Identification of Key Events and Emissions during Thermal Abuse Testing on NCA 18650 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
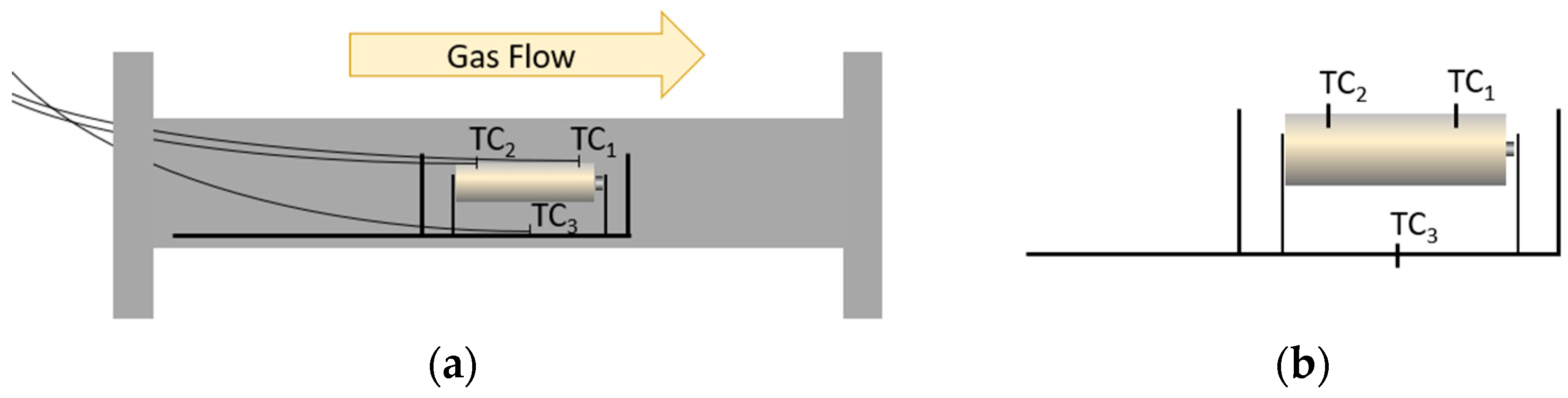
2.2. Lab-Scale Setup: Reactor, Transfer Line, and FT-IR Spectroscopy
2.3. Methods
2.3.1. Thermal Stability Test Conditions
2.3.2. Thermal Abuse Test Conditions
2.3.3. FT-IR
2.3.4. ATR-FT-IR
2.3.5. SEM-EDS
2.3.6. ICP-OES and AAS
3. Results
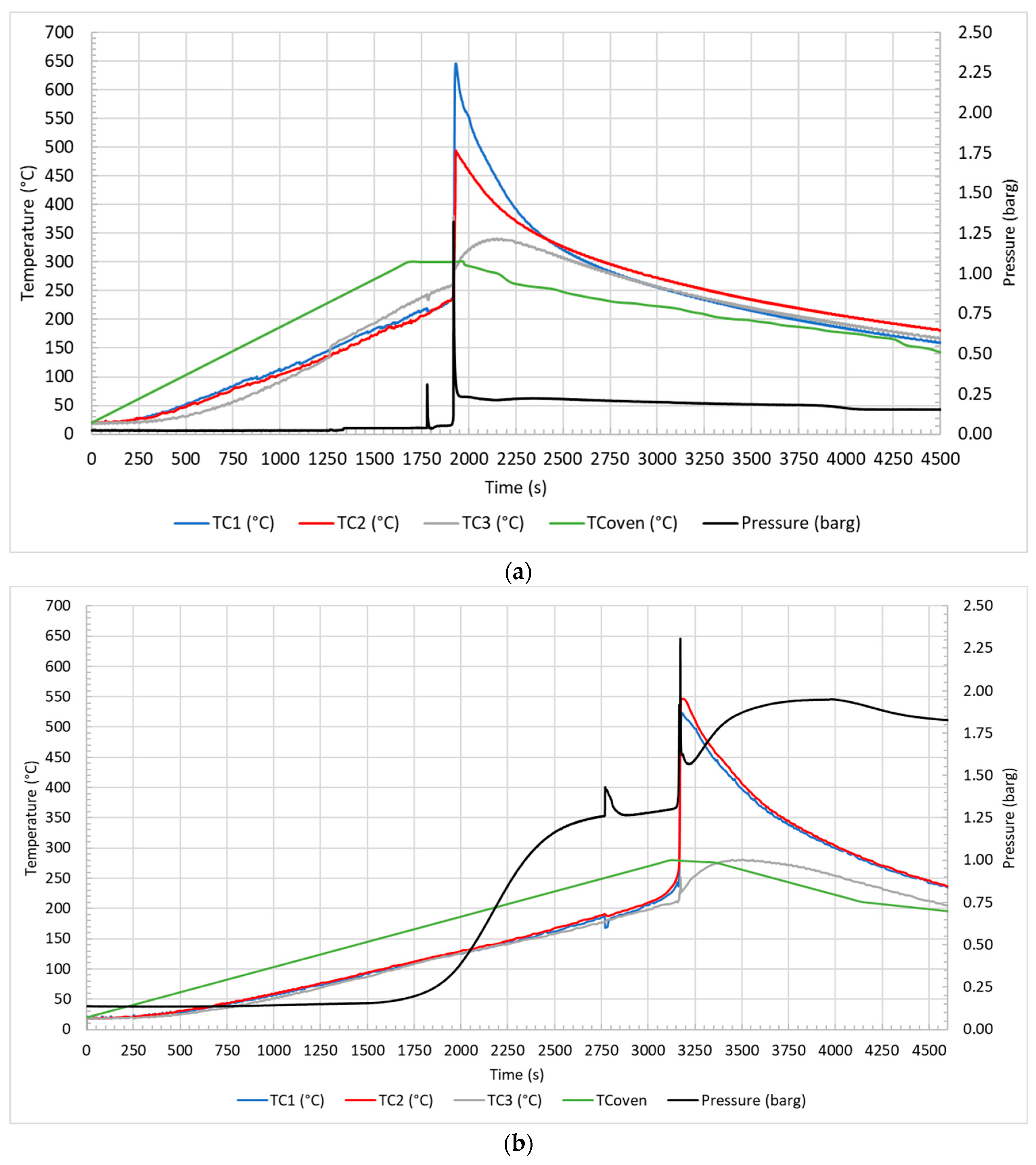
3.1. Thermal Abuse Tests: Temperature and Pressure Profile
3.2. Thermal Abuse Tests: Gas Profile and Quantification
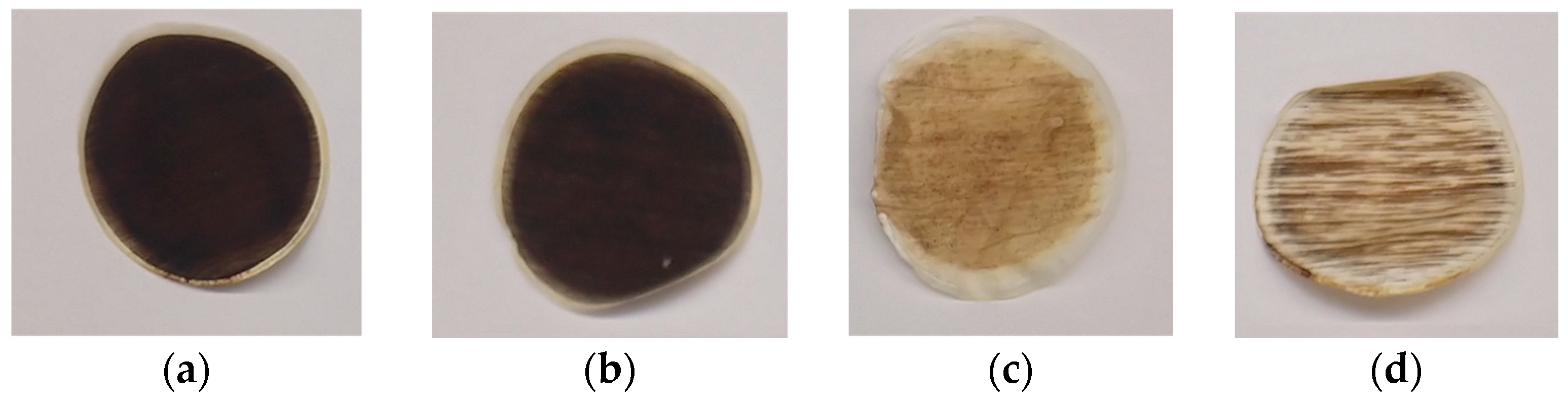
3.3. Thermal Abuse Tests: Condensed-Phase Emissions
3.3.1. Condensed-Phase Characterization: ATR-FT-IR Spectroscopy
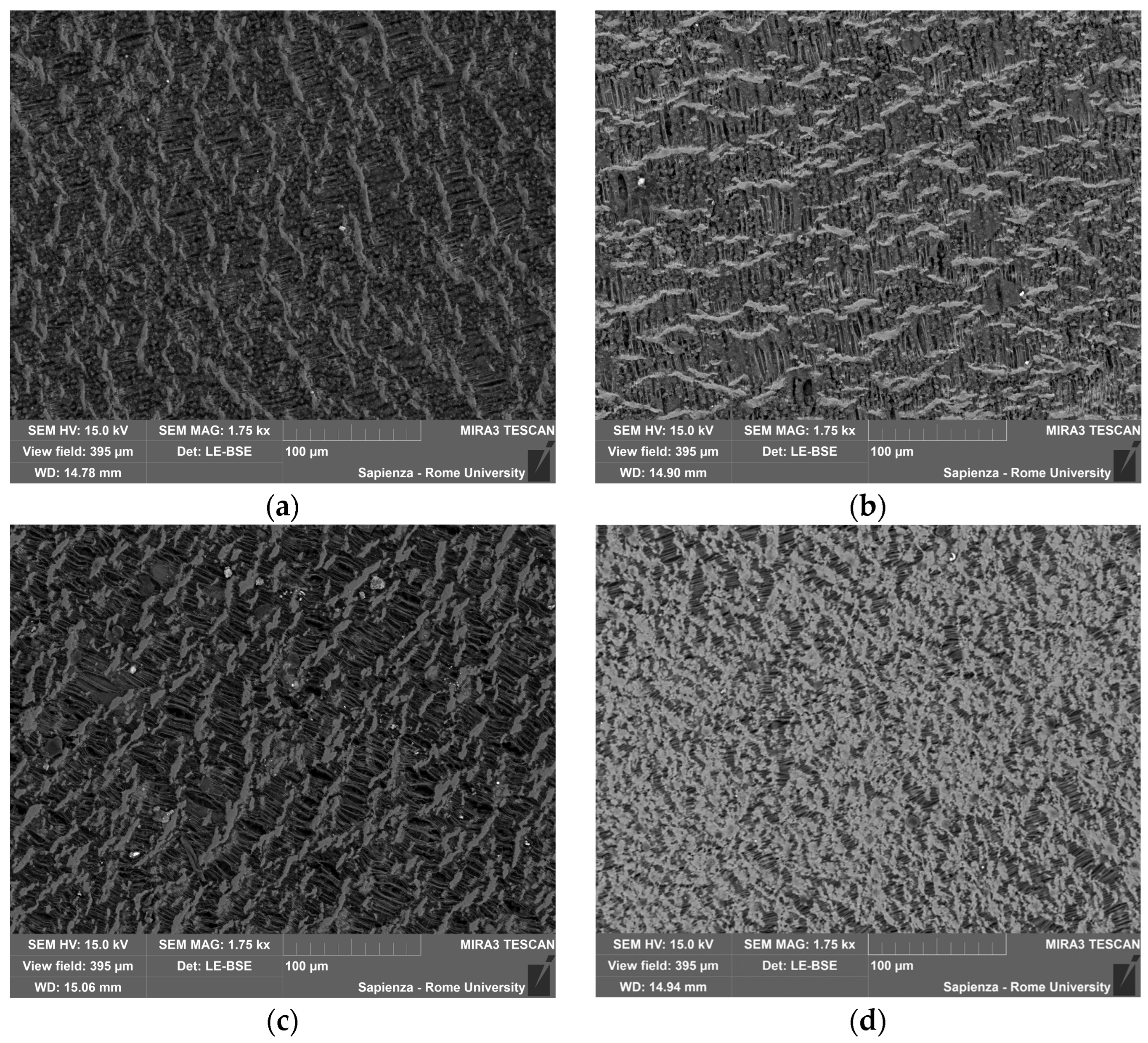
3.3.2. Condensed-Phase Characterization: SEM-EDS
3.3.3. Condensed-Phase Characterization: ICP-OES and AAS
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Armand, M.; Axmann, P.; Bresser, D.; Copley, M.; Edström, K.; Ekberg, C.; Guyomard, D.; Lestriez, B.; Novák, P.; Petranikova, M.; et al. Lithium-ion batteries—Current state of the art and anticipated developments. J. Power Spurces 2020, 479, 228708. [Google Scholar] [CrossRef]
- Huang, W.; Feng, X.; Han, X.; Zhang, W.; Jiang, F. Questions and Answers Relating to Lithium-Ion Battery Safety Issues. Cell Rep. Phys. Sci. 2021, 2, 100285. [Google Scholar] [CrossRef]
