Bioflocculants as Alternative to Synthetic Polymers to Enhance Wastewater Sludge Dewaterability: A Review
Abstract
:1. Introduction
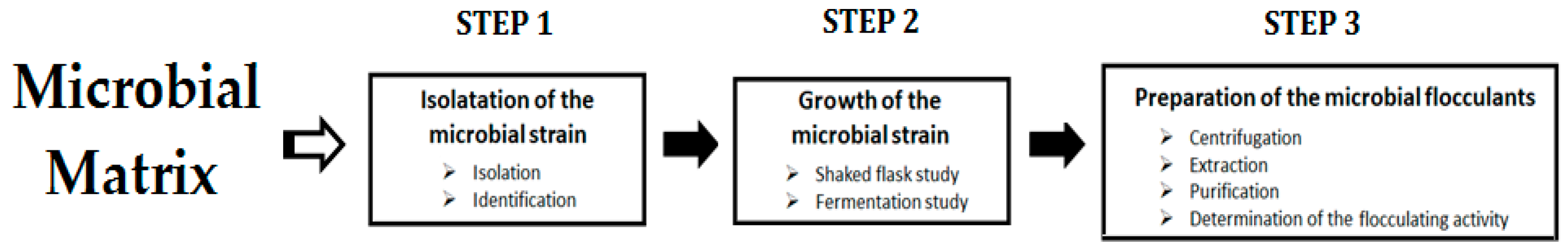
2. Microbial-Based Flocculants
3. Plant-Based Flocculants
4. Animal-Based Flocculants
5. Future Prospective of Bioflocculant for Sludge Dewatering at Large Scale
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kesari, K.K.; Soni, R.; Jamal, Q.M.S.; Tripathi, P.; Lal, J.A.; Jha, N.K.; Siddiqui, M.H.; Kumar, P.; Tripathi, V.; Ruokolainen, J. Wastewater treatment and reuse: A review of its applications and health implications. Water Air Soil Pollut. 2021, 232, 208. [Google Scholar] [CrossRef]
- Elmi, A.; AlOlayan, M. Sewage sludge land application: Balancing act between agronomic benefits and environmental concerns. J. Clean. Prod. 2020, 250, 119512. [Google Scholar] [CrossRef]
- Ekane, N.; Barquet, K.; Rosemarin, A. Resources and risks: Perceptions on the application of sewage sludge on agricultural land in Sweden, a case study. Front. Sustain. Food Syst. 2021, 5, 647780. [Google Scholar] [CrossRef]
- Anjum, M.; Al-Makishah, N.H.; Barakat, M.A. Wastewater sludge stabilization using pre-treatment methods. Process Saf. Environ. Prot. 2016, 102, 615–632. [Google Scholar] [CrossRef]
- Castellanos-Rozo, J.; Galvis-López, J.A.; Castellanos, N.A.M.; Manjarres-Hernández, E.H.; Rojas, A.L. Assessment of two sludge stabilization methods in a wastewater treatment plant in Sotaquirá, Colombia. Univ. Sci. 2020, 25, 17–36. [Google Scholar] [CrossRef]
- Roldán, M.; Bouzas, A.; Seco, A.; Mena, E.; Mayor, Á.; Barat, R. An integral approach to sludge handling in a WWTP operated for EBPR aiming phosphorus recovery: Simulation of alternatives, LCA and LCC analyses. Water Res. 2020, 175, 115647. [Google Scholar] [CrossRef]
- Flores-Alsina, X.; Ramin, E.; Ikumi, D.; Harding, T.; Batstone, D.; Brouckaert, C.; Sotemann, S.; Gernaey, K.V. Assessment of sludge management strategies in wastewater treatment systems using a plant-wide approach. Water Res. 2021, 190, 116714. [Google Scholar] [CrossRef]
- Mowla, D.; Tran, H.N.; Allen, D.G. A review of the properties of biosludge and its relevance to enhanced dewatering processes. Biomass Bioenergy 2013, 58, 365–378. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Champagne, P.; Mabee, W. Overview of current biological and thermo-chemical treatment technologies for sustainable sludge management. Waste Manag. Res. 2014, 32, 586–600. [Google Scholar] [CrossRef]
- Wu, B.; Dai, X.; Chai, X. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations. Water Res. 2020, 180, 115912. [Google Scholar] [CrossRef]
- Zhen, Z.; Jinxiang, Y.; Renhui, D. A review on the physical dewatering methods of sludge pretreatment in recent ten years. IOP Conf. Ser. Earth Environ. Sci. 2020, 455, 012189. [Google Scholar] [CrossRef]
- Wang, H.F.; Hu, H.; Wang, H.J.; Bai, Y.N.; Shen, X.F.; Zhang, W.; Zeng, R.J. Comprehensive investigation of the relationship between organic content and waste activated sludge dewaterability. J. Hazard. Mater. 2020, 394, 122547. [Google Scholar] [CrossRef]
- Xiao, K.; Li, N.; Yang, C.; Zhu, Y.; Yu, Z.; Yu, W.; Liang, S.; Hou, H.; Liu, B.; Hu, J.; et al. Deciphering the impacts of composition of extracellular polymeric substances on sludge dewaterability: An often overlooked role of amino acids. Chemosphere 2021, 284, 131297. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, T.; Lv, L.; Chen, Y.; Tang, W.; Tang, S. Destroying the structure of extracellular polymeric substance to improve the dewatering performance of waste activated sludge by ionic liquid. Water Res. 2021, 199, 117161. [Google Scholar] [CrossRef]
- Meyer, T.; Amin, P.; Allen, D.G.; Tran, H. Dewatering of pulp and paper mill biosludge and primary sludge. J. Environ. Chem. Eng. 2018, 6, 6317–6321. [Google Scholar] [CrossRef]
- Yin, X.; Han, P.; Lu, X.; Wang, Y. A review on the dewaterability of bio-sludge and ultrasound pretreatment. Ultrason. Sonochem. 2004, 11, 337–348. [Google Scholar] [CrossRef]
- Li, Y.B.; Song, J.L.; Yao, Q.J.; Chen, Z.X.; Wei, Y.; Li, H.L.; Wang, M.X.; Wang, B.J.; Zhou, J.M. Effects of dissolved oxygen on the sludge dewaterability and extracellular polymeric substances distribution by bioleaching. Chemosphere 2021, 281, 130906. [Google Scholar]
- Kang, X.; Li, C.; Ding, W.; Ma, Y.; Gao, S.; Zhou, X.; Chen, Y.; Liu, W.; Jiang, G. Optimization of operating conditions in the biological enzymes for efficient waste activated sludge dewatering. Process Saf. Environ. Prot. 2023, 170, 545–552. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, P.; Wu, Y. Enhanced technology for sewage sludge advanced dewatering from an engineering practice perspective: A review. J. Environ. Manag. 2022, 321, 115938. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, T.; Zhang, W.; Wang, D. Enhanced technology based for sewage sludge deep dewatering: A critical review. Water Res. 2021, 189, 116650. [Google Scholar] [CrossRef]
- Guo, J.; Wen, X. Performances and mechanisms of sludge dewatering by a biopolymer from piggery wastewater and application of the dewatered sludge in remediation of Cr (VI)-contaminated soil. J. Environ. Manag. 2020, 259, 109678. [Google Scholar] [CrossRef] [PubMed]
