Theoretical Investigation of Rate Rules for H-Intermigration Reactions for Cyclic Alkylperoxy Radicals
Abstract
1. Introduction
2. Computational Method
3. Results and Discussion
3.1. Potential Energy Surfaces
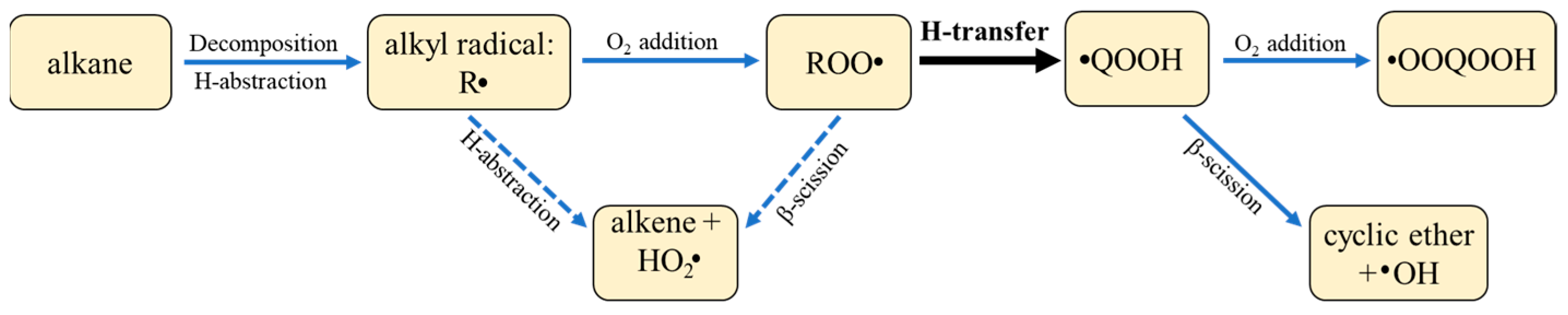
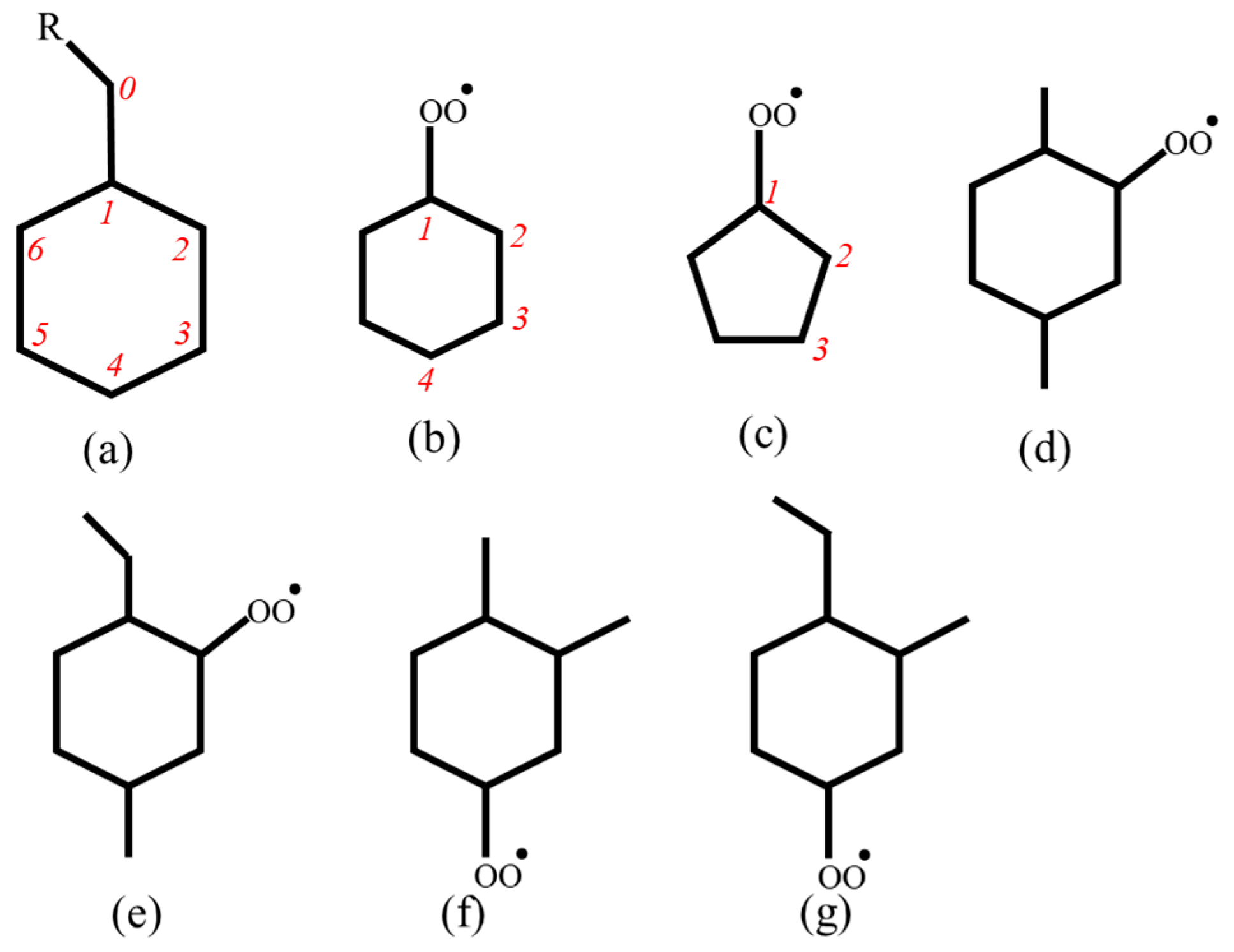
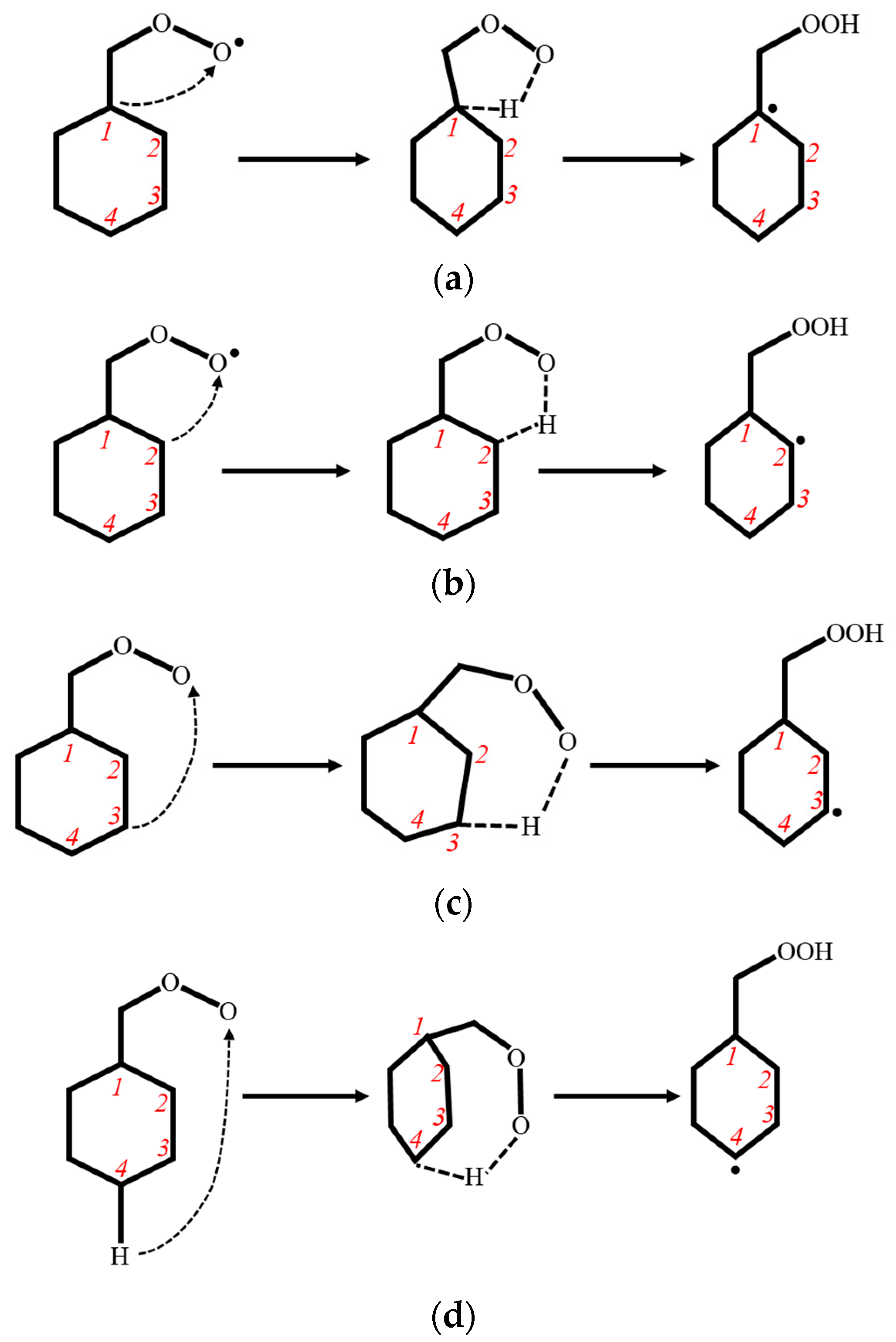
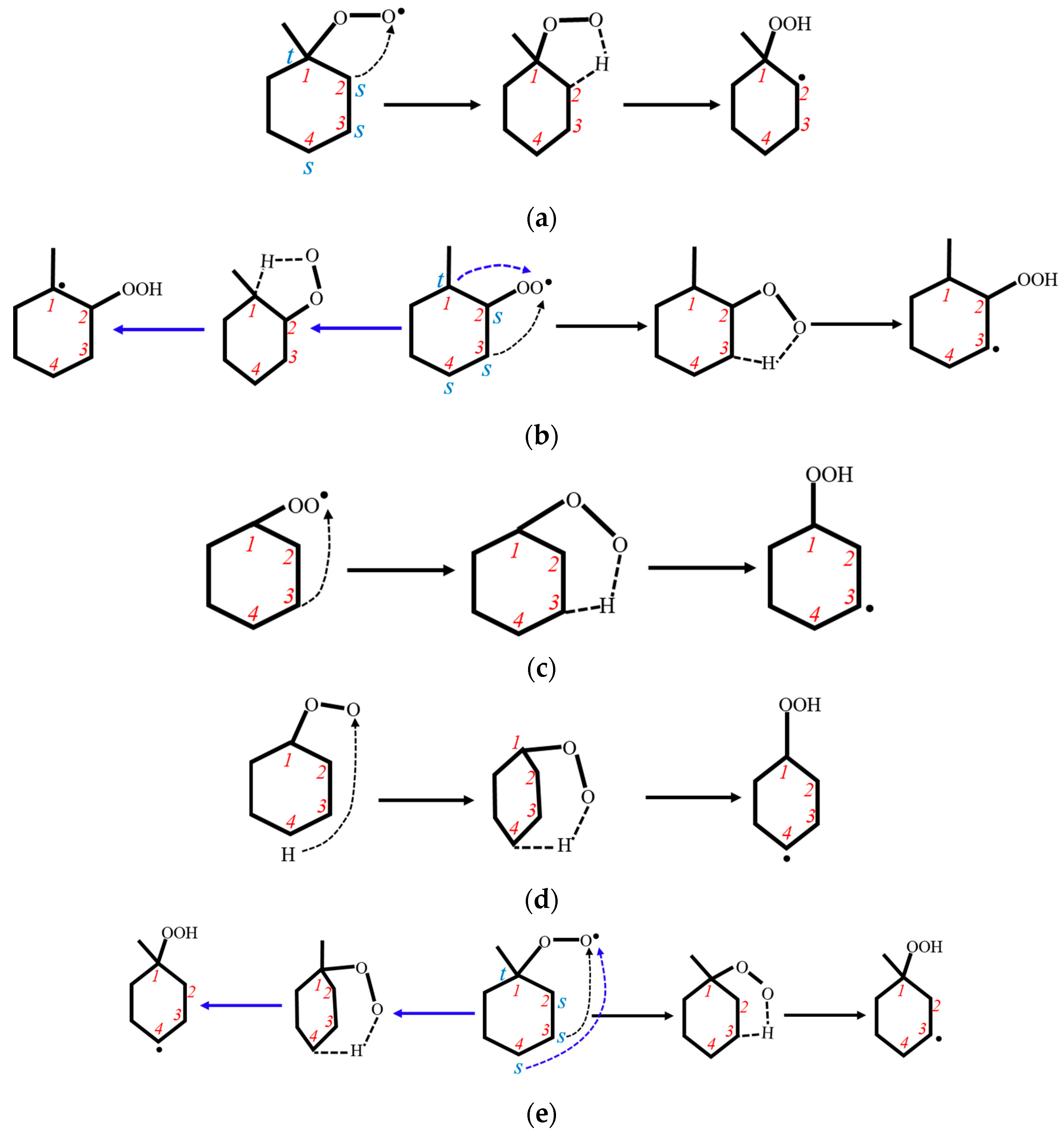
3.1.1. Reaction Types
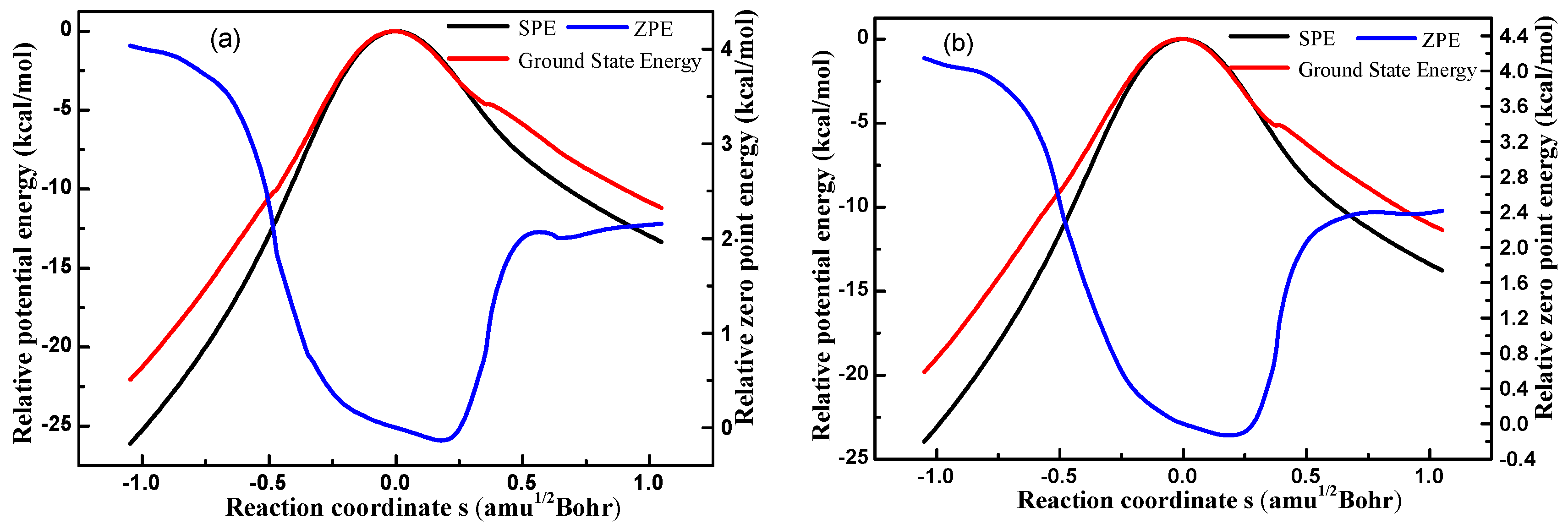
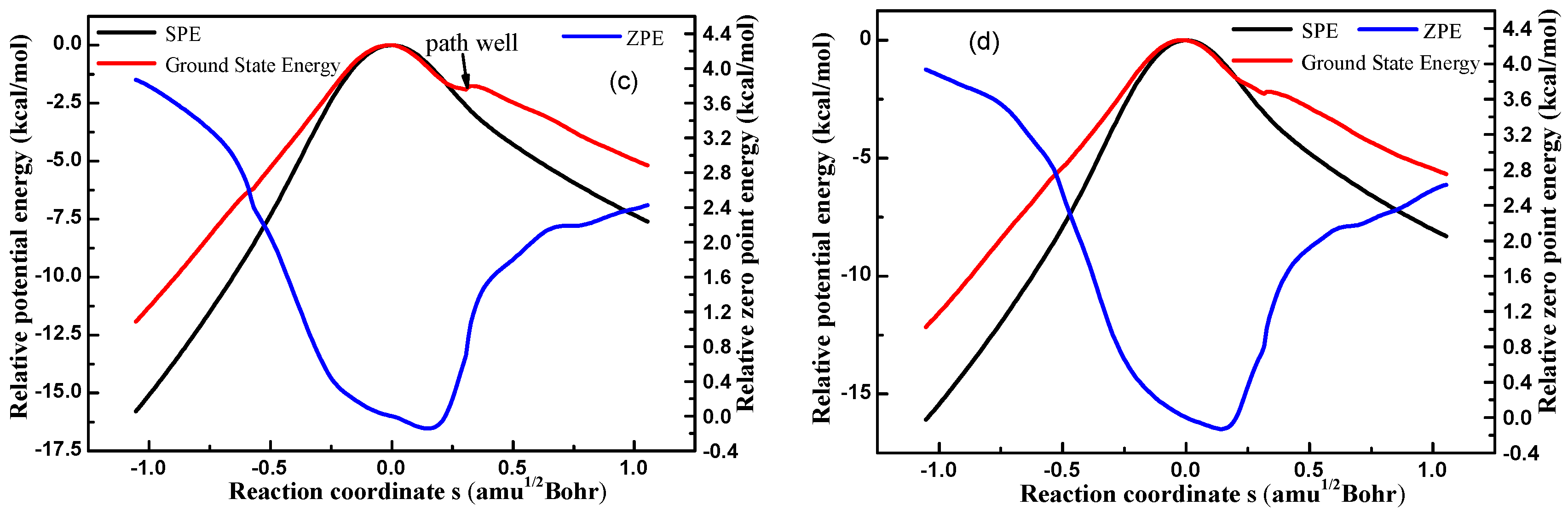
3.1.2. PES Features
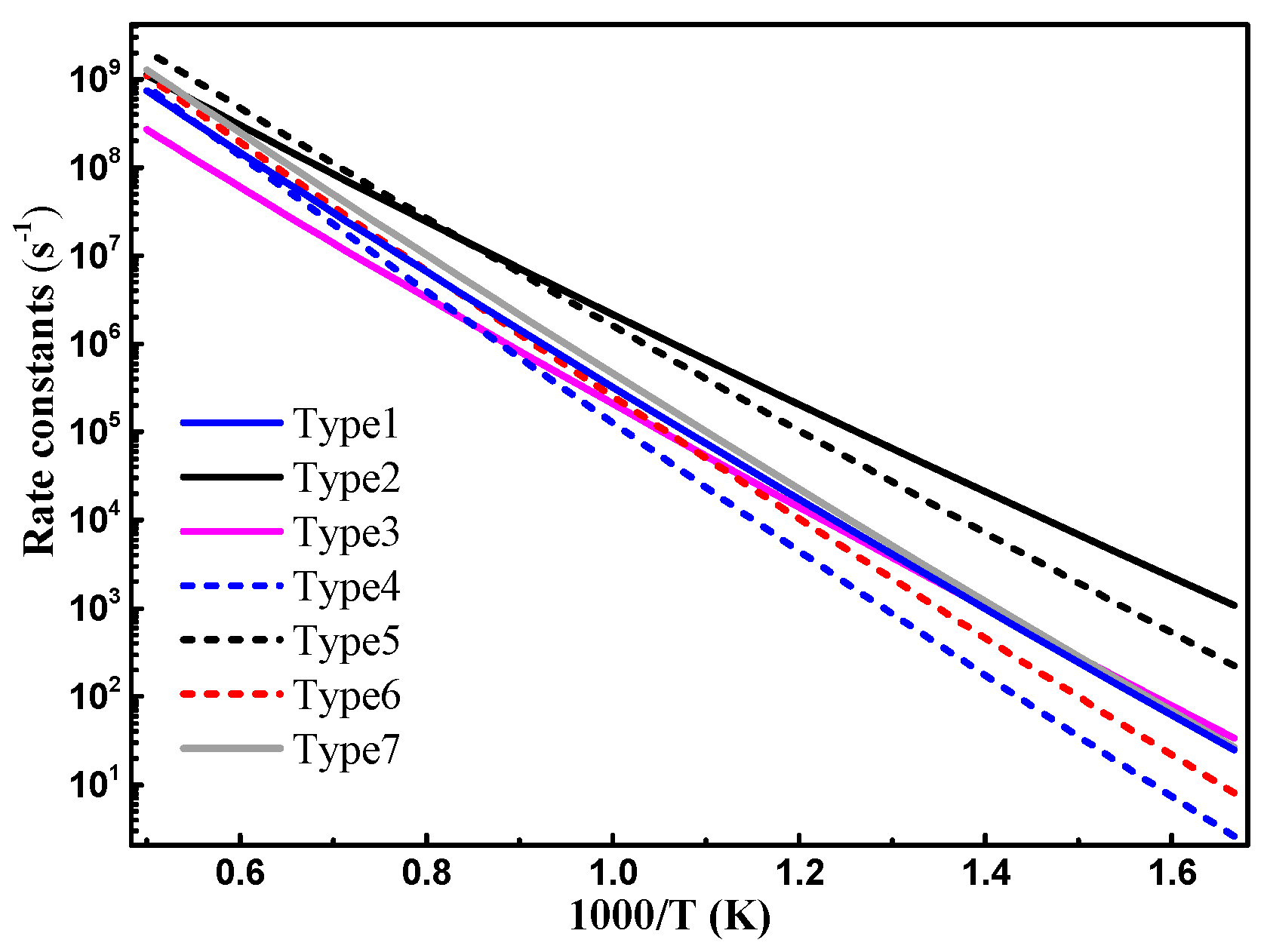
3.2. Reaction Rate Coefficients
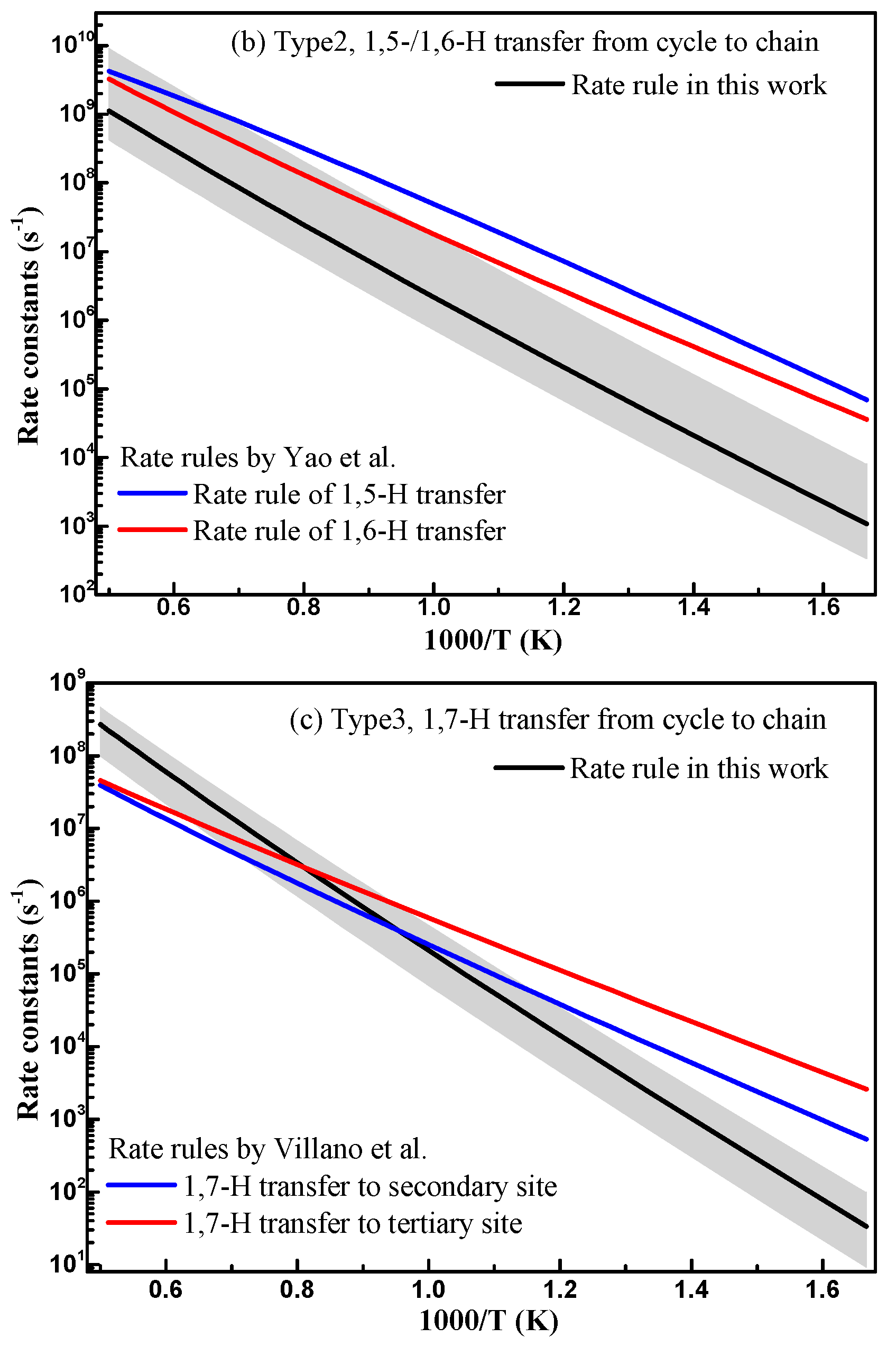
3.2.1. Comparison with Previous Data
3.2.2. High-Pressure Limit Rate Rules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peters, N. Turbulent Combustion. In Turbulent Combustion. Cambridge Monographs on Mechanics Cambridge; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Pitsch, H. Large-eddy simulation of turbulent combustion. Annu. Rev. Fluid Mech. 2005, 38, 453–482. [Google Scholar] [CrossRef]
- Lai, J.Y.W.; Lin, K.C.; Violi, A. Biodiesel combustion: Advances in chemical kinetic modeling. Prog. Energy Combust. Sci. 2011, 37, 1–14. [Google Scholar] [CrossRef]
- Wang, H.; Dames, E.; Sirjean, B.; Sheen, D.A.; Tango, R.; Violi, A.; Lai, J.Y.W.; Egolfopoulos, F.N.; Davidson, D.F.; Hanson, R.K.; et al. A High-Temperature Chemical Kinetic Model of n-Alkane (up to n-Dodecane), Cyclohexane, and Methyl-, Ethyl-, n-Propyl and n-Butyl-Cyclohexane Oxidation at High Temperatures, JetSurF Version 2.0. 2010. Available online: http://web.stanford.edu/group/haiwanglab/JetSurF/JetSurF2.0/index.html (accessed on 1 July 2022).
- Zhou, C.-W.; Li, Y.; Burke, U.; Banyon, C.; Somers, K.P.; Ding, S.; Khan, S.; Hargis, J.W.; Sikes, T.; Mathieu, O.; et al. An experimental and chemical kinetic modeling study of 1,3-butadiene combustion: Ignition delay time and laminar flame speed measurements. Combust. Flame 2018, 197, 423–438. [Google Scholar] [CrossRef]