- Qiu, Y.; Jiang, F. A Review on Passive and Active Strategies of Enhancing the Safety of Lithium-Ion Batteries. Int. J. Heat Mass Transf. 2022, 184, 122288. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal Runaway Caused Fire and Explosion of Lithium Ion Battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Lopez, C.F.; Jeevarajan, J.A.; Mukherjee, P.P. Characterization of Lithium-Ion Battery Thermal Abuse Behavior Using Experimental and Computational Analysis. J. Electrochem. Soc. 2015, 162, A2163–A2173. [Google Scholar] [CrossRef]
- Xu, B.; Kong, L.; Wen, G.; Pecht, M.G. Protection Devices in Commercial 18650 Lithium-Ion Batteries. IEEE Access 2021, 9, 66687–66695. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Blomqvist, P.; Mellander, B.-E. Toxic Fluoride Gas Emissions from Lithium-Ion Battery Fires. Sci. Rep. 2017, 7, 10018. [Google Scholar] [CrossRef]
- Li, W.; Crompton, K.R.; Hacker, C.; Ostanek, J.K. Comparison of Current Interrupt Device and Vent Design for 18650 Format Lithium-Ion Battery Caps. J. Energy Storage 2020, 32, 101890. [Google Scholar] [CrossRef]
- Essl, C.; Golubkov, A.W.; Gasser, E.; Nachtnebel, M.; Zankel, A.; Ewert, E.; Fuchs, A. Comprehensive Hazard Analysis of Failing Automotive Lithium-Ion Batteries in Overtemperature Experiments. Batteries 2020, 6, 30. [Google Scholar] [CrossRef]
- Barkholtz, H.M.; Preger, Y.; Ivanov, S.; Langendorf, J.; Torres-Castro, L.; Lamb, J.; Chalamala, B.; Ferreira, S.R. Multi-Scale Thermal Stability Study of Commercial Lithium-Ion Batteries as a Function of Cathode Chemistry and State-of-Charge. J. Power Sources 2019, 435, 226777. [Google Scholar] [CrossRef]
- Doughty, D.; Roth, E.P. A General Discussion of Li Ion Battery Safety. Electrochem. Soc. Interface 2012, 21, 37. [Google Scholar] [CrossRef]