- Hyrycz, M.; Ochowiak, M.; Krupińska, A.; Włodarczak, S.; Matuszak, M. A review of flocculants as an efficient method for increasing the efficiency of municipal sludge dewatering: Mechanisms, performances, influencing factors and perspectives. Sci. Total Environ. 2022, 820, 153328. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Qin, L.; Gao, J.; Nan, R.; Gao, J. Protein extraction and sludge dewatering performance of ultrasound-assisted enzymatic hydrolysis of excess sludge. Environ. Sci. Pollut. Res. 2020, 27, 18317–18328. [Google Scholar] [CrossRef] [PubMed]
- Tunçal, T.; Mujumdar, A.S. Modern techniques for sludge dewaterability improvement. Drying Technol. 2022, 41, 339–351. [Google Scholar] [CrossRef]
- Hui, K.; Song, L.; Yin, Z.; Song, H.; Wang, Z.; Gao, W.; Xuan, L. Freeze–thaw combined with activated carbon improves electrochemical dewaterability of sludge: Analysis of sludge floc structure and dewatering mechanism. Environ. Sci. Pollut. Res. 2022, 29, 20333–20346. [Google Scholar] [CrossRef]
- Kocbek, E.; Garcia, H.A.; Hooijmans, C.M.; Mijatović, I.; Lah, B.; Brdjanovic, D. Microwave treatment of municipal sewage sludge: Evaluation of the drying performance and energy demand of a pilot-scale microwave drying system. Sci. Total Environ. 2020, 742, 140541. [Google Scholar] [CrossRef]
- Guo, J.; Gao, Q.; Jiang, S. Insight into dewatering behavior and heavy metals transformation during waste activated sludge treatment by thermally-activated sodium persulfate oxidation combined with a skeleton builder—Wheat straw biochar. Chemosphere 2020, 252, 126542. [Google Scholar] [CrossRef]
- Huang, J.; Liang, J.; Yang, X.; Zhou, J.; Liao, X.; Li, S.; Zheng, L.; Sun, S. Ultrasonic coupled bioleaching pretreatment for enhancing sewage sludge dewatering: Simultaneously mitigating antibiotic resistant genes and changing microbial communities. Ecotoxicol. Environ. Saf. 2020, 193, 110349. [Google Scholar] [CrossRef]
- Lin, W.; Liu, X.; Ding, A.; Ngo, H.H.; Zhang, R.; Nan, J.; Ma, J.; Li, G. Advanced oxidation processes (AOPs)-based sludge conditioning for enhanced sludge dewatering and micropollutants removal: A critical review. J. Water Process Eng. 2022, 45, 102468. [Google Scholar] [CrossRef]
- Ge, D.; Wu, W.; Li, G.; Wang, Y.; Li, G.; Dong, Y.; Yuan, H.; Zhu, N. Application of CaO2-enhanced peroxone process to adjust waste activated sludge characteristics for dewaterability amelioration: Molecular transformation of dissolved organic matters and realized mechanism of deep-dewatering. Chem. Eng. J. 2022, 437, 135306. [Google Scholar] [CrossRef]
- Chen, N.; Tao, S.; Xiao, K.; Liang, S.; Yang, J.; Zhang, L. A one-step acidification strategy for sewage sludge dewatering with oxalic acid. Chemosphere 2020, 238, 124598. [Google Scholar] [CrossRef]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/flocculation in dewatering of sludge: A review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef]
- Ma, C.X.; Pei, H.Y.; Hu, W.Y.; Cheng, J.; Xu, H.Z.; Jin, Y. Significantly enhanced dewatering performance of drinking water sludge from a coagulation process using a novel chitosanealuminum chloride composite coagulant in the treatment of cyanobacteria-laden source water. RSC Adv. 2016, 6, 61047–61056. [Google Scholar] [CrossRef]
- Mahmoud, A.; Hoadley, A.F.A.; Citeau, M.; Sorbet, J.M.; Olivier, G.; Vaxelaire, J.; Olivier, J. A comparative study of electro-dewatering process performance for activated and digested wastewater sludge. Water Res. 2018, 129, 66–82. [Google Scholar] [CrossRef]
- Dao, V.H.; Cameron, N.R.; Saito, K. Synthesis, properties and performance of organic olymers employed in flocculation applications. Polym. Chem. 2016, 7, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosanbased flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, K.; Guibal, E.; Jia, S.; Shen, J.; Zhang, X.; Yang, W. Removal of trace nonylphenol from water in the coexistence of suspended inorganic particles and NOMs by using a cellulosebased flocculant. Chemosphere 2016, 161, 482–490. [Google Scholar] [CrossRef]
- Wang, J.P.; Yuan, S.J.; Wang, Y.; Yu, H.Q. Synthesis, characterization and application of a novel starch-based flocculant with high flocculation and dewatering properties. Water Res. 2013, 47, 2643–2648. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.J.; Wang, D.S.; Ma, T.; Bai, R.Y. Enhancement of activated sludge dewatering performance by combined composite enzymatic lysis and chemical re-flocculation with inorganic coagulants: Kinetics of enzymatic reaction and re-flocculation morphology. Water Res. 2015, 83, 367–376. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, H.L.; Guan, Q.Q.; Teng, H.K.; Zhao, C.L.; Zhao, C. Fabricating a flocculant with controllable cationic microblock structure: Characterization and sludge conditioning behavior evaluation. Ind. Eng. Chem. Res. 2016, 55, 2892–2902. [Google Scholar] [CrossRef]
- Cao, B.D.; Zhang, W.J.; Wang, Q.D.; Huang, Y.R.; Meng, C.R.; Wang, D.S. Wastewater sludge dewaterability enhancement using hydroxyl aluminum conditioning: 478 Role of aluminum speciation. Water Res. 2016, 105, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Kameswari, K.S.B.; Kalyanaraman, C.; Varma, V.S.; Porselvam, S.; Thanasekaran, K. Significance of chemical conditioning to improve dewaterability of biosludge generated from tanneries. Clean Technol. Environ. Policy 2013, 15, 945–953. [Google Scholar] [CrossRef]
- Mortula, M.; Bard, S.M.; Walsh, M.E.; Gagnon, G.A. Aluminum toxicity and ecological risk assessment of dried alum residual into surface water disposal. Can. J. Civ. Eng. 2009, 36, 127–136. [Google Scholar] [CrossRef]
