Thermodynamic Analysis of an Innovative Cold Energy Storage System for Auto-Cascade Refrigeration Applications
Abstract
:1. Introduction
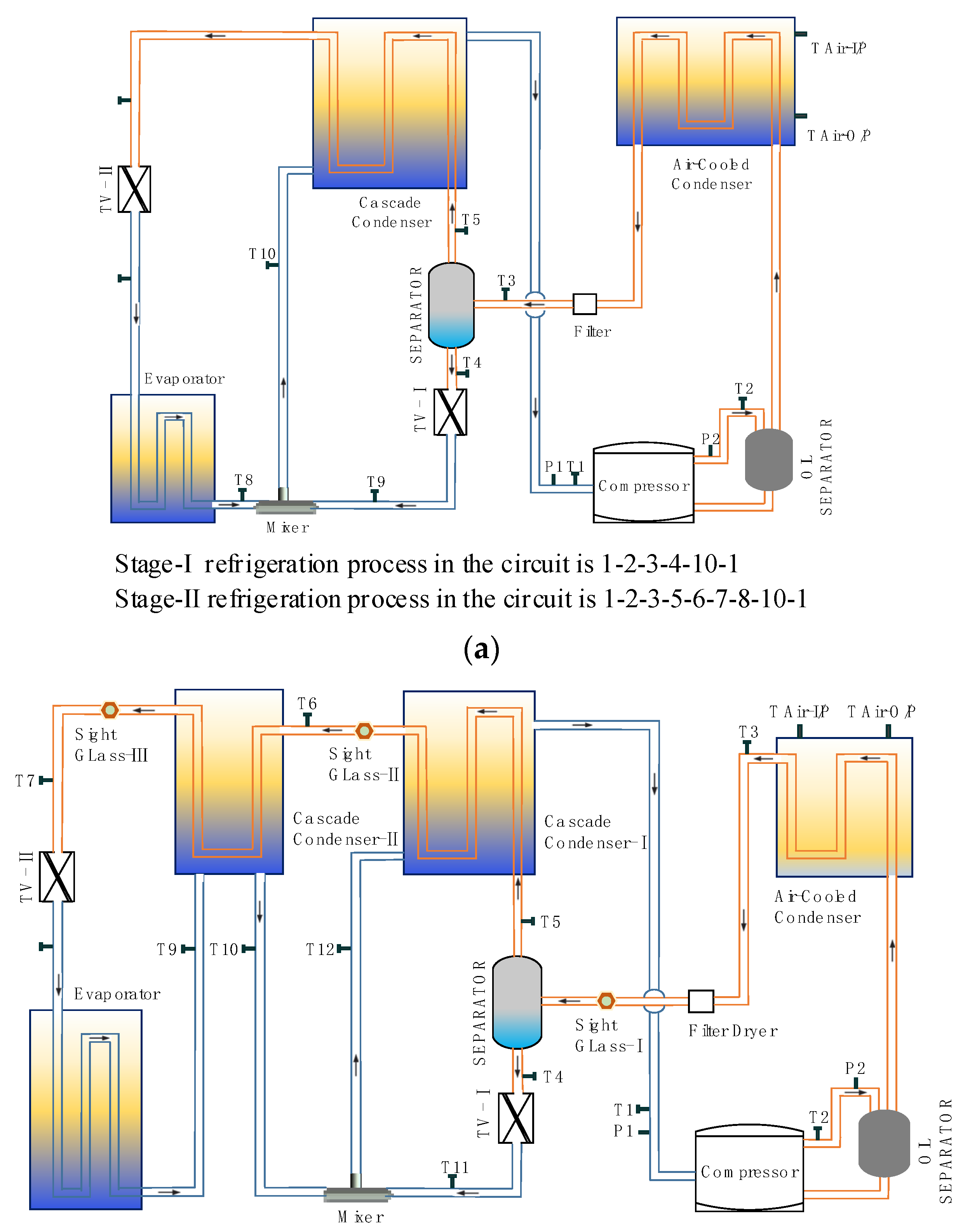
2. Cycle Description and Analysis
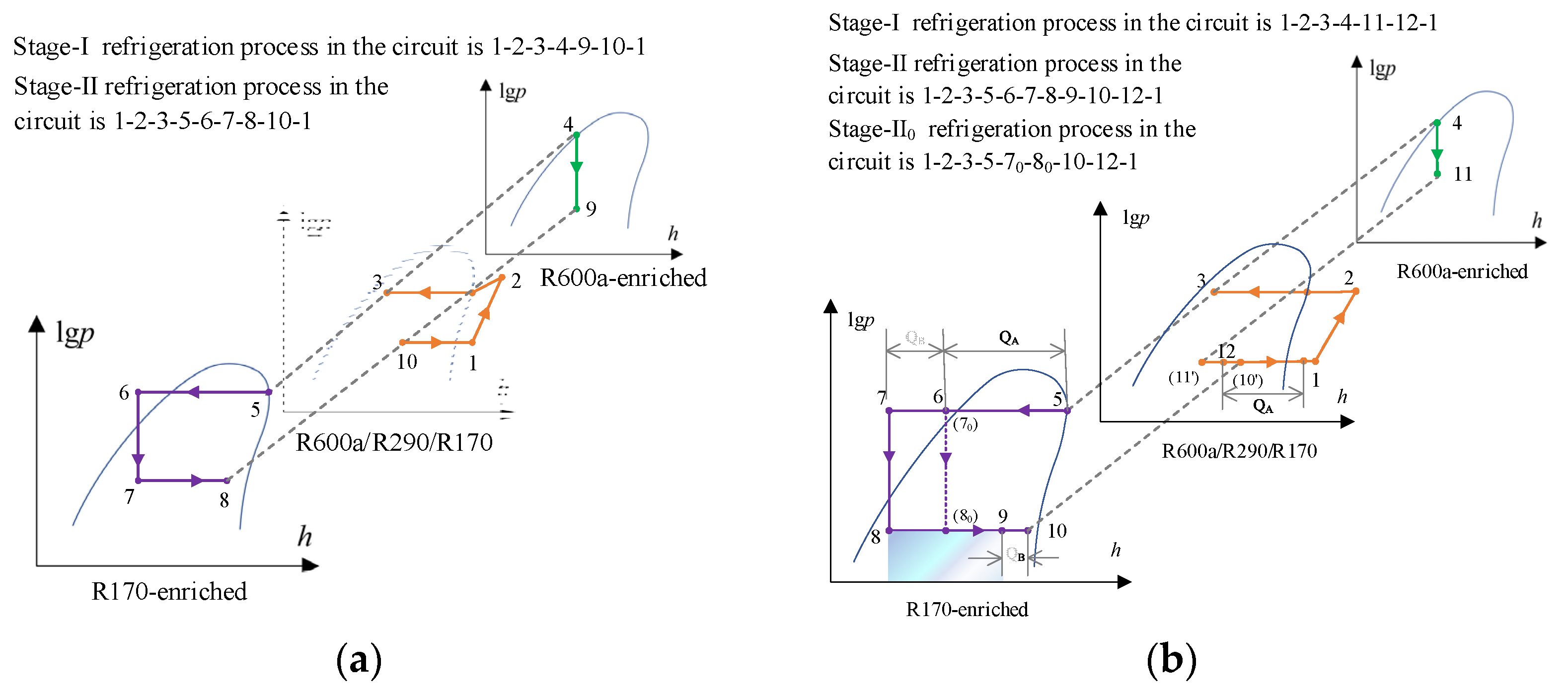
2.1. Cycle Description
2.2. Theoretical Cycle Analysis
3. Thermodynamic Modeling
3.1. Energetic Models
- (1)
- All components are in steady-state operation, and binary flow is assumed homogeneous.