- Russo, P.; Mele, M.L. Li-Ion Batteries: Characterization of the Thermal Runaway Reactions Using a DSC. In Proceedings of the 13th International Symposium on Hazards, Prevention, and Mitigation of Industrial Explosions (ISHPMIE2020), Braunschweig, Germany, 27–31 July 2020. [Google Scholar]
- Fernandes, Y.; Bry, A.; de Persis, S. Identification and Quantification of Gases Emitted during Abuse Tests by Overcharge of a Commercial Li-Ion Battery. J. Power Sources 2018, 389, 106–119. [Google Scholar] [CrossRef]
- Ruiz, V.; Pfrang, A.; Kriston, A.; Omar, N.; Van den Bossche, P.; Boon-Brett, L. A Review of International Abuse Testing Standards and Regulations for Lithium Ion Batteries in Electric and Hybrid Electric Vehicles. Renew. Sustain. Energy Rev. 2018, 81, 1427–1452. [Google Scholar] [CrossRef]
- Doughty, D.; Crafts, C. FreedomCAR: Electrical Energy Storage System Abuse Test Manual for Electric and Hybrid Electric Vehicle Applications; SAND2005-3123, 889934; Sandia National Laboratories (SNL): Albuquerque, NM, USA; Livermore, CA, USA, 2006. [CrossRef]
- Mele, M.L.; Bracciale, M.P.; Ubaldi, S.; Santarelli, M.L.; Mazzaro, M.; Di Bari, C.; Russo, P. Thermal Abuse Tests on 18650 Li-Ion Cells Using a Cone Calorimeter and Cell Residues Analysis. Energies 2022, 15, 2628. [Google Scholar] [CrossRef]
- Russo, P.; Mele, M.L.; Longobardo, G.; Mazzaro, M.; Di Bari, C. Investigation on the Fire Hazards of Li-Ion Cells. Lect. Notes Electr. Eng. 2020, 604, 739–749. [Google Scholar] [CrossRef]