- Curran, H.J.; Gaffuri, P.; Pitz, W.J.; Westbrook, C.K. A Comprehensive Modeling Study of n-Heptane Oxidation. Combust. Flame 1998, 114, 149–177. [Google Scholar] [CrossRef]
- Curran, H.J.; Gaffuri, P.; Pitz, W.J.; Westbrook, C.K. A comprehensive modeling study of iso-octane oxidation. Combust. Flame 2002, 129, 253–280. [Google Scholar] [CrossRef]
- Cai, L.; Pitsch, H. Mechanism optimization based on reaction rate rules. Combust. Flame 2014, 161, 405–415. [Google Scholar] [CrossRef]
- Cai, L.; Pitsch, H. Optimized chemical mechanism for combustion of gasoline surrogate fuels. Combust. Flame 2015, 162, 1623–1637. [Google Scholar] [CrossRef]
- Cai, L.; Pitsch, H.; Mohamed, S.Y.; Raman, V.; Bugler, J.; Curran, H.; Sarathy, S.M. Optimized reaction mechanism rate rules for ignition of normal alkanes. Combust. Flame 2016, 173, 468–482. [Google Scholar] [CrossRef]
- Manente, V.; Johansson, B.; Cannella, W. Gasoline partially premixed combustion, the future of internal combustion engines? Int. J. Engine Res. 2011, 12, 194–208. [Google Scholar] [CrossRef]
- Reitz, R.D.; Duraisamy, G. Review of high efficiency and clean reactivity controlled compression ignition (RCCI) combustion in internal combustion engines. Prog. Energy Combust. Sci. 2015, 46, 12–71. [Google Scholar] [CrossRef]
- Yao, M.; Zheng, Z.; Liu, H. Progress and recent trends in homogeneous charge compression ignition (HCCI) engines. Prog. Energy Combust. Sci. 2009, 35, 398–437. [Google Scholar] [CrossRef]
- Battin-Leclerc, F. Detailed chemical kinetic models for the low-temperature combustion of hydrocarbons with application to gasoline and diesel fuel surrogates. Prog. Energy Combust. Sci. 2008, 34, 440–498. [Google Scholar] [CrossRef]
- Wang, Z.; Herbinet, O.; Hansen, N.; Battin-Leclerc, F. Exploring hydroperoxides in combustion: History, recent advances and perspectives. Prog. Energy Combust. Sci. 2019, 73, 132–181. [Google Scholar] [CrossRef]