- (2)
- The specific enthalpy of the working fluid is consistent for both before and after the throttle valve, and the compression in the compressor and throttling in the throttle valve processes are irreversible and adiabatic.
- (3)
- Only the condenser disperses heat to the surroundings. The pressure drops in the heat exchanger and connecting pipelines are negligible.
- (4)
- Kinetic and potential energy changes are ignored.
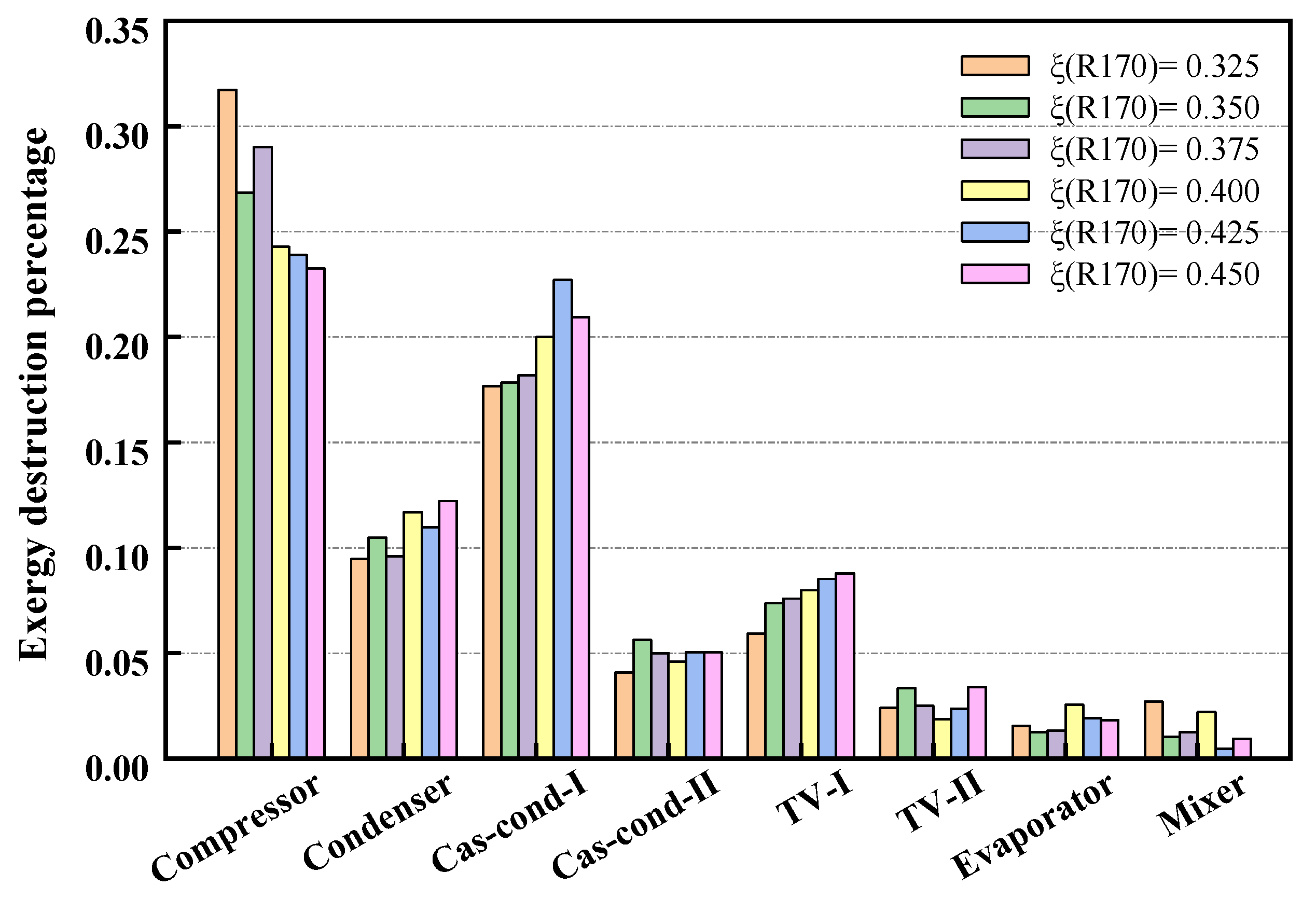
3.2. Exergic Models
4. Results and Discussion
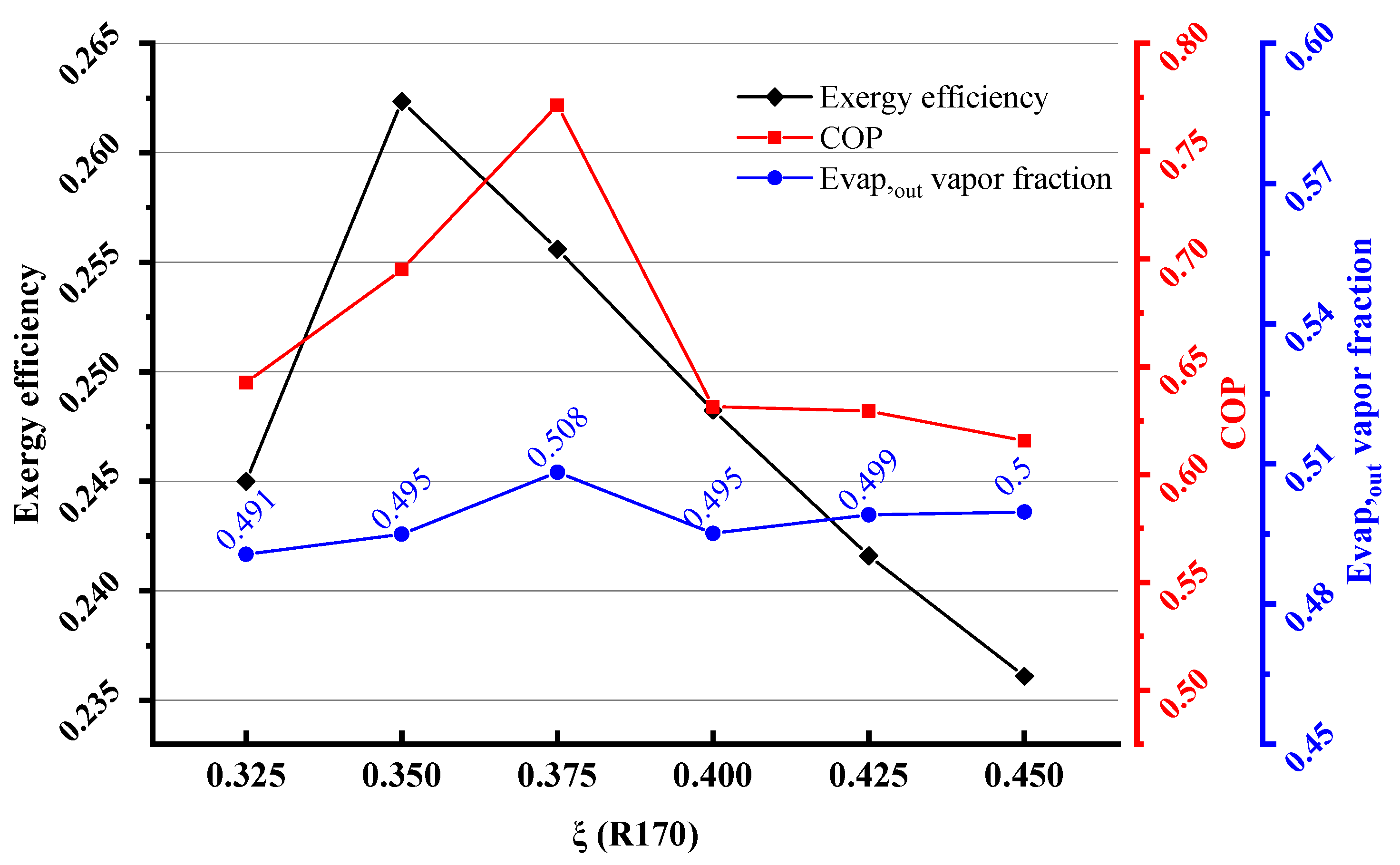