- Larsson, F.; Andersson, P.; Mellander, B.-E. Lithium-Ion Battery Aspects on Fires in Electrified Vehicles on the Basis of Experimental Abuse Tests. Batteries 2016, 2, 9. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, L.; Ju, X.; Liao, B.; Ye, K.; Li, L.; Cao, B.; Ni, Y. A Comprehensive Investigation on the Thermal and Toxic Hazards of Large Format Lithium-Ion Batteries with LiFePO4 Cathode. J. Hazard. Mater. 2020, 381, 120916. [Google Scholar] [CrossRef]
- Kriston, A.; Adanouj, I.; Ruiz, V.; Pfrang, A. Quantification and Simulation of Thermal Decomposition Reactions of Li-Ion Battery Materials by Simultaneous Thermal Analysis Coupled with Gas Analysis. J. Power Sources 2019, 435, 226774. [Google Scholar] [CrossRef]
- Diaz, F.; Wang, Y.; Weyhe, R.; Friedrich, B. Gas Generation Measurement and Evaluation during Mechanical Processing and Thermal Treatment of Spent Li-Ion Batteries. Waste Manag. 2019, 84, 102–111. [Google Scholar] [CrossRef]
- Ribière, P.; Grugeon, S.; Morcrette, M.; Boyanov, S.; Laruelle, S.; Marlair, G. Investigation on the Fire-Induced Hazards of Li-Ion Battery Cells by Fire Calorimetry. Energy Environ. Sci. 2012, 5, 5271–5280. [Google Scholar] [CrossRef]
- Andersson, P.; Blomqvist, P.; Lorén, A.; Larsson, F. Using Fourier Transform Infrared Spectroscopy to Determine Toxic Gases in Fires with Lithium-Ion Batteries: FTIR to Determine Toxic Gases. Fire Mater. 2016, 40, 999–1015. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Yan, W. Identification and Characteristic Analysis of Powder Ejected from a Lithium Ion Battery during Thermal Runaway at Elevated Temperatures. J. Hazard. Mater. 2020, 400, 123169. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef]
