño-Cordero, H.; Juárez-Aguilar, L.G.; Mendoza-Castillo, D.I.; Hernández-Montoya, V.; Bonilla-Petriciolet, A.; Montes-Morán, M.A. Synthesis and Adsorption Properties of Activated Carbons from Biomass of Prunus Domestica and Jacaranda Mimosifolia for the Removal of Heavy Metals and Dyes from Water. Ind. Crops Prod. 2013, 42, 315–323. [Google Scholar] [CrossRef]
- Weng, Z.; van Zwieten, L.; Singh, B.P.; Tavakkoli, E.; Joseph, S.; Macdonald, L.M.; Rose, T.J.; Rose, M.T.; Kimber, S.W.L.; Morris, S.; et al. Biochar Built Soil Carbon over a Decade by Stabilizing Rhizodeposits. Nat. Clim. Chang. 2017, 7, 371–376. [Google Scholar] [CrossRef]
- Shukla, I. Potential of Renewable Agricultural Wastes in the Smart and Sustainable Steelmaking Process. J. Clean. Prod. 2022, 370, 133422. [Google Scholar] [CrossRef]
- Rose, J.; Adams, T.A. Process Design and Techno-Economic Analysis of Biomass Pyrolysis By-Product Utilization in the Ontario and Aichi Steel Industries; Elsevier Masson SAS: Amsterdam, The Netherlands, 2022; Volume 49, ISBN 9780323851596. [Google Scholar]
- Suopajärvi, H.; Dahl, E.; Kemppainen, A.; Gornostayev, S.; Koskela, A.; Fabritius, T. Effect of Charcoal and Kraft-Lignin Addition on Coke Compression Strength and Reactivity. Energies 2017, 10, 1850. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Entchev, E. Ag/Biochar Composite for Supercapacitor Electrodes. Mater. Today Energy 2017, 6, 136–145. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Harfi, K. Co-pyrolysis of olive residue with poly(vinyl chloride) using thermogravimetric analysis. J. Therm. Anal. Calorim. 2009, 95, 1007–1013. [Google Scholar] [CrossRef]
- Czégény, Z.; Jakab, E.; Bozi, J.; Blazsó, M. Pyrolysis of wood—PVC mixtures. Formation of chloromethane from lignocellulosic materials in the presence of PVC. J. Anal. Appl. Pyrol. 2015, 113, 123–132. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.; Meng, A.; Li, Q.; Zhang, Y. Interactions of three municipal solid waste components during co-pyrolysis. J. Anal. Appl. Pyrolysis 2015, 111, 265–271. [Google Scholar] [CrossRef]
- Bittencourt, P.R.S.; Scremin, F. Evolved Gas Analysis of PE: PVC Systems Thermodegradation Under Inert and Oxidizing Atmosphere. J. Polym. Environ. 2019, 27, 612–617. [Google Scholar] [CrossRef]
- Xiu, F.-R.; Lu, Y.; Qi, Y. DEHP degradation and dechlorination of polyvinyl chloride waste in subcritical water with alkali and ethanol: A comparative study. Chemosphere 2020, 249, 126138. [Google Scholar] [CrossRef] [PubMed]
- Nozue, K.; Tagaya, H. Chemical recycling of waste Poly Vinyl Chloride (PVC) by the liquid-phase treatment. J. Mater. Cycles Waste Manag. 2021, 23, 489–504. [Google Scholar] [CrossRef]
- Susa, D.; Haydary, J. Sulphur distribution in the products of waste tire pyrolysis. Chem. Pap. 2013, 67, 1521–1526. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, W.; Wang, L.; Hao, J. Comparative study of nitrogen migration among the products from catalytic pyrolysis and gasification of waste rigid polyurethane foam. J. Anal. Appl. Pyrolysis 2016, 120, 144–153. [Google Scholar] [CrossRef]
- Szargut, J.; Guzik, A.; Górniak, H. Programowany Zbiór Zadań z Termodynamiki Technicznej; PWN: Warszawa, Poland, 1986. [Google Scholar]
- PN-ISO 351:1999; Solid Fuels—Determination of Total Sulphur (High-Temperature Combustion Method). ISO: Geneva, Switzerland. Available online: https://sklep.pkn.pl/pn-iso-351-1999p.html (accessed on 27 July 2023).