- Exley, C.; Clarkson, E. Aluminium in human brain tissue from donors without neurodegenerative disease: A comparison with Alzheimer’s disease, multiple sclerosis and autism. Sci. Rep. 2020, 10, 7770. [Google Scholar] [CrossRef] [PubMed]
- Krupińska, I. Aluminium drinking water treatment residuals and their toxic impact on human health. Molecules 2020, 25, 641. [Google Scholar] [CrossRef]
- Li, R.; Gao, B.; Huang, X.; Dong, H.; Li, X.; Yue, Q.; Wang, Y.; Li, Q. Bioresource Technology Compound bioflocculant and polyaluminum chloride in kaolin-humic acid coagulation: Factors influencing coagulation performance and floc characteristics. Bioresour. Technol. 2014, 172, 8–15. [Google Scholar] [CrossRef]
- Bo, X.; Gao, B.; Peng, N.; Wang, Y.; Yue, Q.; Zhao, Y. Effect of dosing sequence and solution pH on floc properties of the compound bioflocculant-aluminum sulfate dual-coagulant in kaolin-humic acid solution treatment. Bioresour. Technol. 2012, 113, 89–96. [Google Scholar] [CrossRef]
- Lu, Y.; Zheng, G.Y.; Wu, W.Z.; Cui, C.H.; Zhou, L.X. Significances of deflocculated sludge flocs as well as extracellular polymeric substances in influencing the compression dewatering of chemically acidified sludge. Separ. Purif. Technol. 2017, 176, 243–251. [Google Scholar] [CrossRef]
- Novak, J.T. Dewatering of sewage sludge. Dry. Technol. 2006, 24, 1257–1262. [Google Scholar] [CrossRef]
- Pan, J.R.; Huang, C.; Cherng, M.; Li, K.C.; Lin, C.F. Correlation between dewateringindex and dewatering performance of three mechanical dewatering devices. Adv. Environ. Res. 2003, 7, 599–602. [Google Scholar] [CrossRef]
- Sawalha, O.; Scholz, M. Modeling the relationship between capillary suction time and specific resistance to filtration. J. Environ. Eng. 2010, 136, 983–991. [Google Scholar] [CrossRef]
- Scholz, M. Review of recent trends in capillary suction time (CST) dewaterability testing research. Ind. Eng. Chem. Res. 2005, 44, 8157–8163. [Google Scholar] [CrossRef]
- Yukseler, H.; Tosun, I.; Yetis, U. A new approach in assessing slurry filterability. J. Membr. Sci. 2007, 303, 72–79. [Google Scholar] [CrossRef]
- Ben Rebah, F.; Mnif, W.; Siddeeg, S.M. Microbial flocculants as an alternative to synthetic polymers for wastewater treatment: A review. Symmetry 2018, 10, 556. [Google Scholar] [CrossRef] [Green Version]
- Siddeeg, S.M.; Tahoon, M.A.; Rebah, F.B. Agro-industrial waste materials and wastewater as growth media for microbial bioflocculants production: A review. Mater. Res. Express 2019, 7, 012001. [Google Scholar] [CrossRef]
- Kurade, M.B.; Murugesan, K.; Selvam, A.; Yu, S.A.M.; Wong, J.W.C. Sludge conditioning using biogenic flocculant produced by Acidithiobacillus ferrooxidans for enhancement in dewaterability. Bioresour. Technol. 2016, 217, 179–185. [Google Scholar] [CrossRef]
- Guo, J.; Ma, J. Bioflocculant from pre-treated sludge and its applications in sludge dewatering and swine wastewater pretreatment. Bioresour. Technol. 2015, 196, 736–740. [Google Scholar] [CrossRef]
- Guo, J.; Nengzi, K.L.; Zhao, J.; Zhang, Y. Enhanced dewatering of sludge with the composite of bioflocculant MBFGA1 and P(AM-DMC) as a conditioner. Appl. Microbiol. Biotechnol. 2015, 99, 2989–2998. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Zhao, J.; Zhang, Y.; Xiao, X.; Wang, B.; Shu, B. Characterization of a bioflocculant from potato starch wastewater and its application in sludge dewatering. Appl. Microbiol. Biotechnol. 2015, 99, 5429–5437. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; Liu, Y.; Yang, Y.; Yue, D.; Wang, H. Optimized production of a novel bioflocculant M-C11 by Klebsiella sp. and its application in sludge dewatering. J. Environ. Sci. 2014, 26, 2076–2083. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Xia, S.Q.; Zhang, J. Enhanced dewatering of waste sludge with microbial flocculant TJ-F1 as a novel conditioner. Water Res. 2010, 44, 3087–3092. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.W.C.; Murugesan, K.; Yu, S.M.; Kurade, M.B.; Selvam, A. Improved dewatering of CEPT sludge by biogenic flocculant from Acidithiobacillus ferrooxidans. Water Sci. Technol. 2016, 73, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.W.C.; Murugesan, K.; Selvam, A.; Ravindran, B.; Kurade, M.B.; Yu, S.M. Dewatering of saline sewage sludge using iron-oxidizing bacteria: Effect of substrate concentration. Bioresour. Technol. 2016, 213, 31–38. [Google Scholar] [CrossRef]
- Murugesan, K.; Ravindran, B.; Selvam, A.; Kurade, M.B.; Yu, S.M.; Wong, J.W. Enhanced dewaterability of anaerobically digested sewage sludge using Acidithiobacillusferrooxidans culture as sludge conditioner. Bioresour. Technol. 2014, 169, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Kurade, M.B.; Murugesan, K.; Selvam, A.; Yu, S.M.; Wong, J.W.C. Ferric biogenic flocculant produced by Acidithiobacillus ferrooxidans enable rapid dewaterability of municipal sewage sludge: A comparison with commercial cationic polymer. Int. Biodeterior. Biodegrad. 2014, 96, 105–111. [Google Scholar] [CrossRef]
- Murugesan, K.; Selvam, A.; Wong, J.W. Flocculation and dewaterability of chemically enhanced primary treatment sludge by bioaugmentation with filamentous fungi. Bioresour. Technol. 2014, 168, 198–203. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, K.; Liao, D.X.; Li, X.M.; Wang, D.B.; Liu, X.; Zeng, G.M.; Li, X. A novel bioflocculant produced by Klebsiella sp. and its application to sludge dewatering. Water Environ. J. 2012, 26, 560–566. [Google Scholar] [CrossRef]