- Almousa, N.H.; Alotaibi, M.R.; Alsohybani, M.; Radziszewski, D.; AlNoman, S.M.; Alotaibi, B.M.; Khayyat, M.M. Paraffin Wax [As a Phase Changing Material (PCM)] Based Composites Containing Multi-Walled Carbon Nanotubes for Thermal Energy Storage (TES) Development. Crystals 2021, 11, 951. [Google Scholar] [CrossRef]
- Bergeron, C.; Perrier, E.; Potier, A.; Delmas, G. A Study of the Deformation, Network, and Aging of Polyethylene Oxide Films by Infrared Spectroscopy and Calorimetric Measurements. Int. J. Spectrosc. 2012, 2012, 432046. [Google Scholar] [CrossRef]
- Xue, Z.; Heb, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, D.; Sun, M.; Liu, L.; Hu, W.; Jiang, B.; Chu, L.; Li, M. Polyethylene Oxide as a Multifunctional Binder for High-Performance Ternary Layered Cathodes. Polymers 2021, 13, 3992. [Google Scholar] [CrossRef] [PubMed]
- Carbon Monoxide, Immediately Dangerous to Life or Heath Concentrations (IDLH). The National Institute for Occupational Safety and Health (NIOSH). Available online: https://www.cdc.gov/niosh/idlh/630080.html (accessed on 9 January 2023).
- Hydrogen Fluoride (as F), Immediately Dangerous to Life or Heath Concentrations (IDLH). The National Institute for Occupational Safety and Health (NIOSH). Available online: https://www.cdc.gov/niosh/idlh/7664393.html (accessed on 9 January 2023).
- Standard 1926.55Gases, Vapors, Fumes, Dusts, and Mists. Table 1—Permissible Exposure Limits for Airborne Contaminants; Occupational Safety and Health Administration, United States Department of Labor: Washington, DC, USA. Available online: https://www.osha.gov/laws-regs/regulations/standardnumber/1926/1926.55 (accessed on 15 March 2023).