- Miyoshi, A. Systematic Computational Study on the Unimolecular Reactions of Alkylperoxy (RO2), Hydroperoxyalkyl (QOOH), and Hydroperoxyalkylperoxy (O2QOOH) Radicals. J. Phys. Chem. A 2011, 115, 3301–3325. [Google Scholar] [CrossRef]
- Villano, S.M.; Huynh, L.K.; Carstensen, H.-H.; Dean, A.M. High-Pressure Rate Rules for Alkyl + O2 Reactions. 1. The Dissociation, Concerted Elimination, and Isomerization Channels of the Alkyl Peroxy Radical. J. Phys. Chem. A 2011, 115, 13425–13442. [Google Scholar] [CrossRef] [PubMed]
- Villano, S.M.; Huynh, L.K.; Carstensen, H.-H.; Dean, A.M. High-Pressure Rate Rules for Alkyl + O2 Reactions. 2. The Isomerization, Cyclic Ether Formation, and β-Scission Reactions of Hydroperoxy Alkyl Radicals. J. Phys. Chem. A 2012, 116, 5068–5089. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, L.; Qi, F. Ab initio kinetics on low temperature oxidation of iso-pentane: The first oxygen addition. Combust. Flame 2018, 190, 119–132. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, L.; Zhang, F.; Jiang, J. Theoretical kinetic studies for low temperature oxidation of two typical methylcyclohexyl radicals. Combust. Flame 2017, 182, 216–224. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, F.; Zhang, L. Theoretical studies for reaction kinetics of cy-C6H11CH2 radical with O2. Proc. Combust. Inst. 2017, 36, 179–186. [Google Scholar] [CrossRef]
- Yao, X.; Wang, J.; Yao, Q.; Li, Y.; Li, Z.; Li, X. Pressure-dependent rate rules for intramolecular H-migration reactions of normal-alkyl cyclohexylperoxy radicals. Combust. Flame 2019, 204, 176–188. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Parandaman, A.; Perez, J.E.; Sinha, A. Atomospheric decomposition of trifluoromethanol catalyzed by formic acid. J. Phys. Chem. A 2018, 122, 9553–9562. [Google Scholar] [CrossRef] [PubMed]