- Cai, G.; Ebrahimi, M.; Zheng, G.; Kaksonen, A.H.; Morris, C.; O’Hara, I.M.; Zhang, Z. Effect of ferrous iron loading on dewaterability, heavy metal removal and bacterial community of digested sludge by Acidithiobacillus ferrooxidans. J. Environ. Manag. 2021, 295, 113114. [Google Scholar] [CrossRef]
- Guo, J.; Chen, C. Sludge conditioning using the composite of a bioflocculant and PAC for enhancement in dewaterability. Chemosphere 2017, 185, 277–283. [Google Scholar] [CrossRef]
- Liu, H.; Shi, J.; Xu, X.; Zhan, X.; Fu, B.; Li, Y. Enhancement of sludge dewaterability with filamentous fungi Talaromyces flavus S1 by depletion of extracellular polymeric substances or mycelium entrapment. Bioresour. Technol. 2017, 245, 977–983. [Google Scholar] [CrossRef]
- Subramanian, S.B.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances (EPS) producing bacterial strains of municipal wastewater sludge: Isolation, molecular identification, EPS characterization and performance for sludge settling and dewatering. Water Res. 2010, 44, 2253–2266. [Google Scholar] [CrossRef]
- Liu, W.; Hao, Y.; Jiang, J.; Zhu, A.; Zhu, J.; Dong, Z. Production of a bioflocculant from Pseudomonas veronii L918 using the hydrolyzate of peanut hull and its application in the treatment of ash-flushing wastewater generated from coal fired power plant. BioresourTechnol. 2016, 218, 318. [Google Scholar] [CrossRef]
- Houghton, J.J.; Quarmby, J.; Stephenson, T. Municipal wastewater sludge dewaterability and the presence of microbial extracellular polymer. Water Sci.Technol. 2001, 44, 373–379. [Google Scholar] [CrossRef]
- Cetin, S.; Erdincler, A. The role of carbohydrate and protein parts of extracellular polymeric substances on the dewaterability of biological sludges. Water Sci. Technol. 2004, 50, 49–56. [Google Scholar] [CrossRef]
- Sponza, D.T. Extracellular polymer substances and physicochemical properties of flocs in steady and unsteady-state activated sludge systems. Process Biochem. 2002, 37, 983–998. [Google Scholar] [CrossRef]
- Faye, M.C.A.S.; Zhang, K.K.; Sun, P.; Zhang, Y. Sludge dewaterability: The variation of extracellular polymeric substances during sludge conditioning with two natural organic conditioners. J. Environ. Manag. 2019, 251, 109559. [Google Scholar] [CrossRef]
- Nkosi, N.C.; Basson, A.K.; Ntombela, Z.G.; Maliehe, T.S.; Pullabhotla, R.V.S.R. Isolation, Identification and Characterization of Bioflocculant-Producing Bacteria from Activated Sludge of Vulindlela Wastewater Treatment Plant. Appl. Microbiol. 2021, 1, 586–606. [Google Scholar] [CrossRef]
- Li, H.; Wu, S.; Du, C.; Zhong, Y.; Yang, C. Preparation, performances, and mechanisms of microbial flocculants for wastewater treatment. Int. J. Environ. Res. Public Health 2020, 17, 1360. [Google Scholar] [CrossRef] [Green Version]
- Qi, Z.; Zhu, Y.; Guo, H.; Wang, X.; Zhao, Y.; Zhou, Y.; Chen, Y.; Yang, Y.; Qin, W.; Shao, Q. Production of glycoprotein bioflocculant from untreated rice straw by a cazyme-rich bacterium, Pseudomonas sp. HP2. J. Biotechnol. 2019, 306, 185–192. [Google Scholar] [CrossRef]
- Sam, S.; Kucukasik, F.; Yenigun, O.; Nicolaus, B.; Oner, E.T.; Yukselen, M.A. Flocculating performances of exopolysaccharides produced by a halophilic bacterial strain cultivated on agro-industrial waste. Bioresour. Technol. 2011, 102, 1788–1794. [Google Scholar] [CrossRef]
- Liu, W.; Wang, K.; Li, B.; Yuan, H.; Yang, J. Production and characterization of an intracellular bioflocculant by Chryseobacterium daeguense W6 cultured in low nutrition medium. Bioresour. Technol. 2010, 101, 1044–1048. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Song, L.; Li, D.; Qiao, J.; Zhao, T.; Zhao, H. Production, characterization, and flocculation mechanism of cation independent, pH tolerant, and thermally stable bioflocculant from Enterobacter sp. ETH-2. PLoS ONE 2014, 9, e114591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Y.J.; Tian, Z.M.; Tang, W.; Li, L.; Song, L.Y.; Mcelmurry, S.P. Production and characterization of high efficiency bioflocculant isolated from Klebsiella sp. ZZ-3. Bioresour. Technol. 2014, 171, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, P.; Li, Z.; Yu, W.; Wang, Z.; Yao, H.; Wang, Y.; Li, Q.; Deng, X.; He, N. Identification of key genes involved in polysaccharide bioflocculant synthesis in Bacillus licheniformis. Biotechnol. Bioeng. 2017, 114, 645–655. [Google Scholar] [CrossRef]
- Seghosime, A.; Awudza, M.; Akpabla, J.; Richard, B. Comparative studies on proximate composition and phytochemical screening of mango, key lime, African star apple and african pear seeds as possible coagulant aids for water treatment. Am. J. Env. Sci. 2017, 13, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Buenaño, B.; Vera, E.; Aldás, M.B. Study of coagulating/flocculating characteristics of organic polymers extracted from biowaste for water treatment. Ing. Investig. 2019, 39, 24–35. [Google Scholar]
- Benalia, A.; Derbal, K.; Panico, A.; Pirozzi, F. Use of Acorn Leaves as a natural coagulant in a drinking water treatment plant. Water 2019, 11, 57. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.R.; Istalingamurthy, D.B. Natural bio-flocculant in water treatment: Investigation of the performance of cactus extracts as a natural flocculant. Int. J. Innov. Res. Sci. Eng. Technol. 2017, 5, 12609–12614. [Google Scholar]