- Liu, C.; Xu, D.; Weng, J.; Zhou, S.; Li, W.; Wan, Y.; Jiang, S.; Zhou, D.; Wang, J.; Huang, Q. Phase Change Materials Application in Battery Thermal Management System: A Review. Materials 2020, 13, 4622. [Google Scholar] [CrossRef] [PubMed]







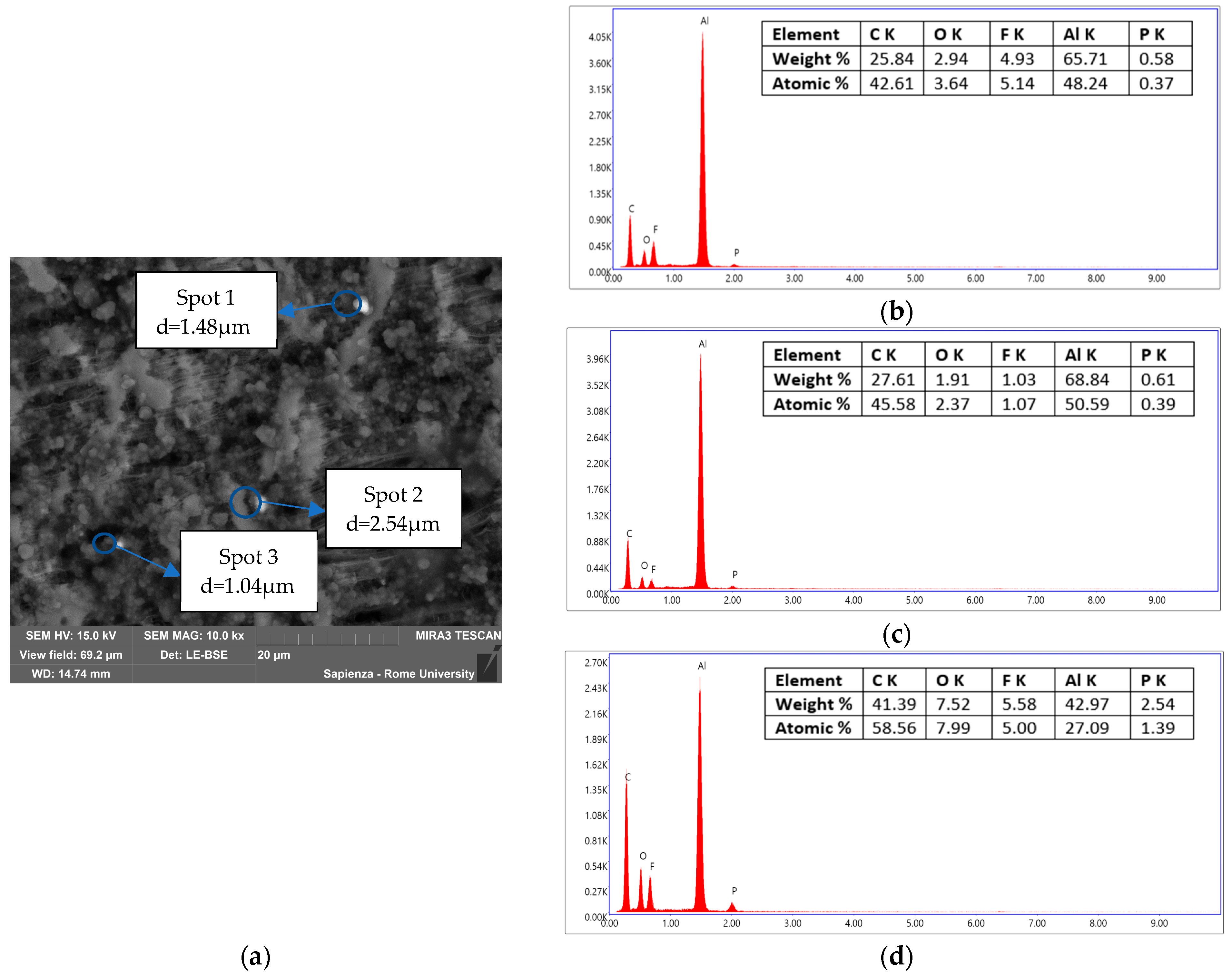
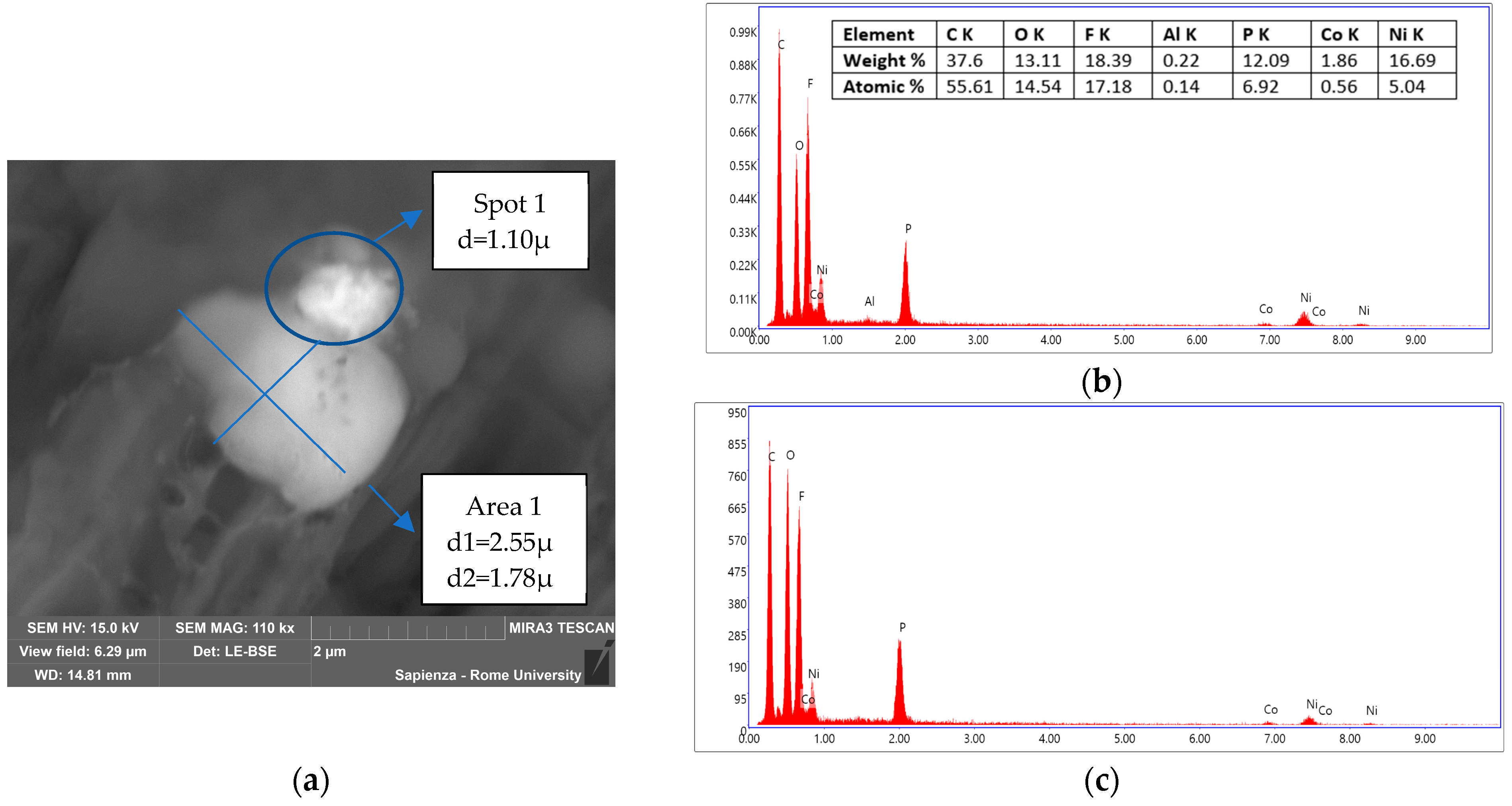
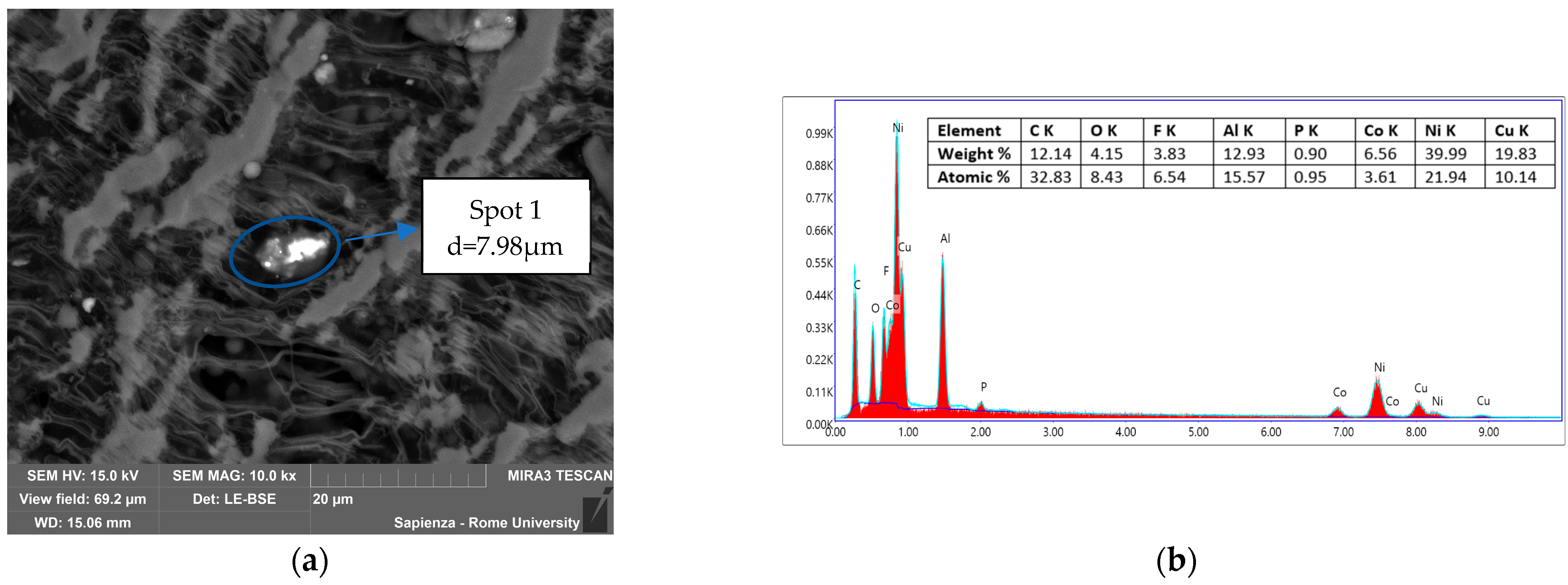
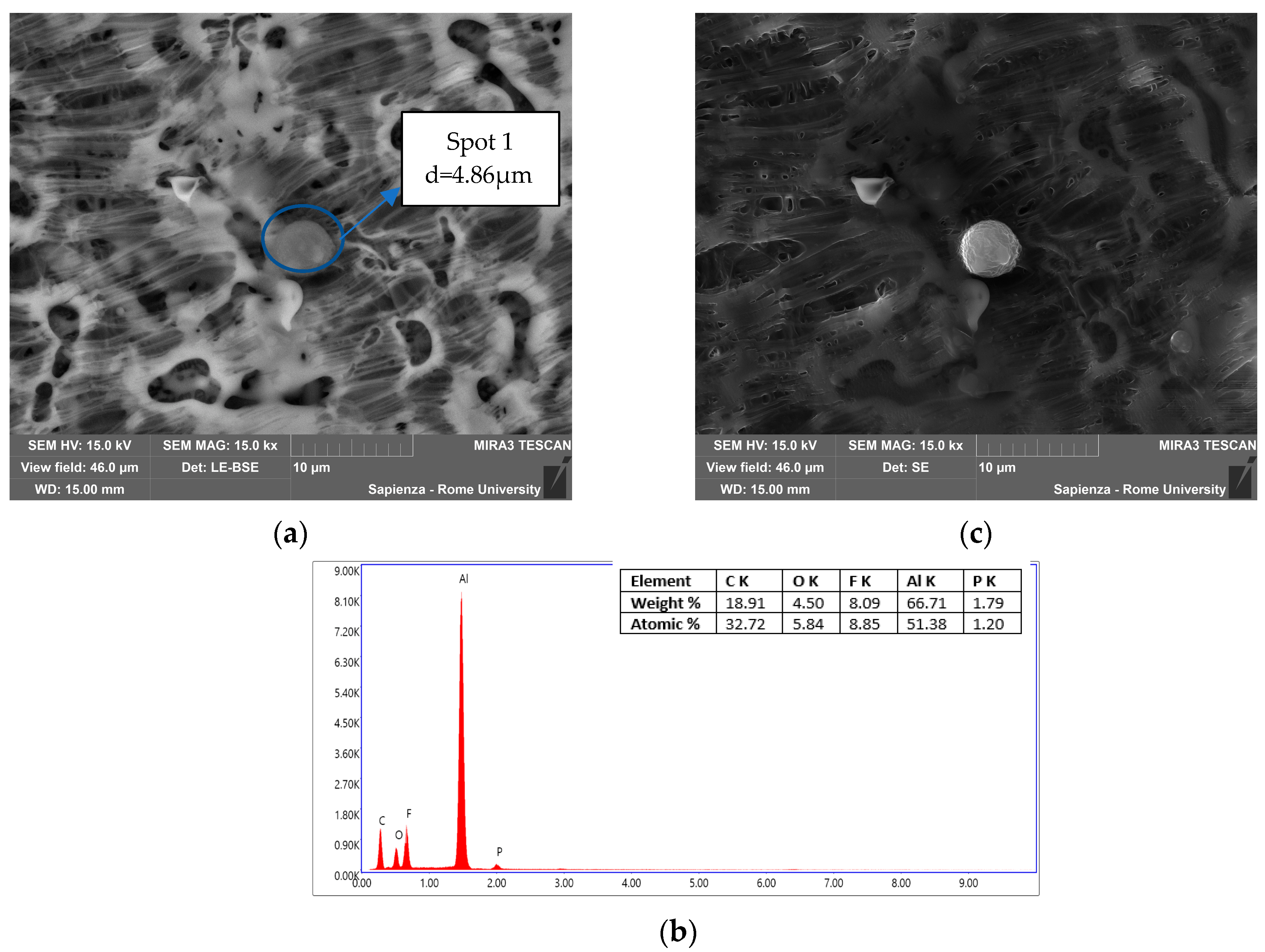

| Instrumentation | Specification |
|---|---|
| Filter unit | Temperature: 180 °C Split: 30% |
| Primary filter | PTFE (5 µm), diameter of 47 mm. |
| Secondary filter | Screen holding a filter with a fine and coarse mesh. |
| Third filter | Cylindric filter in sintered stainless steel (50 µm), diameter of 15 × 25 mm. |
| Pump parameters | Sampling flow: 150 mL/min |
| Sampling tubing | Temperature: 180 °C |
| Gas cell | Volume: 98 mL Path length: 2.0 m Temperature: 180 °C |
| Spectrometer parameters (FT-IR Spectrum 3, Perkin Elmer) | Resolution: 4 cm−1 Spectral range: 4500–400 cm−1 Scans/spectrum: 8 Detector: MCT |
| Software parameters (TimeBase, Perkin Elmer) | Run time: 200 min Delay: 10 min Data collection mode: continuous |
| Compounds | Wavenumber (cm−1) |
|---|---|
| EC | 1079, 1087, 1096, 1122, 1131, 1141, 1385, 1860, 1868, 1876, 3735 |
| DMC | 917, 925, 985, 990, 996, 1295, 1455, 1463; 1768, 1780, 2199 |
| DEC | 791; 862; 1021; 1093; 1258; 1302; 1374; 1409; 1448; 1746; 1742 |
| HF | 4172–4175 (4110); 4202–4203 |
| CO | 2115; 2173 |
| CO2 | 2343; 2360; 3731 |
| CH4 | 2989–2843; 3015; 3224–3029 (3175) |
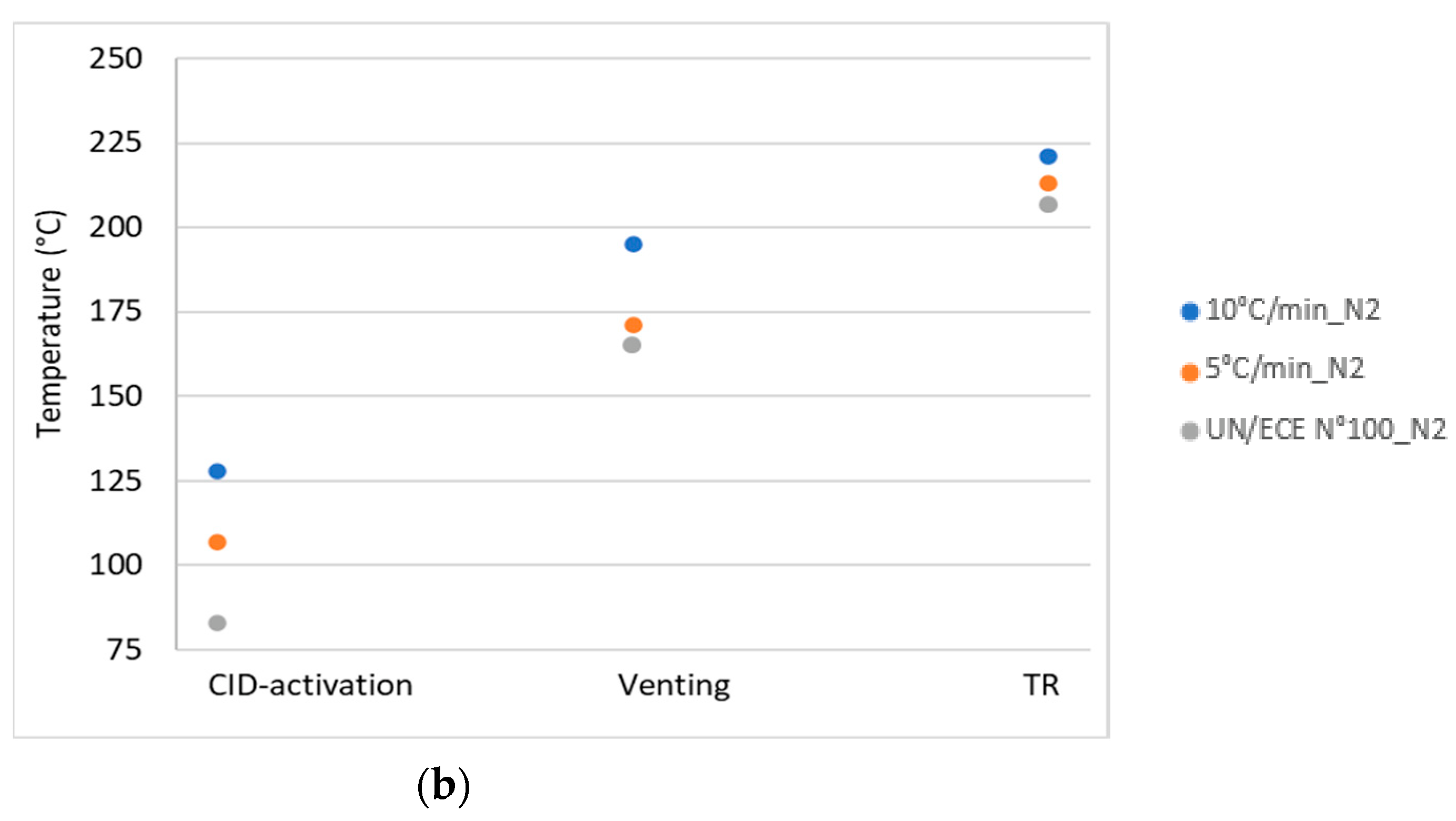
| Key Event | Air—10 °C/min | Air—5 °C/min | N2—10 °C/min | N2—5 °C/min |