- PN-ISO 587:2000; Solid Fuels—Determination of Chlorine Content Using Eschka Mixture. ISO: Geneva, Switzerland. Available online: https://sklep.pkn.pl/pn-iso-587-2000p.html (accessed on 27 July 2023).
- de Marco Rodriguez, I.; Laresgoiti, M.F.; Cabrero, M.A.; Torres, A.; Chomón, M.J.; Caballero, B. Pyrolysis of scrap tyre. Fuel Process. Technol. 2001, 72, 9–22. [Google Scholar] [CrossRef]




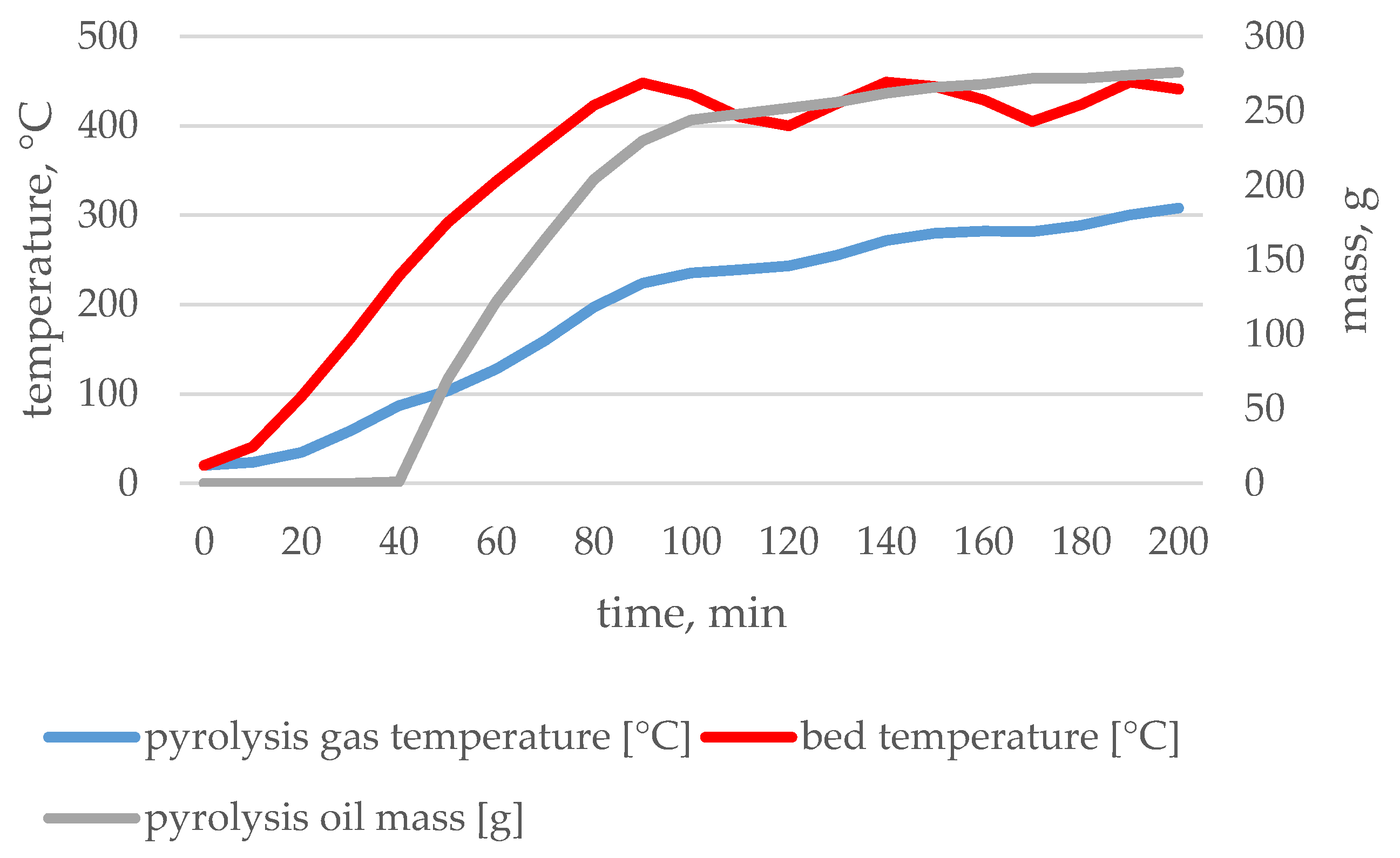
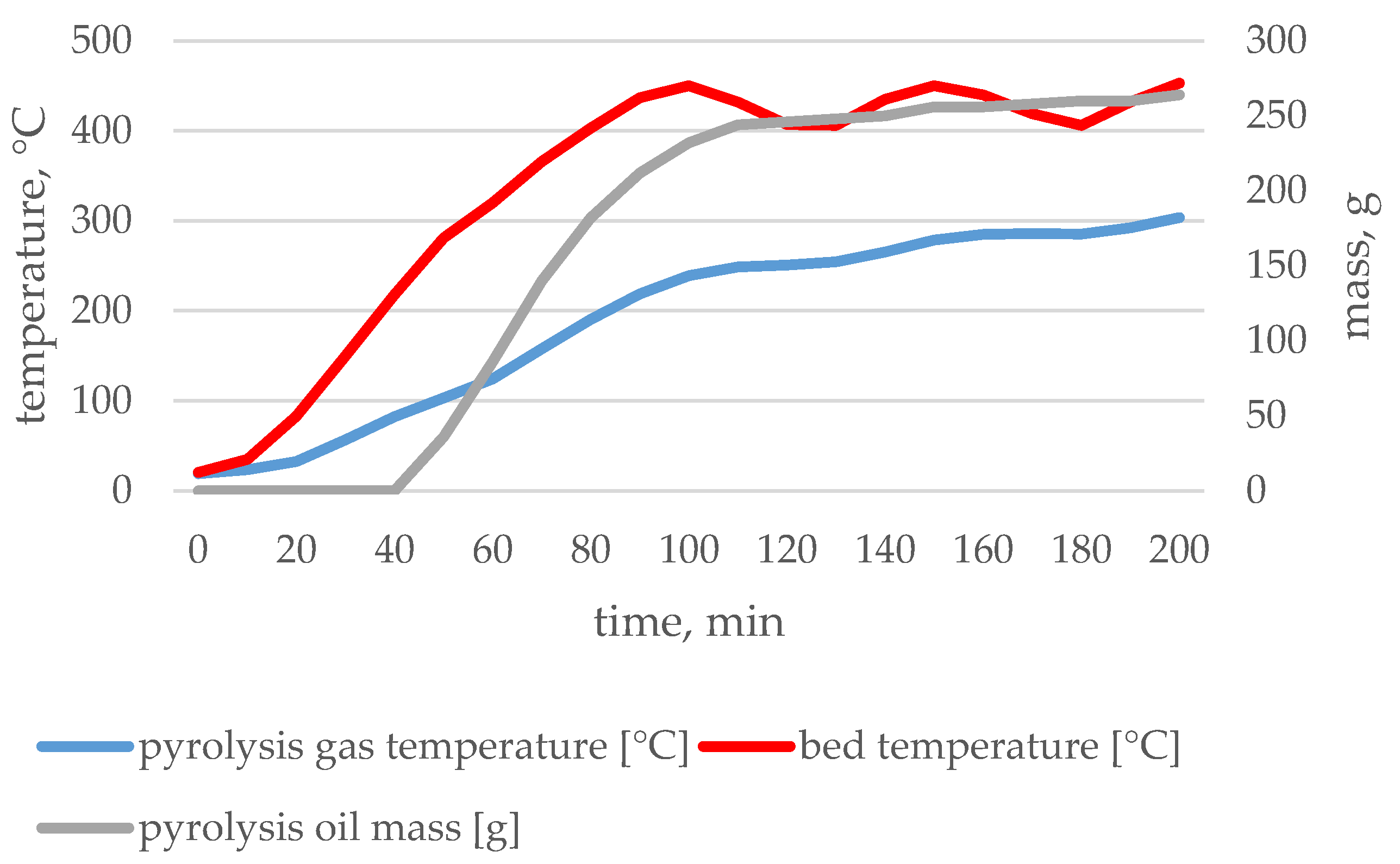
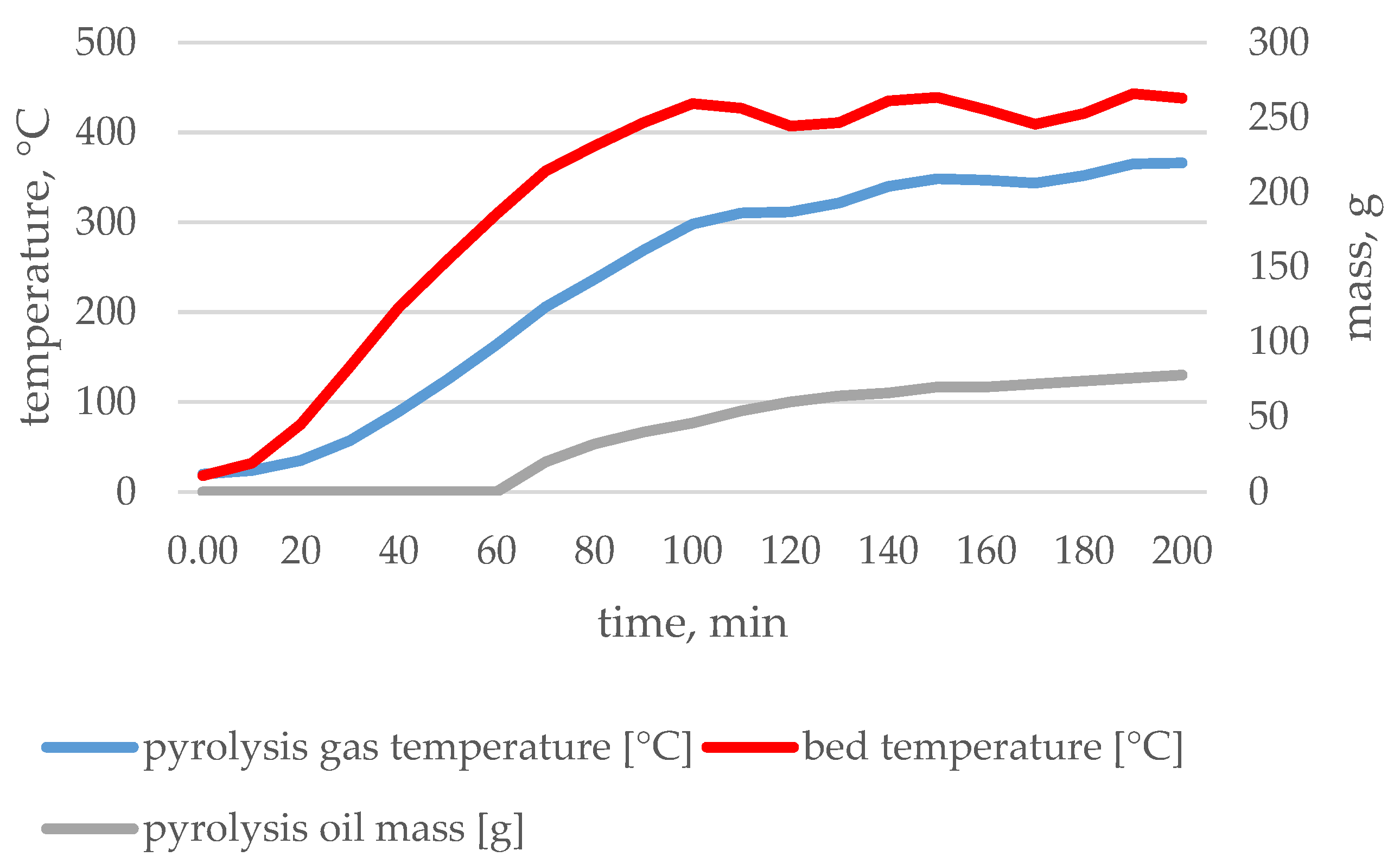
| Type of Waste | Input | Char | Pyrolytic Oil | Pyrolytic Gas | |
|---|---|---|---|---|---|
| Oil-Contaminated Water | Oil | ||||
| HDF floor panel | 1000.00 | 334.00 | 218.76 | 77.24 | 370.00 |
| MDF furniture board | 1000.00 | 418.00 | 192.14 | 31.86 | 358.00 |
| Chipboard furniture | 1000.00 | 356.00 | 242.47 | 41.53 | 360.00 |
| Natural floor plank | 1000.00 | 346.00 | 229.73 | 46.27 | 378.00 |
| Energy willow | 1000.00 | 360.00 | 221.12 | 42.88 | 376.00 |
| Vinyl floor panel | 1000.00 | 684.00 | 98.000 | 23.76 | 194.24 |