- Zaidi, N.S. Potential of fruit peels in becoming natural coagulant for water treatment. Int. J. Integr. Eng. 2019, 11, 140–150. [Google Scholar] [CrossRef]
- Shilpaa, B.S.; Akankshaa, K.; Girish, P. Evaluation of cactus andhyacinth bean peels as natural coagulants. Int. J. Chem. Environ. Eng. 2012, 3, 189–191. [Google Scholar]
- Eichhorn, C.; Weckmüller, S.; Urban, W. Natural Flocculant from a Combination of Moringa Oleifera Seeds and Cactus Cladodes (Opuntia Ficus-Indica) to Optimize Flocculation Properties. Water 2022, 14, 3570. [Google Scholar] [CrossRef]
- Lee, C.S.; Chong, M.F.; Robinson, J.; Binner, E. Optimisation of extraction and sludge dewatering efficiencies of bio-flocculants extracted from Abelmoschus esculentus (okra). J. Environ. Manag. 2015, 157, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Jaouadi, T.; Hajji, M.; Kasmi, M.; Kallel, A.; Chatti, A.; Hamzaoui, H.; Mnif, A.; Tizaoui, C.; Trabelsi, I. Aloe sp. leaf gel and water glass for municipal wastewater sludge treatment and odour removal. Water Sci. Technol. 2020, 81, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Betatache, H.; Aouabed, A.; Drouiche, N.; Lounici, H. Conditioning of sewage sludge by prickly pear cactus (Opuntia ficus Indica) juice. Ecol. Eng. 2014, 70, 465–469. [Google Scholar] [CrossRef]
- Tat, W.K.; Idris, A.; Noor, M.J.M.M.; Mohamed, T.A.; Ghazali, A.H.; Muyibi, S.A. Optimization study on sewage sludge conditioning using Moringa oleifera seeds. Desalination Water Treat. 2010, 16, 402–410. [Google Scholar] [CrossRef]
- Wai, K.T.; Idris, A.; Johari, M.M.N.M.; Mohammad, T.A.; Ghazali, A.H.; Muyibi, S.A. Evaluation on different forms of Moringa oleifera seeds dosing on sewage sludge conditioning. Desalination Water Treat. 2009, 10, 87–94. [Google Scholar] [CrossRef]
- Muyibi, S.A.; Noor, M.J.M.M.; Ong, D.T.; Kai, K.W. Moringa oleifera seeds as a flocculant in waste sludge treatment. Int. J. Environ. Stud. 2001, 58, 185–195. [Google Scholar] [CrossRef]
- Abdulazeez, Q.M.; Jami, M.S.; Alam, M.Z.; Iwata, M. Analysis of the efficiency of sludge dewatering using Moringa oleifera as natural phytocoagulant. Int. J. Res. Chem. Metall. Civ. Eng. 2015, 2, 111–117. [Google Scholar]
- Abdulazeez, Q.M.; Jami, M.S.; Alam, M.Z. Effective sludge dewatering using moringa oleifera seed extract combined with aluminium sulfate. J. Eng. Appl. Sci. 2016, 11, 372–381. [Google Scholar]
- Ghebremichael, K.A.; Hultman, B. Alum sludge dewatering using Moringa oleifera as a conditioner. Water Air Soil Pollut. 2004, 158, 153–167. [Google Scholar] [CrossRef]
- Rebah, F.B.; Siddeeg, S.M. Cactus an eco-friendly material for wastewater treatment: A review. J. Mater. Environ. Sci. 2017, 8, 1770–1782. [Google Scholar]
- Al-Saati, N.H.A.; Hwaidi, E.H.; Jassam, S.H. Comparing cactus (Opuntia spp.) and alum as coagulants for water treatment at Al-Mashroo Canal: A case study. Int. J. Environ. Sci. Technol. 2016, 13, 2875–2882. [Google Scholar] [CrossRef]
- Hamman, J.H. Composition and applications of Aloe veraleaf gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vijayaraghavan, G.; Sivakumar, T.; Kumar, A.V. Application of plant based coagulants for waste watertreatment. Int. J. Adv. Eng. Res. Stud. 2011, 1, 88–92. [Google Scholar]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Camacho, F.P.; Sousa, V.S.; Bergamasco, R.; Teixeira, M.R. The use of Moringa oleifera as a natural coagulant in surface water treatment. Chem. Eng. J. 2017, 313, 226–237. [Google Scholar] [CrossRef]
- Shebek, K.; Schantz, A.B.; Sines, I.; Lauser, K.; Velegol, S.; Kumar, M. The flocculating cationic polypetide from Moringa oleifera seeds damages bacterial cell membranes by causing membrane fusion. Langmuir 2015, 31, 4496–4502. [Google Scholar] [CrossRef]
- Kansal, S.K.; Kumari, A. Potential of M. oleifera for the treatment of water and wastewater. Chem. Rev. 2014, 114, 4993–5010. [Google Scholar] [CrossRef]
- Ademiluyi, J.O.; Eze, R.M. Improving the sludge conditioning potential of moringa seed. Environ. Manag. 1990, 14, 125–129. [Google Scholar] [CrossRef]
- Sellami, M.; Zarai, Z.; Khadhraoui, M.; Jdidi, N.; Leduc, R.; Ben Rebah, F. Cactus juice as bioflocculant in the coagulation–flocculation process for industrial wastewater treatment: A comparative study with polyacrylamide. Water Sci. Technol. 2014, 70, 1175–1181. [Google Scholar] [CrossRef] [Green Version]
- Piazza, G.J.; Garcia, R.A. Meat and bone meal extract and gelatin as renewable flocculants. Bioresour. Technol. 2010, 101, 781–787. [Google Scholar] [CrossRef]
- Vigneshwaran, S.; Karthikeyan, P.; Sirajudheen, P.; Meenakshi, S. Optimization of sustainable chitosan/Moringa. oleifera as coagulant aid for the treatment of synthetic turbid water–A systemic study. Environ. Chem. Ecotoxicol. 2020, 2, 132–140. [Google Scholar] [CrossRef]
- Garcia, R.A.; Bumanlag, L.P.; Piazza, G.J. The relationship between extent of hemoglobin purification and the performance characteristics of a blood-based flocculant. J. Sci. Food Agric. 2017, 97, 4822–4826. [Google Scholar] [CrossRef] [Green Version]
- Piazza, G.J.; Lora, J.H.; Garcia, R.A. Flocculation of kaolin and lignin by bovine blood and hemoglobin. J. Chem. Technol. Biotechnol. 2015, 90, 1419–1425. [Google Scholar] [CrossRef]