4.1. Performance under a Typical Operating Condition with Initial Composition
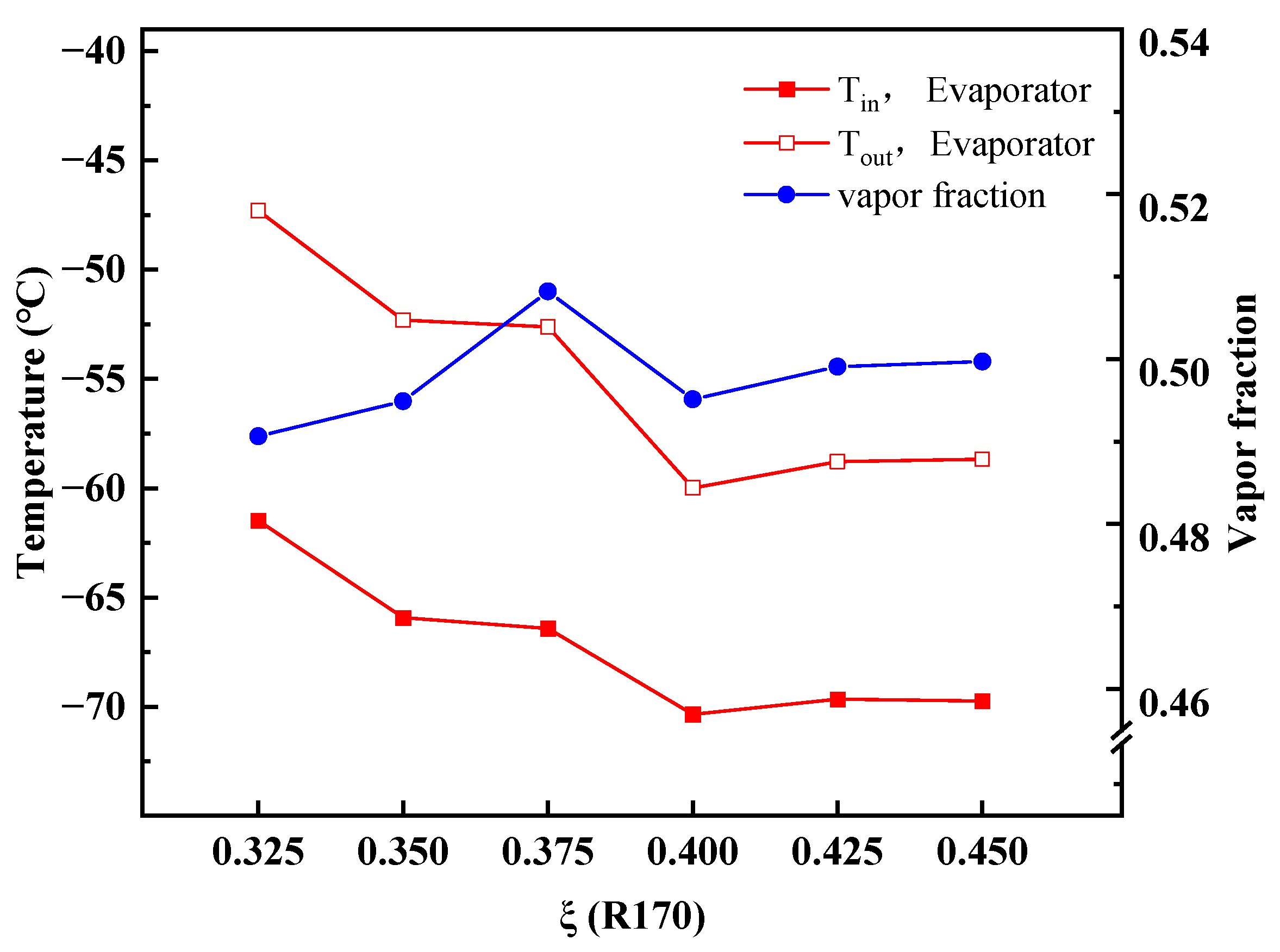
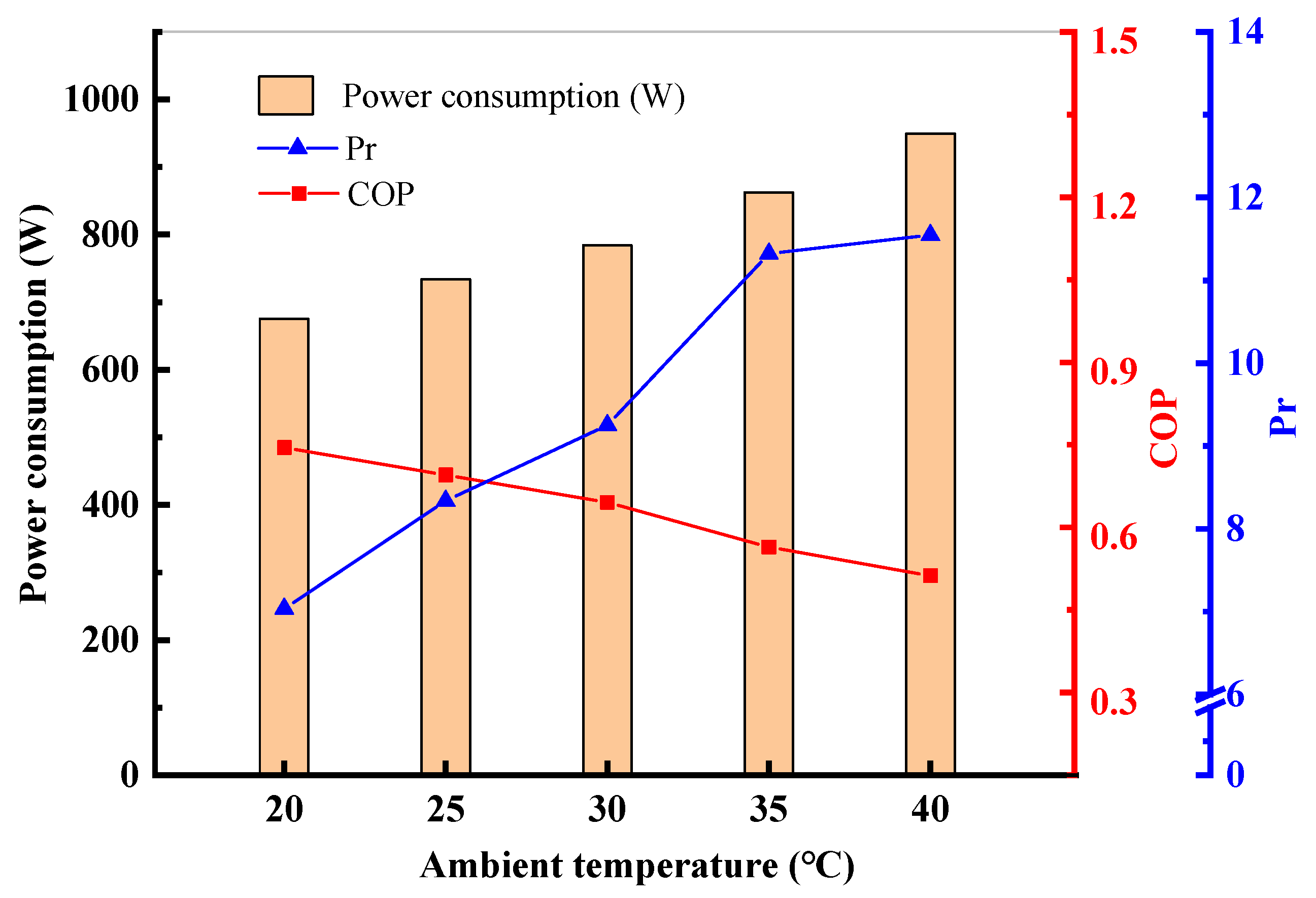
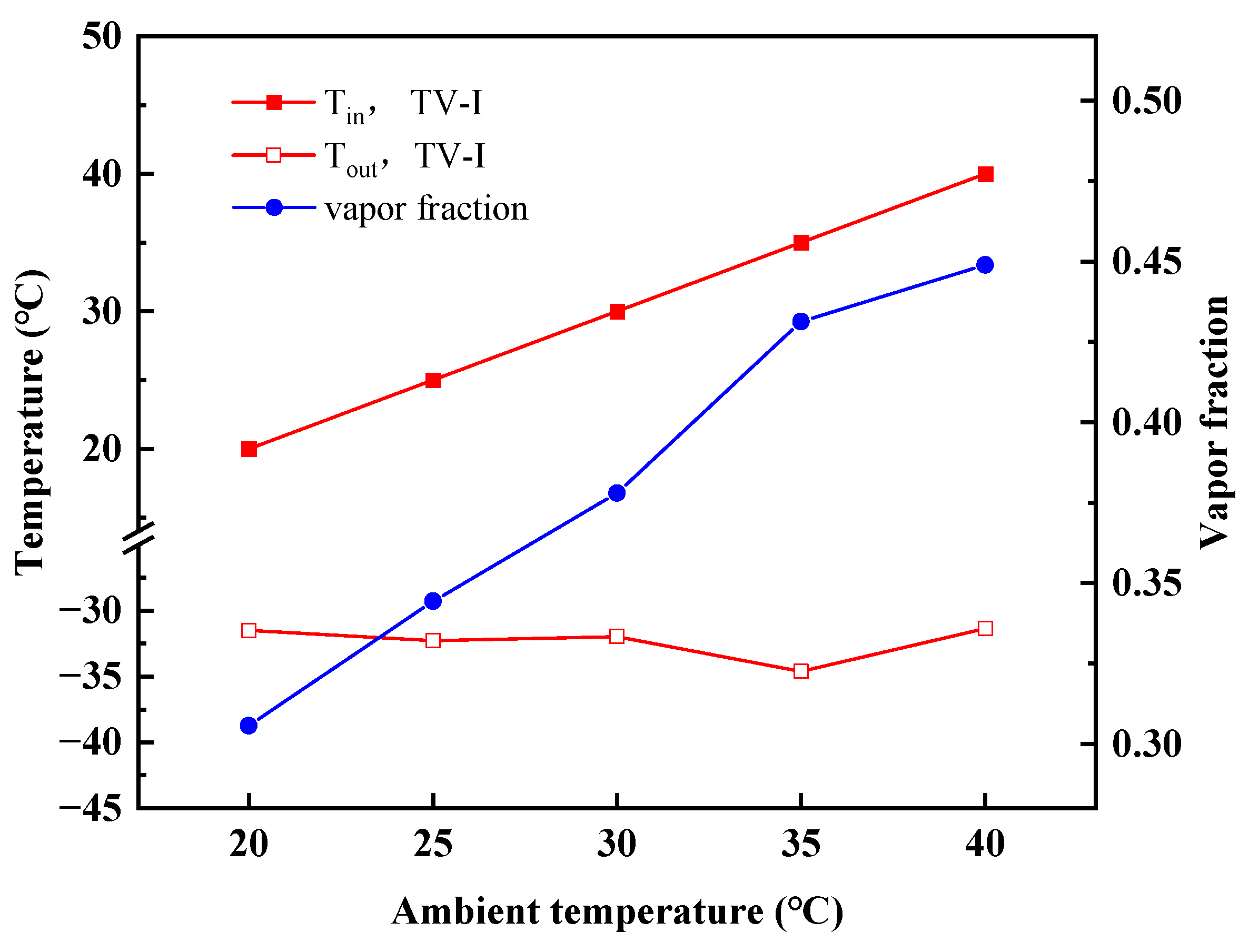
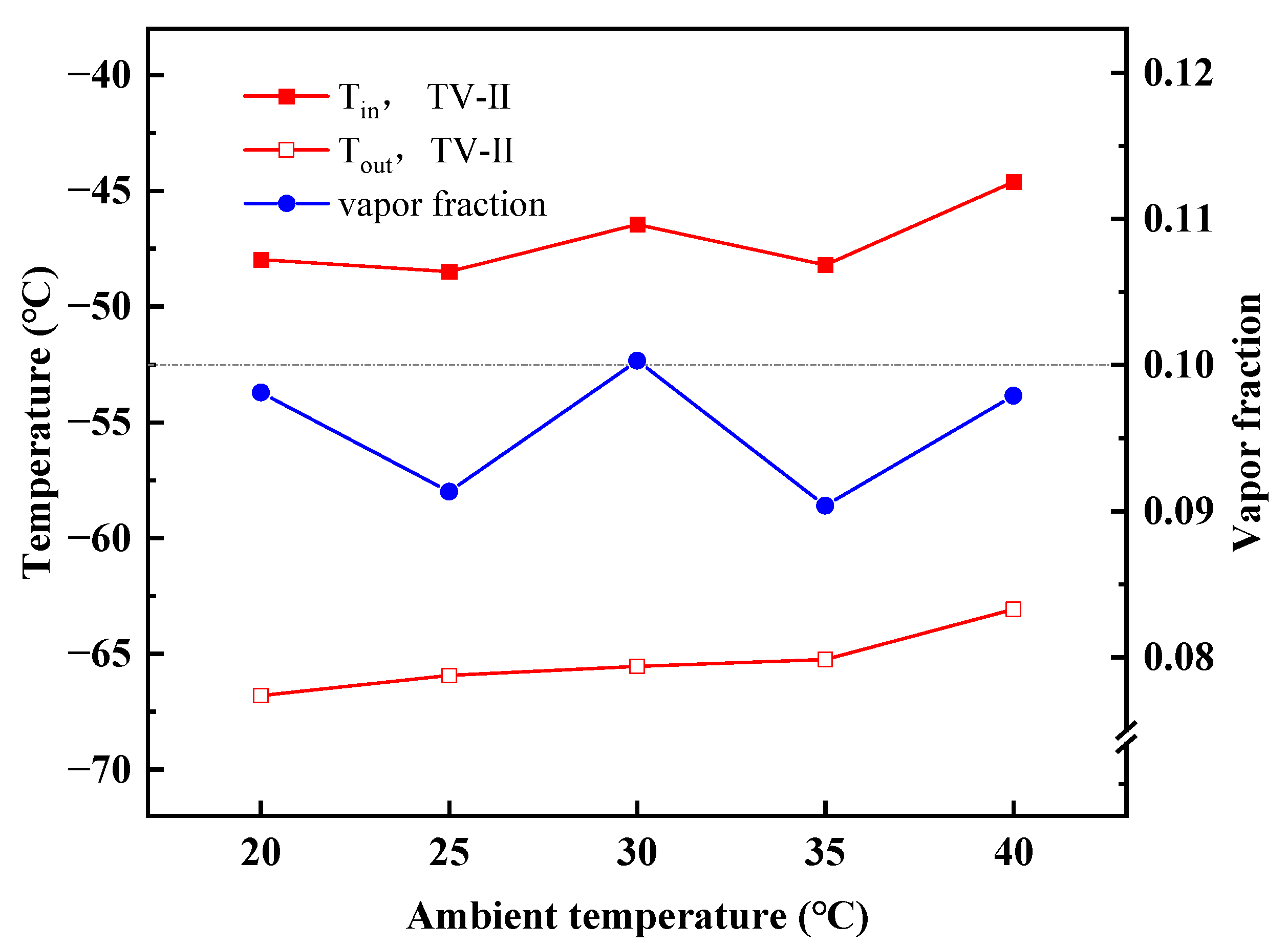