| Type of Waste | Input | Char | Pyrolytic Oil | Pyrolytic Gas | |
|---|---|---|---|---|---|
| Oil-Contaminated Water | Oil | ||||
| HDF floor panel | 100.00 | 33.40 | 21.88 | 7.72 | 37.00 |
| MDF furniture board | 100.00 | 41.80 | 19.21 | 3.19 | 35.80 |
| Chipboard furniture | 100.00 | 35.60 | 24.25 | 4.15 | 36.00 |
| Natural floor plank | 100.00 | 34.60 | 22. 97 | 4.63 | 37.80 |
| Energy willow | 100.00 | 36.00 | 22.11 | 4.29 | 37.60 |
| Vinyl floor panel | 100.00 | 68.40 | 9.80 | 2.38 | 19.42 |
| Type of Waste | Input | Char | Pyrolytic Oil | Pyrolytic Gas | |
|---|---|---|---|---|---|
| Oil-Contaminated Water | Oil | ||||
| HDF floor panel | 0.24 | 0.34 | 0.08 | 0.07 | 0.28 |
| MDF furniture board | 0.45 | 0.22 | 0.05 | 0.08 | 0.95 |
| Chipboard furniture | 0.21 | 0.06 | 0.10 | 0.05 | 0.45 |
| Natural floor plank | 0.65 | 0.015 | 0.03 | 0.03 | 1.69 |
| Energy willow | 0.22 | 0.05 | 0.00 | 0.05 | 0.53 |
| Vinyl floor panel | 0.29 | 0.23 | 0.35 | 3.31 | 0.09 |
| Type of Waste | Input | Char | Pyrolytic Oil | Pyrolytic Gas | |
|---|---|---|---|---|---|
| Oil-Contaminated Water | Oil | ||||