- Garcia, R.A.; Qi, P.X.; Essandoh, M.; Bumanlag, L.P. Enhancement of protein flocculant properties through carboxyl group methylation and the relationship with protein structural changes. J. Dispers. Sci. Technol. 2021, 42, 2063–2074. [Google Scholar] [CrossRef]
- Essandoh, M.; Garcia, R.A.; Strahan, G.D. Methylation of hemoglobin to enhance flocculant performance. J. Chem. Technol. Biotechnol. 2017, 92, 2032–2037. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, H.; Li, L.; Li, D.; Wang, Q.; Xu, Q.; Wang, D. Impact of molecular structure and charge property of chitosan based polymers on flocculation conditioning of advanced anaerobically digested sludge for dewaterability improvement. Sci. Total Environ. 2019, 670, 98–109. [Google Scholar] [CrossRef]
- Lau, S.W.; Sen, T.K.; Chua, H.B.; Ang, H.M. Conditioning of synthetic sludge and anaerobically digested sludge using chitosan, organic polyelectrolytes and inorganic metal cations to enhance sludge dewaterability. Water Air Soil Pollut. 2017, 228, 358. [Google Scholar] [CrossRef]
- Ghazisaidi, H.; Garcia, R.A.; Tran, H.; Yuan, R.; Allen, D.G. Enhancing biosludge dewaterability with hemoglobin from waste blood as a bioflocculant. Polymers 2020, 12, 2755. [Google Scholar] [CrossRef]
- Kumari, S.; Kishor, R. Chitin and chitosan: Origin, properties, and applications. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. [Google Scholar]
- Chang, X.X.; Mubarak, N.M.; Mazari, S.A.; Jatoi, A.S.; Ahmad, A.; Khalid, M.; Walvekar, R.; Abdullah, E.; Karri, R.R.; Siddiqui, M.; et al. A review on the properties and applications of chitosan, cellulose and deep eutectic solvent in green chemistry. J. Ind. Eng. Chem. 2021, 104, 362–380. [Google Scholar] [CrossRef]
- Maliki, S.; Sharma, G.; Kumar, A.; Moral-Zamorano, M.; Moradi, O.; Baselga, J.; Stadler, F.J.; García-Peñas, A. Chitosan as a Tool for Sustainable Development: A Mini Review. Polymers 2022, 14, 1475. [Google Scholar] [CrossRef] [PubMed]
- Meraz, K.A.S.; Vargas, S.M.P.; Maldonado, J.T.L.; Bravo, J.M.C.; Guzman, M.T.O.; Maldonado, E.A.L. Eco-friendly innovation for nejayote coagulation–flocculation process using chitosan: Evaluation through zeta potential measurements. Chem. Eng. J. 2016, 284, 536–542. [Google Scholar] [CrossRef]
- Iber, B.T.; Okomoda, V.T.; Rozaimah, S.A.; Kasan, N.A. Eco-friendly approaches to aquaculture wastewater treatment: Assessment of natural coagulants vis-a-vis chitosan. Bioresour. Technol. Rep. 2021, 15, 100702. [Google Scholar] [CrossRef]
- Wilson, L.D. An overview of coagulation-focculation technology. Water Cond. Purif. Int. Mag. 2014, 56, 28–34. [Google Scholar]
- Christensen, M.L.; Keiding, K.; Halkj, P. Dewatering in biological wastewater treatment: A review. Water Res. 2015, 82, 14–24. [Google Scholar] [CrossRef]
- Othmani, B.; Rasteiro, M.G.; Khadhraoui, M. Toward green technology: A review on some efficient model plant-based coagulants/flocculants for freshwater and wastewater remediation. Clean Technol. Environ. Policy 2020, 22, 1025–1040. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Szopa, D.; Izydorczyk, G.; Moustakas, K.; Witek-Krowiak, A. Management of biological sewage sludge: Fertilizer nitrogen recovery as the solution to fertilizer crisis. J. Environ. Manag. 2023, 326, 116602. [Google Scholar] [CrossRef]
- Engida, T.; Mekonnen, A.; Wu, J.M.; Xu, D.; Wu, Z.B. Review paper on beverage agro-industrial wastewater treatment plant bio-sludge for fertilizer potential in Ethiopia. Appl. Ecol. Environ. Res. 2020, 18, 33–57. [Google Scholar] [CrossRef]
- Khadhraoui, M.; Sellami, M.; Zarai, Z.; Saleh, K.; Ben Rebah, F.; Roland Leduc, R. Cactus juice preparations as bioflocculant: Properties, characteristics and application. Environ. Eng. Manag. J. 2019, 18, 137–146. [Google Scholar]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Suresh, A.; Grygolowicz-Pawlak, E.; Pathak, S.; Poh, L.S.; bin Abdul Majid, M.; Dominiak, D.; Bugge, T.V.; Gao, X.; Ng, W.J. Understanding and optimization of the flocculation process in biological wastewater treatment processes: A review. Chemosphere 2018, 210, 401–416. [Google Scholar] [CrossRef]
- Bakar, S.N.H.A.; Hasan, H.A.; Abdullah, S.R.S.; Kasan, N.A.; Muhamad, M.H.; Kurniawan, S.B. A review of the production process of bacteria-based polymeric flocculants. J. Water Process Eng. 2021, 40, 101915. [Google Scholar] [CrossRef]
- Liu, C.; Sun, D.; Liu, J.; Zhu, J.; Liu, W. Recent advances and perspectives in efforts to reduce the production and application cost of microbial flocculants. Bioresour. Bioprocess. 2021, 8, 51. [Google Scholar] [CrossRef]
- Pi, S.; Qiu, J.; Li, A.; Feng, L.; Wu, D.; Zhao, H.P.; Ma, F. Applied microbiology and biotechnology uncovering the biosynthetic pathway of polysaccharide-based microbial flocculant in Agrobacterium tumefaciens F2. Appl. Microbiol. Biotechnol. 2020, 104, 8479–8488. [Google Scholar] [CrossRef]
- Patchaiyappan, A.; Devipriya, S.P. Application of plant-based natural coagulants in water treatment. In Cost Effective Technologies for Solid Waste and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 51–58. [Google Scholar]
- Keshvardoostchokami, M.; Majidi, M.; Zamani, A.; Liu, B. A review on the use of chitosan and chitosan derivatives as the bio-adsorbents for the water treatment: Removal of nitrogen-containing pollutants. Carbohydr. Polym. 2021, 273, 118625. [Google Scholar] [CrossRef]