- Parandaman, A.; Kumar, M.; Francisco, J.S.; Sinha, A. Organic acid formation from the atmospheric oxidation of gem diols: Reaction mechanism, energetics and rates. J. Phys. Chem. A 2018, 122, 6226–6276. [Google Scholar] [CrossRef] [PubMed]
- Parandaman, A.; Musah, R.A. Computational study investigating the atmospheric oxidation mechanism and kinetics of dipropyl thisulfinate initiated by OH radicals and the fate of propanethiyl radical. J. Phys. Chem. A 2020, 124, 8292–8304. [Google Scholar]
- Zádor, J.; Jasper, A.W.; Miller, J.A. The reaction between propene and hydroxyl. Phys. Chem. Chem. Phys. 2009, 11, 11040–11053. [Google Scholar] [CrossRef]
- Yang, F.; Deng, F.; Pan, Y.; Zhang, Y.; Tang, C.; Huang, Z. Kinetics of Hydrogen Abstraction and Addition Reactions of 3-Hexene by H Radicals. J. Phys. Chem. A 2017, 121, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Liakos, D.G.; Neese, F. Is it possible to obtain coupled cluster quality energies at near density functional theory cost? Domain-based local pair natural orbital coupled cluster vs modern density functional theory. J. Chem. Theory Comput. 2015, 11, 4054–4063. [Google Scholar]
- Yang, F.; Zhang, Y.; Sun, W.; Zhao, Q.; Huang, W.; Qin, X.; Deng, F.; Huang, Z. Theoretical kinetics of hydrogen abstraction and addition reactions of 3-hexene by Ḣ, Ö(3P) and ĊH3. Combust. Flame 2018, 197, 449–462. [Google Scholar] [CrossRef]
- Yin, G.; Hu, E.; Gao, Z.; Yang, F.; Huang, Z. Kinetics of H abstraction and addition reactions of 2,4,4-trimethyl-2-pentene by OH radical. Chem. Phys. Lett. 2018, 696, 125–134. [Google Scholar] [CrossRef]
- Martin, J.M.; Uzan, O. Basis set convergence in second-row compounds. The importance of core polarization functions. Chem. Phys. Lett. 1998, 282, 16–24. [Google Scholar]