|---|---|---|---|---|
| CID activation | 135 ± 7 °C | 106 ± 2 °C | 128 ± 23 °C | 107 ± 4 °C |
| 0.04 barg | 0.175 barg | 0.25 barg | 0.11 barg | |
| Venting | 212 ± 3 °C | 179 ± 16 °C | 195 ± 23 °C | 171 ± 5 °C |
| 0.31 barg | 1.43 barg | 3.70 barg | 0.34 barg | |
| Thermal runaway | 235 ± 1 °C | 230 ± 6 °C | 221 ± 11 °C | 213 ± 6 °C |
| 0.12 barg | 1.50 barg | 4.10 barg | 0.83 barg | |
| Peak | 565 ± 106 °C | 535 ± 17 °C | 497 ± 68 °C | 554 ± 11 °C |
| 1.30 barg | 2.31 barg | 5.10 barg | 1.19 barg |
| Air—10 °C/min | Air—5 °C/min | N2—10 °C/min | N2—5 °C/min | |
|---|---|---|---|---|
| Cell—total mass loss | 5.5754 g | 5.8223 g | 5.7801 g | 5.5794 g |
| Condensed matter | 0.0612 g | 0.0504 g | 0.0183 g | 0.0375 g |
| Identification | Air—10 °C/min | Air—5 °C/min | N2—10 °C/min | N2—5 °C/min | |||||
|---|---|---|---|---|---|---|---|---|---|
| Paraffin oil | s | 2951.76 | m | 2951.19 | m | 2951.70 | m | 2951.14 | m |
| Paraffin oil—PEO | vs | 2917.87 | vs | 2918.01 | vs | 2917.35 | vs | 2918.16 | vs |
| Paraffin oil—PEO | vs | 2849.63 | s | 2849.62 | s | 2849.15 | s | 2849.77 | s |
| Paraffin oil | vw | 2724.01 | vw | 2737.23 | vw | - | - | 2722.61 | vw |
| PEO | w | 1712.88 | m | 1714.37 | m | 1711.40 | w | 1713.75 | w |
| PEO | w | 1644.21 | vw | 1642.61 | vw | 1642.32 | vw | 1642.91 | vw |
| PEO | w | 1614.47 | w | - | - | - | - | 1602.36 | vw |
| Paraffin oil—PEO | s | 1461.48 | s | 1461.66 | s | 1462.57 | m | 1461.97 | m |
| Paraffin oil—PEO | m | 1376.48 | s | 1376.06 | s | 1376.72 | m | 1375.86 | m |
| Paraffin oil | w | 1273.84 | w | 1267.92 | vw | 1201.19 | s | 1212.49 | s |