- Liang, C.; Qi, P.X.; Garcia, R.A.; Lee, C. Molecular basis for the performance and mechanisms of methylated decolorized bovine hemoglobin flocculants. Sep. Purif. Technol. 2022, 292, 121017. [Google Scholar] [CrossRef]
- Suparmaniam, U.; Shaik, N.B.; Lam, M.K.; Lim, J.W.; Uemura, Y.; Shuit, S.H.; Show, P.L.; Tan, I.S.; Lee, K.T. Valorization of fish bone waste as novel bioflocculant for rapid microalgae harvesting: Experimental evaluation and modelling using back propagation artificial neural network. J. Water Process Eng. 2022, 47, 102808. [Google Scholar] [CrossRef]


| Crude Sludge Characteristics | Sludge Characteristics after Bioflocculation | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Sludge | pH | SRF (m/kg) | CST (s) | MC (%) | DS (%) | Flocculation Conditions | SRF (m/kg) | CST (s) | MC (%) | DS (%) | |
| Municipal anaerobically digested sludge | 6.79 | 3.29 × 1013 | 38.70 | Acidithiobacillus ferrooxidans (108 cells/mL, 30 min, 180 rpm) | 0.36 × 1013 | 10.10 | 70.30 | [56] | |||
| Commercial cationic polymer (0.2%) | 1.08 × 1013 | 16.25 | 71.20 | ||||||||
| Municipal secondary sludge | 11.30 × 1012 | 13.20 | Pre-treated sludge flocculant (1.5 g/L), pH 7.5 | 3.40 × 1012 | 22.50 | [57] | |||||
| Al2(SO4)3 (8 g/L, pH 6.5) | 4.70 × 1012 | 15.90 | |||||||||
| PAM (0.15 g/L, pH 7.5) | 3.20 × 1012 | 24.20 | |||||||||
| PAC (4 g/L, pH 7.5) | 3.80 × 1012 | 20.60 | |||||||||
| FeCl3 (8 g/L, pH 6.5) | 4.50 × 1012 | 16.40 | |||||||||
| Municipal secondary sludge | 6.50 | 11.30 × 1012 | 13.20 | Paenibacillus polymyxa flocculant (1.5 g/L, pH 7.5) | 3.60 × 1012 | 21.70 | [58] | ||||
| Secondary sludge | 11.30 × 1012 | 13.20 | Paenibacillus polymyxa flocculant (1.5 g/L, pH 7.5) | 3.90 × 1012 | 20.80 | [59] | |||||
| Secondary sludge | 11.64 × 1012 | Klebsiella pneumoniae (0.1%/wt/v) | 4.66 × 1012 | 59.97 | [60] | ||||||
| Al2(SO4)3 | 6.26 × 1012 | ||||||||||
| PAC | 5.00 × 1012 | ||||||||||
| Secondary sludge | 6.23 | 29.00 × 105 | 3.19 | Proteus mirabilis TJ-1 (7 mg) + CaCl2 (12.5 mg/g Dw), (pH 7.5) | 9.00 × 105 | [61] | |||||
| Chemically treated primary sludge | 6.20 | 71.90 × 1012 | 122.70 | 2.71 | Acidithiobacillus ferrooxidans + Fe2+ (10% v/v) | 5.00 × 1012 | 20.00 | [62] | |||
| Activated sludge | 6.70 | 10.00 × 1012 | 12.60 | 2.08 | Acidithiobacillus ferrooxidans + Fe2+ (10% v/v) | <5.00 × 1012 | 7.90 | [62] | |||
| Anaerobically digested sludge | 7.70 | 8.30 × 1012 | 19.50 | 2.10 | Acidithiobacillus ferrooxidans + Fe2+ (10% v/v) | <3.00 × 1012 | 7.50 | [63] | |||
| Anaerobically digested sludge | 7.45 | 16.10 × 1012 | 30.40 | 2.05 | Acidithiobacillus ferrooxidans + Fe2+ (10% v/v) | <1.00 × 1012 | <20 | [64] | |||
| Chemically treated primary sludge | 6.74 | 111.00 × 1012 | 121.00 | 2.59 | Acidithiobacillus ferrooxidans + Fe2+ (10% v/v) | 11.10 × 1012 | 10.00 | 31.40 | [65] | ||
| Chemically treated primary sludge | 7.03 | 86.90 | 2.00 | Filamentous fungal strains (5% w/v), pH 6.85–7.15 | 35.50 | [66] | |||||
| Secondary sludge | 8.04 | 10.87 × 1012 | 13.10 | Klebsiella sp. (6 mg/g Dw), pH 8 | 3.36 × 1012 | 17.50 | [67] | ||||
| Municipal digested sludge | 7.70 | 339.10 | 82.4 | Acidithiobacillus ferrooxidans ILS-2 + Fe2+ (15% v/v) | 31.30 | 60.10 | [68] | ||||
| Acidithiobacillus ferrooxidans ILS-2 + Fe2+ (21% v/v) | 26.20 | 48.60 | |||||||||
| Secondary activated sludge | 6.40 | 11.30 × 1012 | 12.10 | MBF10 Rhodococcus erythropolis (12 g/kg dry sludge) | 4.80 × 1012 | 19.30 | [69] | ||||
| MBF10 Rhodococcus erythropolis (10.5 g/kg + PAC (19.4 g/kg)) | 3.20 × 1012 | 23.60 | |||||||||
| Municipal activated sludge | 7.43 | 2.76 × 1012 | 21.00 | Talaromyces flavus S1 | 0.83 × 1012 | 12.40 | [70] | ||||
| Application of Abelmoschus esculentus (okra) for Kaolin Sludge Dewatering [92] | |||||||
| Flocculants | Preparation | Dosage (g/L) | SS Removal (%) | Water Recovery (%) | |||
| Aqueous bioflocculant | The pods were removed, sliced (5–10 mm cubes), ground, and extracted with water | 175.00 | >96 | 45–50 | |||
| Dried bioflocculant | The aqueous bioflocculant was dried (40 °C) | 150.00 | >96 | 30–45 | |||
| Application of Aloe vera for municipal wastewater secondary sludge (Chotrana II, Tunis, Tunisia) dewatering [93] | |||||||
| Flocculants | Preparation | Dosage (mL/L) | Turbidity removal (%) | Settling rate (%) | |||
| Aloe vera gel | Leaves washed, skin removed, and the remained filets were mixed, homogenized, and used fresh | 3.00 | 45.00 | 67.50 | |||
| Water glass | SiO2 mixed with Na2CO3 (1:1 M) at 1200 and 1300 °C | 3.00 | 89.00 | ||||
| Aloe vera gel + water glass | 78.00 | 90.00 | |||||
| Untreated sludge | 55.00 | ||||||
| Application of cactus (Opuntia ficus Indica) for municipal wastewater sludge (Beni Messous wastewater treatment plant, Algeria) dewatering [94] | |||||||
| Flocculant | Preparation | Dosage (g/Kg) | SRF (m/Kg) | DC (%) | Filrate turbidity (NTU) | ||
| Cactus juice | Cut, blended, sieved, and the obtained juice was dried (60 °C, 3 days) | 0.40 | 0.13 × 1012 | 20.50 | 2.50 | ||
| Chimfloc C4346 | 8.00 | 0.30 × 1012 | 20.50 | 1.50 | |||
| Sedipur NF 102 | 25.00 | 9.00 × 1012 | 18.50 | 13.50 | |||
| Sedipur NF 400 | 16.00 | 23.00 × 1012 | 10.00 | 5.00 | |||
| FeCl3 | 80.00 | 1.00 × 1012 | 22.00 | 2.40 | |||
| Al2(SO4)3 | 70.00 | 1.00 × 1012 | 21.50 | 2.20 | |||
| Application of Moringa oleifera for municipal wastewater sludge (sewage treatment plant, Kuala Lumpur, Malaysia) dewatering [95,96,97] | |||||||