| HDF floor panel | 2.40 | 1.14 | 0.17 | 0.06 | 1.04 |
| MDF furniture board | 4.49 | 0.94 | 0.10 | 0.03 | 3.40 |
| Chipboard furniture | 2.10 | 0.23 | 0.23 | 0.02 | 1.62 |
| Natural floor plank | 6.50 | 0.05 | 0.06 | 0.02 | 6.37 |
| Energy willow | 2.20 | 0.19 | 0.00 | 0.02 | 1.99 |
| Vinyl floor panel | 2.90 | 1.61 | 0.34 | 0.79 | 0.17 |
| Type of Waste | Input | Char | Pyrolytic Oil | Pyrolytic Gas | |
|---|---|---|---|---|---|
| Oil-Contaminated Water | Oil | ||||
| HDF floor panel | 100.00 | 47.32 | 7.20 | 2.29 | 43.20 |
| MDF furniture board | 100.00 | 20.87 | 2.23 | 0.58 | 76.30 |
| Chipboard furniture | 100.00 | 10.85 | 11.03 | 0.97 | 77.15 |
| Natural floor plank | 100.00 | 0.80 | 0.92 | 0.23 | 98.05 |
| Energy willow | 100.00 | 8.51 | 0.00 | 0.88 | 90.61 |
| Vinyl floor panel | 100.00 | 55.43 | 11.76 | 27.12 | 5.69 |
| Type of Waste | Input | Char | Pyrolytic Oil | Pyrolytic Gas | |
|---|---|---|---|---|---|
| Oil-Contaminated Water | Oil | ||||
| HDF floor panel | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MDF furniture board | 0.67 | 1.18 | 0.06 | 0.09 | 0.44 |