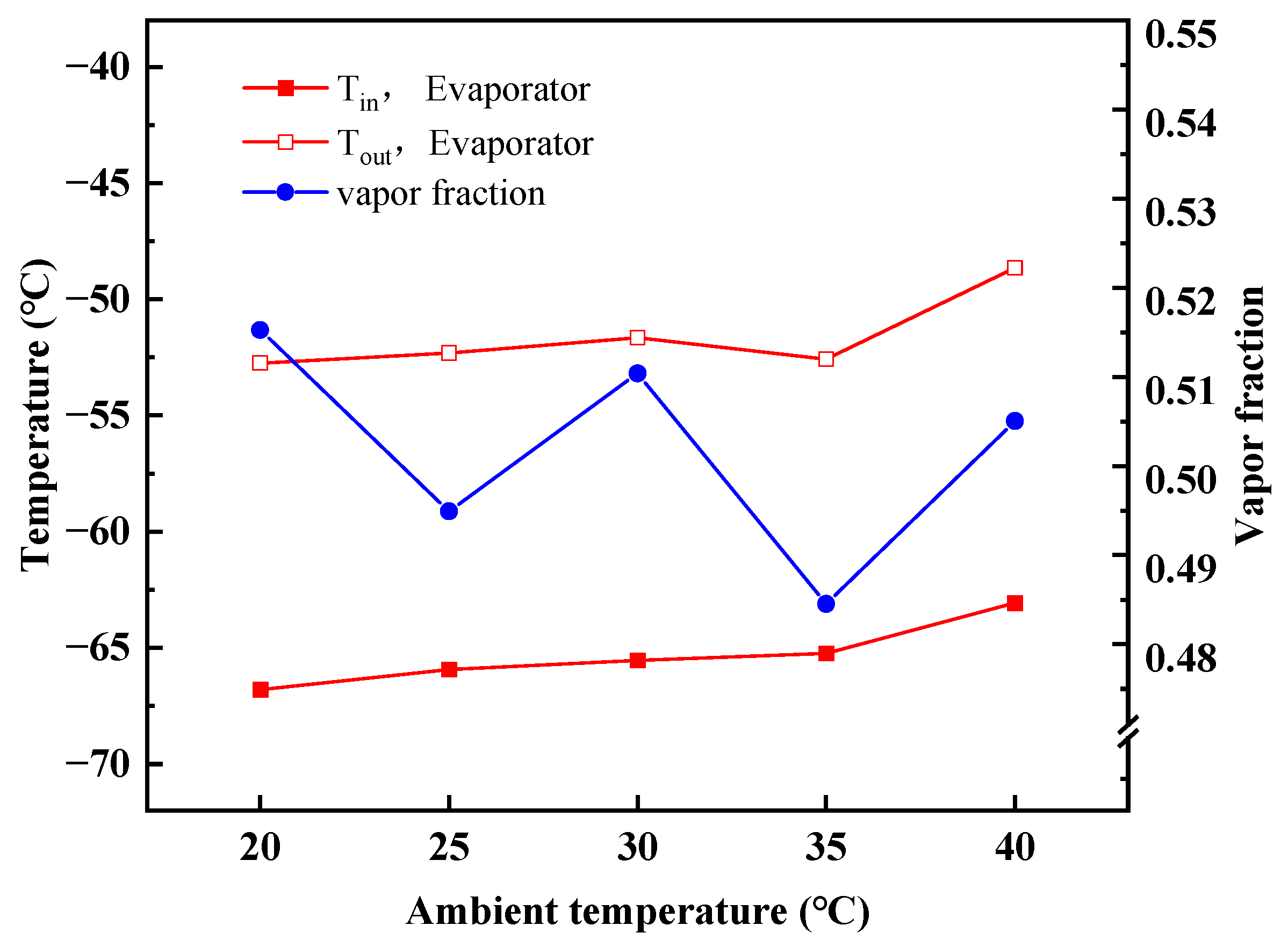
4.2. Performance Variations with Ambient Temperature
5. Conclusions
- (1)
- The MACRC system introduces a subcooled cascade condenser-II, which enables the refrigerant entering stage-II to obtain a greater cooling capacity. This method without complex mechanical components is more suitable for application in practice.
- (2)
- The ternary mixture of R600a/R290/R170 of the mass fraction of 0.25/0.35/0.40 having a coefficient of performance of 0.695 and an exergy efficiency of 0.262 was recommended for the MACRC system.