| Flocculant | Preparation | Dosage (mg/L) | SRF reduction (%) | CST reduction (%) | Enhancement in solid content (%) | Enhancement in Settling rates (%) | |
| Seed dry powder [95] | Seeds dried (45 °C, 24–48 h), ground | 5000 | 24.00 | 93.33 | |||
| Water extract of seeds [95] | Seeds dried (45 °C, 24–48 h), ground, and the obtained powder was extracted with water and filtered (muslin cloth) | 5000 (for SRF), 7000 (for CST) | 31.20 | 92.82 | |||
| Salted water extract of seeds [95] | Seeds dried (45 °C, 24–48 h), ground, and the obtained powder was extracted with NaCl (1N) and filtered (muslin cloth) | 5000 | 10.30 | 83.33 | |||
| Seeds dry powder [96] | Seeds dried (45 °C, 24–48 h) and ground | 2000 (for SRF), 3000 (for CST and SC) | 44.44 | 17.64 | 31.56 | ||
| Water extract of seeds [96] | Seeds dried (45 °C, 24–48 h), ground, and the obtained powder was extracted with water and filtered (muslin cloth) | 4000 (for SRF), 2000 (for CST and SC) | 50.00 | 13.79 | 17.08 | ||
| Salted water extract of seeds [96] | Seeds dried (45 °C, 24–48 h), ground, and the obtained powder was extracted with NaCl (1N) and filtered (muslin cloth) | 2000 (for SRF), 4000 (for CST and SC) | 56.52 | 18.96 | 26.96 | ||
| Zetag 7653 [96] | 50 | 62.96 | 38.98 | 21.92 | |||
| Seed powder [93] | Seeds were shelled and the nuts were ground to obtain powder | 3750 | 41.17 | 66.70 | |||
| Oil extracted seeds powder [97] | Seeds were shelled and the nuts ground. The obtained powder had the oil extracted | 3750–5000 | 47.60 | ||||
| Application of Moringa oleifera for Kaolin sludge dewatering [98,99] | |||||||
| Flocculant | Preparation | Dosage (mg/L) | Vs (cm/min) | Supernatant Turbidity (NTU) | SVI (mL/g) | SRF (m/Kg) | |
| Salted water extract of seeds [94] | Seeds were ground, sieved (212 μm), defatted (hexane), and the obtained defatted powder was extracted with NaCl (1M) and filtered (filtration paper) | 462.80 | 0.93 | 67.20 | 24.70–33.50 | ||
| Salted water extract of defatted seeds [95] | Seeds were ground, sieved (212 μm), defatted (hexane), and the obtained defatted powder was extracted with NaCl (1M) and filtered (filtration paper) | 235.58 | 1.10 × 1011 | ||||
| Mixture (50:50): Alun and M. oleifera seed extract [99] | 1.08 × 1011 | ||||||
| Alun [99] | 1.08 × 1011 | ||||||
| Application of Moringa oleifera for drinking water treatment sludge (Stockholm, Sweden) dewatering [100] | |||||||
| Flocculant | Preparation | Dosage (kg/t dry solids) | SRF reduction (%) | CST reduction (%) | Cake solids (%) | ||
| Salted water extract of seeds | Seeds were shelled and the nuts were ground; the obtained powder was extracted with NaCl solution (1 M) | 125.00 | 34.75 | 57.35 | 4.50 | ||
| Alum | 63.00 | 81.08 | 69.85 | 4.76 | |||
| Praestol 2540 TR | 1.80 | 91.96 | 90.35 | 6.83 | |||
| Praestol 650 TR | 1.80 | 96.83 | 95.21 | 5.95 | |||
| Alum + salted water extract of M. oleifera seeds | 81.08 | 71.42 | 5.95 | ||||
| Application of Chitosan for Anaerobic Digested Sludge (Xiaohongmen Wastewater Treatment Plan, Beijing) Dewatering [117] | |||||
| Flocculant | Preparation | Dosage (mg/gTSS) | SRF Reduction (%) | CST Reduction (%) | Cake MC (%) |
| Chitosan | 57.98 | 83.26 | 88 | ||
| Aminated chitosan | Deacetylated chitosan (90%) is dissolved in acetic acid aqueous solution (3%), heated (30 min), followed by the addition of N2, ceric ammonium nitrate initiator (2% w/w), and dimethyl diallyl ammonium chloride monomer (reaction for 3 h), precipitation of the produced polymer (acetone), purification, and drying (60 °C, 6 h). | 35 | 88.90 | 95.60 | 84 |
| Application of chitosan for anaerobic digested sludge dewatering (Perth, Western Australia) [118] | |||||
| Description | Dosage (g/kg dry solids) | CST reduction (%) | Enhancement in cake solid content (%) | Filrate turbidity (NTU) | |
| Low MW chitosan | MW: 50,000–190,000 Da Deacetylation: >75% | 15–20 | 93–96 | 15.6–16.6 | 35.4–40.6 |
| Medium MW chitosan | MW: 190,000–310,000 Da Deacetylation: 75–85% | 83 | |||
| PAM | 43 | ||||
| EMA 8845 | 41 | ||||
| Application of hemoglobin for secondary sludge (pulp and paper mill) dewatering [119] | |||||
| Preparation | Dosage (%wt) | Enhancement in cake solid content (%) | Decrease in sludge bound water content (%) | ||
| Hemoglobin | 10 | 2.9 | |||
| Methylated hemoglobin | Lyophilized bovine hemoglobin (3% (w/v) is suspended in methanol, followed by the addition of HCl (final concentration 0.8 mol/L), agitation (48 h at room temperature), centrifugation (10,000× g, 15 min), then washing (methanol), suspension (water), and dialysis. | 10 | 47 | 17.30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mnif, W.; Ben Rebah, F. Bioflocculants as Alternative to Synthetic Polymers to Enhance Wastewater Sludge Dewaterability: A Review. Energies 2023, 16, 3392. https://doi.org/10.3390/en16083392
Mnif W, Ben Rebah F. Bioflocculants as Alternative to Synthetic Polymers to Enhance Wastewater Sludge Dewaterability: A Review. Energies. 2023; 16(8):3392. https://doi.org/10.3390/en16083392
Chicago/Turabian StyleMnif, Wissem, and Faouzi Ben Rebah. 2023. "Bioflocculants as Alternative to Synthetic Polymers to Enhance Wastewater Sludge Dewaterability: A Review" Energies 16, no. 8: 3392. https://doi.org/10.3390/en16083392
APA StyleMnif, W., & Ben Rebah, F. (2023). Bioflocculants as Alternative to Synthetic Polymers to Enhance Wastewater Sludge Dewaterability: A Review. Energies, 16(8), 3392. https://doi.org/10.3390/en16083392