| Chipboard furniture | 0.30 | 0.08 | 0.00 | 0.00 | 0.75 |
| Natural floor plank | 0.73 | 0.09 | 0.00 | 0.07 | 1.84 |
| Energy willow | 0.08 | 0.08 | 0.00 | 0.07 | 0.13 |
| Vinyl floor panel | 12.55 | 10.61 | 2.27 | 8.83 | 25.02 |
| Type of Waste | Input | Char | Pyrolytic Oil | Pyrolytic Gas | |
|---|---|---|---|---|---|
| Oil-Contaminated Water | Oil | ||||
| HDF floor panel | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MDF furniture board | 6.66 | 4.93 | 0.12 | 0.03 | 1.58 |
| Chipboard furniture | 3.00 | 0.29 | 0.00 | 0.00 | 2.71 |
| Natural floor plank | 7.30 | 0.32 | 0.00 | 0.03 | 6.95 |
| Energy willow | 0.80 | 0.29 | 0.00 | 0.03 | 0.48 |
| Vinyl floor panel | 125.50 | 72.57 | 2.22 | 2.10 | 48.61 |
| Type of Waste | Input | Char | Pyrolytic Oil | Pyrolytic Gas | |
|---|---|---|---|---|---|
| Oil-Contaminated Water | Oil | ||||
| HDF floor panel | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| MDF furniture board | 100.00 | 74.07 | 1.73 | 0.43 | 23.76 |
| Chipboard furniture | 100.00 | 9.55 | 0.00 | 0.00 | 90.45 |