- Parrish, R.M.; Burns, L.A.; Smith, D.G.A.; Simmonett, A.C.; DePrince, A.E.; Hohenstein, E.G.; Bozkaya, U.; Sokolov, A.Y.; Di Remigio, R.; Richard, R.M.; et al. Psi4 1.1: An Open-Source Electronic Structure Program Emphasizing Automation, Advanced Libraries, and Interoperability. J. Chem. Theory Comput. 2017, 13, 3185–3197. [Google Scholar] [PubMed]
- Neese, F. Software update: The ORCA program system, version 4.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Truhlar, D.G.; Isaacson, A.D.; Garrett, B.C. Generalized Transition State Theory; CRC Press: Boca Raton, FL, USA, 1985; pp. 65–137. [Google Scholar]
- Eckart, C. The penetration of a potential barrier by electrons. Phys. Rev. 1930, 35, 1303. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Gwinn, W.D. Energy levels and thermodynamic functions for molecules with internal rotation: I. Rigid frame with attached tops. In Molecular Structure and Statistical Thermodynamics: Selected Papers of Kenneth S. Pitzer; World Scientific: Singapore, 1993; pp. 33–46. [Google Scholar]
- Pilling, M.J.; Robertson, S.H. Master Equation Models for Chemical Reactions of Importance in Combustion. Annu. Rev. Phys. Chem. 2003, 54, 245–275. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Klippenstein, S.J. Master Equation Methods in Gas Phase Chemical Kinetics. J. Phys. Chem. A 2006, 110, 10528–10544. [Google Scholar] [CrossRef]
- Georgievskii, Y.; Klippenstein, S.J. Transition State Theory for Multichannel Addition Reactions: Multifaceted Dividing Surfaces. J. Phys. Chem. A 2003, 107, 9776–9781. [Google Scholar] [CrossRef]
- Greenwald, E.E.; North, S.W.; Georgievskii, Y.; Klippenstein, S.J. A Two Transition State Model for Radical−Molecule Reactions: A Case Study of the Addition of OH to C2H4. J. Phys. Chem. A 2005, 109, 6031–6044. [Google Scholar] [CrossRef]
- Welty, J.R.; Wicks, C.E. Fundamentals of Momentum, Heat and Mass Transfer, 4th ed.; John Wiley and Sons Ltd.: New York, NY, USA, 2001. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Barker, J.R. Multiple-Well, multiple-path unimolecular reaction systems. I. MultiWell computer program suite. Int. J. Chem. Kinet. 2001, 33, 232–245. [Google Scholar] [CrossRef]