| PEO | w | 1167.09 | vw | 1163.74 | m | 1145.29 | vs | 1154.36 | vs |
| Paraffin oil | vw | 1074.22 | vw | 1068.87 | vw | 1085.88 | vw | 1081.13 | vw |
| PEO | w | 908.95 | w | 909.60 | w | 909.63 | m | 909.28 | m |
| Paraffin oil | w | 887.25 | w | 886.26 | w | 885.48 | m | 887.05 | m |
| Paraffin oil | m | 730.22 | m | 729.99 | m | 729.56 | m | 730.00 | m |
| Paraffin oil—PEO | s | 719.48 | m | 719.27 | m | 719.48 | m | 719.47 | m |
| mg/m3 | Air—10 °C/min | Air—5 °C/min | N2—10 °C/min | N2—5 °C/min |
|---|---|---|---|---|
| Li | 0.04 | 0.07 | 0.17 | 0.02 |
| Ni | <LOD | 0.01 | <LOD | <LOD |
| Co | <LOD | <LOD | <LOD | <LOD |
| Mn | <LOD | <LOD | <LOD | 0.01 |
| Al | 0.14 | 0.34 | 0.20 | 0.14 |
| Cu | <LOD | <LOD | <LOD | <LOD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ubaldi, S.; Conti, M.; Marra, F.; Russo, P. Identification of Key Events and Emissions during Thermal Abuse Testing on NCA 18650 Cells. Energies 2023, 16, 3250. https://doi.org/10.3390/en16073250
Ubaldi S, Conti M, Marra F, Russo P. Identification of Key Events and Emissions during Thermal Abuse Testing on NCA 18650 Cells. Energies. 2023; 16(7):3250. https://doi.org/10.3390/en16073250
Chicago/Turabian StyleUbaldi, Sofia, Marco Conti, Francesco Marra, and Paola Russo. 2023. "Identification of Key Events and Emissions during Thermal Abuse Testing on NCA 18650 Cells" Energies 16, no. 7: 3250. https://doi.org/10.3390/en16073250
APA StyleUbaldi, S., Conti, M., Marra, F., & Russo, P. (2023). Identification of Key Events and Emissions during Thermal Abuse Testing on NCA 18650 Cells. Energies, 16(7), 3250. https://doi.org/10.3390/en16073250