- (3)
- With increasing ambient temperatures, the outlet temperature of TV-I in stage-I is almost constant, whereas the outlet temperature of TV-II in stage-II goes up. The MACRC system can achieve −65 °C when the ambient temperature is less than 35 °C.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Abbreviations | Subscripts | ||
| ULT | ultra-low temperature | in | component inlet side |
| ACRC | auto-cascade refrigeration cycle | out | component outlet side |
| MACRC | modified auto-cascade refrigeration cycle | comp | compressor |
| COP | coefficient of performance | RI | first refrigerant group |
| TV | throttle valve | RII | second refrigerant group |
| NBP | nomal boiling point | evap | evaporator |
| cond | condenser | ||
| Symbols | cas-cond | cascade condenser | |
| Q | heat capacity of heat exchanger (W) | cas-evap | cascade evaporator |
| h | specific enthalpy (J·kg−1) | 0 | reference state |
| s | specific entropy (J·kg−1·K−1) | 1–12 | state points |
| mass flow rate (kg·s−1)) | i | cycle components | |
| ex | specific exergy (J·kg−1) | ||
| q | unit cooling capacity (W) | Greek letters | |
| xd | exergy destruction (W) | ξ | mass fraction |
| W | power consumption (W) | μ | exergy destruction efficiency |
| Pr | pressure ratio of compressor (-) | η | exergy efficiency |
| P | pressure (bar) | ||
| T | temperature (°C) | ||
References
- Miyara, A.; Onaka, Y.; Koyama, S. Ways of Next Generation Refrigerants and Heat Pump/Refrigeration Systems. Int. J. Air-Cond. Refri. 2012, 20, 1130002. [Google Scholar] [CrossRef]
- Li, D.; Wu, Y.; Wang, B.; Liu, C.; Arıcı, M. Optical and thermal performance of glazing units containing PCM in buildings: A review. Constr. Build. Mater. 2020, 233, 117327. [Google Scholar] [CrossRef]
- Tolstorebrov, I.; Eikevik, T.M.; Bantle, M. Effect of low and ultra-low temperature applications during freezing and frozen storage on quality parameters for fish. Int. J. Refrig. 2016, 63, 37–47. [Google Scholar] [CrossRef]
- Mota-Babiloni, A.; Joybari, M.M.; Navarro-Esbrí, J.; Mateu-Royo, C.; Barragán-Cervera, Á.; Amat-Albuixech, M.; Molés, F. Ultralow-temperature refrigeration systems: Configurations and refrigerants to reduce the environmental impact. Int. J. Refrig. 2020, 111, 147–158. [Google Scholar] [CrossRef]
- Bai, T.; Li, D.; Xie, H.; Yan, G.; Yu, J. Experimental research on a Joule-Thomson refrigeration cycle with mixture R170/R290 for −60 °C low-temperature freezer. Appl. Therm. Eng. 2021, 186, 116476. [Google Scholar] [CrossRef]
- Qin, Y.; Li, N.; Zhang, H.; Liu, B. Thermodynamic performance of a modified −150 °C refrigeration system coupled with Linde-Hampson and three-stage auto-cascade using low-GWP refrigerants. Energ. Convers. Manag. 2021, 236, 114093. [Google Scholar] [CrossRef]
- Xu, X.; Liu, J.; Cao, L. Mixed refrigerant composition shift due to throttle valves opening in auto cascade refrigeration system. Chinese. J. Chem. Eng. 2015, 23, 199–204. [Google Scholar] [CrossRef]
- Gao, Y.; He, G.; Cai, D.; Fan, M. Performance evaluation of a modified R290 dual-evaporator refrigeration cycle using two-phase ejector as expansion device. Energy 2020, 212, 118614. [Google Scholar] [CrossRef]
- Bai, T.; Yan, G.; Yu, J. Experimental research on the pull-down performance of an ejector enhanced auto-cascade refrigeration system for low-temperature freezer. Energy 2018, 157, 647–657. [Google Scholar] [CrossRef]
- Bai, T.; Yan, G.; Yu, J. Experimental investigation of an ejector-enhanced auto-cascade refrigeration system. Appl. Therm. Eng. 2018, 129, 792–801. [Google Scholar] [CrossRef]
- Bai, T.; Yan, G.; Yu, J. Experimental investigation on the dynamic malfunction behavior of the two-phase ejector in a modified auto-cascade freezer refrigeration system. Energ. Convers. Manag. 2019, 183, 382–390. [Google Scholar] [CrossRef]
- Yu, M.; Yu, J. Thermodynamic analyses of a flash separation ejector refrigeration cycle with zeotropic mixture for cooling applications. Energ. Convers. Manag. 2021, 229, 113755. [Google Scholar] [CrossRef]
- Elbel, S.; Lawrence, N. Review of recent developments in advanced ejector technology. Int. J. Refrig. 2016, 62, 1–18. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, K.; Sun, T.-F.; Chen, G.-M. Performance of a single-stage auto-cascade refrigerator operating with a rectifying column at the temperature level of −60 °C. J. Zhejiang. Univ.-Sci. A 2011, 12, 139–145. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, R.; Wang, J.; Chen, F.; Han, X.; Chen, G. An investigation of the mixing position in the recuperators on the performance of an auto-cascade refrigerator operating with a rectifying column. Cryogenics 2012, 52, 581–589. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Yan, G.; Yu, J. Theoretical study on a modified single-stage autocascade refrigeration cycle with auxiliary phase separator. Int. J. Refrig. 2021, 122, 181–191. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, S.; Du, P.; Liu, H. Experimental and theoretical investigation on the performance of CO2/propane auto-cascade refrigerator with a fractionation heat exchanger. Appl. Therm. Eng. 2015, 87, 669–677. [Google Scholar] [CrossRef]
- Gurudath Nayak, H.; Venkatarathnam, G. Performance of an auto refrigerant cascade refrigerator operating in liquid refrigerant supply (LRS) mode with different cascade heat exchangers. Cryogenics 2010, 50, 720–727. [Google Scholar] [CrossRef]
- Sobieraj, M.; Rosiński, M. High phase-separation efficiency auto-cascade system working with a blend of carbon dioxide for low-temperature isothermal refrigeration. Appl. Therm. Eng. 2019, 161, 114149. [Google Scholar] [CrossRef]