- Barker, J.R. Energy transfer in master equation simulations: A new approach. Int. J. Chem. Kinet. 2009, 41, 748–763. [Google Scholar] [CrossRef]
- Ning, H.; Gong, C.; Tan, N.; Li, Z.; Li, X. Low- and intermediate-temperature oxidation of ethylcyclohexane: A theoretical study. Combust. Flame 2015, 162, 4167–4182. [Google Scholar] [CrossRef]






| Reaction | A | n | E/cal | Reaction | A | n | E/cal |
|---|---|---|---|---|---|---|---|
| R1 | 5.33 × 10−2 | 3.799 | 22,117.3 | R2 | 1.64 × 102 | 2.664 | 18,747.0 |
| R3 | 1.78 × 101 | 2.846 | 18,042.8 | R4 | 1.35 × 102 | 2.591 | 21,383.4 |
| R5 | 2.94 × 10−1 | 3.635 | 22,149.9 | R6 | 1.41 × 103 | 2.462 | 18,377.6 |
| R7 | 1.32 × 102 | 2.671 | 18,181.3 | R8 | 6.80 × 102 | 2.483 | 21,061.5 |
| R9 | 1.96 × 100 | 3.312 | 20,018.2 | R10 | 3.02 × 103 | 2.324 | 17,491.7 |
| R11 | 2.42 × 102 | 2.531 | 17,444.0 | R12 | 5.55 × 10−2 | 3.632 | 19,672.0 |
| R13 | 7.97 × 10−2 | 3.646 | 22,334.5 | R14 | 3.24 × 102 | 2.597 | 18,408.8 |
| R15 | 3.09 × 101 | 2.775 | 18,135.4 | R16 | 8.39 × 101 | 2.587 | 22,226.7 |
| R17 | 1.79 × 10−3 | 4.567 | 21,782.5 | R18 | 8.39 × 100 | 3.491 | 17,772.9 |
| R19 | 5.43 × 10−1 | 3.652 | 17,851.8 | R20 | 3.05 × 100 | 3.417 | 21,924.4 |
| R21 | 1.79 × 10−6 | 5.107 | 24,557.6 | R22 | 1.28 × 10−2 | 4.163 | 24,748.5 |
| R23 | 9.09 × 103 | 2.460 | 22,315.8 | R24 | 2.27 × 101 | 3.232 | 25,350.7 |
| R25 | 1.93 × 10−2 | 4.092 | 23,930.3 | R26 | 5.71 × 103 | 2.468 | 22,199.5 |
| R27 | 1.28 × 102 | 2.985 | 25,810.1 | R28 | 6.52 × 10−4 | 4.476 | 24,158.1 |
| R29 | 2.33 × 103 | 2.549 | 21,565.1 | R30 | 3.26 × 101 | 3.099 | 24,217.7 |
| R31 | 8.63 × 10−3 | 4.191 | 23,689.1 | R32 | 5.64 × 102 | 2.594 | 21,947.8 |
| R33 | 1.10 × 102 | 3.020 | 25,874.7 | R34 | 3.06 × 102 | 2.614 | 20,375.1 |
| R35 | 9.95 × 10−2 | 3.881 | 24,411.8 | R36 | 2.16 × 10−3 | 4.345 | 24,748.4 |