| Natural floor plank | 100.00 | 4.41 | 0.00 | 0.44 | 95.15 |
| Energy willow | 100.00 | 36.00 | 0.00 | 3.75 | 60.25 |
| Vinyl floor panel | 100.00 | 57.83 | 1.77 | 1.67 | 38.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kajda-Szcześniak, M.; Ścierski, W. Studies on the Migration of Sulphur and Chlorine in the Pyrolysis Products of Floor and Furniture Joinery. Energies 2023, 16, 7446. https://doi.org/10.3390/en16217446
Kajda-Szcześniak M, Ścierski W. Studies on the Migration of Sulphur and Chlorine in the Pyrolysis Products of Floor and Furniture Joinery. Energies. 2023; 16(21):7446. https://doi.org/10.3390/en16217446
Chicago/Turabian StyleKajda-Szcześniak, Małgorzata, and Waldemar Ścierski. 2023. "Studies on the Migration of Sulphur and Chlorine in the Pyrolysis Products of Floor and Furniture Joinery" Energies 16, no. 21: 7446. https://doi.org/10.3390/en16217446
APA StyleKajda-Szcześniak, M., & Ścierski, W. (2023). Studies on the Migration of Sulphur and Chlorine in the Pyrolysis Products of Floor and Furniture Joinery. Energies, 16(21), 7446. https://doi.org/10.3390/en16217446