- Yan, G.; Hu, H.; Yu, J. Performance evaluation on an internal auto-cascade refrigeration cycle with mixture refrigerant R290/R600a. Appl. Therm. Eng. 2015, 75, 994–1000. [Google Scholar] [CrossRef]
- Asgari, S.; Noorpoor, A.R.; Boyaghchi, F.A. Parametric assessment and multi-objective optimization of an internal auto-cascade refrigeration cycle based on advanced exergy and exergoeconomic concepts. Energy 2017, 125, 576–590. [Google Scholar] [CrossRef]
- Boyaghchi, F.A.; Asgari, S. A comparative study on exergetic, exergoeconomic and exergoenvironmental assessments of two internal auto-cascade refrigeration cycles. Appl. Therm. Eng. 2017, 122, 723–737. [Google Scholar] [CrossRef]
- Du, K.; Zhang, S.; Xu, W.; Niu, X. A study on the cycle characteristics of an auto-cascade refrigeration system. Exp. Therm. Fluid. Sci. 2009, 33, 240–245. [Google Scholar] [CrossRef]
- Sivakumar, M.; Somasundaram, P. Exergy and energy analysis of three stage auto refrigerating cascade system using Zeotropic mixture for sustainable development. Energ. Convers. Manag. 2014, 84, 589–596. [Google Scholar] [CrossRef]
- Rui, S.; Zhang, H.; Zhang, B.; Wen, D. Experimental investigation of the performance of a single-stage auto-cascade refrigerator. Heat. Mass. Transfer. 2015, 52, 11–20. [Google Scholar] [CrossRef]
- Park, W.Y.; Shah, N.; Vine, E.; Blake, P.; Holuj, B.; Kim, J.H.; Kim, D.H. Ensuring the climate benefits of the Montreal Protocol: Global governance architecture for cooling efficiency and alternative refrigerants. Energy. Res. Soc. Sci. 2021, 76, 102068. [Google Scholar] [CrossRef]
- Aktemur, C.; Ozturk, I.T.; Cimsit, C. Comparative energy and exergy analysis of a subcritical cascade refrigeration system using low global warming potential refrigerants. Appl. Therm. Eng. 2021, 184, 116254. [Google Scholar] [CrossRef]
- Rodríguez-Jara, E.Á.; Sánchez-de-la-Flor, F.J.; Expósito-Carrillo, J.A.; Salmerón-Lissén, J.M. Thermodynamic analysis of auto-cascade refrigeration cycles, with and without ejector, for ultra low temperature freezing using a mixture of refrigerants R600a and R1150. Appl. Therm. Eng. 2022, 200, 117598. [Google Scholar] [CrossRef]
- Li, D.; Bai, T.; Yu, J. Thermodynamic performance optimization and analysis of an auto-cascade refrigeration cycle with vapor injection for ultra-low temperature freezer. Int. J. Refrig. 2022, 145, 425–435. [Google Scholar] [CrossRef]
- Bai, T.; Yan, G.; Yu, J. Influence of internal heat exchanger position on the performance of ejector-enhanced auto-cascade refrigeration cycle for the low-temperature freezer. Energy 2022, 238, 121803. [Google Scholar] [CrossRef]
- Faruque, M.W.; Uddin, M.R.; Salehin, S.; Ehsan, M.M. A Comprehensive Thermodynamic Assessment of Cascade Refrigeration System Utilizing Low GWP Hydrocarbon Refrigerants. Int. J. Thermofluids 2022, 15, 100177. [Google Scholar] [CrossRef]
- Gill, J.; Singh, J. Adaptive neuro-fuzzy inference system approach to predict the mass flow rate of R-134a/LPG refrigerant for straight and helical coiled adiabatic capillary tubes in the vapor compression refrigeration system. Int. J. Refrig. 2017, 78, 166–175. [Google Scholar] [CrossRef]
- Sobieraj, M. Experimental Investigation of the Effect of a Recuperative Heat Exchanger and Throttles Opening on a CO2/Isobutane Autocascade Refrigeration System. Energies 2020, 13, 5285. [Google Scholar] [CrossRef]
- Wang, J. Modern thermodynamics—New concepts based on the second law of thermodynamics. Prog. Nat. Sci. 2009, 19, 125–135. [Google Scholar] [CrossRef]
- Huber, M.L.; Lemmon, E.W.; Bell, I.H.; McLinden, M.O. The NIST REFPROP Database for Highly Accurate Properties of Industrially Important Fluids. Ind. Eng. Chem. Res. 2022, 61, 15449–15472. [Google Scholar] [CrossRef] [PubMed]


| Refrigerant | Molecular Formula | Molecular Weight (g·mol−1) | ODP | GWP100-yr (CO2-eq) | NBP (K) | Freezing Point (K) | Critical temp. (K) | Critical Press. (MPa) | ASHRAE Safety Group |
|---|---|---|---|---|---|---|---|---|---|
| R600a | C4H10 | 58.12 | 0 | 20 | 261.4 | 113.7 | 407.81 | 3.629 | A3 |
| R290 | CH3CH2CH3 | 44.1 | 0 | 3 | 231.04 | 85.53 | 369.89 | 4.251 | A3 |
| R170 | C2H6 | 30.07 | 0 | 20 | 184.57 | 90.368 | 305.32 | 4.872 | A3 |
| Components (R600a/R290/R170) | 0.25/0.425/0.325 | 0.25/0.40/0.35 | 0.25/0.375/0.375 | 0.25/0.35/0.400 | 0.25/0.325/0.425 | 0.25/0.3/0.450 |
|---|---|---|---|---|---|---|
| COP | 0.634 | 0.695 | 0.771 | 0.631 | 0.629 | 0.626 |
| Exergy efficiency (%) | 0.245 | 0.262 | 0256 | 0.248 | 0.242 | 0.236 |
| Temperature in °C | ||||||
| Compressor inlet | −15.7 | −13.1 | −14.5 | −17.5 | −14.0 | −13.2 |
| Compressor outlet | 93.8 | 96.0 | 90.3 | 102.8 | 100.2 | 105.3 |
| Evaporator inlet | −61.5 | −65.9 | −66.4 | −70.3 | −69.7 | −69.7 |
| Evaporator outlet | −47.3 | −52.3 | −52.6 | −60.0 | −58.8 | −58.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Jiang, J.; Wang, Z.; Zhang, H. Thermodynamic Analysis of an Innovative Cold Energy Storage System for Auto-Cascade Refrigeration Applications. Energies 2023, 16, 2282. https://doi.org/10.3390/en16052282
Liu Z, Jiang J, Wang Z, Zhang H. Thermodynamic Analysis of an Innovative Cold Energy Storage System for Auto-Cascade Refrigeration Applications. Energies. 2023; 16(5):2282. https://doi.org/10.3390/en16052282
Chicago/Turabian StyleLiu, Zhenzhen, Jingde Jiang, Zilong Wang, and Hua Zhang. 2023. "Thermodynamic Analysis of an Innovative Cold Energy Storage System for Auto-Cascade Refrigeration Applications" Energies 16, no. 5: 2282. https://doi.org/10.3390/en16052282
APA StyleLiu, Z., Jiang, J., Wang, Z., & Zhang, H. (2023). Thermodynamic Analysis of an Innovative Cold Energy Storage System for Auto-Cascade Refrigeration Applications. Energies, 16(5), 2282. https://doi.org/10.3390/en16052282