| R37 | 1.40 × 103 | 2.593 | 22,767.5 | R38 | 1.24 × 101 | 3.199 | 26,173.7 |
| R39 | 1.53 × 103 | 2.575 | 22,879.6 | R40 | 7.11 × 10−3 | 4.224 | 23,899.1 |
| R41 | 1.46 × 103 | 2.616 | 21,608.2 | R42 | 2.15 × 101 | 3.188 | 24,879.0 |
| R43 | 1.42 × 103 | 2.618 | 21,327.4 | R44 | 6.90 × 10−3 | 4.246 | 23,479.5 |
| R45 | 8.61 × 102 | 2.675 | 21,234.0 | R46 | 1.07 × 101 | 3.279 | 24,422.2 |
| R47 | 1.03 × 103 | 2.693 | 21,021.8 | R48 | 8.81 × 10−3 | 4.287 | 23,754.1 |
| R49 | 4.16 × 103 | 2.529 | 21,630.1 | R50 | 2.76 × 101 | 3.146 | 24,944.7 |
| R51 | 3.97 × 103 | 2.606 | 21,250.6 | R52 | 1.14 × 10−1 | 3.874 | 24,261.3 |
| R53 | 2.17 × 10−3 | 4.296 | 24,899.8 | R54 | 2.67 × 103 | 2.506 | 21,271.5 |
| R55 | 7.81 × 102 | 2.629 | 22,611.7 | R56 | 5.06 × 10−1 | 3.661 | 24,562.7 |
| R57 | 5.26 × 10−3 | 4.226 | 24,725.1 | R58 | 3.81 × 103 | 2.466 | 20,956.4 |
| R59 | 3.00 × 103 | 2.527 | 22,605.4 | R60 | 1.53 × 100 | 3.213 | 22,847.1 |
| R61 | 5.90 × 100 | 3.338 | 23,189.0 | R62 | 1.56 × 10−4 | 4.695 | 25,028.5 |
| R63 | 7.71 × 10−4 | 4.479 | 24,164.7 | R64 | 1.89 × 102 | 2.850 | 22,302.2 |
| R65 | 1.70 × 10−4 | 4.684 | 25,052.7 | R66 | 2.18 × 101 | 3.209 | 22,853.4 |
| R67 | 6.69 × 10−3 | 4.243 | 24,031.6 | R68 | 3.63 × 10−3 | 4.312 | 24,012.9 |
| R69 | 7.68 × 102 | 2.683 | 22,153.3 | R70 | 1.28 × 101 | 3.301 | 25,423.8 |
| R71 | 7.01 × 100 | 3.293 | 23,179.9 | R72 | 5.85 × 10−4 | 4.525 | 25,199.4 |
| R73 | 3.05 × 10−4 | 4.555 | 23,856.8 | R74 | 4.22 × 102 | 2.780 | 22,153.4 |
| R75 | 2.02 × 102 | 2.922 | 23,838.1 | R76 | 5.67 × 10−4 | 4.503 | 25,440.5 |
| R77 | 1.18 × 10−3 | 4.424 | 22,862.0 | R78 | 7.15 × 102 | 2.700 | 23,045.3 |
| R79 | 1.54 × 10−4 | 4.654 | 25,172.1 | R80 | 3.27 × 102 | 2.769 | 22,732.4 |
| R81 | 2.07 × 100 | 3.452 | 25,812.8 | R82 | 6.22 × 10−6 | 4.923 | 21,781.1 |
| R83 | 2.26 × 10−2 | 3.812 | 24,907.6 | R84 | 7.38 × 100 | 3.030 | 28,626.4 |
| R85 | 3.53 × 101 | 3.081 | 24,096.7 | R86 | 3.86 × 102 | 2.742 | 21,915.3 |
| R87 | 4.97 × 10−4 | 4.509 | 24,852.8 | R88 | 1.93 × 101 | 2.904 | 25,569.5 |
| R89 | 1.63 × 101 | 3.148 | 24,139.8 | R90 | 6.56 × 102 | 2.695 | 21,798.9 |
| R91 | 2.32 × 10−3 | 4.337 | 23,824.0 |
| Reaction Type | A | E (Cal) | Uncertainty |
|---|---|---|---|
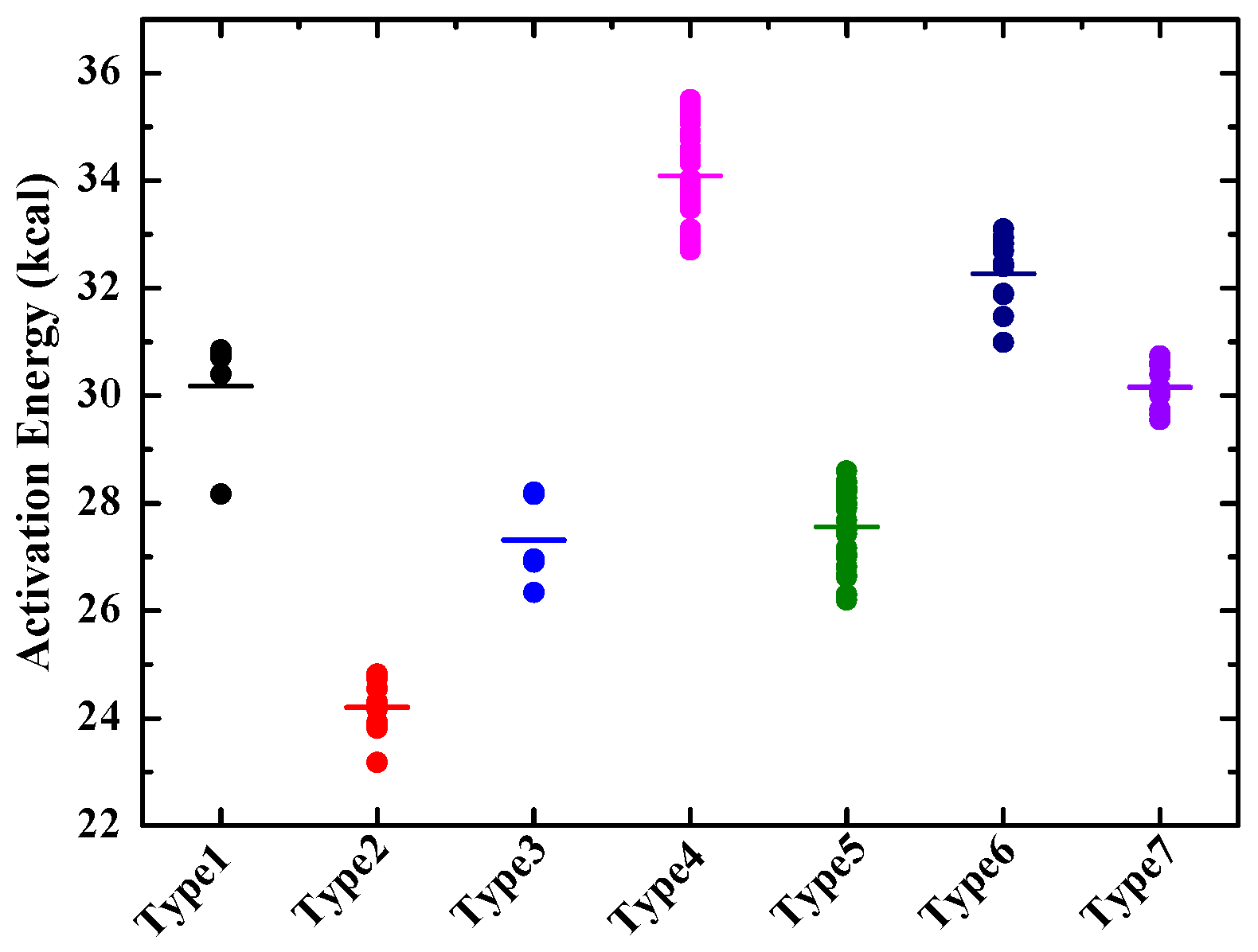
| 1,4-H transfer from the ring to the side chain (type one) | 1.08 × 1012 | 29,575.5 | 5 |
| 1,5-/1,6-H transfer from the ring to the side chain (type two) | 3.86 × 1011 | 23,790.6 | 5 |
| 1,7-H transfer from the ring to the side chain (type three) | 2.18 × 1011 | 27,280.7 | 2.5 |
| 1,4-H transfer to secondary site on the ring (type four) | 3.38 × 1012 | 33,676.1 | 5 |
| 1,5-H transfer to secondary site on the ring (type five) | 1.91 × 1012 | 27,573.3 | 4 |
| 1,6-H transfer to secondary site on the ring (type six) | 3.10 × 1012 | 32,162.0 | 3 |
| 1,5-/1,6-H transfer to secondary site on the ring (type seven) | 2.30 × 1012 | 30,377.6 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Tian, Z.; Li, J.; Yan, Y. Theoretical Investigation of Rate Rules for H-Intermigration Reactions for Cyclic Alkylperoxy Radicals. Energies 2023, 16, 2881. https://doi.org/10.3390/en16062881
Yang K, Tian Z, Li J, Yan Y. Theoretical Investigation of Rate Rules for H-Intermigration Reactions for Cyclic Alkylperoxy Radicals. Energies. 2023; 16(6):2881. https://doi.org/10.3390/en16062881
Chicago/Turabian StyleYang, Kun, Zemin Tian, Jinghua Li, and Yingwen Yan. 2023. "Theoretical Investigation of Rate Rules for H-Intermigration Reactions for Cyclic Alkylperoxy Radicals" Energies 16, no. 6: 2881. https://doi.org/10.3390/en16062881
APA StyleYang, K., Tian, Z., Li, J., & Yan, Y. (2023). Theoretical Investigation of Rate Rules for H-Intermigration Reactions for Cyclic Alkylperoxy Radicals. Energies, 16(6), 2881. https://doi.org/10.3390/en16062881





